Abstract
Puccinia striiformis f. sp. tritici (Pst), the causal agent of wheat stripe rust, is an obligate pathogen that causes significant yield losses worldwide. In order to profile the change of the proteome during uredospore germination of Pst, proteins from uredospores and germtubes (germlings) were isolated and quantified by iTRAQ (isobaric tag for relative and absolute quantitation) technology. Among the 1548 proteins identified, there were 64 and 54 proteins up- and down-regulated during uredospore germination respectively. Proteins involved in catabolic process, energy production and transport were found to be specially accumulated in germlings, which was an indication of metabolic transition from dormancy to germination. While, proteins involved in protein metabolic process were largely down-regulated, suggesting fewer proteins were needed for uredospore germination. Additionally, a number of down-regulated proteins exhibited increasing mRNA level during germination, suggesting relative less correlation between proteins and mRNAs in dormant uredospores.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Puccinia striiformis f. sp. tritici (Pst) is the causal agent of wheat stripe rust, one of the most devastating fungal diseases of wheat, and causes significant yield loss in wheat production worldwide (Hovmoller et al. 2010; Dean et al. 2012). Pst is a macrocyclic and heteroecious rust fungus with five different spore stages and two taxonomically unrelated host species, wheat and barberry (Zhao et al. 2013).
The most common spore form of Pst is uredospore, the asexual spore which infects wheat leaves and causes disease epidemic. Uredospore germination is regarded as the initial step of rust fungus life cycle. During germination, the uredospores break dormancy and transform to the active state, germlings, which recognize and enter into stomata. Several morphological and biological changes, including spore swelling, adhesion, nuclear migration and dynamic of microfibrillar network, have been reported to accompany the process of uredospore germination (Wang and McCallum 2009). Because of its relevant role in the establishment of the fungal disease, the germination process of uredospores has been widely studied. Previous studies mainly focused on the effects of environmental and host on the uredospore germination. These studies revealed that many factors, such as temperature, light, leaf wetness, epicuticular wax, and secondary metabolites, could affect the uredospore germination (Patto and Niks 2001; Bonde et al. 2007; Wang and McCallum 2009; Buck et al. 2010; Johnson and Cummings 2013; Angelotti et al. 2014; Dracatos et al. 2014). While, the molecular basis of uredospore germination of rust fungus is still far from clear. Recently, extensive proteomic analysis of the conidia germination for necrotrophic or hemi-biotrophic fungi has been performed (Gonzalez-Rodriguez et al. 2015; Franck et al. 2013; Rabie El-Akhal et al. 2013; van Leeuwen et al. 2013). However, investigations of the proteome of obligate biotrophic fungus remain still limited. Luster et al. identified 117 predominantly soluble proteins abundant in germlings from Asia soybean rust fungus uredospores by 2-DE and LC-MS/MS (Luster et al. 2010). Since there is no expression data for these proteins during other developmental stages, their specific accumulations during germination still remain unknown. The comparative proteomic analysis of un-germinated and germinated uredopores of bean rust fungus, Uromyces appendiculatus, highlighted the role of proteins involved in energy production and nucleic reorganization during the uredospore germination (Cooper et al. 2006, 2007).
Moreover, in our previous study, we identified 1118 genes expressed in germlings using a cDNA library and found three and seven genes up-regulated and down-regulated respectively during Pst uredospores germination (Zhang et al. 2008; Huang et al. 2011). Recently, we have achieved sequence and annotation of the whole genome of Pst, which provides a powerful resource for the proteomic study of the stripe rust fungus (Zheng et al. 2013). In this study, we used quantitative proteomic profiling to identify the participant proteins and explore the biological processes during Pst uredospore germination by iTRAQ (isobaric tag for relative and absolute quantitation) technology. We also analyzed the correlations between the translational and transcriptional levels of the differentially expressed proteins during uredospore germination. Our results provide proteomic changes of Pst during uredospore germination, allowing the better understanding of Pst development and the advancement of disease management strategies.
