Abstract
This study examined the role of indole-3-acetic acid (IAA) during the early stage of infection of rapeseed (Brassica napus L.) by the obligate biotrophic protist Plasmodiophora brassicae Woron., which causes clubroot disease. Treatment of infected B. napus seedlings with exogenous IAA promoted the development of clubroot disease, including an increase in the number and size of galls, whereas treatment with N-1-naphthylphthalamic acid (NPA) attenuated the disease and altered the location and size of the root nodules, resulting in a reduced disease index. An increased level of free IAA was detected in the roots at 3 and 7 days after inoculation (DAI) compared with the control, suggesting the activation of IAA signaling in the early stage of P. brassicae infection. Moreover, IAA treatment caused no significant change in the germination rate of the resting spores but resulted in enhanced root hair infection. RT–PCR and quantitative real-time PCR (qPCR) were used to analyze the expression of IAA homeostasis-related genes (3, 7, and 10 DAI) to identify gene expression patterns in the roots and leaves of B. napus after inoculation. Five IAA biosynthesis-related genes examined in this study were induced in the roots after inoculation with P. brassicae but not in the leaves. The rapid induction of BnAAO4 expression at 3 DAI may be responsible for the overproduction of IAA during the early stages of infection. This study suggests that IAA acts as a signaling molecule that putatively stimulates root hair infection in the early response of B. napus to P. brassicae infection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Clubroot disease of cruciferous crops is caused by the obligate biotrophic protist Plasmodiophora brassicae (Dixon 2009). Brassica napus L., one of the most important oilseed crops in the world, has suffered from clubroot disease in recent years, posing a serious threat to the production of B. napus in China (Ren et al. 2012). The infection of cruciferous hosts by P. brassicae leads to cell elongation and cell division in the infected roots and hypocotyls, resulting in gall formation, with galls of different sizes forming in the roots (Hwang et al. 2011). Gall formation alters the water and nutrient supply to the plant because the vasculature is destroyed, greatly inhibiting plant growth (Ludwig-Muller 2009b).
Clubroot is a soil-borne disease, and resting spores of P. brassicae can survive in the soil for years, making control of the disease difficult under field conditions once the soil is contaminated (Kageyama and Asano 2009). Crop rotation based on standard agronomic recommendations may not significantly reduce the level of clubroot inoculum in the soil (Niwa et al. 2008). Chemical control can alleviate the disease but cannot reduce the viable resting spores in the soil or completely eradicate the disease. To ensure the yield and quality of the crop, the development of varieties resistant to clubroot disease is the most effective economic management strategy (Deora et al. 2012). Few clubroot-resistant B. napus cultivars are available for commercial production in China, and the pathogen has evolved increasingly virulent pathotypes. Therefore, understanding the molecular mechanisms of clubroot disease development is essential. It may provide a theoretical basis for breeding resistant varieties and aid in the identification of relevant genes for modulation with genetic engineering methods to develop resistant varieties.
The life cycle of the pathogen consists of two distinct phases: the primary phase, when it is restricted to the root hairs of the infected plant, and the secondary phase, which occurs in the cortex and stele of the hypocotyls and roots, leading to abnormal root growth and clubroot formation in susceptible hosts (Feng et al. 2013). The involvement of auxin and cytokinin as pathogenicity factors has been demonstrated by the correlation of clubroot disease symptoms with an increase in the levels of these plant hormones, which cause increased cell division and cell elongation during the late infection stage (Devos et al. 2005; Devos and Prinsen 2006; Jubault et al. 2013; Siemens et al. 2006). It was reported that the free indole-3-acetic acid (IAA) content fluctuated in infected roots at all the time points tested (10–42 days after inoculation [DAI]), although some studies have shown conflicting results. IAA is generally higher in infected roots than in control roots of the same age, which suggests the involvement of IAA accumulation during the late stage of gall formation (Ludwig-Muller 2009a). However, the level of IAA in infected cabbage roots was much lower than uninfected roots when the pathogen produced resting spores at 28 DAI (Kavanagh and Williams 1981). These complicated results may be explained by differences in the interactions between different hosts and the pathogen.
Transcriptomic and proteomic experiments have provided evidence for the involvement of IAA pathway-related genes during gall formation in clubroot disease (Cao et al. 2008; Siemens et al. 2006, 2009; Sundelin et al. 2011). The expression levels of nitrilase (Ando et al. 2008; Grsic-Rausch et al. 2000; Siemens et al. 2006), myrosinase (Searle et al. 1982; Sundelin et al. 2011), aldehyde oxidase (Ando et al. 2006), auxin-conjugate hydrolase (LudwigMuller et al. 1996; Schuller and Ludwig-Muller 2006), and other enzymes involved in auxin homeostasis are induced during the late stage of the infection process. The importance of nitrilase in gall development was shown with a nit1 mutant of the model plant Arabidopsis, and antisense plants have shown reduced gall size that correlated with reduced gall IAA content (Grsic-Rausch et al. 2000; Neuhaus et al. 2000). Thus, the involvement of IAA in gall formation during the secondary phase of the pathogen’s life cycle was demonstrated.
There has been little research on IAA in the primary stage of infection, including the germination of the resting spores, zoospore release and penetration, the formation of the primary plasmodium, and the release of secondary zoospores, which do not produce visible symptoms in the root system. In this study, we focused on the primary stage of the infection process and the initiation of gall formation (3–10 DAI). By treating B. napus with exogenous IAA or N-1-naphthylphthalamic acid (NPA), which inhibits the polar transport of IAA in the plant, we clarified the effect of IAA on clubroot disease. The level of free IAA during the early stage of infection was also investigated. RT-PCR and qPCR were used to examine the expression of IAA-biosynthesis-related genes to determine the gene expression patterns in the roots and leaves of B. napus soon after P. brassicae inoculation (3, 7, and 10 DAI), defined as the ‘root hair infection stage’. The general role of IAA in B. napus clubroot disease was thus clarified and may provide the basis for identifying the molecular mechanism underlying clubroot disease.
