Abstract
The receptor-like cytoplasmic kinases (RLCK family VII) are required for plant defense against various pathogens. Previously, OsPBL1 (ORYZA SATIVA ARABIDOPSIS PBS1-LIKE 1) was isolated from rice as a potential RSV (rice stripe virus) resistant factor, but its physiological roles in plant defense are yet to be investigated. In this study, we demonstrated that OsPBL1increased defense against P. syringae in transgenic Arabidopsis. To ascertain the role of OsPBL1 gene in plant defense, OsPBL1 tagged with HA (i.e. Hemagglutinin) was overexpressed in Arabidopsis and examined for the resistance against Pseudomonas syringae pv. tomato DC3000 (i.e. Pst DC3000). At 3 dpi of Pst DC3000, transgenic Arabidopsis lines exhibited the reduced chlorotic lesion and propagation of P. syringae, compared to wild type. Elevated pathogen resistance of transgenic lines was correlated with increased H2O2 accumulation and callose deposition on the infected leaves. It was also revealed that expression levels of salicylic acid dependent genes such as PR1, PR2, and PR5, were induced higher in transgenic lines than wild type. Taken together, our data suggested that OsPBL1 exerted the role in defense against pathogen attacks in plant via mainly facilitating salicylic acid dependent pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plants have evolved to exert multiple layers of defense mechanisms, such as PAMP triggered immunity (PTI) and effector triggered immunity (ETI), to protect themselves against various pathogen attacks (Jones and Dangl 2006; Dodds and Rathjen 2010). In PTI, pathogen-associated molecular patterns (PAMPs) such as flagellin or fungal chitin are perceived by transmembrane pattern recognition receptors (PRRs) which in turn induce the defense signaling pathways (Zipfel et al. 2004; Wan et al. 2008). However, PTI can be suppressed by bacterial effectors that are secreted through the type III secretion system (T3SS) (He et al. 2006). Plants are also armored with counteracting mechanisms to bacterial effector intrusion, which was referred as ETI (Jones and Dangl 2006). ETI also known as ‘gene-for-gene interaction’ is activated by resistance (R)-genes that recognize pathogenic effector proteins corresponding to avirulence (avr) intruders. R-gene mediated perception of pathogenic effectors trigger local plant defenses, usually associated with programmed plant cell death, known as the hypersensitive responses (HR) (Jones and Dangl 2006). Subsequently, salicylic acid (SA) activates defense mechanisms in the surrounding of infection sites or even distal parts of the plants, leading to the development of SAR (systemic acquired resistance) (Jones and Dangl 2006; Dodds and Rathjen 2010). Based on recent systems biological analyses, PTI and ETI differ in early defense signaling and downstream molecular networks (Dong et al. 2015). However, in physiological aspects, both PTI and ETI induce similar responses such as ion fluxes, oxidative burst, callose deposition and increase in SA level, etc. (Tsuda et al. 2008; Nicaise et al. 2009).
Many receptor-like cytoplasmic kinases (RLCK family VII) have been reported to participate in plant defense mechanisms. Among them, Arabidopsis PBS1 protein (serine/threonine protein kinase, AtPBS1) recognizes the effectors intruded by pathogens which activate the defense reactions (Ade et al. 2007; Zhang et al. 2010; Qi et al. 2014). In more details, Pseudomonas syringae effector protein AvrPphB was shown to penetrate into the cytosol via the T3SS. AvrPphB is a cysteine protease that cleaves AtPBS1 at a single site within the activation loop. The truncated form of AtPBS1 interacted with RPS5 (RESISTANCE TO PSEUDOMONAS SYRINGAE5), an R-protein, and triggered HR responses and the defense pathways (Ade et al. 2007; Zhang et al. 2010; Qi et al. 2014). Two other AtPBS1-like proteins, BIK1 and PBL1, were also shown to be substrates of AvrPphB (Zhang et al. 2010). However, in contrast to AtPBS1, BIK1 and PBL1 were associated with FLS2, a pattern recognition receptor. These findings suggested that the PBS1-like protein family might be involved in PTI as well as ETI.
