Abstract
Colletotrichum spp. cause two important apple diseases, i.e., bitter rot (ABR) and Glomerella leaf spot (GLS). The present study aimed to compare the development of conidial anastomosis tubes (CATs) of strains of Colletotrichum spp. originating from ABR and GLS. For that, conidia were distributed on polystyrene slides and detached apple leaves surfaces, and the development of CATs and pre-infection structures, was microscopically examined. CATs connections were quantified and categorized into three types: conidium-conidium (c-c), conidium-germ tube (c-t) and germ tube-germ tube (t-t). CATs were observed in half of the strains, and Colletotrichum nymphaeae markedly produced more c-c connections at a higher speed. The t-t connections were less often observed in all strains. CATs were also frequently found in strains of Colletotrichum fructicola from fruits, but not in those from leaves, or in Colletotrichum theobromicola. Conidial germlings that produced CATs on polystyrene also did on leaves. Nuclear transference was observed in CATs. Appressoria melanization and CATs development were found to be antagonistic processes. The possible contribution of CATs and consequences for increasing variability of Colletotrichum on apple are discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colletotrichum is one of the most important plant pathogenic genera of fungus infecting fruits and leaves of a broad range of hosts, mainly in the tropics and subtropics (Cannon et al. 2012). On apple (Malus domestica Borkh.) it can cause two distinct diseases, i.e. Apple bitter rot and Glomerella leaf spot. While the former appears in almost every country where apple is commercially grown, Glomerella leaf spot is an emerging disease still restricted to humid subtropical regions like Southern Brazil (Araújo and Stadnik, 2013a; González et al. 2006; Velho et al. 2015).
Several species belonging to Colletotrichum gloeosporioides and Colletotrichum acutatum complexes can infect apple leaves and fruits. In a recent survey, Velho et al. (2015) listed Colletotrichum fructicola and Colletotrichum nymphaeae as the most frequent species associated to apple bitter rot in Brazil, while C. fructicola and Colletotrichum theobromicola in Uruguay. In contrast, Glomerella leaf spot disease is predominantly caused by C. fructicola in all countries where it was reported so far. Nevertheless, a great variability of morphological and physiological characteristics has been observed within each of these Colletotrichum species (González et al. 2006; Velho et al. 2015).
Enhancing genetic variability is essential for evolution of new pathotypes capable of overcoming resistant cultivars, surviving to unfavorable enviromental conditions and evolving resistance to fungicides. Besides sexual reproduction and mutation, the heterokaryosis and parasexual recombination can play a pivotal role in increasing genetic variability within populations of certain economically important plant pathogenic fungi which usually do not reproduce by sexual means, such as Magnaporthe oryzae (Zeigler et al. 1997). There are also some evidences suggesting that parasexual recombination could contribute to generating variability in Colletotrichum spp. (Roca et al. 2005; Ishikawa et al. 2012).
Parasexuality involves cell–cell fusion and ploidy reduction through a stochastic chromosome loss process (Calo et al. 2013). This phenomenon was originally described by Pontecorvo for Aspergillus nidulans (Pontecorvo 1956) and since then it has been documented or suspected to occur in other mitosporic fungi (Zeigler et al. 1997; Roca et al. 2005; Mehrabi et al. 2011; Calo et al. 2013). Parasexual recombination may promote adaptation to less rapidly changing host or environmental niches (Calo et al. 2013), and is likely more efficient in amplifying host range than sexual reproduction (Roca et al. 2004; Mehrabi et al. 2011). By allowing horizontal gene transference, it can provide new virulence genes to fungus, such as those encoding host-selective toxins, gene clusters and pathogenicity chromosomes (Mehrabi et al. 2011).
Anastomosis, or vegetative hyphal fusion, represents the first step of the parasexual cycle, forming a heterokaryotic hypha where distinct nuclei are maintained for an uncertain period of time (Roca et al. 2004, 2005; Mehrabi et al. 2011). This interconnected state can act as a coordinated individual regulating its overall homeostasis by the interchange of nutrients, water, signal molecules and organelles, while allowing genetic exchange (Read et al. 2009). When occurring between conidia, germ tubes or both, fusion connections are specifically called conidial anastomosis tubes (CATs), a term proposed by Roca and coworkers (Roca et al. 2003, 2004, 2005). CATs have been described as being morphologically and physiologically distinct from conidial germ tubes and characterized to be under separate genetic control (Roca et al. 2003, 2004).
