Abstract
This study tested the effectiveness of single and combined applications of Trichoderma and rhizobacterial strains to control white root rot (WRR) caused by Rosellinia necatrix in avocado plants. Three Trichoderma, two T. atroviride and one T. virens monoconidal strains and four bacterial strains (Bacillus subtilis, Pseudomonas pseudoalcaligenes and two P. chlororaphis) were assayed to determine their compatibilities in vitro. In addition, the effects of the bacterial filtrates were evaluated against the Trichoderma strains and reciprocally; these filtrates were applied alone or in combination to determine their effectiveness against R. necatrix. Individual control agents or combinations of them were applied to avocado plants that were artificially inoculated with a virulent R. necatrix strain. Compatibility between the combined Trichoderma applications and the bacterial strains was observed and these combinations significantly improved the control of R. necatrix during the in vitro experiments. A relative protective effect of some Trichoderma and bacteria was observed on the control of avocado WRR when they were applied singly. The combinations of T. atroviride strains with bacterial strains P. chlororaphis and P. pseudoalcaligenes showed a better control of avocado WRR, whereas the rest of Trichoderma and bacteria combinations also reduced significantly the level of disease and induced a delay at the onset of disease with respect to avocado plants inoculated either with Trichoderma or bacteria.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rosellinia necatrix Prillieux (anamorph Dematophora necatrix Hartig) is an ascomycete soil-borne pathogenic fungus that causes white root rot (WRR) (Ten Hoopen and Krauss 2006; Pliego et al. 2012). In fact, WRR is the most important disease that affects avocado plants on the southern coast of Spain (López-Herrera 1998). The effective control of WRR in avocados has been achieved using systemic and contact fungicides (López-Herrera and Zea Bonilla 2007) and soil solarisation (López-Herrera et al. 1998, 1999).
Alternatively, the incorporation of biocontrol agents (BCAs) within an integrated control system reduces the required pesticide dose and minimises the environmental impact (Harman and Kubicek 1998). Several successful attempts for the biological control of R. necatrix in avocado were performed in Israel and Spain using antagonistic microorganisms, such as Trichoderma fungi (Freeman et al. 1986; Ruano-Rosa and López-Herrera 2009), which commonly inhabit soils and occur on plant roots (Harman et al. 2004). Several Trichoderma strains have been isolated in Spain from avocado roots and were selected based on their in vitro and in vivo antagonism against R. necatrix (Ruano-Rosa et al. 2003a, b, 2010). Some of these Trichoderma strains include Trichoderma atroviride P. Karsten, Trichoderma virens (Miller, Gidden and Foster) Arx, Trichoderma harzianum Rifai and Trichoderma cerinum Bissett, Kubicek and Szakacs. The last species produces a novel metabolite called cerinolactone, which was recently discovered by our research group and exhibits activity against certain fungi, including Pythium ultimum, Rhizoctonia solani and Botrytis cinerea (Vinale et al. 2012). Biological control trials of these strains demonstrated better protection when some of them (specifically, CH 101 and CH 304.1 (T. atroviride)) were applied in combination with each other (Ruano-Rosa and López-Herrera 2009).
The use of rhizobacterial strains, including Pseudomonas and Bacillus, is generally conducted in addition to existing plant disease control strategies (Lugtenberg and Kamilova 2009).
Recently, our research group isolated several bacterial strains from the avocado rhizosphere (mainly belonging to Pseudomonas and Bacillus genera), and we demonstrated their individual biocontrol activity against R. necatrix using avocado plantlets (Cazorla et al. 2006, 2007; González-Sánchez et al. 2010; Pliego et al. 2007, 2011). Pseudomonas chlororaphis PCL1601 and PCL1606 strains, and the Bacillus subtilis PCL1608 strain were chosen for their high antagonistic activity, which is related to their antibiotic production and biocontrol activity on avocado plantlets. On the other hand, P. chlororaphis PCL1606 produces the antifungal antibiotic 2- hexyl, 5-propyl resorcinol (HPR), which has an important role in biocontrol against WRR (Calderón et al. 2013).
