Abstract
Plasmodiphora brassicae is a soil-borne obligate parasite. The pathogen has three stages in its life cycle: survival in soil, root hair infection, and cortical infection. Resting spores of P. brassicae have a great ability to survive in soil. These resting spores release primary zoospores. When a zoospore reaches the surface of a root hair, it penetrates through the cell wall. This stage is termed the root hair infection stage. Inside root hairs the pathogen forms primary plasmodia. A number of nuclear divisions occur synchronously in the plasmodia, followed by cleavage into zoosporangia. Later, 4–16 secondary zoospores are formed in each zoosporangium and released into the soil. Secondary zoospores penetrate the cortical tissues of the main roots, a process called cortical infection. Inside invaded roots cells, the pathogen develops into secondary plasmodia which are associated with cellular hypertrophy, followed by gall formation in the tissues. The plasmodia finally develop into a new generation of resting spores, followed by their release back into soil as survival structures. In vitro dual cultures of P. brassicae with hairy root culture and suspension cultures have been developed to provide a way to nondestructively observe the growth of this pathogen within host cells. The development of P. brassicae in the hairy roots was similar to that found in intact plants. The observations of the cortical infection stage suggest that swelling of P. brassicae-infected cells and abnormal cell division of P. brassicae-infected and adjacent cells will induce hypertrophy and that movement of plasmodia by cytoplasmic streaming increases the number of P. brassicae-infected cells during cell division.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Life Cycle In Vivo
Plasmodiphora brassicae is a soil-borne obligate parasite. The pathogen has three stages in its life cycle: survival in soil, root hair infection, and cortical infection (Fig. 1) (Ayers 1944; Ingram and Tommerup 1972; Naiki 1987). Primary inoculum is composed of resting spores dispersed from rotten host tissue into the surrounding soil. The resting spore is about 3 μm in size and subspherical to spherical (Figs. 1a, 2a) (Buczacki and Cadd 1976). The surface of each resting spore is covered with spines (Fig. 2b) (Williams and McNabola 1967; Ikegami and others 1978). A primary zoospore is released from each resting spore, spindle-shaped or pyriform, 2.8–5.9 μm long, and biflagellate (Fig. 1b) (Ayers 1944). The flagellae have two shapes: a shorter flagellum with a blunt end and a longer flagellum with a whiplash or tail piece. When the zoospore reaches the surface of a root hair, it penetrates the cell wall. This stage is termed the root hair infection stage or primary infection stage. In root hairs the pathogen forms primary plasmodia (Fig. 1c). A number of nuclear divisions occur synchronously in the plasmodia, followed by cleaving into zoosporangia. The zoosporangia form clusters in the root hair (Fig. 1d) and sometimes in epidermal cells. Later, 4–16 secondary zoospores are formed in each zoosporangium. After releasing these zoospores, the empty zoosporangia remain in the root hairs (Fig. 1e).
The secondary zoospores cannot be visually differentiated from the primary zoospores. Binucleate zoospores are sometimes found and interpreted as having formed by the fusion of two distinct zoospores, not from division within nuclei (Tommerup and Ingram 1971; Ingram and Tommerup 1972). The secondary zoospores penetrate the cortical tissues, a process called cortical infection or secondary infection stage. Inside infected cells the pathogen develops into secondary plasmodia which proliferate and are associated with cellular hypertrophy (Fig. 1f, g), followed by gall formation in root tissues. After a number of nuclear divisions, the secondary plasmodia contain two nuclei in the early stages of growth and then develop into multinuclear plasmodia (Garber and Aist 1979). In plasmodia with haploid nuclei, the nuclei may fuse forming diploid nuclei. Futhermore, meiotic cleavage may occur in the diploid plasmodia, indicating that the plasmodia return to the haploid state again (Buczacki 1983). This hypothesis is not universally accepted. The plasmodia finally develop into resting spores (Fig. 1h, i) (Ikegayami and others 1982), followed by their release into soil as survival structures. During these complex cleavages, the pathogen produces resting spores and increases its genetic diversity.
Resting-Spore Germination
Resting spores of Plasmodiophora brassicae have a great ability to survive in soil. Gibbs (1931) reported that resting spores may survive without host plants for 5 years. Wallenhammar (1996) found that the half-life of an inoculum was 3–6 years in heavily infested fields and that the level of infestation declined to below a detectable level after a period of 17.3 years. The resting spores remained active when held at 40°C for 24 h but were inactivated by treatment at 30°C for 14 days (White and Buczacki 1979).