Materials and methods
Protein extraction and digestion
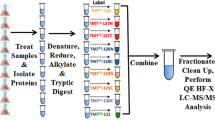
Uredospores of Chinese race CYR32 were collected from infected wheat cultivar Mingxian 169. Germlings were obtained from germinated uredospores in artificial conditions. In details, uredospores were dispersed and suspended in sterile distilled water at 12 °C. After 12 h, germinated uredospores or germlings (checked by microscope) were collected and dried by facial tissue. 100 mg un-germinated uredospores and germlings were pulverized by crushing for 5 min in a mortar and pestle under liquid nitrogen. Cells homogenized were resuspended in 400 μl of ice-cold lysis buffer (10 mM Hepes, pH 7.5, 10 mm KCl, 0.1 mM EDTA, 0.1 mM EGTA, 10 mM DTT, 1 mM PMSF). After incubation on ice for 15 min, the samples were centrifuged at 4 °C and 18,000×g for 20 min. Supernatants were collected and pellets were suspended in lysis buffer and purified twice in the same way. All supernatants were combined and mixed well with four volumes of ice-cold acetone and precipitated at −20 °C overnight. Resulting samples were then centrifuged at 4 °C and 5000×g for 5 min. Protein pellet was resuspended in digestion buffer (100 mM triethylammonium bicarbonate, 0.05 % w/v sodium dodecyl sulfate, Sigma protease inhibitor cocktail) to a final concentration of 1 mg/ml (determined with the Bradford assay using BSA as standard (Protein Assay kit, Bio-Rad)). The protein samples were divided into three parts for the subsequent three technical replicates of iTRAQ annlysis.
Protein digestion and iTRAQ labeling
Equal aliquots (100 μg) from each protein sample were then digested with trypsin (Promega Corporation, Madison, WI; 1:20, w/w) at 37 °C for 14 h and lyophilized. Peptides derived from uredospores and germlings were labeled with isobaric tags 118 and 121 respectively as per manufacturer’s instructions (Applied Biosystems, Foster City, CA). After 1 h incubation, labeled samples were pooled and evaporated to dryness in a vacuum centrifuge and reconstituted in 1 ml of loading buffer (15 mm KH2PO4 in 25 % acetonitrile, pH <3.0) prior to strong cation exchange fractionation. The iTRAQ labeled peptides were fractionated using PolySULFOETHYL ATM SCX column (200 × 4.6 mm, 5 μm particle size, 200 Å pore size) by HPLC system (Shimadzu, Japan) at flow rate 1.0 ml min−1. The 50 min HPLC gradient consisted of 100 % buffer A (10 mM KH2PO4, 25 % acetonitrile, pH 2.85) for 5 min; 0–20 % buffer B (10 mM KH2PO4, 25 % ACN, 500 mM KCL, pH 3.0) for 15 min; 20–40 % buffer B for 10 min; 40–100 % buffer B for 5 min followed by 100 % buffer A for 10 min. The chromatograms were recorded at 218 nm. The collected fractions were desalted with Sep-Pak® Vac C18 cartridges (Waters, Milford, Massachusetts), concentrated to dryness using vacuum centrifuge and reconstituted in 0.1 % formic acid for LC-MS/MS analysis.
The labeled samples were then fractionated by means of two dimensional liquid chromatography as previously described (Ruppen et al. 2010).
MALDI-TOF/TOF tandem MS analysis
The mass spectroscopy analysis was performed using an AB SCIEX TripleTOF™ 5600 mass spectrometer (AB SCIEX, Framingham, MA, USA), coupled with online micro flow HPLC system (Shimadzu, JAPAN) as described before (Qiao et al. 2012). The peptides were separated using nanobored C18 column with a picofrit nanospray tip (75 μm ID × 15 cm, 5 μm particles) (New Objectives, Wubrun, MA). The separation was performed at a constant flow rate of 20 μl min−1, with a splitter to get an effective flow rate of 0.2 μl min−1. The mass spectrometer data were acquired in the positive ion mode, with a selected mass range of 300–2000 m/z. Peptides with +2 to +4 charge states were selected for MS/MS. The three most abundantly charged peptides above a 5 count threshold were selected for MS/MS and dynamically excluded for 30 s with ±30 mDa mass tolerance. Smart information-dependent acquisition (IDA) was activated with automatic collision energy and automatic MS/MS accumulation. The fragment intensity multiplier was set to 20 and maximum accumulation time was 2 s. The peak areas of the iTRAQ reporter ions reflect the relative abundance of the proteins in the samples. For peptide identification, The Triple TOF 5600 mass spectrometer used in this study has high mass accuracy (less than 2 ppm). Other identification parameters used included: fragment mass tolerance: #0.1 Da; mass values: monoisotopic; variable modifications: Gln- > pyro-Glu (N-term Q), oxidation (M), iTRAQ8plex (Y); peptide mass tolerance: 0.05 Da; max missed cleavages: 1; fixed modifications: carbamidomethyl (C), iTRAQ8plex (N-term), iTRAQ8plex (K); other parameters: default.
