Abstract
Ulvan is an algal polysaccharide known for its ability to induce resistance to plant diseases such as the Glomerella leaf spot of apple caused by Colletotrichum gloeosporiodes. This study was aimed at investigating microscopically, in tests in vitro and in vivo, whether ulvan interferes in the development of pre-infective structures of C. gloeosporioides. Conidial germination and appressoria formation were monitored hourly on agar and cellophane, and at 48 h on water- and ulvan-treated susceptible as well as resistant apple leaves. Amendment of agar with ulvan (10 mg ml−1) enhanced the germination and resulted in longer germ tubes at 7 h of incubation. On cellophane it significantly delayed appressoria formation up to 8 h, but later after 14 h increased the number of appressoria per conidium. Spraying of susceptible leaves with ulvan 6 days before inoculation decreased disease severity by 50%. This was associated with inhibition of appressoria formation and stimulus in growth of germ tubes, without interfering with conidial germination, when compared with both water-treated control and resistant plants. Appressorium formation occurred preferentially on anticlinal walls of epidermal cells and its location was not influenced by host resistance or by ulvan treatment. This study suggests a new mode of action for ulvan interfering with appressorium formation that could protect apple plants against C. gloeosporioides infection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colletotrichum gloeosporioides sensu lato is one of the most common and widely distributed plant pathogens in the world and can attack at least 470 different host genera (Sutton 1980). On apple (Malus domestica Borkh.) it causes the Glomerella leaf spot (GLS), an emerging disease that has been reported in regions with a humid subtropical climate, such as southern Brazil (Becker et al. 2000), southeastern USA (González et al. 2006) and, more recently, eastern China (Wang et al. 2012). GLS often occurs during a rainy summer, and under such conditions the disease can result in severe defoliation, reducing yield and weakening apple trees (Becker et al. 2000; González et al. 2006). The ‘Gala’ apple is the most widely grown cultivar in Brazil, but is highly susceptible to GLS, whereas the cultivars descended from the 'Delicious' group, such as ‘Fuji’, are completely resistant to the disease (Becker et al. 2000; Dantas et al. 2009). Resistance has been found to be complete and monogenic recessive (Dantas et al. 2009).
GLS can be caused by both C. gloeosporioides (Penz.) Penz. & Sacc 1884 and Colletotrichum acutatum JH Simmonds, but the former species seems to be more important due to its higher frequency and pathogenic potential (Crusius et al. 2002; González et al. 2006). The first symptoms appear 2 days after inoculation, as reddish-purple spots that evolve into irregularly shaped necrotic lesions. Then, infected leaves turn yellow and usually fall off within a few weeks (Araújo et al. 2008). Young leaves are more susceptible than older ones (Araújo et al. 2008; Araújo & Stadnik 2013a).
The early stages of fungal development during the infection process are essentially the same for all Colletotrichum species (Diéguez-Uribeondo et al. 2005; Wharton and Diéguez-Uribeondo 2004). Through chemical and/or physical signals such as simple sugars and topographic properties of plant tissue surface, respectively, the conidia deposited onto leaves start the process of germination, germ tube elongation and differentiation in a sessile or pedicellate appressorium (Dean 1997; Deising et al. 2000; Gonçalves & Stadnik 2012). Penetration is mainly by the strong osmotic pressure exerted by the infective hyphae, which emerges from a pore of melanized appressorium on the host cuticle, culminating in the colonization of plant tissues and afterwards in formation of reproductive structures (Dean 1997; Mendgen & Hahn 2002; Wharton and Diéguez-Uribeondo 2004).
Two basic colonization strategies have been described for Colletotrichum spp.: (i) intracellular hemibiotrophy (IHB), which is the more common form, and (ii) subcuticular-intramural necrotrophy (SIN). In the IHB strategy, the penetration of infective hyphae occurs in the epidermal cell, whereas in the SIN colonization, the fungus grows beneath the cuticle, within the periclinal and anticlinal walls of epidermal cells (Diéguez-Uribeondo et al. 2005; Mendgen & Hahn 2002; Peres et al. 2005; Wharton and Diéguez-Uribeondo 2004).
Although literature on the infection process of Colletotrichum spp. to apples (Shane & Sutton 1981) and other kinds of fruits (Daykin & Milholland 1984; Gomes et al. 2009; Kolattukudy et al. 2000) is available, knowledge on infection of apple leaves is still scarce, and the interaction between pathogen and host remains poorly understood.