In rice, OsPBL1 (ORYZA SATIVA ARABIDOPSIS PBS1-LIKE 1) was recently identified by reverse genetic screening for resistant factors against rice stripe disease caused by rice stripe virus (RSV) (Lee and Kim 2015). OsPBL1 protein shared the 67 % amino acid identity with AtPBS1 and exhibited the post-translational cleavage by the treatment of SBPH [small brown plant hopper (Laodelphax striatellus)]. The expression of OsPBL1 was rapidly induced by SA treatment and also activated defensive marker genes like PR1b and PR2. The presence of OsPBL1 showed the high level of correlation with cultivar variations in RSV resistance. Considering sequence and functional similarities, OsPBL1 was suggested to regulate plant defense response via an AtPBS1-like mode of action. However, substantial roles of OsPBL1 against RSV or other pathogens are yet to be investigated. Therefore, it will be interesting to determine whether OsPBL1 positively acts in defense response in physiological level. In current study, we examined the roles of OsPBL1 against Pseudomonas syringae DC3000 via heterologous expression in Arabidopsis thaliana. The potential roles of OsPBL1 in defense responses in plants were discussed.
Materials and methods
Plant materials and growth conditions
Arabidopsis thaliana ecotype Columbia-0 (Col-0) was used as the wild type and background for transgenic lines. Seeds were surface-sterilized by exposing to ethanol (70 %) for 5 min, then sodium hypochlorite (2 %) for 7 min and washing with sterile distilled water. For germination, seeds were stratified for 2 to 3 days at 4 °C in the dark and then plated on media containing half-strength Murashige and Skoog (MS) salts with vitamin 0.02 % (w/v) (Sigma-Aldrich, Seoul, Korea), sucrose 1 % (w/v), MES 0.05 % (w/v) and plant agar 0.6 % (w/v) (Sigma-Aldrich, Seoul, Korea), adjusted to pH 5.7. Then, plates were placed at 23 °C under 12 h light/12 h dark cycle. Seven-day old seedlings were transferred to the sterilized soil (gardening soil: vermiculite: perlite = 8: 1: 1) for further growth.
Cloning of OsPBL1 and Arabidopsis transformation
The coding regions of OsPBL1 cDNAs were cloned into a binary vector pCB302ES containing a 35S CaMV promoter, nos-terminator, BASTA (i.e. Phosphinothricin) resistant gene as a selection marker, and hemagglutin (HA) epitope tag (Hwang and Sheen 2001). Then, the OsPBL1 was introduced into Arabidopsis genome using the floral-dipping method mediated by Agrobacterium tumefaciens GV3101 (Clough and Bent 1998). Homozyous T3 lines harboring OsPBL1 were screened by the presence of BASTA (Sigma-Aldrich, Seoul, Korea) and used for further experiments.
Molecular analysis of transgenic Arabidopsis
For expression analysis of transgenic Arabidopsis carrying the OsPBL1 gene, total RNA was isolated from seedlings on half strength MS media (5 day) with TRIZOL reagent (Invitrogen, San Diego, CA, USA). cDNAs were synthesized from 1.0 μg of mRNA using an ImProm-II first-strand cDNA synthesis system (Promega Corp., Madison, WI, USA) with an oligo (dT) 18 primer. An aliquot (1 μL) from cDNAs was used as template in PCR-mediated detection of transgene with the corresponding oligonucleotides (Table 1). The PCR conditions were 95 °C 5 min, once; 95 °C 45 s, 58 °C 1 min, 72 °C 1 min, 30 times; and 72 °C 7 min, once. Actin2 was used as internal control. PCR products were analyzed on agarose gels and visualized using ethidium bromide. For immunodetection of the OsPBL1-HA protein in the transgenic Arabidopsis plants, protein was extracted from rosette leaves using extraction buffer [2 % SDS, 60 mM TRIS (pH 6.8), 14.4 mM β-mercaptoethanol, 10 % glycerol, and 0.1 % (w/v) bromophenol blue]. Total protein concentration was determined using Bio-Rad protein assay reagent (Bio-Rad Laboratories, Hercules, CA, USA) and bovine serum albumin (BSA) as a standard. Fifty μg of protein extracts were separated by 10 % SDS polyacrylamide electrophoresis and transferred to PVDF transfer membrane (Amersham Biosciences, Piscataway, NJ, USA). Then, OsPBL1-HA proteins were detected using anti-HA-HRP IgG antibodies (Cell Signaling, Hitchin, UK). The protein was finally visualized using a SuperSignal® west femto maximum sensitivity substrate kit (Thermo Scientific, Waltham, USA).