Competition between microorganisms in the rhizosphere is an important requirement for the successful biocontrol of diseases (Kloepper 1991). In addition, it would be recommended that biocontrol agents could be combined into a single inoculum for synergism and persistent control of the disease (Deacon 1994). Therefore, strains that will be used in combination should be compatible with one another to combat the disease effectively (Baker 1990). Mendoza García et al. (2003) achieved better control of R. necatrix-induced white rot in cocoa when they used mixtures of antagonists than single strains.
The long-term goal of our research is to rationalize and improve the use of microbial biocontrol agents, including the development of combinations of biocontrol organisms to manage the avocado WRR caused by R. necatrix. Here, we report our observations regarding Trichoderma and bacterial strains, which were previously suggested as possible WRR biocontrol agents when used individually. The aims of this work were to determine: i) the in vitro compatibility between Trichoderma spp. and bacterial strains, and the efficacy of combination of both types of antagonistic microorganisms on the pathogen, R. necatrix; and ii) the effects of combining different biocontrol agents against avocado WRR.
Materials and methods
Fungal and bacterial strains
Three monoconidal strains of two different species of Trichoderma, T. atroviride CH 101 (Ta CH 101) and CH 304.1 (Ta CH 304.1) and T. virens CH 303 (Tv CH 303), isolated from the rhizosphere of avocado escape trees and previously selected as biological control agents for WRR (Ruano-Rosa and López-Herrera 2009) were used in this work. Additionally four bacterial strains, B. subtilis PCL1608 (Bs PCL1608), P. chlororaphis PCL1601 (Pc PCL1601) and PCL1606 (Pc PCL1606), and P. pseudoalcaligenes AVO110 (Pp AVO110), which were previously isolated from the rhizosphere of healthy avocado trees (Cazorla et al. 2006, 2007; Pliego et al. 2007, 2011), were also used (Table 1).
The strain Rn 400 (also named CH 53) of R. necatrix from our culture collection was isolated from avocado rotted roots (Ruano-Rosa et al. 2010) and used previously in vitro and in vivo WRR control experiments (Ruano-Rosa and López-Herrera 2009). This strain has been used in this work as pathogen to artificially inoculate the avocado plants.
In vitro experiments
In vitro dual-culture experiments
The three Trichoderma strains (Ta CH 101, Ta CH 304.1 and Tv CH 303) were evaluated in vitro for antagonism against the four bacterial strains (Bs PCL1608, Pc PCL1601, Pc PCL1606, and Pp AVO110) in dual cultures (Royse and Ries 1978). Petri dishes (90 mm in diam.) containing 20 ml of potato dextrose agar (PDA; Difco Laboratories, Detroit, MI, USA) were each co-inoculated. A 5-mm-diameter mycelial disc containing a 2-day-old culture of Trichoderma grown under chamber conditions (25 °C in darkness) was placed at the centre of the Petri dish, and the bacteria were stab-inoculated 3.5 cm from the Trichoderma mycelial disc edge. The controls were comprised of Trichoderma cultures without bacteria and bacterial cultures without Trichoderma. All treatments were conducted in triplicate. The Trichoderma colony growth was evaluated after 48 h of incubation at 25 °C.
Effect of Trichoderma filtrates on bacterial growth
Trichoderma strains were grown in the dark for 2 days on Petri dishes containing PDA at 24 °C. Next, three mycelial plugs (8 mm) were obtained from the colony edge and placed in a 250 mL Erlenmeyer flask containing 200 mL of two alternative liquid growth media: yeast malt extract (YME) (20 g l−1 malt (Difco) plus 2 g l−1 yeast extract (Difco)), and synthetic medium (SM) (0.2 g l−1 MgS04 · 7H20, 0.9 g l−1 K2HP04, 0.2 g l−1 KCl, 1.0 g l−1 NH4NO3, 1.5 g l−1 glucose, 0.002 g l−1 Fe2+, 0.002 g l−1 Mn2+, 0.002 g l−1 Zn2+ and 0.0001 g l−1 thiamine hydrochloride; Okon et al. 1973). These cultures were shaken at 150 rpm and subjected to 12 h photoperiods for 7 or 2 days, respectively, and then filtered with sterile 0.45 μm Minisart filters (Sartorius Stedim Biotech, Goettingen, Germany).