Materials and methods
Plant material and pathogen isolate
The clubroot-susceptible B. napus cultivar ‘zhongshuang 11’ was used as the plant material. The root galls used in this study were collected from clubroot-infested field plots in Zhi’jiang, Hubei, China. The strain of the resting spores isolated from the galls was identified as pathotype 4 (Ji et al. 2013), according to the differential classification of Williams (1966).
The resting spores of P. brassicae were isolated as the procedure described by Castlebury et al. (1994). Approximately 5 g of fresh galled roots was homogenized in a blender in 50 ml of sterile distilled water (sdH2O) for 2 min at high speed, and the resultant homogenate was filtered through eight layers of cheesecloth. The filtrate was centrifuged at 4 °C in a 50-ml centrifuge tube at 4000 × g for 10 min in a swinging-bucket rotor. The supernatant was discarded, and the pellet was resuspended in 5 ml of 50 % sucrose and centrifuged again at 2000 × g for 10 min. The resulting supernatant was transferred to a new 50-ml tube and diluted with 30 ml of sdH2O before centrifugation at 4000 × g for 10 min. The pellet was resuspended in 5 ml of sdH2O and centrifuged as above to remove any remaining sucrose. The supernatant was removed, and the resulting spore pellet was resuspended in 5 ml of sdH2O. The spore suspension was adjusted to a final concentration of 5 × 106 spores/ml with sdH2O.
Inoculation of resting spores and disease investigation
Seeds of “zhongshuang 11” were sown in commercial sterilized soil (a complex of soil, peat, and composted pine bark) at 22 °C/18 °C day/night temperatures with a photoperiod of 14 h light and 10 h dark. The soil was inoculated with resting spores of P. brassicae 1 week after sowing by the addition of 1 ml of 5 × 106 spores/ml in sdH2O to the soil at the base of each plant. The control plants were not inoculated but were treated with distilled water in the same way.
The disease was investigated by pulling up the seedlings at 35 DAI. Disease severity was assessed qualitatively based on the disease index (Hwang et al. 2012): 0 = no visible root galls; 1 = very slight swelling of the lateral roots; 2 = moderate swelling of the roots; and 3 = presence of large galls on the primary roots. The disease index was calculated from the four-grade scale as follows: disease index = (1n1 + 2n2 + 3n3 + 4n4) × 100/4Nt, where n1–n4 represents the number of plants in the indicated grade and Nt represents the total number of plants tested. The data presented are the mean ± standard errors (SE) of at least three replicates.
Treatment of inoculated seedlings with exogenous IAA or its inhibitor NPA
Rapeseed seedlings were treated with different concentrations (10−7 and 10−10mol/L) of IAA (Sigma-Aldrich, St Louis, MO, USA) by adding IAA to the soil on different days (0, 2, 4, 6, and 8) after inoculation with P. brassicae. Seedlings were treated with NPA (Sigma-Aldrich) (10−7 mol/L) by spraying the leaves after inoculation of the seedlings with P. brassicae. The leaves were covered with film to retain moisture. At least 30 plants were analyzed for each line and each treatment. The disease was investigated as described above.
Treatment of germinating resting spores with exogenous IAA
The adjusted resting spore suspension was disinfected with freshly prepared 2 % (w/v) chloramine-T solution plus 0.05 % (v/v) Tween 20 at room temperature for 25 min and then washed twice with sdH2O by centrifugation, as described previously (Asano et al. 1999). The resulting pellet was suspended in an antibiotic solution containing 1 g/L colistin sulfate, 1 g/L vancomycin hypochlorite, and 6 g/L cefotaxime sodium and incubated at 25 °C in the dark for 1 day. The suspension was then washed twice by centrifugation in sdH2O. Finally, the spores were suspended in sdH2O and adjusted to a concentration of 5 × 106 spores/ml. The optimal conditions of pH 5.5 and temperature of 25 °C were provided for spore generation (Luo et al. 2014). Different concentrations of IAA were continuously added to the disinfected resting spore suspension on different days (0, 2, 4, 6, and 8) after inoculation at final concentrations of 10−7, 10−8, and 10−9 mol/L.
Spore germination was analyzed every second day from 1 to 9 DAI. A droplet of spore suspension was placed on a microscope slide and stained with orcein. The proportion of germinated spores was determined under a light microscope by counting the empty spores (Friberg et al. 2005). The statuses of 200 spores (germinated or non-germinated) on three different microscope slides were analyzed for each replicate.
Treatment of root hair infection with exogenous IAA
An improved solution culture technique was employed to observe root hair infection (Luo et al. 2014). Seeds of rapeseed were germinated on wet filter paper for 3 days (25 °C, 16 h photoperiod). Culture solution was added to a10 × 20 × 35 cm plastic tray and consisted of half strength Hoagland nutrient solution. A 0.5 cm thick plank with holes (2 cm diameter) covered the plastic tray. The seedlings were fastened to round pieces of foam (1 cm thick), which were placed in the holes. The spore suspension was added to the solution to give a final concentration of 106 spores/ml. IAA was then added to the solution on different days (0, 2, 4, 6 and 8) after inoculation to give a final concentration of 10−7 mol/L.
Roots were rinsed clean with tap water, and a segment (0.5 cm long) was cut from the top 0 to 1 cm of taproot at different days (3, 5, 7 and 9 days) after inoculation. The segment was stained with FAA Phloxine B and was mounted on glass with a coverslip to be observed and imaged with an optical microscope with a × 20 objective lens (Donald et al. 2008). The number of total root hairs and root hairs with primary infection were counted in a field of view at 7 DAI. At least 20 segments were analyzed for each treatment.