Pseudomonas syringae pv. tomato DC3000 and plant inoculation
Cultures of Pseudomonas syringae pv. tomato strain DC3000 (i.e. Pst DC3000 were grown to middle to late log phase in Luria-Bertani (LB) broth supplemented with rifampicin (50 mg/ml) at 30 °C and 180 rpm. For bacterial inoculation, fully expanded healthy leaves of plants (10 days) were subjected to syringe-mediated infiltration at the abaxial side with 50 μl of Pst DC3000 (1 × 108 bacteria/ml) or 10 mM MgCl2 as mock treatment (Whalen et al. 1991).
Bacterial growth assay
The bacterial growth in infected plants was monitored within leaf tissue at 4 h post inoculation (hpi) and 3 days post inoculation (dpi). Leaves were surface sterilized with 70 % (v/v) ethanol and then washed in sterile water for 1 min before carrying out bacterial counts at each time point. The leaves were placed in 1 ml solution of 10 mM MgCl2, and were grinded to release the bacteria and plated on LB media (Katagiri et al. 2002). The plates were incubated at 30 °C for 48 h prior to counting of the colonies.
The percentage of disease area was measured at 3 dpi from digital photographs by comparing the number of brown pixels (diseased area) relative to the total number of pixels covering plant material, using Photoshop CS5.1 software. Average disease area measurements were based on 10 photographs from different seedlings and were analyzed for statistical differences by student’s t-tests.
Hydrogen peroxide detection assay
The leaves were detached from the plants at 0 hpi, 4 hpi and 3 dpi, and immersed for 2 h at room temperature in 1 mg/ml of 3,3′-diaminobenzidine (DAB, Sigma-Aldrich, St Louis, MO) (pH 3.8). Stained leaves were then bleached in acetic acid-glycerol-ethanol (1/1/3) (v/v/v) solution at 95 °C for 15 min, and then stored in glycerol-ethanol (1/4) (v/v) solution until photographs was taken.
Quantification of H2O2 contents was done following the method of Kotchoni et al. (2006). The DAB-stained leaves were ground in liquid nitrogen. The resulting powder was homogenized in 0.2 M HClO4 (1 ml), and then centrifuged for 10 min at 12,000 × g. The absorbance was immediately measured at 450 nm and compared with a standard curve containing 5 to 50 μM of H2O2 in 0.2 M HClO4-DAB. Experiments were conducted taking 10 leaves per transgenic line.
Callose deposition assay
For callose staining, leaf samples were collected at 4 hpi and 3 dpi, fixed in 3:1 ethanol-to glacial acetic acid on a shaker with several changes of fixative until leaves appeared slightly translucent. Then the leaf samples were rehydrated sequentially in 70 and 50 % ethanol solution each for over 2 h. After washing twice with water, the leaf samples were left in water overnight on a shaker. The leaf samples were then incubated in 150 mM K2HPO4 (pH 9.5) solution containing 0.01 % aniline blue for over 4 h (Weigel and Glazebrook 2002). The samples were mounted on slides with 50 % glycerol and the presence of callose was detected based on fluorescence emitted at 460 nm of wavelength under UV illumination using ZEISS Axio Scope. A1 (Carl Zeiss, Oberkochen, Germany).
Quantitative measurement of callose content was done according to method given by Shedletzky et al. (1997). Briefly, plant samples were extracted with 1 N NaOH at 80 °C. A mixture of 142 μl of aniline blue (0.1 %), 75 μl of 1 M HCl, and 210 μl of 1 M glycine/NaOH (buffer solution, pH 9.5) was added to 71 μl of the supernatant fluid. The mixture was placed in a water bath at 50 °C water for 20 min and then cooled. Fluorescence was quantified with BMG Labtech Microplate Reader (Cape cod, MA, USA). Excitation wavelength was 400 nm/slit widths 30 and emission wavelength was 460 nm/slit widths 40. Change in emission intensity of Pst DC3000 infected leaf samples over mock inoculated leaves was calculated as an index for the comparison of callose contents.