Control of GLS has been achieved by preventive applications of synthetic fungicides (Becker et al. 2000; Crusius et al. 2002). However, both environmental and health concerns have fostered the development of eco-friendly technologies for plant protection, such as compounds that can induce plant resistance. In this context, one compound showing potential to induce resistance in plants is ulvan, a water-soluble heteropolysaccharide obtained from the cell walls of green algae Ulva spp. Delile 1813 (Cluzet et al. 2004; Paulert et al. 2009).
Ulvan represents the major polymeric fraction of the cell walls and is composed of rhamnose, xylose, uronic acids and other small amounts of simple sugars (Paulert et al. 2009). Although no direct effect on fungi has been reported (Araújo et al. 2008; Paulert et al. 2009), spraying with ulvan can frequently protect crop plants against different kinds of pathogens (Cluzet et al. 2004; Delgado et al. 2013; Freitas & Stadnik 2012). Furthermore, molecular studies have shown that ulvan can induce the expression of jasmonate genes related to plant defense responses in Arabidopsis (Jaulneau et al. 2010). In apple plants, ulvan can confer systemic protection, reducing GLS severity by half, when sprayed 6 days before inoculation (Araújo et al. 2008). Despite these promising results, it is still unknown whether and how the fungal infection process is affected by ulvan-induced resistance to GLS. Thus, the objective was to determine microscopically whether ulvan can interfere with the development of pre-infective structures of C. gloeosporioides in tests in vitro and in vivo.
Materials and methods
Obtaining ulvan
Ulvan was obtained as described by Paulert et al. (2009) with modifications. Briefly, 100 g of dried Ulva fasciata seaweed was autoclaved in 1 l of distilled water at 110°C for 2 h. The resulting aqueous solution was filtered through cotton cloth and the polysaccharides were precipitated with three volumes of ethanol (92.6%) at –20°C for 48 h. Then, the precipitates were discarded and the supernatant was kept under the conditions described earlier to obtain a second precipitation. Polysaccharides (ulvans) obtained from the second precipitation were collected with filter paper (25 μm porosity) on a Büchner filter under suction. Ulvans were dried in an air-forced oven at 45ºC for 48 h, ground in an analytical grinder (model Q298A21, Quimis; Diadema, Brazil) and passed through a 0.42-mm metallic sieve. Ulvan powder was stored at –20ºC until its use in the biological assays. The concentration of ulvan (10 mg ml−1) was based on previous studies (Araújo et al. 2008; Paulert et al. 2009). Before its use, ulvan was completely dissolved in distilled water at room temperature using a magnetic stirrer.
Conidia production
The pathogenic strain MANE147 of C. gloeosporioides was maintained on potato dextrose agar (PDA) medium (Araújo & Stadnik 2011, 2013a,b). To obtain conidia, 8 mm discs of fungal cultures were transferred to petri dishes with PDA and incubated at 25°C and 12 h photoperiod. After 10 days, the colonies were flooded with sterile distilled water, lightly scraped, and the concentration of the conidial suspension was determined using a Neubauer’s counting chamber, as specified in each experiment.
Conidial germination assay on agar
Water-agar medium (2%), amended with ulvan (10 mg ml−1) or not (control), was poured into 9-cm petri dishes (5 ml per dish), in a total of four dishes per treatment. A volume of 100 μl of conidial suspension at a concentration of 1.0 x 106 conidia ml−1 was spread with a Drigalsky spatula over the agar surface, and then incubated at 25°C and 12 h photoperiod. At every hour (1–7 h), three 8-mm discs were sampled from agar medium for examination under a microscope (FWL1500, Feldmann Wild Leitz; Manaus, AM, Brazil). Discs were mounted on glass slides and fungus growth was stopped by adding lactophenol-cotton dye (lactic acid, crystalline phenol, distilled water at the proportion of 1:1:1; v/w/v, and 0.2% cotton blue). Each replicate consisted of one glass slide with a set of three media discs. Two discs per replicate were randomly chosen and 100 conidia on each were monitored arbitrarily. Germinated conidia (germlings) were considered those with germ-tubes longer than their width, or presenting an appressorium (Morin et al. 1996). Germ tube length was measured at 7 h incubation time. Germ tubes were classified according to length as (i) <25 μm; (ii) ≥25 μm and <50 μm; (iii) ≥50 μm. For germlings with two or more germ tubes, only the length of the longer one was considered.
Conidial germination and appressorium formation on cellophane
Conidial germination and appressorium formation were examined using the cellophane membrane technique (Shane & Sutton 1981). For that, transparent cellophane membranes (RMV-Realce®) were cut into 76 x 36 mm2 pieces and settled on glass slides. One 10-μl drop of an aqueous conidial suspension (2x105 conidia ml−1) containing ulvan (10 mg ml−1) or not, was distributed on the membrane surface and incubated at 25°C and 100% relative humidity. Every 2 h and over a 16-h period, fungal development was stopped by adding 10 μl of lactophenol–cotton blue dye and 100 conidia were microscopically monitored. Each replicate consisted of one drop of conidial suspension on a cellophane membrane.