The Trichoderma filtrate fractions (10 ml) were mixed with 10 ml of the water-agar (WA, 25 g agar-agar l−1) and poured into Petri dishes. Each of the four bacterial strains mentioned above were streaked onto these plates and incubated at 24 °C in the dark. The controls comprised bacterial cultures without Trichoderma filtrates in the media YME + WA or SM + WA. All treatments were conducted in quadruplicate. Since the in vitro bacterial growth was not uniform, this was evaluated qualitatively by comparison with the control growth during five consecutive days.
Effect of bacterial filtrates on Trichoderma growth
The Trichoderma and bacterial strains that were previously assayed in vitro in dual-culture experiments were also used in this experiment. The bacterial strains were grown in 200 mL of King’s B liquid medium (King et al. 1954) in 1-l Erlenmeyer flasks. These flasks were shaken for 2 days at 150 rpm and 24 °C in the dark. The bacterial cells were then removed by centrifugation (4500 rpm, 15 min), and the supernatants were filtered through 0.22 μm filters (Millipore Corporation, Bedford, USA). A 10 ml aliquot of each filtrate was mixed with 10 ml of WA (25 g agar-agar l−1) before pouring into Petri dishes. The Trichoderma strains were simultaneously grown on PDA plates and incubated at 24 °C in the dark for 2 days. Later, mycelial discs (5 mm diameter) from the colony edge were placed in the centre of the plates containing the medium and bacterial filtrates. The controls comprised Trichoderma cultures without bacteria filtrates in WA medium. All treatments were conducted in quadruplicate. The effects on the Trichoderma colony growth were assessed with daily colony diameter measurements along two perpendicular lines until the control plates were completely covered. These data were expressed as the area under the accumulated growth curve (AUAGC) over the growth period.
This experiment was performed using a completely randomised experimental design. In addition, an analysis of variance (ANOVA) was conducted on the four Trichoderma strain replicates. The average of the treatments was compared by using Fisher’s LSD test to separate the means (P < 0.05) (Steel and Torrie 1985).
Effect of bacterial and/or Trichoderma filtrates on R. necatrix
The effects of the previously selected bacterial and Trichoderma strains on the growth of R. necatrix were evaluated. The filtrates of these strains were obtained using the same methodology described above. In this experiment, the effect of individual applications was evaluated by pouring 5 ml of the corresponding bacterial or Trichoderma filtrate, 5 ml of sterilised water and 10 ml of WA into Petri dishes. In contrast, the combined applications consisted of 5 ml of bacterial filtrate, 5 ml of Trichoderma filtrate and 10 ml of WA in each Petri dish.
The R. necatrix strains were simultaneously grown in Petri dishes containing PDA and incubated at 24 °C in the dark for 7 days. Later, mycelial discs were placed 5 mm from the colony edge and were placed into the centre of the plates that contained medium with bacterial and/or Trichoderma filtrates. The controls comprised R. necatrix cultures grown on PDA without Trichoderma and bacteria filtrates. All treatments were conducted in quadruplicate. The effects on R. necatrix colony growth were assessed by measuring the colony diameter daily along two perpendicular lines until the controls completely covered the plates. These data were expressed as the AUAGC over the growth period.
This experiment was performed using a completely randomised experimental design. In addition, an ANOVA was conducted for the average of four replicates. The averages of the treatments were compared by using Fisher’s LSD test to separate the means (P < 0.05) (Steel and Torrie 1985).