Extraction and determination of free IAA
Infected and control roots and leaves were harvested at 3, 7, and 10 DAI, washed thoroughly, and dried on filter paper. Approximately 0.1 g of fresh roots and leaves was frozen in liquid nitrogen and ground into powder. Free IAA was extracted and quantified as previously described (Li et al. 2011). A 0.5 ml aliquot of 1-propanol:H2O:concentrated HCl (2:1:0.002, v/v/v) was added, and the solution was shaken for 30 min at 4 °C. Dichloromethane (1 ml) was added, the solution was shaken again for another 30 min, and centrifuged at 12,000 × g for 10 min. After centrifugation, the bottom organic phase (approximately 1 ml) was transferred to a 10-ml tube and evaporated to dryness with a constant stream of nitrogen. Each sample was resolubilized in 80 % methanol (1 ml), vortexed for 5 min, and then extracted using a C18 SPE cartridge (CNWBOND HC-C18, 500 mg, 3 ml). The elutate (2 ml) was evaporated to dryness under vacuum in a water bath at 35 °C, and finally dissolved in 200 μL of methanol:0.05 % formic acid (1:1, v/v). The solution was filtered through a 0.45-μm microfilter before analysis.
The samples were separated by reverse-phase high performance liquid chromatography (HPLC) and scanned with electrospray ionization–tandem mass spectrometry (RP-HPLC/ESI–MS/MS) (ABI 4000 Q-Trap, Applied Biosystems, Foster City, CA, USA) in the MRM mode, with a 50 ms dwell time in the enhanced product ion scan mode; precursor ions were fragmented with a collision energy of −25 V, and the products were scanned in the m/z range of 80–355.
Identification of IAA-related sequences in B. napus
Coding sequences (CDS) of Arabidopsis IAA biosynthesis and regulatory genes were used to search for B. napus homologs obtained from the NCBI database (http://www.ncbi.nlm.nih.gov).Target sequences with the highest identity with the identified genes in Arabidopsis were counted as the same gene.
RNA isolation and RT–PCR
The seedlings were dug from the soil at 3, 7, and 10 DAI, and the roots were washed with sdH2O, cut at the root collar, and immediately frozen in liquid nitrogen. The root and leaf samples were stored at −80 °C until RNA extraction. Approximately 30 seedlings were collected for each treatment. To check that the inoculation was successful, clubroot susceptibility was also evaluated on randomly selected samples from each treatment group at 35 DAI to confirm the presence or absence of clubroot disease.
Total RNA was isolated from control and infected roots and leaves of B. napus at different times after inoculation (3, 7, and 10 DAI) with TRIzol Reagent (Invitrogen, Karlsruhe, Germany), according to the manufacturer’s instructions. First-strand cDNA was generated from 3 μg total by using the Superscript first-strand synthesis system (Invitrogen, Foster City, CA, USA). The primers used for real-time PCR (RT–PCR) and quantitative real-time PCR (qPCR) were designed with Primer Premier 5.0 software and synthesized commercially (BGI, Shenzhen, China), as shown in Table 1. The standard PCR cycles for RT–PCR were as follows: one cycle at 94 °C for 5 min, followed by 32 cycles at 94 °C for 30 s, 57 °C for 30 s, and 72 °C for 30 s, and a final extension step at 72 °C for 5 min. The Actin1 gene was used as the control. The PCR products were separated on 1.2 % agarose gel and stained with ethidium bromide.
For qPCR, 20 μL qPCR samples were run in three technical replicates on an ABI 7500 Real Time PCR System (Applied Biosystems, Foster,CA, USA) using 5 μL first-strand cDNA and SYBR Green PCR Master Mix (Applied Biosystems). PCR cycles were as follows: 1 cycle of 1 min at 94 °C, followed by 40 cycles at 94 °C for 15 s and 58 °C for 45 s. Following amplification, all products were subjected to melt curve analysis. The Actin1 gene was used as the reference gene to normalize the total amount of cDNA in each reaction. There were three biological replicates for these study, and relative fold changes were calculated with 2−ΔΔCt using the SDS software from the 7500 Real Time PCR System (Applied Biosystems) (Rieu and Powers 2009).
Results
Effects of IAA and NPA on clubroot disease
To better understand the physiological effects of IAA in clubroot disease, IAA and its inhibitor NPA were applied to infected plants and examined at 35 DAI (Table 2 and Fig. 1). The data confirmed that the infection was successful, and control plants did not develop the disease. The typical symptoms of clubroot disease include the formation of differently sized galls on the primary root and/or lateral roots (Fig. 1). Higher concentrations of IAA did not alter the locations of the galls on the primary root but increased the plants’ susceptibility to P. brassicae, increasing the number and size of galls, which increased the disease index. Conversely, treatment of P. brassicae-inoculated B. napus seedlings with NPA, an inhibitor of the polar transport of IAA, resulted in a reduced disease rate and disease index and altered locations and sizes of the root galls.
Determination of free IAA content
The concentrations of endogenous free IAA were determined in the control plants and plants infected with P. brassicae for 3, 7, and 10 DAI (Fig. 2). The production of IAA was significantly increased in the inoculated roots at 3 DAI. The IAA content then decreased to nearly that of the control at 10 DAI. The free IAA was higher in the leaves after P. brassicae inoculation than in the control. A higher content of IAA was detected in the leaves than in the roots.