Plant material and growing conditions
Seedlings were obtained from seeds according to Araújo & Stadnik (2011, 2013a). Briefly, ‘Gala’ apple seeds were distributed in plastic germination boxes between layers of moistened cotton and then stored at 5ºC for 50 days to break dormancy. Germinated seeds were transferred to polystyrene boxes containing soil and maintained under greenhouse conditions. Seedlings were transplanted into individual plastic pots containing 1 l of a mixture of organic compost and loamy soil (1:2, v/v) and were grown for 45 days until they had from 10 to 15 expanded leaves. Seedlings were manually irrigated as needed and individually fertilized with 0.25 g of monoammonium phosphate (12-61-0/ N-P-K). New leaves developing after the treatment were identified with a tag attached to the last fully expanded leaf (designated as the 1st leaf). Since seedlings originated from the seeds of ‘Gala’ pollinated by ‘Fuji’ (pollen donor), segregating resistant and susceptible individuals to GLS were first identified by means of a detached leaf test (Araújo & Stadnik 2011, 2013a).
Plant treatment, inoculation and sampling
Six days before the fungal inoculation, 45-day-old susceptible plants were sprayed with the ulvan solution (10 mg ml−1) until runoff using a manual sprayer (Griffin, Italy) coupled to an air compressor (25 psi, Schulz; Campos dos Goytacazes (RJ), Brazil) and delivering ~4 ml per plant. A set of resistant and susceptible plants were sprayed with distilled water serving as controls. All seedlings were kept on greenhouse benches until the inoculation.
Apple seedlings were inoculated 6 days after treatment by spraying a volume of ~4 ml per plant at 3 x 105 conidia ml−1. All inoculated plants were maintained in the dark at 26°C and nearly 100% relative humidity for 24 h. After that, they were returned to the greenhouse benches until the disease evaluation.
At 48 h after inoculation (HAI), four foliar discs (8 mm diam) were taken from the 2nd and 3rd uppermost expanded leaves of each seedling to examine the infection process of C. gloeosporioides under a microscope.
Disease evaluation
GLS severity was assessed in the upper leaves (1st and 4th inoculated leaf) on the 10th day post-inoculation using image analyses. For that, leaves were detached, mounted between two transparent glass plates (28 x 21 x 0.8 cm) with a black sheet as background, and then scanned. The percent necrotic area for each leaf image was determined using Quant® software (v.1.01; 2003, Federal University of Viçosa). A replicate consisted of one seedling per pot.
Germination and appressorium formation on apple leaves
Four leaf discs were collected per seedling for microscopic analysis, and were bleached and stained according to Araújo & Stadnik (2011, 2013b). Briefly, discs of five seedlings per treatment were immediately placed with the upper side in an ethanol and acetic acid (3:1; v/v) solution to fix and bleach the tissues; the solution was changed periodically during a 3-day period. Finally, the solution was replaced by a conservation solution containing lactic acid, glycerol and water (1:1:1; v/v/v). Microscopic analyses were performed using a light microscope. To visualize the structures of the fungus, leaf discs were mounted on glass slides and stained with lactophenol–cotton blue dye. The percentages of germination and appressorium formation were randomly determined on 100 conidia per leaf disc. C. gloeosporioides germlings were classified into five categories, according to the structures emitted: (i) one germ tube; (ii) two germ tubes; (iii) one appressorium; (iv) two appressoria; and (v) one appressorium and one germ tube. For germinated conidia with one appressorium, the germ tube was classified into two categories, according to its length: (i) short (≤10 μm), or (ii) long (>10 μm). Additionally, the frequency of appressoria on the surface of epidermal cells or anticlinal walls of epidermal cells was determined in 250 germlings. An experimental unit consisted of four discs per seedling.
Experimental design and statistical analysis
Experiments were arranged in a completely randomized design with four and five replicates per treatment for in vitro assays and in planta tests, respectively. The experiments were repeated twice. Analyses of variance for disease evaluation and infection process of leaves were followed by Tukey’s separation test (P ≤ 0.05), while for in vitro assays the t-test (P ≤ 0.05) was used. Statistical analysis was performed using the software Statistica 6.0 - Stat Soft®.
Results
Conidial germination and germ tube elongation on water agar
Conidia of C. gloeosporioides started to germinate ~1 h after contact with the agar surface. Germination occurred faster on ulvan-amended water agar than on unamended medium until 7 h, when a difference was no longer observed (Fig. 1). Furthermore, with the addition of ulvan, germlings with germ tubes longer than 50 μm and between 25 μm and 50 μm were 67% and 39% more frequent, respectively, compared with water control (Fig. 2 a,b; Table 1). No appressoria were observed on agar medium (Fig. 2 a,b).