Resting-spore germination is the first step in the life cycle. Knowledge of the environmental conditions that affect germination is key to controlling clubroot disease (see Dixon, this issue; Donald and Porter, this issue). The release of calcium ions reputedly triggers the germination of the resting spores (Yano and others 1991). These authors found that a reabsorption of calcium ions was necessary for successful germination. They used a combination of inhibitors of calcium ion release and absorption in their study (personal communication of unpublished data). Macfarlane (1970) found that the resting spores from old rotten galls had higher germinability than those extracted from young hard galls. The work of Yano and others (1991) agreed with that of Macfarlane (1970). Scanning electron microscopy (SEM) demonstrated that the young spores were covered with fibrous materials (Fig. 2a), whereas the mature spores possessed a number of spines but the fibrous material was not present (Fig. 2b). Mature spores germinated regardless of the presence of calcium ions, whereas immature young spores required the presence of calcium ions (Fig. 3). The results suggest that populations of resting spores have different levels of maturation and that their ability to germinate is associated with the level of maturation of the spores. The germination of mature resting spores is affected by environmental factors such as pH, humidity, temperature, and other inorganic ions and by biological factors (Takahashi 1994a; Friberg and others 2005) (see Dixon, this issue).
The zoospores released from resting spores survive for relatively short periods of time (Suzuki and others 1992; Takahashi 1994b), suggesting that induction of spontaneous germination would provide a valuable opportunity to reduce the inoculum potential of this pathogen. Germination may be triggered by host root exudates as well as those from nonhosts (Narita and Nishiyama 1955; Bochow 1965; Kroll and others 1983; Ikegami 1985; Suzuki and others 1992; Friberg and others 2005). Root hair infection has also been observed in nonhost plants such as Lolium perenne, Reseda odorata, and Tropæolum majus but apparently does not result in cortical infection (Macfarlane 1952). Therefore, use of decoy or trap plants could be a valuable tactic for disease control. Murakami and others (2000, 2001) demonstrated that leafy daikon (Raphanus sativus), spinach, and oats when grown on infested land reduced the pathogen population density and resulted in a parallel decline in disease severity.
Root Hair Infection
Although the primary zoospores infect root hairs, zoosporangial clusters with many zoospores are formed within the root hairs. Furthermore, Naiki and others (1984a) found that secondary zoospores released from zoosporangia in root hairs reinfected them. These events suggest that the pathogen may proliferate in a short cycle around root hairs and epidermal cells and this enhances the infection of cortical tissues during the root hair infection stage of the life cycle.
As described above, root hair infection is observed in nonhost plants as well as host plants. It is not clear whether the secondary zoospores from nonhost plant roots will infect the roots of Brassica hosts. Host specificity may be less in the root hairs compared with the cortical cells. Further study is necessary to understand the role of root hair infection of nonhost plants.
The estimation of infestation levels in fields was based on the relationship between population densities of the pathogen and the frequencies of root hair infections (Samuel and Garrett 1945). Naiki and others (1978) reported, however, that no root hair infection was observed when there were fewer than 103 resting spores/g soil. Clubroot formation occurred above this concentration (Fig. 4a). This level of sensitivity is not enough for practical diagnosis (see Faggian and Strelkov, this issue).
Calcium concentration may be related to root hair infection as well as spore germination. Webster and Dixon (1991) found that increasing calcium concentration around roots reduced the development of zoosporangia in root hairs and slowed the release of the secondary zoospores from zoosporangia. These effects can be associated with successful control of the disease by liming the soil (see Donald and Porter, this issue). Liming could have two effects: the addition of calcium ions and the increase of soil alkalinity (Webster and Dixon 1991).
Cortical Infection
The cortical infection stage is induced by secondary zoospores released from zoosporangia in root hairs. The primary zoospores will not infect cortex tissues (Dobson and Gabrielson 1983). The infection processes resulting in cortical infection are still not clear. Whether the zoospores are released from the root hair into the soil or move inside the root hair or both has yet to be clarified. Stages of movement within the host were demonstrated in a dual culture of the host and pathogen as described below.