Expression profiling of pathogen responsive genes
Total RNA was isolated from leaves of Col-0 and transgenic lines at 0, 3 and 6 hpi with Pst DC3000. RNA was isolated with TRIZOL reagent (Invitrogen, San Diego, CA, USA). cDNA was synthesized from 1.0 μg of mRNA using an ImProm-II first-strand cDNA synthesis system (Promega Corp., Madison, WI, USA) with an oligo (dT) 18 primer. Real-time RT-PCR was performed with SYBR Green PCR Master Mix (Applied Biosystems, San Diego, CA) and an ABI StepOnePlus thermocycler (Applied Biosystems, San Diego, CA). Cycling conditions were: 95 °C for 10 min, followed by 40 cycles of 95 °C for 30 s, 52 °C for 25 s, and 72 °C for 30 s. Each experiment was performed three times. The primers were designed specifically for pathogenesis/defense related genes (Table 1). Actin2 transcripts were amplified as an internal control for normalization.
Results
Transgenic Arabidopsis thaliana expressing OsPBL1
To investigate the function of OsPBL1 in planta defense responses against bacterial pathogen, we first generated the transgenic Arabidopsis overexpressing OsPBL1 and selected two independent T3 homozygous lines for OsPBL1. Under normal growth condition, both line #7 and #10 exhibited the similar growth behavior to Col-0, a background wild type control (Fig. 1a). No transcript of OsPBL1 was detected in Col-0 (Fig. 1b). However, transgenic lines clearly exhibited the expression of OsPBL1. Among selected transgenic lines, line #10 exhibited higher expression level of OsPBL1 than line #7 (Fig. 1b). Both Col-0 and transgenic lines showed no significant difference in transcript levels of AtPBS1, a potential ortholog of OsPBL1 (Fig. S1). On the other hand, correlated with transcript levels, no OsPBL1-HA protein was accumulated in Col-0 background (Fig. 1c). Noticeably, OsPBL1-HA proteins were detected in transgenic lines. Among transgenic lines, the accumulation of OsPBL1-HA was higher in #10 than #7 (Fig. 1c).
Characterization of transgenic Arabidopsis thaliana expressing OsPBL1. a. Morphological phenotypes of Col-0 and OsPBL1 expressing lines. Images were taken from the seedlings grown in soil for 3 weeks. b. Steady-state levels of OsPBL1 mRNA in Col-0, and transgenic lines by RT-PCR. Total RNA was extracted from 5 day old seedlings and reverse transcribed for cDNA synthesis. The housekeeping gene Actin2 was used as internal control. Lane L, DNA size ladder. c. Immunodetection of OsPBL1-HA proteins in transgenic Arabidopsis lines. Col-0 was used as a negative control. The coomassie stained Rubisco large subunit (RBL) was indicated to reveal the equal loading of protein among lanes. Lane L, DNA size ladder
OsPBL1 conferred the increased resistance to P. syringae DC3000 in Arabidopsis
To investigate whether OsPBL1 expressing Arabidopsis altered the response to pathogenic attacks, we treated rosette leaves with Pst DC3000 by syringe-mediated infiltration. According to bacterial infection, the onset leaves of Col-0, line #7, and #10 exhibited the severe chlorotic lesions (data not shown). As the time was elapsed, the leaves were recovered from the lesion for which area varied among Arabidopsis lines. At 3 dpi, the area of the lesion was measured as 53 % of infected leave in Col-0 (Fig. 2a and b). However, the lesions of the leaves in line #7 and #10 were 46 and 43 %, respectively. On the other hand, the residual population of Pst DC3000 also varied among the Arabidopsis lines. At 3 dpi, density of Pst DC3000 in the onset leaves of Col-0 was reached to 7.6 × 104 CFU/g fresh weight (Fig. 2c). Similarly, line #7 revealed slightly reduced population of Pst DC3000 (6.1 × 104), compared to Col-0. Noticeably, however, line #10 exhibited significantly lower population of bacteria (7.0 × 103) than Col-0. Compared to Col-0, reduction rate of bacteria population in line #10 was estimated as 9.21 %.
