Abstract
One of the most important soilborne diseases affecting avocado (Persea americana Mill.) crops is white root rot, caused by the fungus Rosellinia necatrix. In this study we investigated the metabolic responses elicited by white root rot in the aerial part of the plant with special focus on the potential applications of imaging technique (including chlorophyll fluorescence (Chl-F), blue-green fluorescence and thermography) in early detection of the disease on leaves. The results show that leaf metabolism was significantly affected by the infection only when symptoms started to appear, which was probably related to the loss of root functionality. However, changes in some Chl-F parameters provided early indications of stress even prior to the development of symptoms. We suggest that the combinatorial analysis of several Chl-F parameters could be used as a method for early detection of stress related to white root rot, and might prove useful as a general indicator of biotic and abiotic stress in avocado plants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
One of the most important diseases affecting avocado (Persea americana Mill.) plantations is white root rot, caused by the ascomycete fungus Rosellinia necatrix (Pliego et al. 2009, 2012; Sztejnberg and Madar 1980). This disease also cause great economic losses in other woody crops, including ornamental and fruit trees (Pliego et al. 2012). Symptoms of white root rot include rotting of the roots and yellowing of the leaves that eventually wilt. The tree eventually dies within a few weeks after the appearance of the first foliar symptoms. Efforts have been made over the past three decades to achieve control of R. necatrix by different strategies, including the use of fungicides (Kanadani et al. 1998), soil solarisation (López-Herrera et al. 1998), biological control (Calderón et al. 2013; Cazorla et al. 2006; Freeman et al. 1986; Pliego et al. 2007, 2008), or their combination (Ruano-Rosa et al. 2014). Nevertheless, the economic losses caused by R. necatrix in crop fields are increasing worldwide, as reviewed by Pliego et al. (2012).
Imaging techniques applied to remote sensing are powerful tools for use in crop protection (Mahlein et al. 2012), and their application in white root rot infected avocado orchards could have great potential as a non-expensive and quick method for the detection and control of the disease. Several imaging techniques, including autofluorescence techniques and thermography, could be applied for that purpose. Analysis of Chl-F is a widely used method in the study of photosynthesis, since it measures indirectly the activity of the chloroplast electron transport chain. The photosynthetic apparatus is a primary target of stress, particularly the chloroplast thylakoid membrane. In this membrane, photosystem II catalyses the production of O2 and is the main source of reactive oxygen species that can cause oxidative damage. An important defence mechanism of the chloroplast is the dissipation of energy in a safe form, mainly as heat and fluorescence. Therefore, Chl-F parameters such as the maximum efficiency of photosystem II (FV/FM) and non-photochemical quenching (NPQ) can be sensitive indicators of plant stress. Indeed, Chl-F imaging has been used in the study of many plant-pathogen interactions, and in many cases proved useful as a tool for pre-symptomatic detection of disease, as reviewed by Gorbe and Calatayud (2012) and Rolfe and Scholes (2010).
Multicolour fluorescence imaging consists of capturing fluorescence images in several spectral bands to characterize the response of plant secondary metabolism to stress (Bilger et al. 2001; Cerovic et al. 1999). Of particular interest is the autofluorescence in the blue and green regions which is emitted by phenolic compounds with a wide range of roles in plant defence. Hence, this method provides a useful tool to discriminate stressed plants from healthy ones (Buschmann and Lichtenthaler 1998).
Thermal imaging provides measurements of leaf temperature, which is an indicator of transpiration and stomatal conductance (Chaerle and Van der Straeten 2000; Glenn 2012). In many cases of water deficit or pathogen infection, plants (including avocado) activate stomatal closure resulting in restricted transpiration and hence an increase in leaf temperature (Chartzoulakis et al. 2002; Ploetz and Schaffer 1989).
The aim of this work was to study the effects of R. necatrix infection on leaf metabolism of avocado plants using a combination of fluorescence and thermal imaging techniques. Furthermore, we wanted to explore the potential applications of these techniques in pre-symptomatic detection of stress related to white root rot.
Materials and methods
Biological materials
Virulent R. necatrix strain CH53-GFP was grown at 25 °C on potato dextrose agar (PDA; Difco Laboratories, Detroit, MI) or tryptone-pentone-glycerol (TPG) agar plates (Calderón et al. 2013; Pliego et al. 2009) and stored in TPG at 4 °C as previously described (Gutiérrez-Barranquero et al. 2012).