The development of CATs has been described for several species of Colletotrichum originating from different crop plants (Latunde-Dada et al. 1999; Roca et al. 2004; Wharton and Diéguez-Uribeondo, 2004). The migration of nuclei has been successfully monitored by means of staining with 4′,6-diamidino-2-phenylindole (DAPI) and propidium iodide (Roca et al. 2003, 2004), and nuclear labelling of Colletotrichum with fluorescent proteins (Nesher et al. 2008, Ishikawa et al. 2012). Interspecific nuclear transference through CATs has been also observed between Colletotrichum lindemuthianum and Colletotrichum gossypii (Roca et al. 2004). On apple leaves, Araújo and Stadnik (2013b) reported the formation of multiple appressoria and CATs by C. fructicola. However, detailed quantitative studies on these processes remain lacking. Thus, the present manuscript aimed to compare the development of pre-infection structures and CATs undergone by different strains and species of Colletotrichum on artificial surface as well as apple leaf.
Materials and methods
Strains
Ten Colletotrichum strains of apple fruits and leaves were obtained as monosporic culture from the mycological collections of the Federal University of Santa Catarina-Brazil (MANE40, MANE55, MANE70, MANE143, MANE144, MANE147) and the University of Republic-Uruguay (C15, C21, C29 e C38) (Table 1). Strains were previously identified as C. fructicola, C. nymphaeae and C. theobromicola (Velho et al. 2015).
Conidia production
Conidia were obtained from 20-day-old colonies cultivated in PDA medium (potato dextrose agar) at 25 °C and 12 h photoperiod under fluorescent light. Plates were flooded with sterile distilled water and slightly rubbed with a Drigalski spatula. Resultant suspension was filtered through sterile double layer cheesecloth and successively washed twice by centrifugation at 25 °C and 8228×g for 15 min. Conidial concentration was adjusted to 1 × 106 conidia/mL and used in both slide and detached leaf assays.
Polystyrene slide assays
Assays were performed on sterile colorless polystyrene 800-μm-thick slides (Euro Signs®, São Paulo), according to Araújo et al. (2014), with modifications. Three drops of 10 μL of conidial suspension were equidistantly distributed on slides and incubated at 25 °C, 12 h photoperiod and 100 % RH (relative humidity). Cool white fluorescent light was used giving a photon flux density of 160 μE m−2 s−1. The formation of fungal pre-infection structures and CATs development were observed at 24 h of incubation (Ishikawa et al. 2010), using brightfield- (Feldman Wild Leitz FWL 1500T, Brazil ) and fluorescence microscope (Nikon- Eclipse50i, Nikon®, Melville, NY) at a magnification of 400×. For brightfield microscopy, fungus structures were stained with trypan blue solution (Sigma-Aldrich®, USA) (0.1 mg/mL lactophenol). For fluorescence microscopy, structures were stained with a solution containing KOH (10 %) and calcofluor white (Sigma-Aldrich®, EUA) at a 1:1 ratio (v/v), according to the modified methodology of Roca et al. (2004). Fluorescent signal was detected at 380–420 nm. Staining solutions were filtered through cellulose membrane of 0.2 μm of porosity and kept at room temperature in the dark.
Microscopic preparations stained with trypan blue were used for quantitative assessment of the following variables: i) Conidial germination; Percentage of germlings with ii) appressoria, and iii) melanized appressoria. Germinated conidia were considered those with a germ tube longer than its width or with an apressorium (Gonçalves and Stadnik 2012). CATs were quantified and classified according to the type of connection as: i) conidium-conidium (c-c), ii) conidium-germ tube (c-t) and iii) germ tube-germ tube (t-t). CATs were characterized as thin connecting tubes (≤ 2 μm) that emerged from conidia or germ tubes (Roca et al. 2003, Read et al. 2009), and were not considered for assessing the conidial germination.