Proteomic data analysis
The MS data were processed using Proteome Discoverer software (Version 1.2.0.208, Thermo Scientific) with default parameters for generating peak list. The data acquisition was performed with Analyst QS 2.0 software (Applied Biosystems/ MDS SCIEX). Protein identification and quantification were performed using Mascot 2.3.02 (Matrix Science, London, United Kingdom) (Charbonneau et al. 2007). Each MS/MS spectrum was searched against the protein database derived from whole genome database of Pst CYR32 obtained recently (Zheng et al. 2013).
For iTRAQ quantification, the peptide for quantification was automatically selected by the algorithm to calculate the reporter peak area (using default parameters in Mascot Software package). The resulting data set was auto bias-corrected to get rid of any variations imparted due to the unequal mixing during combining differently labeled samples. Proteins with 1.5 fold change or above between germlings and uredospores and p-value of statistical evaluation less than 0.05 were determined as differentially expressed proteins. The quantitation was performed at the peptide level by following procedures described in http://www.matrixscience.com/help/quant_statistics_help.html. The student’s t-test was performed using the Mascot 2.3.02 software. The functional annotation of the all proteins identified was initially assigned using the Protein Center software. Three main types of annotations were obtained from the gene ontology consortium web site: www.geneontology.org. Proteins up- and down-regulated during uredospore germination were further categorized and mapped to KEGG pathway by blast2GO software (version 3.0) (Conesa et al. 2005; Götz et al. 2008)
Relative quantification of mRNA
Total RNA was isolated from 100 mg un-germinated uredospores or germlings produced as described above using RNeasy kit (Qiagen, Doncaster, VIC, Australia) according to the manufacturer’s instruction, and genomic DNA contamination was removed by DNase I treatment. First-strand cDNAs were synthesized from 2 μg of total RNA in a 20-μl reaction volume using the RevertAid First-Strand cDNA Synthesis kit (Thermo Scientific, Waltham, MA, USA) in accordance with the manufacturer’s instructions. For gene mRNA level analysis, SYBR green qRT-PCR assays were performed and Pst β-tubulin gene TUBB (Genbank accession No. EG374306) was used as endogenous control according to our previous results (Huang et al. 2012). Primers (see Supplementary Table 1) were designed to anneal specifically to each of the selected genes and TUBB.
Reactions were performed on a 7500 Real-Time PCR System (Life Technologies, Grand Island, NY, USA) and relative gene quantification was calculated by the comparative 2–ΔΔCt method (Livak and Schmittgen 2001) and normalized to the corresponding expression level of the TUBB. The experiments were performed in triplicate.
Results
Proteins identified by iTRAQ
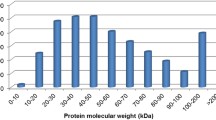
To investigate proteomic changes during the germination of uredospores, we suspended freshly harvested uredospores of Pst in sterile distilled water at 12 °C. After incubating for 12 h, about 90 % of uredospores germinated and produced long, unbranched germ tubes (Fig. 1). Peptides derived from un-germinated uredospores and germlings were labeled with iTRAQ tags 118 and 121, respectively. In our analysis of proteins extracted from uredospores and germlings, we detected 42,728 peptides, which represented 1548 proteins with sequence coverages ranging from 4.2 to 80.5 % and at 95 % or better confidence level. Most of them (74.5 %) were identified by more than one peptide. Complete list and details of the proteins identified is available as Supplementary Table 2.
Proteins up- or down-regulated during uredospore germination
Using a threshold of 1.5 fold, we identified 118 proteins whose abundance was significantly changed during uredospore germination. Among them, 54 proteins were up-regulated (Table 1) and 64 proteins were down-regulated (Table 2) during uredospore germination.