Artificial inoculations to reproduce avocado white root rot were performed using the P. americana/R. necatrix system as described previously (Cazorla et al. 2006). Commercial six-month-old avocado plants (cv. Walter hole) were obtained from Brokaw nurseries (Brokaw España, S.L., Vélez-Málaga, Spain). The plants were placed into pots containing 300 g wet potting soil (Jongkind Grond B.V., Aalsmeer, The Netherlands) and infested with R. necatrix grown on four infected wheat grains per pot as described previously (Freeman et al. 1986). Control plants were mock-infested with four healthy wheat grains per pot. Then the plants were maintained at 16/8 h light⁄dark regime at 24/18 °C, 60 % relative humidity and 200 μmol photon m−2 s−1. All plants were kept well watered at all times to avoid any possible shortage of water supply to the roots.
The plants were analysed at mid-photophase every 2–3 days during the presymptomatic phase, and then every 4 days when symptoms developed (from 28 days post-inoculation [dpi]), and representative data are shown. For each experiment, at least five plants per treatment were analysed and data are presented as means ± sd (n = 5). In the case of small differences between infected and control plants that was not clear from the standard deviations, a t-test was performed. The experiment was repeated six times with same results.
Chlorophyll fluorescence imaging
Chl-F imaging was carried out with a customised Open FluorCam FC 800-O (PSI, Brno, Czech Republic) kinetic imaging fluorometer controlled by FluorCam7 (PSI) software. Measuring light flashes (10 μs) for modulated Chl-F excitation were generated by a pair of red LED panels (λmax ~ 618 nm), and saturating light pulses (1 s, ~2000 μmol m−2 s−1) and actinic light by a pair of blue LED panels (λmax ~ 455 nm). Chl-F kinetics were captured by a charge-coupled device camera with 12-bit and 96 pixels per inch resolution, taking 10 images per second. Reflected radiation was blocked by a far-red filter (RG697, Schott, Mainz, Germany). Measurements of minimum fluorescence (F0) on 30 min dark-adapted leaves and maximum fluorescence (FM) after a saturating light pulse were used to determine maximum quantum yield by the formula FV/FM = (FM − F0)/FM. Changes in maximum fluorescence during actinic light (400 μmol photon m−2 s−1) adaptation were followed during a period of 5 min with saturating light pulses applied after 10 s, 2.5 min and 5 min. Measurements of maximum fluorescence in 5 min light-adapted leaves (F’M) were used to determine steady-state NPQ in the light by NPQ = (FM − F’M)/F’M. The measuring data were also used to determine other Chl-F parameters that are potential stress indicators, including FM/F0, F’V/F’M, F’M/F’0 and F’V/F’0 (where F’V = F’M − F’0).
Blue-green fluorescence imaging
Blue-green fluorescence images were captured by the same imaging system using UV (360 nm) excitation light. Fluorescence images in the blue (F440) and green (F520) regions of the spectrum were acquired sequentially using single-band bandpass filters of 442/46 nm and 525/39 nm (Semrock, Illinois, USA), respectively (Pérez-Bueno et al. 2014). The acquisition of each fluorescence image required the accumulation of 9 images captured during 18 s, generating images of 640 × 480 pixels with a resolution of 96 pixels per inch.
Thermal imaging
Infrared images of plant leaves were recorded in the growth chamber with a Photon 640 camera (FLIR Systems Wilsonville, USA) vertically positioned approximately 0.5 m above the leaves. The Photon 640 is a long-wavelength (7.5–13.5 μm) uncooled microbolometer camera with a resolution of 640 × 512 pixels (25 μm) and NEdT performance <50mK at f/1.0. Digital video data (14-bit serial LVDS) were transmitted through an ethernet adapter to a computer and calibrated by FluorCam7 software (PSI). Infected plants and control plants were positioned for simultaneous imaging of one leaf from each plant, and the images were used to determine differences in average leaf temperature between treatments (ΔT = TR-TC, where TR is the temperature of the infected plant leaf and TC is the temperature of the control plant leaf).
Results
Development of fungal infection in plant host
Initial symptoms of R. necatrix infection in P. americana plants appeared at 28 dpi in the form of wilt, similar to symptoms of severe water deficit (Fig. 1). A few days later (32–36 dpi), plant leaves were desiccated and dead.