The resistance of Arabidopsis against Pseudomonas syringae pv. tomato DC3000. a. The representative images of disease symptoms in leaves of Arabidopsis lines. Ten day-old leaves were infected by Pst DC3000 using syringe infiltration with 50 μl cell suspension (1 × 108 bacteria). The images were taken at 3 days post inoculation (dpi). b. Leaf area showing disease symptoms caused by Pst DC3000 at 3 dpi. Disease area was presented as average values (n = 10) of brown pixels in leaves relative to the number of pixels covering total leaf material. Error bars indicate the standard deviation (SD) of the means. c. Population density of Pst DC3000 in the leaves of Arabidopsis lines at 3 dpi. The numbers of colonies counted from five leaves were averaged and transformed to log scale. Statistically significant difference in population density and diseased area on leaves of transgenic lines was compared to the Col-0 (student t-test) (*, p < 0.05)
OsPBL1 facilitated the accumulation of H2O2 and callose in P. syringae infected leaves
Oxidative burst and cell wall composition changes are key signatures during host-pathogen interactions. In details, H2O2 level and callose deposition are highly correlated with the activation status of defense in plants (Zhang et al. 2004). To investigate whether OsPBL1 increased defense responses in physiological level, we primarily measured the H2O2 accumulation in infected leaves among Arabidopsis lines using DAB-dependent staining of peroxides (Fig. 3a and b). When leaves were intact without treatment of Pst DC3000, the marginal accumulation of H2O2 was detected in Col-0 (Fig. 3a and b). Interestingly, under the same condition, transgenic line #7 and #10 exhibited the higher accumulation of H2O2 than Col-0. On the other hand, when the leaves were treated with Pst DC3000, all of Arabidopsis lines exhibited the severe lesions around the position of pathogen infiltration on the leaves (Fig. 3a). Noticeably, both line #7 and #10 accumulated much higher amount of peroxide than Col-0 (Fig. 3b). Especially, in transgenic line #10 (85 μM), the production of H2O2 was 2.65 fold higher than Col-0 (32 μM) at 3 dpi.
Measurement of hydrogen peroxide (H2O2) and callose accumulation. a. The representative images of leaves stained with DAB in the absence or presence of Pst DC3000. b. The quantification of H2O2 on the infected leaves in Arabidopsis lines. Error bars indicate the standard deviation of the means (n = 10). The microscopic images of aniline blue stained leaves of Pst DC3000 untreated and treated conditions. At 4 hpi and 3 dpi of Pst DC3000 treatment, leaves were excised and stained with aniline blue. Statistically significant difference in leaves of transgenic lines was compared to the Col-0 using Student t-test (*, P < 0.05)
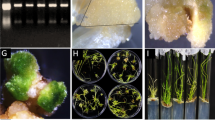
To examine the changes in cell wall composition which is a classical marker for defense response, we also compared callose deposition on the leaves infected with Pst DC3000 among Arabidopsis lines (Fig. 4a and b). In case of Col-0, according to the treatment of Pst DC3000, the accumulation of callose was increased as the function of time (Fig. 4a and b). In a similar way, both transgenic lines expressing OsPBL1 also increased callose deposition after exposure to Pst DC3000. Notably, however, the callose deposition was significantly higher in transgenic lines than Col-0 both in presence and absence of Pst DC3000 (Fig. 4b). Especially after 3 dpi, the leaves of line #10 exhibited 1.85 fold higher in callose-driven fluorescence emission, which indicated OsPBL1 conferred the more callose deposition in Arabidopsis.
Measurement of callose deposition on the leaf epidermal cells. a. Fluorescent microscopic observation of Pst DC3000 induced callose deposition in Col-0 and OsPBL1 expressing transgenic lines. At 4hpi and 3dpi, inoculated leaves were excised and stained with aniline blue. Fluorescence was observed using a ZEISS Axio Scope. A1 fluorescent microscope. Bars represent 100 μm. b. The relative comparison of callose-driven fluorescence on the infected leaves. Error bars indicated standard deviation (n = 10). Statistically significant difference in leaves of transgenic lines was compared to the Col-0 using Student t-test (*, P < 0.05)
OsPBL1 activated salicylic acid responsive genes
To systematically examine the differences in defense responses among Col-0 and OsPBL1 overexpressing transgenic lines, we investigated the expression patterns of several known marker genes for salicylic acid (SA)- and jasmonic acid (JA)- mediated defense signaling pathways (Fig. 5). PR1, PR2 and PR5 were commonly used as molecular markers for SA-dependent SAR signaling, whereas PDF1.2 often reflected the intensity of JA-dependent SAR signaling (Uknes et al. 1993; Cao et al. 1994; Seo et al. 2008). In Col-0, the expressions of PR1, PR2 and PR5 genes were maintained to be minimal in the absence of Pst DC3000 (Fig. 5a, b, and c). However, according to Pst DC3000 treatment, the expression of PR1, PR2 and PR5 were gradually increased in Col-0 at 3 and 6 hpi. OsPBL1 expressing line #7 also exhibited the similar patterns to Col-0, but with greater fold in the induction level than wild type. Surprisingly, the expression levels of PR1, PR2 and PR5 in line #10 were already activated even in the absence of Pst DC3000. However, the inductions of PR1, PR2 and PR5 in line #10 were reduced at 6 hpi (Fig. 5a, b, and c).