Light micrographs of Colletotrichum gloeosporioides following deposition of conidia on agar and apple leaves. Germination and germ tube (GT) of conidia (CN) treated with water (a) and ulvan (b) after 7 h of incubation. Conidia and infection structures developed on leaf surface at 48 h after inoculation (c, d, e and f). Germlings with: two germ tubes (a); a long germ tube (>50 μm) (b); a short germ tube (≤10 μm) and one appressorium (AP) located on epidermal cells (EC) (c); a long germ tube (>10 μm) and one appressorium located on anticlinal walls (AW) of EC (d); one germ tube and one appressorium located on AW of EC (e); and two appressoria located on AW of EC (f). Bars: 10 μm
Conidial germination and appressorium differentiation on cellophane membrane
Conidial germination started on cellophane at 2 h of incubation and occurred similarly in both treatments, except at 8 h when a slightly higher germination was significantly recorded for ulvan-treated conidia, in comparison with water control (Fig. 3a). Appressorium formation started about 4 h after deposition of conidia on cellophane paper. During the first 8 h, appressorium formation was 3–4-fold significantly higher in the water control than in the ulvan treatment, and appressoria were mostly sessile. From 10 up to 12 h, appressoria formation exhibited no difference between water and ulvan treatment, and almost all germlings had one appressorium. Later, at 14 and 16 h incubation time, the number of appressoria per germling was ~15% and 25% higher, respectively, in ulvan treatment than in the water control. Conidia treated with ulvan had their germ tubes more stimulated to form a second appressorium, usually at the end of long germ tubes (Fig. 3b).
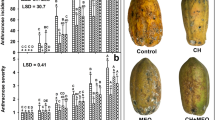
Disease severity
Resistant seedlings inoculated with C. gloeosporioides never showed symptoms, whereas susceptible plants exhibited variable leaf lesions (Fig. 4). Spraying ulvan in upper leaves reduced the necrotic leaf area by 49% (Fig. 4), similarly to as found by Araújo et al. (2008).
Severity of Glomerella leaf spot (%) at 10 days after inoculation of apple seedlings with Colletotrichum gloeosporioides. Plants (resistant and susceptible) were sprayed with water or ulvan 6 days prior to the inoculation. Within columns, different lowercase letters indicate significant differences (P ≤ 0.05) between mean values
Conidial germination and appressorium formation on apple leaves
No statistical difference in the percentage of conidial germination of C. gloeosporioides on the apple leaves was observed among treatments (Table 2). Germlings with and without appressoria showed longer germ tubes on ulvan-treated leaf surfaces than in the susceptible control or resistant leaves (Fig. 2 c,d; Table 2).
At 48 HAI, conidia formed germ tubes and appressoria on the upper leaf surface. Pre-treatment of leaves with ulvan significantly increased the percentage of germlings without appressoria of C. gloeosporioides by 175%, in comparison with water control (Fig. 5a). The total number of germlings without appressoria on water-sprayed susceptible plants did not differ significantly from resistant ones (Fig. 5a). For germlings with more than one germ tube, no statistical differences were detected among treatments (Fig. 5a).
Frequency of classes of germlings without (a) or with (b) appressoria of Colletotrichum gloeosporioides on leaves of susceptible apple seedlings, treated with water or ulvan, and resistant seedlings at 48 h after inoculation (HAI). Categories of germlings with: one (1 GT) or two germ tubes (>1 GT); one (1AP) or two appressoria (2AP) and one appressorium and one germ tube (1 AP + 1 TG). Bars with the same lowercase letters indicate no statistical difference. *Indicates significant difference of class compared with the control (P ≤ 0.05)
Ulvan also reduced the percentage of germlings with appressoria of C. gloeosporioides by 24% (Fig. 5b). When compared with water control, germlings grown on ulvan-treated leaves exhibited a lower percentage of classes with only one appressorium, but a higher percentage of conidia with both one appressorium and one germ tube (Figs. 2 c,d,e; 5b). For conidia with two appressoria, there were no significant differences between treatments (Figs. 2f; 5b).
The appressoria formation site of C. gloeosporioides germlings was about threefold higher on anticlinal walls (Fig. 2 d,e,f; Table 2) than on the surface of epidermal cells in all experiments (Fig. 2c; Table 2).