Effects of fungal infection on host photosynthesis
Effects of the infection on photosynthetic parameters such as FV/FM and NPQ were analysed by Chl-F imaging (Fig. 2). FV/FM remained constant at 0.78 in the leaves of infected plants during the pre-symptomatic phase, and then decreased dramatically when symptoms developed, declining by 50 % at 28 dpi. Already in the late pre-symptomatic phase (25 dpi) the data suggested a small decrease that, although not statistically significant (p = 0.11), was consistent in all experiments carried out. NPQ was stable in the range of 1.5–1.8 during the pre-symptomatic phase, and then decreased dramatically when the first symptoms appeared, declining by 75 % at 28 dpi.
Chl-F images of FV/FM in leaves of R. necatrix-infected avocado plant (R) and non-infected control plant (C) at 25 dpi and 28 dpi (a), and average FV/FM values throughout the infection (b); Chl-F images of NPQ in leaves of infected plant and control plant at 25 dpi and 28 dpi (c), and average NPQ values throughout the infection (d); means ± sd, n = 5
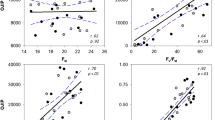
Some other Chl-F parameters that are potential stress indicators were also assessed,. Among these, FM/F0, F’V/F’M and F’V/F’0 showed significant declines in the leaves of infected plants already at the pre-symptomatic stage (25 dpi), and further decreased when symptoms appeared (28 dpi, Fig. 3, Suppl. Fig. S1). Another parameter, F’M/F’0, only showed a significant decrease in the leaves of infected plants after symptoms appeared (28 dpi).
Effects of fungal infection on host secondary metabolism
Effects of the infection on host secondary metabolism were assessed by blue-green fluorescence imaging (Fig. 4). A significant increase in blue (F440) and green (F520) fluorescence in the leaves of infected plants were only observed at the late-symptomatic stage (32–36 dpi), which is probably caused by an alteration in the optical properties of the leaves due to desiccation (Cerovic et al. 1999).
Blue fluorescence (F440) images of leaves of R. necatrix-infected avocado plant (R) and non-infected control plant (C) at 25 dpi and 28 dpi (a), and average F440 values throughout the infection (b); Green fluorescence (F520) images of leaves of infected plant and control plant at 25 dpi and 28 dpi (c), and average F520 values throughout the infection (d); means ± sd, n = 5
Effects of fungal infection on host transpiration
Effects of the infection on host transpiration were assessed by thermal imaging (Fig. 5). A significant increase in the leaf temperature (0.5–1.0 °C) of infected plants was recorded at the early to late-symptomatic stage (28–32 dpi), indicating stomatal closure in response to the infection.
Discussion
R. necatrix is a filamentous ascomycete causing white root rot in a wide range of perennial plants (Pliego et al. 2012). When mycelial inoculum in the dormant stage encounters roots, mycelial masses and strands start to propagate on the roots, penetrate through the lenticels or at the junction between epidermal cells, and then invade the epidermis and xylem of the roots, forming fan-shaped mycelial strands (Pliego et al. 2009). Symptoms in the aerial parts of the plants cannot be recognized in early stages of root infection and, to our best knowledge, there is no study describing early detection of disease effects on leaves. In this study, the system P. americana/R. necatrix was used as a model to explore the applicability of imaging techniques for the detection of metabolic stress on avocado leaves.
Root rot has been reported to result in water deficit in the aerial part of the plant (Ploetz and Schaffer 1989), a phenomenon also observed for other biotic and abiotic stresses (Chartzoulakis et al. 2002; Sade et al. 2012). Additionally, the involvement of toxins in the development of symptoms has been described for the P. americana/R. necatrix interaction (Pérez-Jiménez 2006; ten Hoopen and Krauss 2006). Quite unexpectedly, the photosynthetic parameters FV/FM and NPQ, the blue and green autofluorescence and the transpiration rate, which otherwise are good stress indicators (Buschmann and Lichtenthaler 1998; Mahlein et al. 2012; Rolfe and Scholes 2010), showed no significant differences between infected and healthy plants before the appearance of symptoms in our study. Therefore, it can be concluded that the disease had little impact on leaf metabolism in the pre-symptomatic phase. This provides an explanation why, up to this date, the only methods available for early detection of the disease are still based on direct observation of infected roots or on the use of molecular tools (Kondo et al. 2013), and therefore rather complicated and laborious.