The relationship between root hair infection and cortical infection leading to gall formation is complex (Naiki and others 1984b). The susceptibility of root hairs could not be correlated with that of cortical cells and resultant gall formation. For example, in Fig. 5, the cultivar represented by the symbol (●) showed high levels of root hair infection but no gall formation when exposed to the pathogen physiologic race ECD20/15/12. In addition, the balance of root hair infection and gall formation varied when different pathogen races were used. The cultivar represented by (□) showed moderate root hair infection and low gall formation when exposed to the race ECD20/15/12, whereas the cultivar showed low root hair infection and high gall formation when exposed to the race ECD20/31/31.
Using highly susceptible cultivars of Chinese cabbage, the population density of resting spores was significantly correlated with gall formation (Fig. 4b) (Naiki and others 1978). Based on the regression curve of this relationship, the minimum population density for gall formation was calculated as 3.5 resting spores/g soil. There are two pathogenic strategies in each life cycle: root hair infection forming zoosporangial clusters with many zoospores and cortical infection forming a number of resting spores. Therefore, even if the infestation level is low and does not affect the yield of a susceptible crop, the population density will increase substantially for the next cropping season. Actually, there are several reports that single resting spores induced gall formation (Tinggal and Webster 1981; Jones and others 1982; Scott 1985; Kageyama and others 1995; Narisawa and others 1996).
Life Cycle In Vitro Dual Culture
Although several investigations have used dual cultures of P. brassicae with callus culture (Strandberg and others 1966; Ingram 1969; Tommerup and Ingram 1971; Dekhuijzen 1975; Buczacki 1980; Ikegami 1992), there remain difficulties in observing this pathogen within host tissues. In vitro dual cultures of P. brassicae with hairy root culture (Asano and others 1999, 2000) and suspension cultures (Asano and Kageyama 2006) were developed to provide a way to nondestructively observe the growth of this pathogen within host cells. New views of the life cycle of P. brassicae were obtained from these cultural techniques.
Germination of Surface-Disinfected Resting Spores
Satisfactory surface disinfection of resting spores is one of the most important techniques needed when establishing a dual culture of P. brassicae in hairy root cultures. Resting spores of P. brassicae were surface-disinfected with 2% (w/v) cloramine-T solution for 20 min and an antibiotic solution (1 μg ml−1 of colistin sulfate, 1 μg ml−1 of vancomycin hydrochloride, and 6 μg ml−1 of cefotaxime sodium) for 1 day (Asano and others 1999).
The germination of surface-disinfected resting spores started 2 days after incubation commenced, increased steadily for 6 days, and then stopped. On the other hand, the germination of nondisinfected resting spores was delayed by 2 days and the percentage of successful germination was lower. At the end of the experiment, the germination rate of surface-disinfected resting spores (12.0%) was significantly higher than that of nondisinfected resting spores (6.7%). These results suggest that the germination process is identical with one for intact resting spores but other biological factors will affect the germinability of resting spores.
Growth of P. brassicae in Root Hairs Using Hairy Root Cultures
Previous studies reported that the age of root hairs might affect infection by P. brassicae (Samuel and Garrett 1945; Naiki and others 1978). Serial observation of root hair infection by resting spores was difficult when using traditional methods of soil and hydroponic culture. By contrast, dual culture of hairy roots and P. brassicae under axenic conditions allowed successful serial observation of root hair infection in a sample (Asano and others 2000) over a period of time.
In dual culture, the number of infections was high in 5–6-day-old root hairs, indicating that the age of root hairs influenced successful infection. Infection began about 3 days after inoculation, increased up to 6 days, and continued increasing more slowly until 10–12 days after inoculation. The germination of resting spores and root hair infections occurred synchronously with a delay of 2 days between these events. This might be due to a time lag in infection by primary zoospores following resting-spore germination.
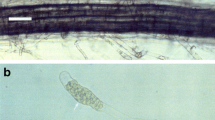
The growth of P. brassicae in a single root hair in turnip hairy root culture was serially observed for 8 days after inoculation. An amorphous primary plasmodium (Fig. 6a) expanded to fill as much as one-quarter of the volume of the root hair in 1 day (Fig. 6b). The protoplasm of the plasmodium became tuberculate in shape, and each tubercle subsequently became bounded with a membrane and developed zoosporangia (Fig. 6c). The cytoplasm within these zoosporangia cleaved and then differentiated into mature zoosporangia containing secondary zoospores. Secondary zoospores were released from zoosporangia (Fig. 6d). In dual cultures of P. brassicae and turnip hairy roots, swimming secondary zoospores were observed 6–8 days after inoculation. The process of secondary infection could not be observed, however, in dual culture. The development of P. brassicae in hairy roots was apparently similar to that found in intact plants and reported by previous workers (Katsura and others 1970; Ingram and Tommerup 1972), despite the fact that hairy roots without shoots may differ physiologically and biochemically from those of intact plants.