Discussion
Conidial germination of C. gloeosporioides occurred promptly on both water-agar and cellophane, reaching its highest value at 7 h (almost 100%) and 10 h (100%) after coming into contact with those surfaces, respectively. In contrast, germination of conidia on apple leaves, at 48 HAI, was lower than that observed on both inert surfaces. This is in accordance with Shane & Sutton (1981), who recorded higher germination of C. gloeosporioides-conidia on cellophane than on apple cuticle.
Ulvan did not interfere with the germination ability of conidia on the leaf surface but accelerated their germination on agar and less dramatically on cellophane. On the agar surface this stimulus persisted until 7 h, and was also confirmed by the increase in length of germ tubes. Possibly, the presence of nutrients and sugars stimulated germ tube elongation on agar and leaves. Sugar monomers are known to stimulate growth of Colletotrichum spp. (Chaturvedi 1964; Hugovieux et al. 1997) and can be released from the algal polysaccharide into the medium by both physical and enzymatic processes (Paulert et al . 2009). Rhamnose, one of the main components of ulvan, is for instance involved in pathogenesis, enhancing expression of endopolygalacturonase gene in C. lindemuthianum (Hugovieux et al. 1997). Ulvan is also rich in sulphur (Cluzet et al. 2004; Paulert et al. 2009), an essential element for growing C. gloeosporioides in media (Chaturvedi 1964). Araújo et al. (2008) reported that the addition of ulvan to culture medium at concentrations of 1–20 mg ml−1 increased the mycelial growth of C. gloeosporioides. Finally, it cannot be excluded that trace elements, present in the polysaccharide, might have also contributed to accelerating the conidial germination.
Following a well slide test, Paulert et al. (2009) reported that the addition of ulvan at concentrations of 0.1–10 mg ml−1 increased the conidial germination of C. lindemuthianum from 29% to 42%, respectively. Thus, ulvan seems to be able to improve conidial germination in cases when it is low (Paulert et al. 2009). In contrast, in our work the germination ability of conidia was high (97%), and therefore no difference could be recorded any longer at 7 h incubation on water agar. Interestingly, changes in germination rate were not observed for several hours of incubation on cellophane, where fungus rapidly started to form appressoria. At the first hours of incubation on cellophane, conidia germinated forming mostly sessile appressoria, typical for C. gloeosporioides (Oh et al. 1998; Shisler et al. 1991). This is a common strategy of a hemibiotrophic fungus to penetrate its host as soon as possible, since the process of emission and elongation of germ tubes is energetically expensive, quickly draining scarce reserves from the conidia (Ferreira et al. 2006). Hence, in the absence of exogenous nutrients, fungus is stimulated to produce appressoria, in order to obtain them directly from its host (Dean 1997; Shisler et al. 1991). On the contrary, while providing some nutrients, ulvan stimulated germ tube growth and delayed its differentiation into appressoria on cellophane. Curiously, later at 14 and 16 h of incubation, ulvan had the opposite effect, strongly increasing appressoria formation, mostly on extremities of long secondary germ tubes. This can be explained possibly by the formation of a second appressorium as the result of better nourished C. gloeosporioides-germlings. In accordance with this finding, it has also been demonstrated that melanization of primary appressoria is delayed by ulvan, whereas growth of germ tubes is stimulated on cellophane membrane (Gonçalves & Stadnik 2012). Thus, under in vitro conditions, the better nutritional status of fungus at the beginning of germination promotes elongation of germ tubes, which later differentiate into a higher number of appressoria per conidium, when nutrients become scarce.
Spraying leaves with ulvan stimulated fungus growth over the apple leaf surface, delaying the formation of appressoria and, consequently, the penetration of plant tissue. It is known that C. gloeosporioides is able to grow saprophytically on organic substrates as well (Peres et al . 2005; Wharton and Diéguez-Uribeondo 2004). However, when growing over plant surfaces, fungus survival on the phylloplane may be affected by epiphytical colonization of other microorganisms, including antagonists (Waipara et al. 2002). Also UV-light and temperature oscillations affect leaf surfaces more dramatically than its inner parts (Kinkel 1997). All these factors together can make a leaf outdoors a hostile environment for Colletotrichum spp. As a result, the sooner the fungus penetrates into the plant, the safer will be its growth (Kinkel 1997).
Early studies have demonstrated the absence of an inhibitory effect of ulvan against Colletotrichum spp. (Araújo et al. 2008; Paulert et al. 2009). This, together with results from physiological and molecular investigations, have led to the only conclusion that ulvan treatment induces systemic resistance, providing partial control of fungal diseases in both local and distant plant tissues (Araújo et al. 2008; Cluzet et al. 2004; Freitas & Stadnik 2012; Jaulneau et al. 2010). However, the effect has been found to be stronger locally than systemically in apple (Araújo et al. 2008) and bean (Freitas & Stadnik 2012) plants. Our study provided clear evidence of a possible new mode of action for ulvan on fungal pre-infective structures that, in turn, could explain its better effectiveness in directly treated leaves. The contribution of each mode of action to disease control by ulvan still remains an open question for further investigation.