Effect of IAA treatment on the germination rate of resting spores
The germination of the resting spores started at1 DAI and increased steadily from 3 to 9 DAI before reaching a constant rate (Fig. 3). At 11 DAI, the germination rate of the resting spores was 61 %. Treatment with different concentrations of IAA did not significantly alter the germination rate of the resting spores compared to control spores.
Effect of IAA treatment on root hair infection
An improved solution culture technique was employed to study the role of IAA on root hair infection. To better understand the role of IAA on root hair infection, the number of root hairs, including infected and uninfected hairs, was calculated every 2 days after treatment. In this study, infection was first observed in root hairs at 3 DAI, and maximal root hair infection was observed at 9 DAI, which corresponds to the germination timeline of resting spores. Considering that the IAA content increased from 3 to 7 DAI, we focused on the 7 DAI time point (Fig. 4a–d). Pathogen inoculation enhanced the number of total root hairs compared to the control, but there was no significant difference between control and inoculated plants under IAA treatment. Furthermore, IAA treatment resulted in an increased number of total root hairs and infected root hairs, which subsequently exhibited a higher infection rate at 7 DAI compared to infection without IAA (57.69 % versus 43.24 %) (Fig. 4e).
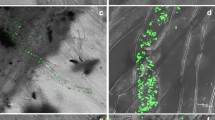
Root hair infection of B. napus roots at 9 DAI with IAA treatment. Root segments were stained by Phloxine B. Staining in root hair indicated the presence of root hair infection. Control (a) and inoculated (b) roots not treated with IAA. Control (c) and inoculated (d) roots treated with IAA. Numbers of infected and uninfected root hairs are shown in (e). Different letters above the bars indicate that the differences are significant (P < 0.05)
Expression patterns of IAA-related genes during the early response of B. napus L. to P. brassicae
In this study, we looked for IAA-related gene sequences of B. napus in the GenBank database to identify sequences with homology to Arabidopsis genes. Eighteen sequences with high homology to IAA synthesis-related genes in Arabidopsis were identified, which encoded proteins involved in the major pathways of IAA synthesis, including the indole-3-acetaldoxime (IAOx), indole-3-pyruvate (IPyA), and tryptamine pathways (Fig. 5a). No genes in the indole-3-acetamide (IAM) pathway were identified.
Summary of the proposed IAA biosynthetic pathway in plants (a) and the expression patterns of genes involved in this pathway after infection with P. brassicae by RT–PCR (b) and qPCR (c). Enzyme names shown in bold indicate genes induced by P. brassicae infection. Different letters above the bars indicate that the differences are significant (P < 0.05). IAM indole-3-acetamide, IPyA indole-3-pyruvic acid, TAM tryptamine, IAOx indole-3-acetaldoxime, IAAld indole-3-acetaldehyde, IAN indole-3-acetonitrile, GSL glucosinolates, CYP83B cytochrome P450 mono-oxygenase, FMO flavin monooxygenase-like, AAO aldehyde oxidase, Nit nitrilase, GH3 Gretchen Hagen 3
To determine the role of IAA in the regulation of the early infection stage of clubroot disease, the expression patterns of IAA-related genes in B. napus at early time points (3, 7, and 10 DAI) after inoculation were analyzed by RT–PCR (Fig. 5b). Five genes were identified after P. brassicae inoculation by semi-quantitative RT–PCR. Considering that RT-PCR cannot detect gene expression levels quantitatively, further study was conducted using qPCR (Fig. 5c). Through qPCR analysis, detailed relative gene expression levels were determined, especially for genes with low expression levels such as BnNit4. In general, the qPCR measurements were well correlated with the RT–PCR results. The expression of BnAAO4 (aldehyde oxidase) showed a transient increase at 3 DAI in the inoculated roots. The expression of BnFMO5 (flavin monooxygenase-like) and BnGH3-1 (Gretchen Hagen 3) increased consistently in inoculated roots compared with the control roots during the first stage of infection (3–10 DAI). The expression of BnCYP83B1 (cytochrome P450 mono-oxygenase) increased significantly at 3 and 7 DAI in the infected roots. BnNit4 (nitrilase) expression, which was extremely low in the roots, increased significantly at 10 DAI in the roots. All the genes investigated displayed higher expression in the leaves than in the roots and did not show significant changes in leaves compared with the control.
Discussion
Recent research has addressed the role of IAA in gall formation during the late stage of clubroot disease (Devos and Prinsen 2006). The initiation of cell division and cell enlargement, which are responsible for gall formation, are possibly regulated by changes in IAA balance (Ludwig-Muller and Schuller 2008). The gall formation observed in the late infection stage can be considered a secondary effect of the altered morphology of the host; disease development is triggered in the early stage. A rapid increase in free IAA levels is detected after P. brassicae inoculation in B. napus. Therefore, we focus on the primary stage of the infection process (3–10 DAI) to determine the regulatory role of IAA in clubroot disease.
IAA promotes the development of clubroot disease in B. napus L
Treatment with a higher concentration of IAA results in an increased disease index, including the number of galls on the lateral roots and the size of the galls on the primary roots, suggesting that early treatment with IAA promotes the development of clubroot disease in B. napus.
Auxin is the only plant hormone with polar transport characteristics, and its polar transport allows its broad distribution to control differences in plant growth and development (Hayashi 2012). This fact suggests that the transport of IAA from the stem apex to the root is required for gall development. It is also demonstrated in a study of the Arabidopsis mutant alh1, which showed inhibited auxin transport and had a defect in the crosstalk between ethylene and auxin (Vandenbussche et al. 2003). The mutant shows attenuated disease symptoms after P. brassicae inoculation, whereas the addition of 1-naphthaleneacetic acid (NAA) to the infected mutant does not rescue the mutant phenotype (Devos et al. 2006). However, infected wild-type plants show an increased infection rate when treated with NAA. These results also suggest that IAA transport plays a central role in gall development.