Growth of Plasmodiophora brassicae within a single root hair of turnip hairy root photographed (a) 4, (b) 5, (c) 6, and (d) 8 days after inoculation with resting spores. a Amorphous primary plasmodium. b Young zoosporangia developed from primary plasmodium. c Mature zoosporangia containing secondary zoospores. d Partly evacuated zoosporangia. Arrows indicate empty zoosporangium. Scale bar = 50 μm
Asano and others (1999) also established gall formation in hairy root cultures. Therefore, dual culture appears to have some advantages over traditional methods of using soil or other substrates. The dual-culture method allows for the investigation of P. brassicae under carefully defined chemical and physical conditions such as temperature, humidity, pH, and nutrients without the effects of other microorganisms being present. This method can be used to study the effect of environmental and biological factors on the infection and growth of P. brassicae (see Dixon, this issue).
Growth of Secondary Plasmodia Using Suspension Culture
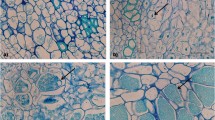
Asano and Kageyama (2006) developed dual cultures of P. brassicae and turnip using suspension cell cultures. The suspension culture of P. brassicae-infected turnip cells was derived from P. brassicae-infected callus in MS liquid medium with 0.1 mg 2,4-D L−1 and 0.02 mg kinetin L−1. Propagated suspension cells were spherical to cylindrical or filamentous in shape (Fig. 7a–d). Plasmodiophora brassicae-infected cells were significantly larger than uninfected cells, in accordance with the earlier findings of Williams and others (1969) and Gustafsson and others (1986) who used infected cells and intact gall tissues. Abnormally shaped cells (Fig. 7d) were sometimes observed among the infected cells, but not among uninfected cells. These observations led to speculation that P. brassicae-infected cells are induced to produce auxin following invasion by this pathogen (Ludwig-Miller 1999) (see Ludwig-Müller and others, this issue). Stimulated auxin production was thought to be a cause of the swelling of infected cells and result in hypertrophy of infected tissues. The fact that uninfected cells were not significantly hypertrophied suggests that the cell-stimulating activity of auxin was localized within infected cells. Because secondary plasmodia of P. brassicae may be able to synthesize cytokinins (Müller and Hilgenberg 1986), P. brassicae may promote cell division in gall tissue, leading to hyperplasia (Gustafsson and others 1986; Kobelt and others 2000). Although the concentrations of kinetin added to the medium were low or even nil, both infected and uninfected cells propagated vigorously under these experimental conditions. These results suggest that swelling of P. brassicae-infected cells and abnormal cell division of P. brassicae-infected and adjacent cells induce the hypertrophy and hyperplasia required to form gall tissue.
Plasmodiophora brassicae-infected or -noninfected turnip suspension cell morphology. a P. brassicae-noninfected suspension cells. b Cylindrical cells containing secondary plasmodia (arrows). c Filamentous cells containing secondary plasmodia (arrows). d An abnormally growing cell containing secondary plasmodia (arrow). Scale bars = 20 μm
Plasmodiophora brassicae grew into secondary plasmodia in suspension-culture cells (Asano and Kageyama 2006). The characteristics of secondary plasmodia in the cells were identical with those in naturally infected galls. All the growth stages of secondary plasmodia were observed (Fig. 8a–f). Spherical or subspherical young plasmodia (Fig. 8a) divided into numerous small, spherical plasmodia (Fig. 8b). The small plasmodia developed (Fig. 8c), and then formed a cluster by repeated division (Fig. 8d). Plasmodia fused with each other (Fig. 8e), followed by development of vegetative plasmodia (Fig. 8f). These observations are in agreement with the scanning electron microscopy (SEM) observations of Ikegami and others (1978).