The parameter FV/FM decreased dramatically when the first symptoms appeared at 28 dpi (Fig. 2). The decrease in this parameter suggests an oxidative damage to photosystem II, probably due to the water deficit caused by the white root rot. By contrast, some other Chl-F parameters (FM/F0, F’V/F’M and F’V/F’0), revealed significant differences between healthy and stressed plants at an earlier stage of the infection, which suggest that the photosynthetic apparatus is already affected in the pre-symptomatic phase.
Chl-F imaging is an inexpensive, non-destructive tool widely used in the study of plant stress. Based on the findings reported here, we suggest that the combined analysis of various Chl-F parameters, some of which show differences in the pre-symptomatic phase (FM/F0, F’V/F’M and F’V/F’0), could be used as a method for early detection of stress related to R. necatrix infection. This method could be used to screen for potentially infected or tolerant plants in breeding programs or to complement the diagnosis of the disease by conventional methods. Additionally, these techniques could be useful as an objective method for quantitative analysis of experimental infections, and have more general applications in the detection of biotic and abiotic stress conditions in avocado plants.
References
Bilger, W., Johnsen, T., & Schreiber, U. (2001). UV-excited chlorophyll fluorescence as a tool for the assessment of UV-protection by the epidermis of plants. Journal of Experimental Botany, 52, 2007–2014.
Buschmann, C., & Lichtenthaler, H. K. (1998). Principles and characteristics of multi-colour fluorescence imaging of plants. Journal of Plant Physiology, 152, 297–314.
Calderón, C. E., Pérez-García, A., de Vicente, A., & Cazorla, F. M. (2013). The dar genes of Pseudomonas chlororaphis PCL1606 are crucial for biocontrol activity via production of the antifungal compound 2-hexyl, 5-propyl resorcinol. Molecular Plant-Microbe Interactions, 26, 554–565.
Cazorla, F. M., Duckett, S. B., Bergström, E. T., Noreen, S., Odijk, R., Lugtenberg, B. J. J., et al. (2006). Biocontrol of avocado Dematophora root rot by antagonistic Pseudomonas fluorescens PCL1606 correlates with the production of 2-hexyl 5-propyl resorcinol. Molecular Plant-Microbe Interactions, 19, 418–428.
Cerovic, Z. G., Samson, G., Morales, F., Tremblay, N., & Moya, I. (1999). Ultraviolet-induced fluorescence for plant monitoring: present state and prospects. Agronomie, 19, 543–578.
Chaerle, L., & Van der Straeten, D. (2000). Imaging techniques and the early detection of plant stress. Trends in Plant Science, 5, 495–501.
Chartzoulakis, K., Patakas, A., Kofidis, G., Bosabalidis, A., & Nastou, A. (2002). Water stress affects leaf anatomy, gas exchange, water relations and growth of two avocado cultivars. Scientia Horticulturae, 95, 39–50.
Freeman, S., Sztejnberg, A., & Chet, I. (1986). Evaluation of Trichoderma as a biocontrol agent for Rosellinia necatrix. Plant and Soil, 94, 163–170.
Glenn, D. M. (2012). Infrared and chlorophyll fluorescence imaging methods for stress evaluation. HortScience, 47, 697–698.
Gorbe, E., & Calatayud, A. (2012). Applications of chlorophyll fluorescence imaging technique in horticultural research: a review. Scientia Horticulturae, 138, 24–35.
Gutiérrez-Barranquero, J. A., Pliego, C., Bonilla, N., Calderón, C. E., Pérez-García, A., de Vicente, A., et al. (2012). Sclerotization as a long-term preservation method for Rosellinia necatrix strains. Mycoscience, 53, 460–465.
Kanadani, G., Date, H., & Nasu, H. (1998). Effect of fluazinam soil-drench on white root rot of grapevine. Japanese Journal of Phytopathology, 64, 139–141.
Kondo, H., Kanematsu, S., & Suzuki, N. (2013). Viruses of the white root rot fungus, Rosellinia necatrix. In A. G. Said (Ed.), Advances in virus research (pp. 177–214). USA: Academic.
López-Herrera, C. J., Pérez-Jiménez, R. M., Zea-Bonilla, T., Basallote-Ureba, M. J., & Melero-Vara, J. M. (1998). Soil solarization in established avocado trees for control of Dematophora necatrix. Plant Disease, 82, 1088–1092.
Mahlein, A. K., Oerke, E. C., Steiner, U., & Dehne, H. W. (2012). Recent advances in sensing plant diseases for precision crop protection. European Journal of Plant Pathology, 133, 197–209.