In a follow-up assay, C. fructicola (C29) and C. nymphaeae (MANE143) strain were used to monitor the development of CATs over time. Incubation conditions were as stated previously. Three polystyrene culture slides were taken every two hours from 14 to 24 h of incubation, stained with trypan blue, and observed at brightfield microscope. Data were used to calculate the daily connection rate (DCR) for each type of connection (c-c, c-t and t-t), based on linear regression model.
Nuclear staining
Nuclear migration through CATs was observed after 24 h of incubation using DAPI and propidium iodide staining (Sigma-Aldrich®, EUA), according to the modified methodology of Roca et al. (2003) and James et al. (1995) as follows: For DAPI staining, 20 μL of sodium phosphate buffer at a concentration of 50 μM, pH 7.0 and 10 μl of DAPI solution (500 μg/mL) was pipetted on slide cultures. Fluorescent signal was detected at 380–420 nm. For propidium iodide staining a mixture containing 10 μL of ethanol 70 % and 10 μL propidium iodide solution (10 μg/mL) was added to the slide culture and then, observed using a fluorescence microscope at 510–560 nm and 1000× magnification. All the staining solutions were prepared in sterile distilled water and kept in the dark at 4 °C.
Detached leaf assay
The strains C29, MANE147 (C. fructicola), MANE143 (C. nymphaeae) and C15 (C. theobromicola) were used in a complementary assay to evaluate their capacity in generating CATs fusions on detached apple leaves.
Susceptible leaves were obtained from 90-day-old apple plants according to Araújo and Stadnik (2013a). The last expanded leaves were collected, washed off, air-dried, and individually placed into each Petri dish with their petiole wrapped in moist cotton.
Detached leaves were inoculated by pipetting two 20 μL-drops of aqueous conidial suspension onto the adaxial leaf surface and then incubated at 25 °C, 100 % UR and 12 h of photoperiod. After 48 h, two 8-mm foliar discs were excised from inoculated areas for brightfield and fluorescence microscopy.
To bleach tissues, leaf discs were carefully placed in a Petri dish on filter paper soaked in chloral hydrate (2.5 g/mL) for four days. Foliar discs were transferred to glass slides and observed under both brightfield and fluorescence microscopy. For the former, a volume of 20 μL of trypan blue solution was added onto translucent foliar disc surface and then observed at a magnification of 400×. For fluorescence microscopy, a mix solution containing 10 μL of KOH (10 %) and 10 μL of calcofluor white was added to discs and then examined at 380–420 nm filter. The conidial germination and CAT formation was assessed as described previously.
Experimental design and statistical analyses
Polystyrene slide assays were constituted by three replicates (slides). For detached leaves, five replicates (discs) were used. In average, 640 conidia were examined for each replication.
Homogeneity of variances was verified by Levene’s test (p ≥ 0.05). Scott-Knott (p ≥ 0.05) test was used to pinpoint differences and patterns of development between strains. Statistical analyses were performed using the software STATISTICA 6.0. For verifying the significance of the correlation values, t test was used (p ≥ 0.01). The determination of DCR was based on the linear regression model. All experiments were repeated twice, with similar results, and values presented at this study consist in the average of them.
Results
Formation of pre-infection structures and CATs on polystyrene slides
The conidial germination rate varied greatly among Colletotrichum strains. All the three leaf strains of C. fructicola abundantly emitted germ tubes and successfully formed melanized appressoria on polystyrene slides (Table 1).
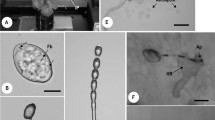
At 24 h of incubation, no CATs were observed in strains of C. fructicola from leaves and C. theobromicola. Only fruit strains of C. fructicola and C. nymphaeae formed these structures (Fig 2a, c and e). Strains of C. nymphaeae exhibited the highest number of CATs fusions, being in average two and half times more frequent than that recorded for C. fructicola strains from fruits. CATs of C. nymphaeae and C. fructicola varied in width, and particularly in length.
In comparison to other types, the t-t connections were less frequent representing up to 20 % of the total number of connections. The c-c connection type was highly frequent in C. nymphaeae strains (Fig 1 and Fig 2e). The c-t connection was more frequent in fruit strains of C. fructicola, which also presented higher number of t-t connections. Both were positively correlated (Table 2).