Except for hypothetical proteins, striiformis_Gene9990, a 60S ribosomal protein has the greatest fold change in abundance (decrease by 87.1-fold during germination). An exp1-like protein (striiformis_Gene20207), which has been generally considered as an architectural component of chromatin that has a general role in regulating chromosomal functions, showed a decrease by about 12-fold in abundance during germination. Other proteins exhibiting significantly decreased abundance included a blue light-inducible protein, a Dnajc2-prov protein, a fasciclin domain family protein, an elastinolytic metalloproteinase, a small glutamine-rich tetratricopeptide repeat-containing protein, and a glutamine synthetase, which decreased by 10.6, 10.9, 8.4, 7.9, 7.9 and 6.5-folds, respectively (Table 1).
On the other hand, proteins up-regulated exhibited more apparent changes in abundance. Our analysis revealed that seven proteins, including CaaX prenyl protease, duf1690 domain-containing protein, uracil phosphoribosyltransferase, mitochondrial-processing peptidase subunit alpha, C2 domain protein, and two unknown proteins, were induced most distinctly during germination with 87.9-fold change in abundance (Table 2).
Functional classification of differentially expressed proteins
The differentially expressed proteins were grouped by GO Biological Process and the proportions were compared for each group (Fig. 2; Supplementary Table 4). The total amount of proteins involved in “biosynthetic process”, “cellular metabolic process”, “cellular response to stimulus”, “cytoskeleton organization”, “metabolic process” and organic substance metabolic process remained relatively static.
While, proteins involved in ATP metabolic process were apparently induced during uredospore germination (Fig. 2). All 5 proteins fell into this group were found to be up-regulated (Supplementary Table 4). ATP is one of the main components of energy metabolism. Accumulation of ATP metabolic related proteins implies activation of energy production. This was consistent with the KEGG pathway analysis which revealed three up-regulated proteins were mapped to oxidative phosphorylation pathway (Supplementary Figure 1). Among them, striiformis_Gene10701 (NADH-ubiquinone oxidoreductase subunit), striiformis_Gene20539 (UcrQ family protein) and striiformis_Gene27232 (ATP synthase subunit beta) belonged to the oxidative phosphorylation complex I, III and V separately.
Proteins related to “transport” were also accumulated with the germination of uredospores. Among nine differentially expressed proteins assigned to the class “transport”, seven proteins were up-regulated while only two were down-regulated (Supplementary Table 4). Additionally, up-regulated proteins were dominant in some other biological processes such as biological regulation, catabolic process, cellular lipid metabolic process, nitrogen compound metabolic process, nucleotide metabolic process, organic substance catabolic process, regulation of biological process and small molecule metabolic process (Fig. 2).
However, abundance of most proteins involved in protein and nucleic acid metabolic process decreased during germination (Fig. 2). Take proteins attributed to “translation” (GO: 0006412) as an example, nine proteins, including striiformis_Gene24015 (eukaryotic initiation factor 4f subunit), striiformis_Gene12023 (polyadenylate-binding protein), striiformis_Gene15162 (threonyl-trna synthetase) and six ribosomal proteins were down-regulated, versus three proteins were up-regulated. A large part of differentially expressed proteins contributed to “protein folding” and “protein modification” processes were also down-regulated. The expression level of striiformis_Gene9884, a ribosome associated chaperone zuotin, decreased by 11-fold after germination. Moreover, more proteolysis related proteins were up-regulated, indicating protein degradation during uredospore germination, the early stage of Pst infection.
Generally speaking, during the germination of uredospores, proteome was obviously skewed toward energy production and dissipation represented by catabolic process and transport. This was also validated by another GO analysis for the molecular functions of these differentially expressed proteins. For example, all seven proteins that fell into the molecular function of “ATPase activity (GO: 0016887)” were up-regulated during the germination of uredospores (Supplementary Table 4).