Growth stages of Plasmodiophora brassicae in transformed turnip suspension cells. a Very small (arrowheads) and small secondary plasmodia (arrows). b Proliferation of small secondary plasmodia. c Small cluster of secondary plasmodia (arrow). d Large cluster of secondary plasmodia with numerous subunits (arrow). e Vegetative plasmodium with several spherical bodies (arrow). f Vegetative plasmodium (arrow). Scale bars = 20 μm
Observation made of a single suspension cell over time using light microscopy with Nomarski optics revealed that secondary plasmodia moved in transformed suspension cells (Fig. 9a–d). Spherical plasmodia and starch grains separated from a cluster of plasmodia (Fig. 9a) and in 20 min they had moved to the opposite sides of host cells by means of cytoplasmic streaming (Fig. 9b–d). Light microscopy revealed that spreading mechanisms of secondary plasmodia within host cells were accompanied by division of the latter (Fig. 10). This observation resembles that reported by Buczacki (1983). His observations suggested that vegetative plasmodia were clustered at the time when infected cells divided and gave rise to two daughter cells. The probability of two daughter cells forming with plasmodia was low. If plasmodia were actively moved during host cell division into infected cells (Fig. 9a–d), then the probability of two daughter cells containing plasmodia would be much higher. This suggests that the movement of plasmodia by cytoplasmic streaming may increase the number of P. brassicae-infected cells during cell division.
Mithen and Magrath (1992) and Kobelt and others (2000) showed cell-to-cell movement of myxamoebae via cell wall breakage by pseudopodia-like structures in the outer cortex of host roots. On the other hand, distribution of secondary plasmodia in the inner cortex and medullar rays increased following host-cell division (Dekhuijzen 1976). Asano and Kageyama (2006) made continuous nondestructive observations of intracellular plasmodial movement and division. Their observations led to the proposition that there are several stages in the growth of P. brassicae during the cortical infection phase. Secondary zoospores infect cortical cells, become myxamoebae, and then invade internal root tissues. Myxamoebae penetrate and migrate from the epidermis to the vascular stele, then become secondary plasmodia and grow in the cortical cells. The secondary plasmodia in the inner cortical cells are distributed by cytoplasmic streaming and host-cell division rather than by disruption of the intact cell wall.
The similarity of growth characteristics in in vitro culture to those in intact P. brassicae-infected plants strongly suggests that the use of hairy root culture and suspension culture is a realistic method for growth studies of P. brassicae and brassicaceous plants.
References
Asano T, Kageyama K (2006) Growth and movement of secondary plasmodia of Plasmodiophora brassicae in turnip suspension-culture cells. Plant Pathol 55:145–151
Asano T, Kageyama K, Hyakumachi M (1999) Surface disinfestation of resting spores of Plasmodiophora brassicae used to infect hairy roots of Brassicae spp. Phytopathology 89:314–319
Asano T, Kageyama K, Hyakumachi M (2000) Germination of surface-disinfected resting spores of Plasmodiophora brassicae and their root hair infection in turnip hairy roots. Mycoscience 41:49–54
Ayers GW (1944) Studies on the life history of the club root organism, Plasmodiophora brassicae. Can J Res Sect C 32:143–149
Bochow H (1965) The effect of root diffusates of host and non-host plants on the resting spore germination of Plasmodiophora brassicae Wor. In: Macura J, Vancura V (eds) Plant microbes relationships. Publishing House of the Czechoslovak Academy of Prague, Prague, pp 296–299
Buczacki ST (1980) Culture of Plasmodiophora brassicae in host callus tissue. In: Ingram DS, Helgeson JP (eds) Tissue culture methods for plant pathologists. Blackwell Scientific, Oxford, pp 145–150