Pre-infection structures, conidial anastomosis tubes (CATs) and nuclear migration through CATs of Colletotrichum spp. after 24 h of incubation on polystyrene slides. a Network formed by CAT connections in C. fructicola (C29), and b Pre-infection structures of C. fructicola (C29) by trypan blue bright-field microscopy; c Network by CATs of C. fructicola (C29), and d Germlings of C. fructicola (MANE70) with melanized appressoria, and e Germling of C. nymphaeae (MANE143) without appressorium and c-c connection by calcofluor white fluorescence microscopy; f Nuclear migration through CATs of C. fructicola (C29) by propidium iodide fluorescence microscopy; g Nuclear migration and CATs of C. nymphaeae (MANE143) by DAPI fluorescence microscopy. Arrows indicate CATS. Abbreviations: a = appressorium; c = conidium; n = nucleus; gt = germ tube; c-c = conidium-conidium; c-t = conidium-germ tube and t-t = germ tube-germ tube. Bars = 10 μm
Strains without CATs, such as those of C. fructicola from leaves and C. theobromicola exhibited a high percentage of melanized appressoria (Table 1 and Fig 2b and d). CATs and melanized appressoria formation were negatively correlated (Table 2).
Conidial germination rate was positively correlated with the formation of appressoria, but not with their melanization. However, both appressorial formation and melanization were significantly correlated. The total number of CATs was positively correlated with c-c and c-t connections, but not with t-t (Table 2).
Except for t-t, further CAT types were already noticeable at 14 h (Fig 3). The number of these connections continuously increased during the period of evaluation. In relation to C. fructicola (C29), the DCR for c-c and c-t of C. nymphaeae (MANE143) was 3.5-fold higher and 2-fold lower, respectively (Fig 3a and b). Both strains exhibited similar DCR for the t-t connections (Fig 3c) which started to be formed two days later in C. nymphaeae strain.
Nuclear migration through CATs on polystyrene slides
DAPI and propidium iodide staining showed high affinity for genetic material in all tested Colletotrichum strains. Most conidia that did not undergo neither CAT fusion nor germination, presented a single centralized round-shaped nucleus. In contrast, germinated conidia (with sessile appressorium or germ tube) or those with CATs usually exihibited two nuclei (Fig 2f and g). Genetic material crossing through CATs was observed (Fig 2f).
Formation of appressoria and CATs on detached leaves
At 48 h of incubation on leaf surface, MANE147 and C15 strains formed melanized appressoria, but no CAT. In contrast, MANE143 and C29 developed many CATs, especially those of c-c type, and fewer appressoria (Table 3). Extensive networks composed of many interconnected conidia were formed on detached apple leaves. Longer CATs were observed frequently on apple leaves (Fig 4a and b).
Pre-infection structures and conidial anastomosis tubes (CATs) of a C. fructicola (C29) by calcofluor white fluorescence, and b C. nymphaeae (MANE 143) by trypan blue bright-field microscopy, at 48 h of incubation on detached apple leaves. Arrows indicate CATs. Abbreviations: a = appressorium; c = conidium; gt = germ tube. Bars = 10 μm
Discussion
The formation of CATs has been reported to occur in several species within the genus Colletotrichum (Roca et al. 2003; 2004, 2005; Ishikawa et al. 2010, 2012; Latunde-Dada et al. 1999; Wharton and Diéguez-Uribeondo 2004), including C. fructicola on apple leaves (Araujo & Stadnik 2013b), and it has been thought to be genetically determined, but also environmentaly affected (Ishikawa et al. 2010). Accordingly, CATs were observed in half of our strains, and C. nymphaeae markedly produced more c-c connections at a higher speed. In contrast, C. theobromicola and leaf strains of C. fructicola did not form any CATs, but conidia of these non-CAT-forming strains exihibited high ability to germinate and produce melanized appressoria. Previously, Araujo and Stadnik (2013b) registered the occurrence of CATs on apple leaves by the MANE147 monoconidial strain of C. fructicola, the same one that did not form such structures in our work. Although most experimental conditions were very similar or even identical, light exposure may have been the main distinct factor. In contrast to our study, where conidia were initially exposed for 12 h to fluorescent light, Araujo and Stadnik (2013b) kept them in the dark. Therefore, it would be interesting to further investigate whether and how light can affect CAT formation in C. fructicola.