The expression patterns of SA-mediated and JA-mediated marker genes in Pst DC3000 treated Arabidopsis lines. The quantitative realtime RT-PCR was conducted using RNA from samples harvested at 0, 3, and 6 h post-inoculation, respectively. Target transcripts accumulation was quantified relative to that of the housekeeping gene Actin2, following △Ct method [△Ct = 2-(Ct target transcript – Ct Actin2)]. Bars indicate standard deviation (n = 3). Statistically significant differences (Student t-test) between transgenic lines and Col-0 were indicated (*, p < 0.05; **, p < 0.01)
On the other hand, the expression of PDF1.2 exhibited the quite different profiles from the PR1, PR2, and PR5 genes among Arabidopsis lines (Fig. 5d). In Col-0, the expression of PDF1.2 was greatly induced according to the treatment of Pst DC3000. However, in both line #7 and #10, the expression of PDF1.2 were minimally activated at 3 hpi and 6 hpi. Taken together, based on the expression profiles of several molecular reporters, OsPBL1 appeared to mainly facilitate the SA-dependent pathways.
Discussion
Receptor-like cytoplasmic kinases (RLCK family VII) have been reported to participate in plant defense mechanisms. Among them, Arabidopsis PBS1 and its family genes (e.g. PBL1, PBL2, BIK1) exert the critical roles both in PTI and ETI. In this study, we firstly demonstrated that OsPBL1, a rice ortholog of Arabidopsis PBS1, also conferred the increase in resistance of plants against pathogenic attacks. Transgenic Arabidopsis lines expressing OsPBL1 revealed the suppression of Pst DC3000 propagation in host plant (Fig. 2), increased hypersensitive responses (Fig. 3) and enhanced cell wall barriers by callose deposition (Fig. 4). In addition, OsPBL1 drastically induced SA-responsive marker genes like PR1, PR2, and PR5 under steady state and early time courses within 3 h of pathogen treatment (Fig. 5). Combined all, we suggested that OsPBL1 increased the innate immunity of Arabidopsis against Pst DC3000 mainly via SA dependent pathway.
Interestingly, OsPBL1 expressing lines exhibited the high level of H2O2 and callose accumulation, even without treatment of Pst DC3000 (Fig. 3). The expression of pathogen related genes were also activated in OsPBL1 lines (Fig. 4). Especially, transgenic line #10 revealed the high expressions of PR genes both bacteria-free conditions and Pst DC3000 treated conditions. Therefore, overexpression of OsPBL1 might be correlated with the increase in resistance against Pst DC3000 in onset tissues. These indicated that the defense capability of transgenic Arabidopsis was determined by the expression level of OsPBL1, further suggesting that the activity of OsPBL1 might be regulated at transcriptional or post-transcriptional levels. Physiologically, such constitutive activations of defense mechanisms and activation of PR genes in OsPBL1 expressing lines likely maintained the basal defense as high in plants (Ahmad et al. 2010).
Noticeably, however, OsPBL1 expressing lines showed the negative regulation in transcript level of PR genes at 6 hpi of Pst DC3000 (Fig. 5). The results implicated that a pathogen triggered feedback regulation restrained the prolonged activation of PR genes and possibly other defense-related responses including HR cell death in response to pathogen. Such feedback regulations were previously reported to contribute to the modulation of intensity in defense signaling and physiological adaptation processes, which might be important to minimize the lesion of programmed cell death in systemic tissues (Zhang et al. 2004). Devadas and Raina (2002) also demonstrated that the high level of basal defense or intrinsic resistance was correlated with a negative feedback loop to regulate HR-associated cell death in Arabidopsis mutant. In addition, the rapid degradation of R-protein RPS5 soon after HR initiation strongly suggested the existence of a negative feedback loop modulating the expression of PR genes (Ade et al. 2007). Nevertheless, the identification of negative regulators of OsPBL1 or its downstream components will be of interest to examine substantial roles of feedback regulation of PR genes.