Buczacki ST (1983) Plasmodiophora: an inter-relationship between biological and practical problems. In: Buczacki ST (ed) Zoosporic plant pathogens. Academic Press, London, pp 161–191
Buczacki ST, Cadd SE (1976) Size distribution of the clubroot organism, Plasmodiophora brassicae. Tran Br Mycol Soc 67:133–136
Dekhuijzen HM (1975) The enzymatic isolation of secondary vegetative plasmodia of Plasmodiophora brassicae from callus tissue of Brassica campestris. Physiol Plant Pathol 6:187–192
Dekhuijzen HM (1976) Microscopical studies on the cortex and medullary rays of Brassica campestris var. rapa infected with Plasmodiophora brassicae. Phytopathol Z 87:171–186
Dobson RL, Gabrielson RL (1983) Role of primary and secondary zoospores of Plasmodiophora brassicae in the development of clubroot in Chinese cabbage. Phytopathology 73:559–561
Friberg H, Lagerlöf J, Rämert B (2005) Germination of Plasmodiophora brassicae resting spores stimulated by a non-host plant. Eur J Plant Pathol 113:275–281
Garber RC, Aist JR (1979) The ultrastructure of mitosis in Plasmodiophora brassicae (Plasmodiophorales). J Cell Bot 57:2509–2518
Gibbs JG (1931) Dissemination of clubroot in the dung from stock. NZ J Agric 42:193–198
Gustafsson M, Liljeroth E, Gunnarsson M, Lundborg T (1986) Effects of infection by Plasmodiophora brassicae on root anatomy of rape. J Phytopathol 117:144–151
Ikegami H (1985) Disease of clubroot fungus by cultivation of different crops in heavily infested soil. Res Bull Fac Agric Gifu Univ 50:19–32 [in Japanese, abstract in English]
Ikegami H (1992) Studies on the clubroot of cruciferous plants. X. Proliferation and pathogenicity of Plasmodiophora brassicae in infected callus tissue. Proc Kansai Plant Prot 34:17–28
Ikegami H, Mukobata H, Naiki T (1978) Scanning electron microscopy of Plasmodiophora brassicae in diseased root cells of turnip and Chinese cabbage (Studies on the Clubroot of Cruciferous Plants III). Ann Phytopathol Soc Jpn 44:456–464
Ikegayami H, Naiki T, Ito T, Imuro Y (1982) Ultrastructural growth process of Plasmodiophora brassicae in infected cells of Chinese cabbage and turnip (Studies on the Clubroot of Cruciferous Plants IV). Res Bull Fac Agric Gifu Univ 46:9–19
Ingram DS (1969) Growth of Plasmodiophora brassicae in host callus. J Gen Microbiol 55:9–18
Ingram DS, Tommerup IC (1972) The life history of Plasmodiophora brassicae Woron. Proc R Soc Lond Ser B 180:103–112
Jones DR, Ingram DS, Dixon GR (1982) Characterization of isolates derived from single resting spores of Plasmodiophora brassicae and studies of their interaction. Plant Pathol 31:229–238
Kageyama K, Kamimura Y, Hyakumachi M (1995) A simple inoculation method with a single resting spore of Plasmodiophora brassicae. Ann Phytopathol Soc Jpn 61:415–418
Katsura K, Tamura M, Yamaguchi N (1970) Some observations on the infection of Plasmodiophora brassicae Woronin in root hair of Cruciferae plants. Proc Kansai Plant Prot 12:23–29 [in Japanese]
Kobelt P, Simens J, Sacristan MD (2000) Histological characterization of the incompatible interaction between Arabidopsis thaliana and the obligate biotrophic pathogen Plasmodiophora brassicae. Mycol Res 104:220–225
Kroll TK, Lacy GH, Moore LD (1983) A quantitative description of the colonization of susceptible and resistant radish plants by Plasmodiophora brassicae. Phytopathol Z 108:97–105
Ludwig-Miller J (1999) Plasmodiophora brassicae, the causal agent of clubroot disease: a review on molecular and biochemical events in pathogenesis. J Plant Dis Prott 106:109–127
Macfarlane I (1952) Factors affecting the survival of Plasmodiophora brassicae Worin the soil and its assessment by a host test. Ann Appl Biol 39:239–256
Macfarlane I (1970) Germination of resting spores of Plasmodiophora brassicae. Trans Br Mycol Soc 55:97–112
Mithen R, Magrath R (1992) A contribution to the life history of Plasmodiophora brassicae: secondary plasmodia development in root galls of Arabidopsis thaliana. Mycol Res 96:877–885
Müller P, Hilgenberg W (1986) Isomers of zeatin and zeatin riboside in clubroot tissue: evidence for trans-zeatin biosynthesis by Plasmodiophora brassicae. Physiol Planta 66:245–250