Melanin accumulation in appressoria is essential for successful infection of fungi such as Colletotrichum that penetrate the host cuticle directly (Ludwig et al. 2014). A fast formation and melanization of appressoria is an efficient strategy to quickly obtain nutrients from host and to establish inside the host tissues (Araujo et al. 2014). On the other hand, CAT-forming strains may possibly increase chances to survive longer on the leaf surface while sharing their nutrients. Hence, the capacity to form CATs is likely not necessary, at least in terms of nutrition, for those fungus genotypes that form melanized appressoria and rapidly infect its host. Interestingly, exogenous nutrients inhibit the formation of both CATs (Ishikawa et al. 2010) and appressoria (Gonçalves and Stadnik 2012) in Colletotrichum.
Similar interconnected conidia were previously reported for Colletotrichum on cowpea leaves (Latunde-Dada et al. 1999) and Fusarium oxysporum on tomato root surface (Ruiz-Roldán et al. 2010). These conidial networks apparently downregulate the additional formation and melanization of appressoria in Colletotrichum. It seems that Colletotrichum germlings may have a self-regulation mechanism to inhibit the appressorium development once the conidial interconnection is established. This phenomenon involving the microbial cell-to-cell communication is known as quorum sensing, and has been observed in bacteria and to a lesser extent in fungi (Albuquerque and Casadevall 2012). In this sense, Roca et al. (2005) showed that the formation of CATs in Neurospora crassa is dramatically reduced at low conidial concentrations, and that MAP kinase signaling is possibly involved in the process. MAP kinases play a key role in the appressorium formation and melanin biosynthesis (Takano et al. 2000), and in the induction of CATs during conidial germination (Pandey et al. 2004). In our study, the appressorial melanization compromised the formation of CATs, or vice versa. In accordance to our findings, Roca et al. (2012) reported for Botrytis cinerea that once the cellular parasitic program is triggered, to give origin to germ tube and appressoria, the germling fusion competence is blocked. Studying such antagonistic interferences at physiological and molecular level will be an exciting challenge for future research in the Colletotrichum- apple interaction.
Most ungerminated conidia exhibited only a single nucleus, whereas those that germinated usually presented two, or less frequently one. Mitosis has been considered not essential for germination tube and appressorium formation in many Colletotrichum species (Nesher et al. 2008, Ishikawa et al. 2012, 2013), but in other plant pathogen fungi (e.g. M. oryzae) it is (Kershaw and Talbot 2009). For Colletotrichum of apple, two nuclei can apparently remain in the germinated conidium or one of them migrates into germ tube. Applying of live-cell imaging approaches in this biological system may help answering these open questions on nuclear dynamics.
CATs have been characterized as thinner (≤ 2 μm), shorter (≤ 15 μm), unbranched, nonseptate tubes, exhibiting determinate growth with positive chemotropism towards each other, when compared to germ tubes that show negative chemotropism (Roca et al. 2003, 2005). Although most CATs fulfilled all these criteria, we also observed tubes of larger dimensions that connected at their tips, possibly by means of inconspicuous CATs. What probably happened is that the germ tube grew towards a conidium or another tube, and when it was very close its tip differentiated into a very short CAT which could be detected sometimes as a thinning at tip end. This problem with interpretation may be solved with the developing of CATs-specific markers. In comparison to N. crassa, CATs in Colletotrichum have been more difficult to be distinguished from germ tubes because the process takes longer (Ishikawa et al. 2010).
Generation of variability in Colletotrichum spp. on apple is not fully understood yet. In nature, GLS-strains frequently form perithecia on leaves as well as culture media, indicating the occurrence of sexual reproduction and generation of genetic variability in apple orchards (Velho et al. 2015). In contrast, reproduction of ABR-strains seems to be preferentially or exclusivelly assexual, since perithecia have not been observed in associtation with apple bitter rot (González et al. 2006). In this situation, CATs may play an important role to generate variability in strains adapted to infect apple fruits, making possible to create variability by parasexual reproduction. In fact, high variability of different traits has been reported for Colletotrichum spp. originating from rotten apple fruits (González et al. 2006; Velho et al. 2015).