In planta experiments
Three experiments were conducted to study the effects of the three monoconidial Trichoderma strains and the four bacterial strains on WRR control. These strains were tested individually and in different combinations on avocado plants that were inoculated with R. necatrix Rn 400. The first experiments consisted of bacterial and Trichoderma treatments that were applied individually, with 10 plants per treatment; these experiments were performed three times. Additionally, a separate experiment was conducted in which one of each Trichoderma strains and one of the two bacterial strains (PCL1601 and AVO110), were combined. For this experiment, 10 plants were used for each combined treatment in each replicate; this experiment was performed three times.
Plant material
Prior to inoculation, avocado plants (Hass scion grafted onto Duke 7 clonal rootstock), from the Brokaw-Spain nursery were grown for 24 months in 5-l pots containing sand, peat and silt (1:2:1 v/v/v) under controlled greenhouse conditions of 18–26 °C and 56–80 % RH.
Preparation and application of bacterial inoculum
The bacterial strains were grown on KB media, as previously described (González-Sánchez et al. 2010). Briefly, 24 h liquid bacterial cultures were adjusted to an optical density of 0.8 at 600 nm (approximately 109 cfu ml−1). These bacteria were applied to individual plants by watering with 20 ml of these cultures every 15 days.
Fungal inoculum preparations
The Trichoderma strains and R. necatrix inocula were composed by sterilised wheat seeds that were colonised by a single fungal strain (Sztejnberg and Madar 1980). Briefly, 200 g of wheat seeds were transferred to a flask containing 1 l of distilled water and soaked for 24 h. The excess water was removed, and the flasks were autoclaved at 121 °C on two consecutive days. Each flask of sterile seeds was inoculated with eight discs (6 mm in diameter) from R. necatrix or Trichoderma mycelia that were grown on PDA and incubated for 15 days at 24 °C in the dark.
Fungal inoculations of avocado plants
The inoculations were performed by adding 10 g l−1 of the colonised wheat seeds for each of the Trichoderma strains (approximately 109 spores per l substrate) or 3.75 g seeds l−1 of the colonised wheat seeds for R. necatrix Rn 400 to the plant substrate at different depths and distances from the plant stem.
The Trichoderma inoculations were performed at 0 and 30 days from the beginning of the experiment to maintain sufficient populations of the antagonist in the soil and to favour biological control (Knudsen et al. 1991; Sallam et al. 2009). The pathogen was inoculated at 14 days after the initial Trichoderma treatment, as previously described (Ruano-Rosa and López-Herrera 2009).
The aerial symptoms of avocado WRR disease were assessed every 3 days or weekly, depending on the experiment. The following scale of 1–5 was used for these assessments: 1, healthy plant; 2, plant expressing the first symptoms of wilt; 3, wilted plant; 4, wilted plant with the first symptoms of leaf desiccation and 5, completely desiccated and dead plant. The standardized area under disease progress curve (SAUDPC, Campbell and Madden 1990) for the data collected every 3 days or weekly were calculated for every experiment and treatment. In addition, the average SAUPDC values were obtained based on the number of replicates within each experiment. An ANOVA was conducted, and the treatment averages were compared using Fisher’s LSD test to separate the means (P < 0.01) (Steel and Torrie 1985).
Results
Compatibility of Trichoderma and bacterial strains in dual culture
The three Trichoderma strains were evaluated for in vitro antagonism against four bacterial strains in dual culture (Table 2 ). It is worth to note that Pc PCL1601 bacterial strain was compatible with all three Trichoderma strains; however, Pp AVO110 was incompatible with all assayed Trichoderma strains.
Effect of Trichoderma filtrates on bacterial growth
All the assayed bacteria showed growth in presence of the Trichoderma filtrates, in any or both of the culture media, with the unique exception of Pp AVO110 against Ta CH 101 filtrates. In both culture media assayed (SM and YME), the addition of Ta CH 304.1 filtrate did not inhibit the growth of any of the four bacterial strains. However, the growth of Pc PCL1606 did co-occur with the Trichoderma strain filtrates (Table 3).