In summary, treatment with IAA resulted in enhanced disease severity and inhibited IAA transport, causing attenuated disease symptoms. These results emphasize the significance of IAA in clubroot disease in B. napus.
Rapid increase in free IAA after inoculation with P. brassicae
Reports have shown that the IAA content of the cruciferous plants A. thaliana and B. rapa is dramatically altered during clubroot disease (Grsic et al. 1999; Ludwig-Muller et al. 2009). However, it is generally accepted that increased levels of IAA during the later infection stage play an important role in clubroot disease (Devos et al. 2006; Devos and Prinsen 2006; Ludwig-Muller and Schuller 2008; Ludwig-Muller 2009a).
In this study, we detect a rapid increase in IAA production in the root at 3 and 7 DAI with P. brassicae. The result is supported by the rapidly induced expression of IAA biosynthesis-related genes, which shows that de novo IAA synthesis is elevated quickly after P. brassicae inoculation. Devos et al. (2005) detect a rapid increase in IAAconjugate levels but not in IAA synthesis during primary infection at 6 DAI. Further study suggests that the induction of IAA correlates with an increase in seedling growth and xyloglucan endotransferase/hydrolase, leading to wall loosening. There has been little research that focused on early infection after inoculation, especially at 3 DAI. Complex perception, transduction, and exchange of signals happen at this time point. In light of these phenomena, IAA is proposed to be a signal molecule or involved in the induction of plant development involved in the early response of B. napus to P. brassicae. Taken together, these results are interesting and prompt the study of the exact role of IAA during early infection.
Treatment with IAA promoted root hair infection
In the life cycle of P. brassicae, the primary infection stage occurs in root hair. The resting spores germinate and then release primary zoospores, which can penetrate through the cell walls of root hairs (Kageyama and Asano 2009). Resting spores germinate not only in the presence of a host plant but also spontaneously (Friberg et al. 2005; Rashid et al. 2013). Spontaneous germination is affected by different environmental conditions. The production of IAA is significantly increased at 3–7 DAI, which coincide with the germination of most resting spores and infection of zoospores. In light of this fact, different concentrations of IAA are added to suspensions of resting spores under optimum germination conditions. However, treatment with different concentrations of IAA does not significantly alter the germination rate of the resting spores.
Then, root hair infection is investigated by dynamic observation of infected roots. It has been suggested that treatment with IAA results in increased numbers of both total root hairs and infected root hairs. It is well known that a low concentration of exogenous IAA triggers an increased density of lateral roots and root hairs (Saini et al. 2013). Therefore, it is speculated that the increased total number of root hairs is due to the increased IAA content. A previous study suggests that IAA increases plant disease susceptibility to plant–pathogen interactions, leading to the loosening of the plant cell wall, thus weakening its role in the plant defense against pathogens (Bari and Jones 2009). The rapid induction of IAA likely facilitates the primary infection phase by altering the cell wall structure and allowing penetration by zoospores.
It can be deduced from these data that exogenous IAA treatment resulted in higher root hair infection. However, determining whether it directly correlated to the increased disease index and disease rate requires more study. Further research is required to establish the cytoarchitecture and cytochemistry of P. brassicae-inoculated roots and to determine the exact role of IAA during the early infection stage of clubroot disease.
Regulation of IAA homeostasis during the early stage of disease development
Nitrilase is known to play a key role in several biosynthetic pathways in the Brassicaceae family because it catalyzes the last step in the IAOx pathway of IAA biosynthesis (Mashiguchi et al. 2011). The expression of NIT1 is induced during the later stage of infection (14, 28, and 35 DAI) in A. thaliana and B. rapa, which indicates a major role for nitrilase in gall formation (Devos et al. 2005; Devos et al. 2006; Grsic-Rausch et al. 2000; Siemens et al. 2006). A NIT1 mutant (nit1) displays smaller root galls and lower free IAA levels in the clubbed roots of infected plants, emphasizing the relevance of enhanced auxin synthesis in pathogenicity (Neuhaus et al. 2000). In this study, the expression of BnNit4 is extremely low at the earlier time points and is significantly induced at 10 DAI, when cortex infection is initiated. These findings correlate with the results of the present study, in that the increased production of IAA via the nitrilase pathway likely affects gall formation during the later stage of infection. Taken together, these studies show that BnNit4 may not be directly responsible for the enhanced content of IAA at 3–7 DAI.
Indole glucosinolates are precursors for IAA biosynthesis, and they have been found to correlate with disease severity and indole glucosinolate content (Ludwig-Muller 2009a; Ludwig-Muller et al. 1999). CYP83B1, which converts IAOx to indole glucosinolates, is upregulated at 10 DAI and then downregulated at 23 DAI in Arabidopsis, according to a microarray analysis (Siemens et al. 2006). Similarly, the expression of BnCYP83B1 is slightly increased at 3 and 7 DAI but is not induced at 10 DAI. Despite the increased expression of BnCYP83B1 at the earlier time points, BnNit4, which is directly involved in IAA synthesis, was not induced at those times. Therefore, BnCYP83B1 may not be a direct regulator of the increase in IAA in response to P. brassicae infection, and indole glucosinolates may not be the primary source of IAA.