Comparison of translational and transcriptional levels of differentially expressed proteins
To investigate the relationship between the transcriptional levels and the translational levels of these differentially expressed proteins, we examined their gene expression levels in dormant uredospores and germlings 6, 12 and 16 h post germination. Fifty two genes with known functions that displayed significant changes in abundance at the translational level were selected for further analyses. For most of the 24 genes whose proteins were up-regulated during germination, their mRNA levels kept increasing with the development of the uredospores, which was consistent with the changes at the protein level. Striiformis_Gene19006, a CaaX prenyl protease whose protein abundance was 87.9-fold higher in germlings than in uredospores, displayed the highest mRNA level at 16 h post germination. In addition, mRNA levels of five genes increased as early as 6 h post germination (above 2-fold increase). For instance, the gene expression levels of striiformis_Gene28371, a Glucan-beta-glucosidase, were increased by 4.3, 110.0 and 274.0 folds at 6, 12, and 16 h post germination respectively. However, the transcriptional levels of most other proteins (15 proteins) began to increase apparently until 12 h post germination. A uracil phosphoribosyl transferase, striiformis_Gene17762 was the only one exceptional protein up-regulated instead with a decreasing mRNA level. On the other hand, for proteins down-regulated during germination, the protein levels did not always agree with their mRNA levels. We found 13 proteins that were down-regulated at the protein levels but had higher mRNA levels 16 h post germination. For example, the abundance of striiformis_Gene9884, a Dnajc2-prov protein, increased by 11-fold at protein level but decreased by 5.7-fold on mRNA level during the germination of uredospores.
Discussion
Our study has provided a comprehensive profiling that documents the dynamics of the proteome during Pst uredospore germination and the relationship between mRNA and protein patterns. The results showed that the uredospores of Pst were subject to many proteome changes during germination.
High-throughput iTRAQ has become a robust method for proteomic quantification (Shadforth et al. 2005). In our analysis of the proteome of Pst uredospores and germlings by iTRAQ, 1548 proteins at 95 % or more confidence level were detected and 118 differentially expressed proteins between uredospores and germlings were identified, which was more efficient than gel based technology (Luster et al. 2010).
Germination from dormant uredospores is a complex process combining organics metabolism and energy conversion. It is presumed that germination of uredospores is fuelled entirely by endogenous resources and energy reserves since the fungus does not intake nutrients from the host until haustoria formation (Götz and Boyle 1998). The most apparent change was the increase of catabolic metabolism which would provide energy and some intermediates. This was evidenced by the accumulation of eight catabolic process related proteins during germination (Fig. 2 and Supplementary Table 4). Catabolic metabolism may provide sufficient energy for the growth of germtubes, which was consistent with the accumulation of numerous proteins involved in ATP metabolic process during germination. This observation was supported by a previous study that several proteins involved in energy production such as ATP:ADP transporters and acetyl-CoA acytyltransferase were accumulated in germinated urediospores of Uromyces appendiculatus (Cooper et al. 2007). It was further noted that proteins functioning in nucleotide but not in nucleic acid metabolism were up-regulated during uredospore germination, suggesting that nucleotides were used for energy production rather than nucleic acids synthesis. This differs from what happens in plant seeds germination where nucleotides involved both energy production and nucleic acids synthesis (Stasolla et al. 2002).
It was unexpected that the abundance of proteins involved in protein metabolism, such as translation, protein folding and modification, declined during germination (Fig. 2). Seven translation-related proteins were down-regulated during germination, indicating they were relatively abundant in un-germinated uredospores. This was different with previous studies which found that translation-related proteins were overaccumulated in germinated conidia of some necrotrophic or saprophytic filamentous fungi such as Botrytis cinerea, Fusarium solani, Neurospora crassa and Aspergillus nidulans (Gonzalez-Rodriguez et al. 2015; Osherov and May 2001). Biotrophic pathogens such as powdery mildews and the rusts, establish long-term relationships with host to fulfill their life cycles. In contrast to necrotrophs and saprophytes, they need to contend with the defense mechanisms of the plant to develop within the host and feed on living cells, especially at the later stage of infection (Valent and Khang 2010). The accumulation of translation-related proteins in Pst uredospores implies the synthesis and pre-stored of proteins needed for germination during the uredospore formation. Limited energy was preferentially used for synthesis those proteins essential for germtube growth and primary/initial stage of infection, such as striiformis_Gene15225 (a chitin deacetylase essential for fungal wall synthesis) and striiformis_Gene21611 (a pectinesterase essential for degradation of host cell wall).