Salicylic acid (SA) is the small phenolic compound critical for defense signaling and SA accumulation is induced significantly upon pathogen infection. The expression levels of PR1, PR2, and PR5 have been heavily documented to reflect the intensity of SA mediated SAR. Therefore, in our study, even though the roles of OsPBL1 were investigated only in primary tissues treated with Pst DC3000, such activations of PR genes implied that OsPBL1 could also affect the SAR mediated by SA. On the other hand, SA was known to inhibit JA dependent defense signaling pathway (Kunkel and Brooks 2002). In our study, suppressed activation of PDF1.2 in OsPBL1 expressing Arabidopsis lines (Fig. 5) supported the inhibitory interaction between SA and JA dependent pathway. Combined all, OsPBL1 might mainly act in SA dependent pathways rather than in JA-related responses.
Previously, the presence of OsPBL1 was correlated with the resistance against rice stripe disease caused by plant-hopper and RSV (Lee and Kim 2015). Even though direct function of OsPBL1 against rice stripe disease is still unknown, our data strongly suggested that OsPBL1 might regulate the RSV resistance in a similar way to the roles against PstDC3000 in transgenic Arabidopsis. In conclusion, the OsPBL1 gene is notable because it can confer broad-spectrum disease resistance against viral and bacterial pathogen. Nevertheless, the roles of OsPBL1 in RSV resistance must be investigated in near future. In addition, whether OsPBL1 behaves similarly to Arabidopsis PBS1 in defense responses such as PTI or ETI should be prompted in the mechanistic level. Well-designed physiological, genetic and molecular approaches to deepen the insight into the function of OsPBL1 will contribute to extend our knowledge for molecular basis of resistance signaling pathways against broad spectrum of diseases.
References
Ade, J., DeYoung, B. J., Golstein, C., & Innes, R. W. (2007). Indirect activation of a plant nucleotide binding site-leucine-rich repeat protein by a bacterial protease. Proceedings of National Academy of Science USA, 104, 2531–2536.
Ahmad, S., Gordon-Weeks, R., Pickett, J., & Ton, J. (2010). Natural variation in priming of basal resistance: from evolutionary origin to agricultural exploitation. Molecular Plant Pathology, 11, 817–827.
Cao, H., Bowling, S. A., Gordon, A. S., & Dong, X. (1994). Characterization of an Arabidopsis mutant that is nonresponsive to inducers of systemic acquired resistance. Plant Cell, 6, 1583–1592.
Clough, S. J., & Bent, A. F. (1998). Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant Journal, 16(6), 735–743.
Devadas, S. K., & Raina, R. (2002). Preexisting systemic acquired resistance suppresses hypersensitive response-associated cell death in Arabidopsis hrl1 mutant1. Plant Physiology, 128, 1234–1244.
Dodds, P. N., & Rathjen, J. P. (2010). Plant immunity: towards an integrated view of plant-pathogen interactions. Nature Reviews Genetics, 11, 539–548.
Dong, X., Jiang, Z., Peng, Y. L., & Zhang, Z. (2015). Revealing shared and distinct gene network organization in Arabidopsis immune responses by integrative analysis. Plant Physiology, 167, 1186–1203.
He, P., Shan, L., Lin, N. C., Martin, G. B., Kemmerling, B., Nurnberger, T., & Sheen, J. (2006). Specific bacterial suppressors of MAMP signaling upstream of MAPKKK in Arabidopsis innate immunity. Cell, 125, 563–575.
Hwang, I., & Sheen, J. (2001). Two-component circuitry in Arabidopsis cytokinin signal transduction. Nature, 413, 383–389.
Jones, J. D. G., & Dangl, J. L. (2006). The plant immune system. Nature, 444, 323–329.
Katagiri, F., Thilmony, R., & He, S. Y. (2002). The Arabidopsis thaliana – Pseudomonas syringae interaction. In E. M. Meyerowitz & C. R. Somerville (Eds.), The Arabidopsis book (pp. 1–35). Rockville: Am Soc Plant Biol.
Kotchoni, S. O., Kuhns, C., Ditzer, A., Kirch, H. H., & Bartels, D. (2006). Overexpression of different aldehyde dehydrogenase genes in Arabidopsis thaliana confers tolerance to abiotic stress and protects plants against lipid peroxidation and oxidative stress. Plant Cell and Environment, 29(6), 1033–1048.
Kunkel, B. N., & Brooks, D. M. (2002). Cross talk between signaling pathways in pathogen defense. Current Opinion in Plant Biology, 5, 325–331.