Aldehyde oxidase (AAO) is presumed to be responsible for the conversion of indole-3-acetaldehyde (IAAld) to IAA, and it is involved in IAA biosynthesis via the IAOx and TAM pathways (Mashiguchi et al. 2011). In this study, the rapidly induced expression of BnAAO4 at 3 DAI may be responsible for the overproduction of IAA at 3 and 7 DAI during the first stage of infection. Of the two AAO genes identified in B. rapa (BrAAO1 and BrAAO2), the expression of BrAAO1 is enhanced in the infected roots from 15 DAI, when clubroot is still undetectable, and expression of BrAAO2 is repressed (Ando et al. 2006). These different expression patterns also indicate the diverse roles of the AAO gene family in the plant response to clubroot disease. However, AAO has also been reported to be involved in the biosynthesis of abscisic acid (ABA) (Seo and Koshiba 2002). AAO3 plays an important role in ABA biosynthesis in the leaves and seeds of Arabidopsis (Seo et al. 2004). Whether the early increase in expression of BnAAO4 is involved in the biosynthesis of ABA has not been determined. To date, little attention has been paid to the involvement of the AAO proteins in clubroot disease. However, these results suggest a promising research direction for clubroot disease.
Yucca-encoded flavin monooxygenase-like proteins (FMOs) have been implicated in the conversion of IPyA to IAA in Arabidopsis, which is the main IAA biosynthetic pathway in Arabidopsis (Mashiguchi et al. 2011). The expression of BnFMO5 is induced at 3–10 DAI in pathogen-inoculated roots, which suggests the involvement of BnFMO5 in the response of B. napus to P. brassicae. However, due to the constitutively induced expression of BnFMO5 in the early infection stage, BnFMO5 cannot be directly linked to the increase in IAA levels at 3 to 7 DAI. Although the YUC genes participate in plant development and different stress responses, there is limited information about their involvement in disease responses (Kim et al. 2011). Because it is involved in the major IAA biosynthetic pathway, a detailed investigation of BnFMO5 will be key to understanding the regulation of IPyA-dependent IAA biosynthesis in the development of clubroot disease.
The GH3 genes encode IAA-amido synthetases, which are involved in the regulation of IAA homeostasis by conjugating IAA to amino acids by adenylation (Park et al. 2007). Most of the total auxin in plants is found in the conjugated form, and the formation of auxin conjugates is one of the important mechanisms regulating the activation or inactivation of IAA. GH3 proteins play roles in the plant defense responses of Arabidopsis and rice (Oryza sativa). Overexpression of GH3-8 in rice resulted in the increased accumulation of conjugated IAA (IAA–Asp) and reduced levels of free IAA compared to wild-type plants, which enhanced the plants’ resistance to Xanthomonas oryzae pv. Oryzae (Ding et al. 2008). The increased expression of several members of the GH3 gene family is observed at 10 DAI in Arabidopsis with an ATH1 genome array, whereas BnGH3-1 shows transiently increased expression at 3 DAI, but not at 10 DAI, in B. napus (Siemens et al. 2006). The different expression patterns in Arabidopsis and Brassica species, in terms of gene regulation or enzymatic activity, must be considered. The expression of BnGH3-1 increases from 3 to 10 DAI in the study. Because the GH3 family may markedly contribute to maintaining the level of IAA when excess amounts of IAA are produced, the induced expression of BnGH3-1 is consistent with the rapidly elevated production of free IAA content at 3–7 DAI. It has been speculated that not only the level of free IAA but also the level of total auxin are involved in the regulation of the early response of B. napus to P. brassicae.
In summary, the detailed relative expression levels, which were determined by qPCR, provide a better understanding of the role of IAA during early infection of P. brassicae in B. napus. The genes that are differentially expressed after pathogen infection were considered to respond directly to this infection. However, taking the IAA synthesis pathway into consideration, BnAAO4 may be directly responsible for the overproduction of IAA at 3 and 7 DAI during the first stage of infection.
Induced IAA signaling in the early response to P. brassicae in roots but not leaves
The genes investigated show higher expression in the leaves than in the roots, which confirms that the majority of IAA was generated in leaves. Despite this finding, most of the genes are induced by P. brassicae infection in the roots but not in the leaves. The slightly and quickly induced expression of IAA-biosynthesis-related genes in the root suggests that IAA acts as a signal molecule, regulating the early response to P. brassicae infection.
In conclusion, the IAA response is likely rapidly activated by pathogen inoculation and is involved in regulating the early response of B. napus to P. brassicae. The induced production of IAA results in an increased number of root hairs, which likely induces infection and disease development. However, the local changes induced at the cytochemical level early after infection must be examined to determine the precise influence of IAA on the early response. It is also suggested that BnAAO4 plays a crucial role in the early response of B. napus to P. brassicae, and further functional characterization is required to clarify the regulatory role of IAA in the development of this disease.
References
Ando, S., Tsushima, S., Tagiri, A., Kamachi, S., Konagaya, K., Hagio, T., et al. (2006). Increase in BrAO1 gene expression and aldehyde oxidase activity during clubroot development in Chinese cabbage (Brassica rapa L.). Molecular Plant Pathology, 7, 223–234.
Ando, S., Tsushima, S., Kamachi, S., Konagaya, K., & Tabei, Y. (2008). Alternative transcription initiation of the nitrilase gene (BrNIT2) caused by infection with Plasmodiophora brassicae Woron. in Chinese cabbage (Brassica rapa L.). Plant Molecular Biology, 68, 557–569.
Asano, T., Kageyama, K., & Hyakumachi, M. (1999). Surface disinfestation of resting spores of Plasmodiophora brassicae used to infect hairy roots of Brassica spp. Phytopathology, 89, 314–319.
Bari, R., & Jones, J. D. (2009). Role of plant hormones in plant defence responses. Plant Molecular Biology, 69, 473–488.
Cao, T., Srivastava, S., Rahman, M. H., Kav, N. N. V., Hotte, N., Deyholos, M. K., et al. (2008). Proteome-level changes in the roots of Brassica napus as a result of Plasmodiophora brassicae infection. Plant Science, 174, 97–115.