Pérez-Bueno, M. L., Pineda, M., Díaz-Casado, M. E., & Barón, M. (2014). Spatial and temporal dynamics of primary and secondary metabolism in Phaseolus vulgaris challenged by Pseudomonas syringae. Physiologia Plantarum, 153, 161–174.
Pérez-Jiménez, R. M. (2006). A review of the biology and pathogenicity of rosellinia necatrix – the cause of white root rot disease of fruit trees and other plants. Journal of Phytopathology, 154, 257–266.
Pliego, C., Cazorla, F. M., González-Sánchez, M. A., Pérez-Jiménez, R. M., de Vicente, A., & Ramos, C. (2007). Selection for biocontrol bacteria antagonistic toward Rosellinia necatrix by enrichment of competitive avocado root tip colonizers. Research in Microbiology, 158, 463–70.
Pliego, C., de Weert, S., Lamers, G., de Vicente, A., Bloemberg, G., Cazorla, F. M., et al. (2008). Two similar enhanced root-colonizing Pseudomonas strains differ largely in their colonization strategies of avocado roots and Rosellinia necatrix hyphae. Environmental Microbiology, 10, 3295–304.
Pliego, C., Kanematsu, S., Ruano-Rosa, D., de Vicente, A., López-Herrera, C., Cazorla, F. M., et al. (2009). GFP sheds light on the infection process of avocado roots by Rosellinia necatrix. Fungal Genetics and Biology, 46, 137–45.
Pliego, C., López-Herrera, C., Ramos, C., & Cazorla, F. M. (2012). Developing tools to unravel the biological secrets of Rosellinia necatrix, an emergent threat to woody crops. Molecular Plant Pathology, 13, 226–39.
Ploetz, R., & Schaffer, B. (1989). Effects of flooding and phytophthora root rot on net gas exchange and growth of avocado. Phytopathology, 79, 204–208.
Rolfe, S. A., & Scholes, J. D. (2010). Chlorophyll fluorescence imaging of plant-pathogen interactions. Protoplasma, 247, 163–75.
Ruano-Rosa, D., Cazorla, F. M., Bonilla, N., Martín-Pérez, R., Vicente, A., & López-Herrera, C. J. (2014). Biological control of avocado white root rot with combined applications of Trichoderma spp. and rhizobacteria. European Journal of Plant Pathology, 138, 751–762.
Sade, N., Gebremedhin, A., & Moshelion, M. (2012). Risk-taking plants. Plant Signaling & Behavior, 7, 767–770.
Sztejnberg, A., & Madar, Z. (1980). Host range of Dematophora necatrix, the cause of white root rot disease in fruit trees. Plant Disease, 64, 662–664.
ten Hoopen, G. M., & Krauss, U. (2006). Biology and control of Rosellinia bunodes, Rosellinia necatrix and Rosellinia pepo: A review. Crop Protection, 25, 89–107.
Acknowledgments
This work was supported by grants from CICE-Junta de Andalucía (Proyectos de Excelencia P08-CVI-03475, P10-AGR-5797 and P12-AGR-0370), Plan Nacional de I + D + I del Ministerio de Ciencia e Innovación, Spain (AGL2011-30354C0201) cofinanced by FEDER, EU and RECUPERA 2020/20134R060 (Ministerio de Economía y Competitividad-CSIC, Feder funds). E. Granum was recipient of a JAE-Doc contract funded by CSIC, Spain. C. E. Calderón was supported by a grant from FPI, Ministerio de Ciencia e Innovación, Spain.
Compliance with Ethical Standards
The Authors declare that the present manuscript complies with the Ethical Rules of good scientific practice applicable for the European Journal of Plant Pathology.
Conflict of Interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Suppl. Fig 1
Average values of Chl-F parameters FM/F0 (a), F’M/F’0 (b), F’V/F’M (c) and F’V/F’0 (d) in leaves of R. necatrix-infected avocado plants (R) and non-infected control plants (C) throughout the infection; means ± sd, n = 5. (GIF 68 kb)
Rights and permissions
About this article
Cite this article
Granum, E., Pérez-Bueno, M.L., Calderón, C.E. et al. Metabolic responses of avocado plants to stress induced by Rosellinia necatrix analysed by fluorescence and thermal imaging. Eur J Plant Pathol 142, 625–632 (2015). https://doi.org/10.1007/s10658-015-0640-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-015-0640-9