The formation of appressoria is pivotal to penetrating the cuticle and establishing a pathogenic relationship with the host (Deising et al. 2000; Mendgen & Hahn 2002). Consequently, impairment of appressorium formation reduces the aggressiveness of the fungal pathogen to the host. Therefore, we may reasonably speculate that impairment of appressorium formation on leaf surfaces previously treated with ulvan can contribute to reducing disease severity in apple plants. Indeed, reduction in appressorium formation of Colletotrichum spp. has been confirmed to decrease anthracnose severity in different pathosystems (Basavaraju et al. 2009; Bentes & Matsuoka 2002; Diéguez-Uribeondo et al. 2005; Munaut & Maraite 1998). Moreover, greater elongation of the germ tube of C. acutatum has been related to a delayed onset of disease symptoms in leaves of almond (Diéguez-Uribeondo et al. 2005).
The initial events of penetration, i.e., C. gloeosporioides-germ tube elongation and appressorium formation, are similar in both susceptible and resistant plants (Araújo & Stadnik 2011, 2013b). Conidia emit germ tubes that form globular and melanized appressoria. From beneath the appressorium a penetration peg emerges and grows through the cuticle (Bentes & Matsuoka 2002; Daykin & Milholland 1984; Gomes et al. 2009; Wharton et al. 2001). In general, differences in resistance to Colletotrichum appear only during colonization of the infective hyphae in epidermal and parenchyma cells (Bentes & Matsuoka 2002; Gomes et al. 2009; Wharton et al. 2001; Wharton and Diéguez-Uribeondo 2004).
Most C. gloeosporioides-conidia germinated and formed appressoria on anticlinal walls of epidermal cells of apple and this pattern was not significantly changed by host resistance or application of ulvan. Although species of Colletotrichum usually colonize the host through the IHB, the SIN pattern or the use of the two strategies of colonization has been reported for some species (Diéguez-Uribeondo et al. 2005; Mendgen & Hahn 2002; Peres et al. 2005; Wharton and Diéguez-Uribeondo 2004). In the SIN strategy, the interaction is largely necrotrophic and the biotrophic phase is too short, or even non-existent (Wharton and Diéguez-Uribeondo 2004). Accordingly, plants of apple infected with C. gloeosporioides exhibit disease symptoms as early as 45 HAI (Becker et al. 2000), indicating that the fungus follows SIN-strategy of tissue colonization. Thus, our results suggest that C. gloeosporioides colonizes apple leaves preferentially by SIN strategy. However, it is important to emphasize that only the location of appressoria on anticlinal walls was microscopically determined and, therefore, further studies with ultrastructural microscopy techniques are needed to confirm our findings.
In conclusion, it was demonstrated that ulvan stimulates transiently the germination and germ tube elongation of conidia, whereas it delays appressorial differentiation of C. gloeosporioides in vitro and inhibits it on apple leaves. The development of infection structures in resistant and susceptible plants was similar and appressoria were preferentially located on the anticlinal cell walls, suggesting that the fungus colonizes apple leaves through a SIN strategy. This location pattern was not affected by host resistance or ulvan treatment. The reduction of disease severity in ulvan-sprayed apple leaves was associated with an increase of germ tube elongation and an inhibition of appressorium formation. This study reports a possible new mode of action for ulvan interfering with the appressorium formation that could potentially have a role in the protection of apple plants against C. gloeosporioides infection. Investigating the ways such interference occurs on leaves will be an exciting challenge for future research.
References
Araújo, L., Borsato, L. C., Valdebenito-Sanhueza, R. M., & Stadnik, M. J. (2008). Fosfito de potássio e ulvana no controle da mancha foliar da gala em macieira. [Potassium phosphite and ulvan in the control of ‘Gala’ leaf spot on apple.]. Tropical Plant Pathology, 33, 74–80.
Araújo, L., & Stadnik, M. J. (2011). Processo infeccioso e atividade de enzimas em plântulas de macieira de genótipo resistente ou suscetível à mancha foliar de Glomerella causada por Colletotrichum gloeosporioides. [Infection process and activity of enzymes in apple seedlings of genotype resistant or susceptible to Glomerella leaf spot caused by Colletotrichum gloeosporioides.]. Tropical Plant Pathology, 36, 241–248.
Araújo, L., & Stadnik, M. J. (2013a). Cultivar-specific and ulvan-induced resistance of apple plants to Glomerella leaf spot are associated with enhanced activity of peroxidases. Acta Scientiarum Agronomy, 35, 287–293.