The use of novel techniques, such as live-cell microscopy, allow to narrowly observe CATs growth and nuclear dynamics in time course through this cell-cell connections (Read et al. 2009; Roca et al. 2005; Ishikawa et al. 2010, 2012). Applying these techniques to apple-Colletotrichum spp. system may be important for further studying the antagonism between CATs and melanized appressoria as well as the exchange of genetic material inter- and intraspecifically.
References
Albuquerque, P., & Casadevall, A. (2012). Quorum sensing in fungi – a review. Medical Mycology, 50(4), 337–345.
Araújo, L., & Stadnik, M. J. (2013a). Cultivar-specific and ulvan-induced resistance of apple plants to Glomerella leaf spot are associated with enhanced activity of peroxidases. Acta Scientiarum, 35(3), 287–293.
Araújo, L., & Stadnik, M. J. (2013b). Múltiplos apressórios e tubos de anastomoses conidiais no processo infeccioso de Colletotrichum gloeosporioides em macieira. Bragantia, 72(2), 180–183.
Araújo, L., Gonçalves, A. E., & Stadnik, M. J. (2014). Ulvan effect on germination and appressoria formation of Colletotrichum gloeosporiodes. Phytoparasitica, 42(5), 631–640.
Calo, S., Billmyre, R. B., & Heitman, J. (2013). Generators of phenothypic diversity in the evolution of pathogenic microorganisms. PLoS Pathogens, 9(3), e1003181.
Cannon, P. F., Damm, U., Johnston, P. R., & Weir, B. S. (2012). Colletotrichum-current status and future directions. Studies in Mycology, 73(1), 181–213.
Gonçalves, A. E., & Stadnik, M. J. (2012). Interferência de ulvana na formação melanização de apressórios de Colletotrichum gloeosporioides. Tropical Plant Pathology, 37(6), 431–437.
González, E., Sutton, T. B., & Correll, J. C. (2006). Clarification of the etiology of Glomerella leaf spot and bitter rot of apple caused by Colletotrichum spp. based on morphology and genetic, molecular, and pathogenicity tests. Phytopathology, 96(9), 982–992.
Ishikawa, F. H., Souza, E. A., Read, N. D., & Roca, M. G. (2010). Live-cell imaging of conidial fusion in the bean pathogen. Colletotrichum lindemuthianum, Fungal Biology, 114(1), 2–9.
Ishikawa, F. H., Souza, E. A., Shoji, J. Y., Connolly, L., Freitag, M., Read, N. D., & Roca, M. G. (2012). Heterokaryon incompatibility is suppressed following conidial anastomosis tube fusion in a fungal plant pathogen. PloS One, 7(2), e31175.
James, S. W., Mirabito, P. M., Scacheri, P. C., & Morris, N. R. (1995). The Aspergillus nidulans bimE (blocked-in-mitosis) gene encodes multiple cell cycle functions involved in mitotic checkpoint control and mitosis. Journal of Cell Science, 108(11), 3485–3499.
Kershaw, M. J., & Talbot, N. J. (2009). Genome-wide functional analysis reveals that infection-associated fungal autophagy is necessary for rice blast disease. Proceedings of the National Academy of Sciences of the United States of America, 106(37), 15967–15972.
Latunde-Dada, A. O., O’Connell, R. J., Nash, C., & Lucas, J. A. (1999). Stomatal penetration of cowpea (Vigna unguiculata) leaves by a Colletotrichum species causing latent anthracnose. Plant Pathology, 48(6), 777–785.
Ludwig, N., Löhrer, M., Hempel, M., Mathea, S., Schliebner, I., Menezel, M., Kiesow, A., Schaffrath, U., Deising, H. B., & Horbach, F. (2014). Melanin is not required for turgor generation but enhances cell-wall rigidity in appressoria of the corn pathogen Colletotrichum graminicola. Molecular Plant-Microbe Interactions, 27(4), 315–327.