Castlebury, L. A., Maddox, J. V., & Glawe, D. A. (1994). A technique for the extraction and purification of viable Plasmodiophora brassicae resting spores from host root tissue. Mycologia, 86, 13.
Deora, A., Gossen, B. D., & Mcdonald, M. R. (2012). Effect of host resistance on infection by Plasmodiophora brassicae in canola. Canadian Journal of Plant Pathology, 34, 329–330.
Devos, S., & Prinsen, E. (2006). Plant hormones: a key in clubroot development. Communications of Agricultural &Appied Biological Science, 71, 869–872.
Devos, S., Vissenberg, K., Verbelen, J. P., & Prinsen, E. (2005). Infection of Chinese cabbage by Plasmodiophora brassicae leads to a stimulation of plant growth: impacts on cell wall metabolism and hormone balance. New Phytologist, 166, 241–250.
Devos, S., Laukens, K., Deckers, P., Van Der Straeten, D., Beeckman, T., Inze, D., et al. (2006). A hormone and proteome approach to picturing the initial metabolic events during Plasmodiophora brassicae infection on Arabidopsis. Molecular Plant Microbe Interaction, 19, 1431–1443.
Ding, X., Cao, Y., Huang, L., Zhao, J., Xu, C., Li, X., et al. (2008). Activation of the indole-3-acetic acid-amido synthetase GH3-8 suppresses expansin expression and promotes salicylate- and jasmonate-independent basal immunity in rice. Plant Cell, 20, 228–240.
Dixon, G. R. (2009). The occurrence and economic impact of Plasmodiophora brassicae and clubroot disease. Journal of Plant Growth Regulation, 28, 194–202.
Donald, E. C., Jaudzems, G., & Porter, I. J. (2008). Pathology of cortical invasion by Plasmodiophora brassicae in clubroot resistant and susceptible Brassica oleracea hosts. Plant Pathology, 57, 201–209.
Feng, J., Hwang, S. F., & Strelkov, S. E. (2013). Studies into primary and secondary infection processes by Plasmodiophora brassicae on canola. Plant Pathology, 62, 177–183.
Friberg, H., Lagerlof, J., & Ramert, B. (2005). Germination of Plasmodiophora brassicae resting spores stimulated by a non-host plant. European Journal of Plant Pathology, 113, 275–281.
Grsic, S., Kirchheim, B., Pieper, K., Fritsch, M., Hilgenberg, W., & Ludwig-Muller, J. (1999). Induction of auxin biosynthetic enzymes by jasmonic acid and in clubroot diseased Chinese cabbage plants. Physiologia Plantarum, 105, 521–531.
Grsic-Rausch, S., Kobelt, P., Siemens, J. M., Bischoff, M., & Ludwig-Muller, J. (2000). Expression and localization of nitrilase during symptom development of the clubroot disease in Arabidopsis. Plant Physiology, 122, 369–378.
Hayashi, K. (2012). The interaction and integration of auxin signaling components. Plant Cell Physiology, 53, 965–975.
Hwang, S. F., Ahmed, H. U., Zhou, Q., Strelkov, S. E., Gossen, B. D., Peng, G., et al. (2011). Influence of cultivar resistance and inoculum density on root hair infection of canola (Brassica napus) by Plasmodiophora brassicae. Plant Pathology, 60, 820–829.
Hwang, S. F., Ahmed, H. U., Zhou, Q., Strelkov, S. E., Gossen, B. D., Peng, G., et al. (2012). Assessment of the impact of resistant and susceptible canola on Plasmodiophora brassicae inoculum potential. Plant Pathology, 61, 945–952.
Ji, H. W., Ren, L., Chen, K. R., Xu, L., Liu, F., Sun, C. C., et al. (2013). Identification of physiological races of clubroot and resistance of rape cultivars to Plasmodiophora brassicae. Chinese Journal of Crop Science, 35, 301–306.
Jubault, M., Lariagon, C., Taconnat, L., Renou, J. P., Gravot, A., Delourme, R., et al. (2013). Partial resistance to clubroot in Arabidopsis is based on changes in the host primary metabolism and targeted cell division and expansion capacity. Functional & Integrative Genomics, 13, 191–205.
Kageyama, K., & Asano, T. (2009). Life cycle of Plasmodiophora brassicae. Journal of Plant Growth Regulation, 28, 203–211.
Kavanagh, J. A., & Williams, P. H. (1981). Indole auxins in Plasmodiophora infected cabbage roots and hypocotyls. Transactions of the British Mycological Society, 77, 125–129.
Kim, J. I., Murphy, A. S., Baek, D., Lee, S. W., Yun, D. J., Bressan, R. A., et al. (2011). YUCCA6 over-expression demonstrates auxin function in delaying leaf senescence in Arabidopsis thaliana. Journal of Experimental Botany, 62, 3981–3992.
Li, Y. H., Wei, F., Dong, X. Y., Peng, J. H., Liu, S. Y., & Chen, H. (2011). Simultaneous analysis of multiple endogenous plant hormones in leaf tissue of oilseed rape by solid-phase extraction coupled with high-performance liquid chromatography-electrospray ionisation tandem mass spectrometry. Phytochemical Analysis, 22, 442–449.
Ludwig-Muller, J. (2009a). Glucosinolates and the clubroot disease: defense compounds or auxin precursors? Phytochemistry Reviews, 8, 135–148.
Ludwig-Muller, J. (2009b). Plant defence - what can we learn from clubroots? Australasian Journal of Plant Pathology, 38, 318–324.
Ludwig-Muller, J., & Schuller, A. (2008). What can we learn from clubroots: alterations in host roots and hormone homeostasis caused by Plasmodiophora brassicae. European Journal of Plant Pathology, 121, 291–302.