Murakami H, Tsushima S, Akimoto T, Murakami K, Goto I, Shishido Y (2000) Effects of growing leafy daikon (Raphanus sativus) on populations of Plasmodiophora brassicae (clubroot). Plant Pathol 49:584–589
Murakami H, Tsushima S, Akimoto T, Shishido Y (2001) Reduction of spore density of Plasmodiophora brassicae in soil by decoy plants. J Gen Plant Pathol 67:65–88
Naiki T (1987) Life cycle and control of Plasmodiophora brassicae, causing clubroot disease of cruciferous plants. Soil Microorganisms 29:23–37
Naiki T, Kageyama K, Ikegami H (1978) The relation of spore density of Plasmodiophora brassicae Wor. to the root hair infection and club formation in Chinese cabbage (Studies on the Clubroot of Cruciferous Plant II). Ann Phytopathol Soc Jpn 44:432–439
Naiki T, Kawaguchi C, Ikegami H (1984a) Root hair reinfection in Chinese cabbage seedlings by the secondary zoospores of Plasmodiophora brassicae Woronin. Ann Phytopathol Soc Jpn 50:216–220
Naiki T, Tanahashi K, Kageyama K (1984b) The relationship between root hair infection with Plasmodiophora brassicae Wor. and subsequent club formation among cruciferous species. Ann Phytopathol Soc Jpn 50:211–215
Narisawa K, Kageyama K, Hashiba T (1996) Efficient root infection with single resting spores of Plasmodiophora brassicae. Mycol Res 100:855–858
Narita T, Nishiyama Y (1955) Some observation on the germination of the resting spore of Plasmodiophora brassicae Wor. In: Jubilee Publication in Commemoration of the Sixtieth Birthday of Prof. Yoshihiko Tochinai and Prof. Teikichi Fukushi. Sapporo, Japan, pp 309–315 [in Japanese, abstract in English]
Samuel G, Garrett SD (1945) The infected root-hair count for estimation of the activity of Plasmodiophora brassicae Woron. in the soil. Ann Appl Biol 32:96–101
Scott ES (1985) Production and characterization of single-spore isolates of Plasmodiophora brassicae. Plant Pathol 34:287–292
Strandberg JO, Williams PH, Yukawa Y (1966) Monoxenic culture of Plasmodiophora brassicae with cabbage tissue. Phytopathology 56:903 [abstract]
Suzuki K, Matumiya E, Ueno Y, Mizutani J (1992) Some properties of germination-stimulating factor from plants for resting spores of Plasmodiophora brassicae. Ann Phytopathol Soc Jpn 58:699–705
Takahashi K (1994a) Influences of some environmental factors on the viability of resting spores of Plasmodiophora brassicae Wor. incubated in sterile soil. Ann Phytopathol Soc Jpn 60:658–666
Takahashi K (1994b) Biological agents affecting the viability of resting spores of Plasmodiophora brassicae Wor. in soil without host roots. Ann Phytopathol Soc Jpn 60:667–674
Tinggal SH, Webster J (1981) Technique for single spore infection by Plasmodiophora brassicae. Tran Br Mycol Soc 76:187–190
Tommerup IC, Ingram DS (1971) The life-cycle of Plasmodiophora brassicae Wor. in Brassica tissue cultures and in intact roots. New Phytol 70:327–332
Wallenhammar AC (1996) Prevalence of Plasmodiophora brassicae in a spring oilseed rape growing area in central Sweden and factors influencing soil infestation level. Plant Pathol 45:710–719
Webster MA, Dixon GR (1991) Calcium, pH and inoculum concentration influencing colonization by Plasmodiophora brassicae. Mycol Res 95:64–73
White JG, Buczacki ST (1979) Observations on suppression of clubroot by artificial or natural heating of soil. Trans Br Mycol Soc 73:271–275
Williams PH, McNabola SS (1967) Fine structure of Plasmodiophora brassicae in sporogenesis. Can J Bot 45:1665–1669
Williams PH, Reddy MN, Strandberg JO (1969) Growth of noninfected and Plasmodiophora brassicae infected cabbage callus in culture. Can J Bot 47:1217–1221
Yano S, Tanaka S, Kameya-Iwaki M, Katumoto K (1991) Relation of Ca2+ efflux to germination of resting spores of clubroot fungus. Bull Fac Agric Yamaguchi Univ 39:105–112
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kageyama, K., Asano, T. Life Cycle of Plasmodiophora brassicae . J Plant Growth Regul 28, 203–211 (2009). https://doi.org/10.1007/s00344-009-9101-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-009-9101-z