Lee, K. J., & Kim, K. (2015). The rice serine/threonine protein kinase OsPBL1 (ORYZA SATIVA ARABIDOPSIS PBS1-LIKE 1) is potentially involved in resistance to rice stripe disease. Plant Growth Regulation, 77(1), 67–75.
Nicaise, V., Roux, M., & Zipfel, C. (2009). Recent advances in PAMP-triggered immunity against bacteria: pattern recognition on receptors watch over and raise the alarm. Plant Physiology, 150, 1638–1647.
Qi, D., Dubiella, U., Kim, S. H., Sloss, D. I., Dowen, R. H., Dixon, J. E., & Innes, R. W. (2014). Recognition of the protein kinase AVRPPHB SUSCEPIBLE1 by the disease resistance protein RESISTANCE TO PSEUDOMONAS SYRINGAE5 is dependent on S-acylation and an exposed loop in AVRPPHB SUSCEPTIBLE1. Plant Physiology, 164, 340–351.
Seo, P. J., Lee, A. K., Xiang, F. N., & Park, C. M. (2008). Molecular and functional profiling of Arabidopsis pathogenesis-related genes: insights into their roles in salt response of seed germination. Plant Cell Physiology, 49, 334–344.
Shedletzky, E., Unger, C., & Delmer, D. P. (1997). A microtiter-based fluorescence assay for (1,3)-beta-glucan synthases. Analytical Biochemistry, 249, 88–93.
Tsuda, K., Sato, M., Glazebrook, J., Cohen, J. D., & Katagiri, F. (2008). Interplay between MAMP-triggered and SA-mediated defense responses. Plant Journal, 53, 763–775.
Uknes, S., Winter, A. M., Delaney, T., Vernooij, B., Morse, A., Friedrich, L., Nye, G., Potter, S., Ward, E., & Ryals, J. (1993). Biological induction of systemic acquired resistance in Arabidopsis. Molecular Plant-Microbe Interactions, 6, 692–698.
Wan, J. R., Zhang, X. C., Neece, D., Ramonell, K. M., Clough, S., Kim, S. Y., Stacey, M. G., & Stacey, G. (2008). A LysM receptor-like kinase plays a critical role in chitin signaling and fungal resistance in Arabidopsis. Plant Cell, 20, 471–481.
Weigel, D., & Glazebrook, J. (2002). Arabidopsis: A laboratory manual (p. 85). New York: Cold Spring Harbor Laboratory Press.
Whalen, M. C., Innes, R. W., Bent, A. F., & Staskawicz, B. J. (1991). Identification of Pseudomonas syringae pathogens of Arabidopsis and a bacterial locus determining avirulence on both Arabidopsis and soybean. Plant Cell, 3, 49–59.
Zhang, C., Gutsche, A. T., & Shapiro, A. D. (2004). Feedback control of the Arabidopsis hypersensitive response. Molecular Plant-Microbe Interactions, 17(4), 357–365.
Zhang, J., Li, W., Xiang, T., Liu, Z., Laluk, K., Ding, X., Zou, Y., Gao, M., Zhang, X., Chen, S., Mengiste, T., Zhang, Y., & Zhou, J. M. (2010). Receptor-like cytoplasmic kinases integrate signaling from multiple plant immune receptors and are targeted by a Pseudomonas syringae effector. Cell Host & Microbe, 7, 290–301.
Zipfel, C., Robatzek, S., Navarro, L., Oakeley, E. J., Jones, J. D. G., Felix, G., & Boller, T. (2004). Bacterial disease resistance in Arabidopsis through flagellin perception. Nature, 428, 764–767.
Acknowledgments
This research was supported by grants from the National Research Foundation of Korea, which is funded by the government (MEST) (NRF-2012R1A1A2042533, No.2011-0020202), and the Cooperative Research Program for Agriculture Science & Technology Development (PJ010042).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 34 kb)
Rights and permissions
About this article
Cite this article
Khare, E., Kim, K. & Lee, KJ. Rice OsPBL1 (ORYZA SATIVA ARABIDOPSIS PBS1-LIKE 1) enhanced defense of Arabidopsis against Pseudomonas syringae DC3000. Eur J Plant Pathol 146, 901–910 (2016). https://doi.org/10.1007/s10658-016-0968-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-016-0968-9