LudwigMuller, J., Epstein, E., & Hilgenberg, W. (1996). Auxin-conjugate hydrolysis in Chinese cabbage: characterization of an amidohydrolase and its role during infection with clubroot disease. Physiologia Plantarum, 97, 627–634.
Ludwig-Muller, J., Pieper, K., Ruppel, M., Cohen, J. D., Epstein, E., Kiddle, G., et al. (1999). Indole glucosinolate and auxin biosynthesis in Arabidopsis thaliana (L.) Heynh. glucosinolate mutants and the development of clubroot disease. Planta, 208, 409–419.
Ludwig-Muller, J., Prinsen, E., Rolfe, S. A., & Scholes, J. D. (2009). Metabolism and plant hormone action during clubroot disease. Journal of Plant Growth Regulation, 28, 229–244.
Luo, H. C., Chen, G. K., Liu, C. P., Huang, Y., & Xiao, C. G. (2014). An improved culture solution technique for Plasmodiophora brassicae infection and the dynamic infection in the root hair. Australasian Journal of Plant Pathology, 43, 53–60.
Mashiguchi, K., Tanaka, K., Sakai, T., Sugawara, S., Kawaide, H., Natsume, M., et al. (2011). The main auxin biosynthesis pathway in Arabidopsis. Proceedings of the National Academy of Sciences of the United States of America, 108, 18512–18517.
Neuhaus, K., Grsic-Rausch, S., Sauerteig, S., & Ludwig-Muller, J. (2000). Arabidopsis plants transformed with nitrilase 1 or 2 in antisense direction are delayed in clubroot development. Journal of Plant Physiology, 156, 756–761.
Niwa, R., Nomura, Y., Osaki, M., & Ezawa, T. (2008). Suppression of clubroot disease under neutral pH caused by inhibition of spore germination of Plasmodiophora brassicae in the rhizosphere. Plant Pathology, 57, 445–452.
Park, J. E., Park, J. Y., Kim, Y. S., Staswick, P. E., Jeon, J., Yun, J., et al. (2007). GH3-mediated auxin homeostasis links growth regulation with stress adaptation response in Arabidopsis. Journal of Biological Chemistry, 282, 10036–10046.
Rashid, A., Ahmed, H. U., Xiao, Q., Hwang, S. F., & Strelkov, S. E. (2013). Effects of root exudates and pH on Plasmodiophora brassicae resting spore germination and infection of canola (Brassica napus L.) root hairs. Crop Protection, 48, 16–23.
Ren, L., Jia, J. G., Li, M., Liu, F., Cheng, Y. G., Chen, K. R., et al. (2012). Distribution of rapeseed clubroot disease in Hubei Province and evaluation of yield loss. Agricultural of Science & Technology, 13, 775–777.
Rieu, I., & Powers, S. J. (2009). Real-time quantitative RT-PCR: design, calculations, and statistics. Plant Cell, 21, 1031–1033.
Saini, S., Sharma, I., Kaur, N., & Pati, P. K. (2013). Auxin: a master regulator in plant root development. Plant Cell Report, 32, 741–757.
Schuller, A., & Ludwig-Muller, J. (2006). A family of auxin conjugate hydrolases from Brassica rapa: characterization and expression during clubroot disease. New Phytologist, 171, 145–157.
Searle, L. M., Chamberlain, K., Rausch, T., & Butcher, D. N. (1982). The conversion of 3-indolemethylglucosinolate to 3-indoleacetonitrile by myrosinase and its relevance to the clubroot disease of the cruciferae. Journal of Experimental Botany, 33, 935–942.
Seo, M., & Koshiba, T. (2002). Complex regulation of ABA biosynthesis in plants. Trends of Plant Science, 7, 41–48.
Seo, M., Aoki, H., Koiwai, H., Kamiya, Y., Nambara, E., & Koshiba, T. (2004). Comparative studies on the Arabidopsis aldehyde oxidase (AAO) gene family revealed a major role of AAO3 in ABA biosynthesis in seeds. Plant and Cell Physiology, 45, 1694–1703.
Siemens, J., Keller, I., Sarx, J., Kunz, S., Schuller, A., Nagel, W., et al. (2006). Transcriptome analysis of Arabidopsis clubroots indicate a key role for cytokinins in disease development. Molecular Plant Microbe Interaction, 19, 480–494.
Siemens, J., Graf, H., Bulman, S., In, O., & Ludwig-Muller, J. (2009). Monitoring expression of selected Plasmodiophora brassicae genes during clubroot development in Arabidopsis thaliana. Plant Pathology, 58, 130–136.
Sundelin, T., Jensen, D. F., & Lubeck, M. (2011). Identification of expressed genes during infection of Chinese cabbage (Brassica rapa subsp. pekinensis) by Plasmodiophora brassicae. Journal of Eukaryotic Microbiology, 58, 310–314.
Vandenbussche, F., Smalle, J., Le, J., Saibo, N. J., De Paepe, A., Chaerle, L., et al. (2003). The Arabidopsis mutant alh1 illustrates a cross talk between ethylene and auxin. Plant Physiology, 131, 1228–1238.
Williams, P. H. (1966). A system for the determination of races of Plasmodiophora brassicae that infect cabbage and rutabaga. Phytopathology, 56, 624–626.
Acknowledgments
Financial support from the National Project for Agricultural Technology System (CARS-13) and the Natural Science Foundation of China grants (31401720 and 31501617) is greatly appreciated.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xu, L., Ren, L., Chen, K. et al. Putative role of IAA during the early response of Brassica napus L. to Plasmodiophora brassicae . Eur J Plant Pathol 145, 601–613 (2016). https://doi.org/10.1007/s10658-016-0877-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-016-0877-y