Mehrabi, R., Bahkali, A. H., Abd-Elsalam, K. A., Moslem, M., M’barek, S. B., Gohari, A. M., Jashni, M. K., Stergiopoulos, L., Kema, G. H. J., & Wit, P. J. G. M. (2011). Horizontal gene and chromosome transfer in plant pathogenic fungi affecting host range. FEMS Microbiology Review, 35(3), 542–554.
Nesher, I., Barhoom, S. & Sharon, A. 2008. Cell cycle and cell death are not necessary for appressorium formation and plant infection in the fungal plant pathogen Colletotrichum gloeosporioides. BMC Biology, (6)9, 1–11.
Pandey, A., Roca, M. G., Read, N. D., & Glass, N. L. (2004). Role of a mitogen-activated protein kinase pathway during conidial germination and hyphal fusion in Neurospora crassa. Eukaryotic Cell, 3(2), 348–358.
Pontecorvo, G. (1956). The parasexual cycle in fungi. Annual Review of Microbiology, 10(1), 393–400.
Read, N. D., Lichius, A., Shoji, J., & Goryachev, A. B. (2009). Self-signaling and self-fusion in filamentous fungi. Current Opinion in Microbiology, 12(6), 608–615.
Roca, M. G., Davide, L. C., Mendes-Costa & M. C. & Wheals, A. (2003). Conidial anastomosis tubes in Colleotrichum. Fungal Genetics and Biology, 40(2), 138–145.
Roca, M. G., Davide, L. C., Davide, L. M. C., Mendes-Costa, M. C., Schwan, R. F., & Wheals, A. E. (2004). Conidial anastomosis fusion between Colletotrichum species. Mycological Research, 108(11), 1320–1326.
Roca, M. G., Read, N. D., & Wheals, A. E. (2005). Conidial anastomosis tubes in filamentous fungi. FEMS Microbiology Letters, 249(2), 191–198.
Roca, M. G., Wheichert, M., Siegmund, U., Tudzynski, P., & Fleißner, A. (2012). Germling fusion via conidial anastomosis tubes in the grey mould Botrytis cinerea requires NADPH oxidase activity. Fungal Biology, 116(3), 379–387.
Ruiz-Roldán, M. C., Köhli, M., Roncero, M. I., Philippsen, P., Di Pietro, A., & Espeso, E. A. (2010). Nuclear dynamics during germination, conidiation, and hyphal fusion of Fusarium oxysporum. Eukaryotic Cell, 9(8), 1216–1224.
Takano, Y., Kikuchi, T., Kubo, Y., Hamer, J. E., Mise, K., & Furusawa, Y. (2000). The Colletotrichum lagenarium MAP kinase Gene CMK1 regulates diverse aspects of fungal pathogenesis. Molecular Plant-Microbe Interactions, 13(4), 374–383.
Velho, A. C., Alaniz, S., Casanova, L., Mondino, P., & Stadnik, M. J. (2015). New insights into the characterization of Colletotrichum species associated with apple diseases in Southern Brazil and Uruguay. Fungal Biology, 119(4), 229–244.
Wharton, P. S., & Diéguez-Uribeondo, J. (2004). The biology of Colletotrichum acutatum. Anales del Jardin Botánico de Madrid, 61(1), 3–22.
Zeigler, R. S., Scott, R. P., Leung, H., Bordeos, A. A., Kumar, J., & Nelson, R. J. (1997). Evidence of parasexual exchange of DNA in the rice blast fungus challenges its exclusive clonality. Phytopathology, 87(3), 284–294.
Acknowledgments
The authors thank the Brazilian Ministry of Education Agency for Graduate Studies (CAPES) for granting the M.Sc.-scholarship to the first author. M.J.S is a research member of the National Council for Scientific and Technological Development (CNPq). We are also grateful to Dr. M.B. de Freitas (UFSC) for critical reviewing of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gonçalves, A.E., Velho, A.C. & Stadnik, M.J. Formation of conidial anastomosis tubes and melanization of appressoria are antagonistic processes in Colletotrichum spp. from apple. Eur J Plant Pathol 146, 497–506 (2016). https://doi.org/10.1007/s10658-016-0934-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-016-0934-6