Araújo, L., & Stadnik, M. J. (2013b). Múltiplos apressórios e tubos de anastomoses conidiais no processo infeccioso de Colletotrichum gloeosporioides em macieira. [Multiple appressoria and conidial anastomosis tubes in the infection process of Colletotrichum gloeosporioides on apple.]. Bragantia, 72, 180–183.
Basavaraju, P., Shetty, N. P., Shetty, H. S., Neergaard, E., & Jørgensen, H. J. L. (2009). Infection biology and defence responses in sorghum against Colletotrichum sublineolum. Journal of Applied Microbiology, 107, 404–415.
Becker, W. F., Katsurayama, Y., & Boneti, J. I. S. (2000). Mancha foliar da gala: principal doença de verão da cultura da macieira. [Gala leaf spot: Major summer disease of apple crop]. Agropecuária Catarinense, 13, 14–20.
Bentes, J. L. S., & Matsuoka, K. (2002). Histologia da interação Colletotrichum guaranicola e Paullinia cupana var. sorbilis em clones resistente e suscetível. [Histology of Colletotrichum guaranicola and Paullinia cupana var. sorbilis on resistant and susceptible clones.]. Fitopatologia Brasileira, 27, 71–77.
Chaturvedi, C. (1964). Nutritional studies on Colletotrichum gloeosporioides Penz. Mycopathologia et Mycologia Applicata, 27, 265–272.
Cluzet, S., Torregrosa, C., Jacquet, C., Lafite, C., Fournier, J., Mercier, L., et al. (2004). Gene expression profiling and protection of Medicago truncatula against a fungal infection in response to an elicitor from green algae Ulva spp. Plant, Cell and Environment, 27, 917–928.
Crusius, L. U., Forcelini, C. A., Sanhueza, R. M. V., & Fernandes, J. M. C. (2002). Epidemiology of apple leaf spot. Fitopatologia Brasileira, 27, 65–70.
Dantas, A. C. M., Silva, M. F., & Nodari, R. O. (2009). Avanços genéticos da macieira no controle de doenças. [Genetic advances in the control of apple diseases.]. In M. J. Stadnik (Ed.), Manejo integrado de doenças da macieira. [Integrated management of apple diseases.] (pp. 127–152). Florianópolis: CCA-UFSC.
Daykin, M. E., & Milholland, R. D. (1984). Histopathology of ripe rot caused by Colletotrichum gloeosporioides on Muscadine grape. Phytopathology, 74, 1339–1341.
Dean, R. A. (1997). Signal pathways and appressorium morphogenesis. Annual Review of Phytopathology, 35, 211–234.
Deising, H. B., Werner, S., & Wernitz, M. (2000). The role of fungal appressoria in plant infection: Review. Microbes and Infection, 2, 1631–1641.
Delgado, D. Z., de Freitas, M. B., & Stadnik, M. J. (2013). Effectiveness of saccharin and ulvan as resistance inducers against rust and angular leaf spot in bean plants (Phaseolus vulgaris). Crop Protection, 47, 67–73.
Diéguez-Uribeondo, J., Förster, H., Soto-Estrada, A., & Adaskaveg, J. E. (2005). Subcuticular-intracellular hemibiotrophic and intercellular necrotrophic development of Colletotrichum acutatum on almond. Phytopathology, 95, 751–758.
Ferreira, E. M., Alfenas, A. C., Mafia, L. A., & Maia, R. G. (2006). Eficiência de fungicidas sistêmicos para o controle de Cylindrocladium candelabrum em eucalipto. [Efficiency of systemic fungicides for control of Cylindrocladium candelabrum in eucalypt.]. Fitopatologia Brasileira, 31, 469–475.
Freitas, M. B., & Stadnik, M. J. (2012). Race-specific and ulvan-induced defense responses in bean (Phaseolus vulgaris) against Colletotrichum lindemuthianum. Physiological and Molecular Plant Pathology, 77, 1–6.
Gomes, S., Prieto, P., Martins-Lopes, P., Carvalho, T., Martin, A., & Guedes-Pinto, H. (2009). Development of Colletotrichum acutatum on tolerant and susceptible Olea europaea L. cultivars: a microscopic analysis. Mycopathologia, 168, 203–211.
Gonçalves, A. E., & Stadnik, M. J. (2012). Interferência de ulvana no desenvolvimento e melanização de apressórios de Colletotrichum gloeosporioides. [Interference of ulvan on appressoria development and melanization of Colletotrichum gloeosporioides.]. Tropical Plant Pathology, 37, 431–437.