In our study, most up-regulated proteins during germination had corresponding increasing mRNA level except for striiformis_Gene17762, a uracil phosphoribosyl transferase (UPRT). Its relatively high transcriptional level in uredopores probably due to pre-packaged mRNA, which were synthesized during the formation of uredospores. The ability of fungal spores to store pre-packaged mRNA has been observed in N. crassa, S. cerevisae and A. fumigatus (Brengues et al. 2002; Osherov and May 2001; Lamarre et al. 2008). These pre-packaged mRNA are primed for rapid activation which allowed the protein biosynthesis immediately after the spore germination. UPRT synthesizes Uridine 5′-monophosphate, a common precursor of all pyrimidine nucleotides. In Candida albicans, UPRT plays a key role in the resistance to flucytosine, an antifungal reagent (Hope et al. 2004). These results indicated that UPRT may be a promising target for fungicide design. On the other hand, we also found mRNA levels of 13 down-regulated proteins increased during germination. Although the changes in protein abundance observed in proteomic studies fail to correlate well with changes in gene expression in some cases (Schwanhausser et al. 2011; Franck et al. 2013), it was more likely that the lack of protein synthesis element in germlings mentioned above would be responsible for the paradox between protein and mRNA levels. Further evidence for this point of view was that mRNA levels of most of these proteins did not begin increasing until 12 h post germination, the later stage of Pst uredospore germination when nutrition has been almost depleted. Another plausible explanation would be that more proteolysis proteins accumulated during germination might speed up the degradations of these proteins.
In our proteomic study, in order to maximize the germination rates and get enough germlings, we chose to use water surface as the place for uredospores germination. Future germination studies can be improved either by germinating on plant surfaces or in media made from plant extracts, which may mimic the chemical composition of the real plant infection.
In conclusion, the iTRAQ analysis of an in vitro assay of rust uredospore germination has been proved to be a valuable and relevant tool in the analysis of global protein expression of a pathogen species. These specific results will provide convincing and accurate leads towards further studies of biological events related to Pst pathogenicity and be helpful to specific fungicides development.
References
Angelotti, F., Scapin, C. R., Tessmann, D. J., Vida, J. B., & Canteri, M. G. (2014). The effect of temperature, leaf wetness and light on development of grapevine rust. Australasian Plant Pathology, 43(1), 9–13.
Bonde, M. R., Berner, D. K., Nester, S. E., & Frederick, R. D. (2007). Effects of temperature on urediniospore germination, germ tube growth, and initiation of infection in soybean by Phakopsora isolates. Phytopathology, 97(8), 997–1003.
Brengues, M., Pintard, L., & Lapeyre, B. (2002). mRNA decay is rapidly induced after spore germination of Saccharomyces cerevisiae. Journal of Biological Chemistry, 277(43), 40505–40512.
Buck, J. W., Dong, W. B., & Mueller, D. S. (2010). Effect of light exposure on in vitro germination and germ tube growth of eight species of rust fungi. Mycologia, 102(5), 1134–1140.
Charbonneau, M. E., Girard, V., Nikolakakis, A., Campos, M., Berthiaume, F., Dumas, F., Lepine, F., & Mourez, M. (2007). O-linked glycosylation ensures the normal conformation of the autotransporter adhesin involved in diffuse adherence. Journal of Bacteriology, 189(24), 8880–8889.
Conesa, A., Götz, S., Garcia-Gomez, J. M., Terol, J., Talon, M., & Robles, M. (2005). Blast2GO: a universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics, 21(18), 3674–3676.
Cooper, B., Garrett, W. M., & Campbell, K. B. (2006). Shotgun identification of proteins from uredospores of the bean rust Uromyces appendiculatus. Proteomics, 6(8), 2477–2484.
Cooper, B., Neelam, A., Campbell, K. B., Lee, J., Liu, G., Garrett, W. M., Scheffler, B., & Tucker, M. L. (2007). Protein accumulation in the germinating Uromyces appendiculatus uredospore. Molecular Plant-Microbe Interactions, 20(7), 857–866.
Dean, R., Van Kan, J. A., Pretorius, Z. A., Hammond-Kosack, K. E., Di Pietro, A., Spanu, P. D., Rudd, J. J., Dickman, M., Kahmann, R., Ellis, J., & Foster, G. D. (2012). The top 10 fungal pathogens in molecular plant pathology. Molecular Plant Pathology, 13(4), 414–430.
Dracatos, P. M., Van der Weerden, N. L., Carroll, K. T., Johnson, E. D., Plummer, K. M., & Anderson, M. A. (2014). Inhibition of cereal rust fungi by both class I and II defensins derived from the flowers of Nicotiana alata. Molecular Plant Pathology, 15(1), 67–79.