González, E., Sutton, T. B., & Correll, J. C. (2006). Clarification of the etiology of Glomerella leaf spot and bitter rot of apple caused by Colletotrichum spp. based on morphology and genetic, molecular, and pathogenicity tests. Phytopathology, 96, 982–992.
Hugovieux, V., Centis, S., Lafitte, C., & Esquerre-Tugaye, M. T. (1997). Induction by a-L-arabinose and a-L-rhamnose of endopolygalacturonase gene expression in Colletotrichum lindemuthianum. Applied and Environmental Microbiology, 63, 2287–2292.
Jaulneau, V., Lafitte, C., Jacquet, C., Fournier, S., Salamagne, S., Briand, X., et al. (2010). Ulvan, a sulfated polysaccharide from green algae, activates plant immunity through the jasmonic acid signaling pathway. Journal of Biomedicine and Biotechnology. doi:10.1155/2010/525291.
Kinkel, L. L. (1997). Microbial population dynamics on leaves. Annual Review of Phytopathology, 35, 327–347.
Kolattukudy, P. E., Kim, Y. K., Li, D., Liu, Z. M., & Rogers, L. (2000). Early molecular communication between Colletotrichum gloeosporioides and its host. pp. 78-98. In D. Prusky, S. Freeman, & M. B. Dickman (Eds.), Colletotrichum: Host specificity, pathology and host–pathogen interaction. St. Paul, MN, USA: The American Phytopathological Society.
Mendgen, K., & Hahn, M. (2002). Plant infection and the establishment of fungal biotrophy: Review. Trends in Plant Science, 10, 1–5.
Morin, L., Derby, J. A. L., & Kokko, E. G. (1996). Infection process of Colletotrichum gloeosporioides f. sp. malvae on Malvaceae weeds. Mycological Research, 100, 165–172.
Munaut, F., & Maraite, H. (1998). Conidium germination and appressorium penetration of Colletotrichum gloeosporioides on Stylosanthes guianensis. Phytopathology, 146, 19–26.
Oh, B., Kim, K. D., & Kim, Y. S. (1998). A microscopic characterization of the infection of green and red pepper fruits by an isolate of Colletotrichum gloeosporioides. Journal of Phytopathology, 146, 301–303.
Paulert, R., Talamini, V., Cassolato, J. E. F., Duarte, M. E. R., Noseda, M. D., Smania, J. A., et al. (2009). Effects of sulfated polysaccharide and alcoholic extracts from green seaweed Ulva fasciata on anthracnose severity and growth of common bean (Phaseolus vulgaris L.). Journal of Plant Diseases and Protection, 116, 263–270.
Peres, N. A., Timmer, L. W., Adaskaveg, J. E., & Correll, J. C. (2005). Lifestyles of Colletotrichum acutatum: Review. Plant Disease, 89, 784–796.
Shane, W. W., & Sutton, T. B. (1981). Germination, appressorium formation, and infection of immature and mature apple fruit by Glomerella cingulata. Phytopathology, 71, 454–457.
Shisler, D. A., Jackson, M. A., & Bothast, R. J. (1991). Influence of nutrition during conidiation of Colletotrichum truncatum on conidial germination and efficacy in inciting disease in Sesbania exaltata. Phytopathology, 81, 458–461.
Sutton, B. C. (1980). The Coelomycetes. London, UK: Commonwealth Mycological Institute.
Waipara, N. W., Obanor, F. O., & Walter, M. (2002). Impact of phylloplane management on microbial populations. New Zealand Plant Protection, 55, 125–128.
Wang, C. X., Zhang, Z. F., Li, B. H., Wang, H. Y., & Dong, X. L. (2012). First report of Glomerella leaf spot of apple caused by Glomerella cingulata in China. Plant Disease, 96, 912.
Wharton, P. S., & Diéguez-Uribeondo, J. (2004). The biology of Colletotrichum acutatum. Anales del Jardín Botánico de Madrid, 61, 3–22.
Wharton, P. S., Julian, A. M., & O’Connell, R. J. (2001). Ultrastructure of the infection of Sorghum bicolor by Colletotrichum sublineolum. Phytopathology, 91, 149–158.
Acknowledgments
The authors thank the Brazilian Ministry of Education Agency for Graduate Studies (CAPES) for granting the M.Sc.-scholarship to the first author. M.J.S is a research member of the National Council for Scientific and Technological Development (CNPq).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Araújo, L., Gonçalves, A.E. & Stadnik, M.J. Ulvan effect on conidial germination and appressoria formation of Colletotrichum gloeosporioides . Phytoparasitica 42, 631–640 (2014). https://doi.org/10.1007/s12600-014-0404-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12600-014-0404-7