Franck, W. L., Gokce, E., Oh, Y., Muddiman, D. C., & Dean, R. A. (2013). Temporal analysis of the magnaporthe oryzae proteome during conidial germination and cyclic AMP (cAMP)-mediated appressorium formation. Molecular and Cell Proteomics, 12(8), 2249–2265.
Gonzalez-Rodriguez, V. E., Lineiro, E., Colby, T., Harzen, A., Garrido, C., Manuel Cantoral, J., Schmidt, J., & Javier Fernandez-Acero, F. (2015). Proteomic profiling of botrytis cinerea conidial germination. Archives of Microbiology, 197, 117–133.
Götz, M., & Boyle, C. (1998). Changes in metabolite pools in host and pathogen during the uredinio and teliospore development of the bean rust fungus Uromyces appendiculatus. Journal of Phytopathology, 146(11–12), 599–607.
Götz, S., Garcia-Gomez, J. M., Terol, J., Williams, T. D., Nagaraj, S. H., Nueda, M. J., Robles, M., Talon, M., Dopazo, J., & Conesa, A. (2008). High-throughput functional annotation and data mining with the Blast2GO suite. Nucleic Acids Research, 36(10), 3420–3435.
Hope, W. W., Tabernero, L., Denning, D. W., & Anderson, M. J. (2004). Molecular mechanisms of primary resistance to flucytosine in Candida albicans. Antimicrobial Agents and Chemotherapy, 48(11), 4377–4386.
Hovmoller, M. S., Walter, S., & Justesen, A. F. (2010). Escalating threat of wheat rusts. Science, 29(5990), 369.
Huang, X., Chen, X., Coram, T., Wang, M., & Kang, Z. (2011). Gene expression profiling of Puccinia striiformis f. sp. tritici during development reveals a highly dynamic transcriptome. Journal of Genetics and Genomics, 38(8), 357–371.
Huang, X. L., Feng, H., & Kang, Z. S. (2012). Selection of reference genes for quantitative real-time PCR normalization in Puccinia Striiformis f.sp. tritici. Journal of Agricultural Biotechnology, 20(2), 181–187.
Johnson, D. A., & Cummings, T. F. (2013). Effects of temperature on rust development on mint infected with strains of Puccinia menthae. Canadian Journal of Plant Pathology, 35(4), 469–475.
Lamarre, C., Sokol, S., Debeaupuis, J. P., Henry, C., Lacroix, C., Glaser, P., Coppee, J. Y., Francois, J. M., & Latge, J. P. (2008). Transcriptomic analysis of the exit from dormancy of Aspergillus fumigatus conidia. BMC Genomics, 9, 417. doi:10.1186/1471-2164-9-417.
Livak, K. J., & Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(−Delta Delta C) method. Methods, 25(4), 402–408.
Luster, D. G., McMahon, M. B., Carter, M. L., Fortis, L. L., & Nunez, A. (2010). Proteomic analysis of germinating urediniospores of Phakopsora pachyrhizi, causal agent of Asian soybean rust. Proteomics, 10(19), 3549–3557.
Osherov, N., & May, G. S. (2001). The molecular mechanisms of conidial germination. FEMS Microbiology Letters, 199(2), 153–160.
Patto, M. C. V., & Niks, R. E. (2001). Leaf wax layer may prevent appressorium differentiation but does not influence orientation of the leaf rust fungus Puccinia hordei on Hordeum chilense leaves. European Journal of Plant Pathology, 107(8), 795–803.
Qiao, J. J., Wang, J. X., Chen, L., Tian, X. X., Huang, S. Q., Ren, X. Y., & Zhang, W. W. (2012). Quantitative iTRAQ LC-MS/MS proteomics reveals metabolic responses to biofuel ethanol in cyanobacterial synechocystis sp PCC 6803. Journal of Proteome Research, 11, 5286–5300.
Rabie El-Akhal, M., Colby, T., Cantoral, J. M., Harzen, A., Schmidt, J., & Javier Fernandez-Acero, F. (2013). Proteomic analysis of conidia germination in Colletotrichum acutatum. Archives of Microbiology, 195, 227–246.
Ruppen, I., Grau, L., Orenes-Piñero, E., Ashman, K., Gil, M., Algaba, F., Bellmunt, J., & Sánchez-Carbayo, M. (2010). Differential protein expression profiling by iTRAQ-two-dimensional LC-MS/MS of human bladder cancer EJ138 cells transfected with the metastasis suppressor KiSS-1 gene. Molecular & Cellular Proteomics, 9(10), 2276–2291.
Schwanhausser, B., Busse, D., Li, N., Dittmar, G., Schuchhardt, J., Wolf, J., Chen, W., & Selbach, M. (2011). Global quantification of mammalian gene expression control. Nature, 473(7347), 337–342.
Shadforth, I. P., Dunkley, T. P. J., Lilley, K. S., & Bessant, C. (2005). i-Tracker: for quantitative proteomics using iTRAQ (TM). BMC Genomics. doi:10.1186/1471-2164-6-145.
Stasolla, C., Loukanina, N., Ashihara, H., Yeung, E. C., & Thorpe, T. A. (2002). Pyrimidine nucleotide and nucleic acid synthesis in embryos and megagametophytes of white spruce (Picea glauca) during germination. Physiologia Plantarum, 115(1), 155–165.
Valent, B., & Khang, C. H. (2010). Recent advances in rice blast effector research. Current Opinion in Plant Biology, 13(4), 434–441.
van Leeuwen, M. R., Krijgsheld, P., Bleichrodt, R., Menke, H., Stam, H., Stark, J., Wosten, H. A. B., & Dijksterhuis, J. (2013). Germination of conidia of Aspergillus niger is accompanied by major changes in RNA profiles. Studies in Mycology, 74, 59–70.
Wang, X. B., & McCallum, B. (2009). Fusion body formation, germ tube anastomosis, and nuclear migration during the germination of urediniospores of the wheat leaf rust fungus, Puccinia triticina. Phytopathology, 99(12), 1355–1364.
Zhang, Y., Qu, Z., Zheng, W., Liu, B., Wang, X., Xue, X., Xu, L., Huang, L., Han, Q., Zhao, J., & Kang, Z. (2008). Stage-specific gene expression during urediniospore germination in Puccinia striiformis f. sp tritici. BMC Genomics. doi:10.1186/1471-2164-9-203.
Zhao, J., Wang, L., Wang, Z., Chen, X., Zhang, H., Yao, J., Zhan, G., Chen, W., Huang, L., & Kang, Z. (2013). Identification of eighteen Berberis species as alternate hosts of Puccinia striiformis f. sp. tritici and virulence variation in the pathogen isolates from natural infection of barberry plants in China. Phytopathology, 103(9), 927–934.
Zheng, W. M., Huang, L. L., Huang, J. Q., Wang, X. J., Chen, X. M., Zhao, J., Guo, J., Zhuang, H., Qiu, C. Z., Liu, J., Liu, H. Q., Huang, X. L., Pei, G. L., Zhan, G. M., Tang, C. L., Cheng, Y. L., Liu, M., Zhang, J. S., Zhao, Z. T., Zhang, S. J., Han, Q. M., Han, D. J., Zhang, H. C., Zhao, J., Gao, X. N., Wang, J. F., Ni, P. X., Dong, W., Yang, L. F., Yang, H. M., Xu, J. R., Zhang, G. Y., & Kang, Z. S. (2013). High genome heterozygosity and endemic genetic recombination in the wheat stripe rust fungus. Nature Communications. doi:10.1186/1471-2164-9-203.
Acknowledgments
We are grateful to BGI (Shenzhen, China) for their excellent iTRAQ technical assistance and constructive discussions.
Funding
This work was supported by grant from the National Basic Research Program of China (National “973” program, No.2013CB127700), Modern Agro-industry Technology Research System in China (CARS-3-1-11), the 111 Project from the Ministry of Education of China (No.B07049).
Author information
Authors and Affiliations
Corresponding author
Additional information
Jing Zhao and Hua Zhuang contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Figure 1
(PPT 237 kb)
Supplementary Table 1
(XLS 20 kb)
Supplementary Table 2
(XLS 1468 kb)
Supplementary Table 3
(XLS 18 kb)
Supplementary Table 4
(XLS 212 kb)
Rights and permissions
About this article
Cite this article
Zhao, J., Zhuang, H., Zhan, G. et al. Proteomic analysis of Puccina striiformis f. sp. tritici (Pst) during uredospore germination. Eur J Plant Pathol 144, 121–132 (2016). https://doi.org/10.1007/s10658-015-0756-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-015-0756-y