Effect of bacterial filtrates on the Trichoderma strains
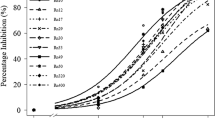
The growth of the Trichoderma strains was significantly reduced by the bacterial filtrates, although the Bs PCL1608 filtrate showed the less inhibitory effect, so it did not inhibit the growth of Tv CH 303 relative to the control. In contrast, PCL1606 and PCL1601 filtrates strongly inhibit the growth of Trichoderma strains, but Tv CH 303 was less reduced, approximately 45 % (F = 42.91; df = 4, 15; P < 0.01). The growth of the T. atroviride CH 101 and CH 304.1 strains was limited to approximately 65 % of their normal growth by Bs PCL1608 and was strongly inhibited by the Pc PCL1601 and Pf PCL1606 filtrates (F = 97.65; df = 4, 15; P = 0.01 and F = 279.27; df = 4, 15; P < 0.01, for the two T. atroviride strains, respectively). Lastly, the Pp AVO110 filtrate almost completely limited the growth of all the Trichoderma strains (Table 4).
Effect of bacterial and/or Trichoderma filtrates on R. necatrix growth
The Bs PCL1608 filtrates significantly reduced the in vitro growth of R. necatrix alone or in combination with filtrates of each Trichoderma strain (Table 5). The rest of the bacterial or Trichoderma filtrates did not significantly reduce that R. necatrix growth when they were applied separately. However, the filtrate combinations with Pc PCL1601 or Pc PCL1606 and Ta CH 304.1 significantly reduced R. necatrix growth relative to when the filtrates were applied individually. Furthermore, combinations of Pc PCL1606 with Ta CH 101.1 and Tv CH 303 significantly also improved the control of R. necatrix (Table 5) (F = 47.20; df = 19, 60; P < 0.01).
In planta experiments
The SAUPDC values obtained the different treatments of three experiments is shown in Table 6. The application of T. atroviride CH 304.1 significantly reduced (F = 9.28; df = 4, 45; P < 0.01) WRR when it was applied individually to the avocado plants inoculated with R. necatrix (Fig. 1). Treatment with Pc PCL1601, Pc PCL1606 and Bs PCL1608 also reduced significantly (F = 2.69; df = 5, 21; P < 0.01) the disease (Fig. 2). Five combinations of Trichoderma and bacterial strains were assayed, including combinations considered as compatible or incompatible in vitro. To determine whether the least effective bacteria showed increased biocontrol activity when combined with Trichoderma, PCL 1601 and AVO 110 bacteria strains were focused on in these biocontrol experiments. In the combinations, Pp AVO 110 plus Ta CH 304.1, and Pc PCL1601 plus Ta CH101 completely nullified the disease, and Pp AVO110 plus Tv CH 303 and Pc PCL1601 plus Ta CH 304.1 also reduced significantly the level of disease (F = 4.42; df = 6, 21; P < 0.01) (Fig. 3). Besides the combination Pp AVO110 plus Tv CH 303 delayed 43 and 23 days the onset of symptoms with respect to Tv CH303 (Figs. 1 and 3) and Pp AVO 110, (Figs. 2 and 3), respectively when they were applied individually. Moreover Pc PCL1601 plus Ta CH 304.1 it delayed 35 days with respect to Ta CH 304.1 when applied singly (Figs. 1 and 3).
In vivo effect of applying three Trichoderma strains on the biological control of avocado white root rot. This experiment was conducted on 24-month-old avocado plants (Hass scion grafted onto Duke 7 clonal rootstock) inoculated under greenhouse conditions (18–26 °C and 56–88 % RH). Above-ground symptoms were evaluated every 3 days along a period of 51 days after inoculation using a 1–5 scale, where 1 is a healthy plant, 2 is a plant with the first symptoms of wilt, 3 is a wilted plant, 4 is a plant wilted that shows the first symptoms of leaf desiccation and 5 is a plant that is completely desiccated and dead. The data are the means of three independent experiments
In vivo effect of applying four bacterial strains on the biological control of avocado white root rot. This experiment was conducted on 24-month-old avocado plants (Hass scion grafted onto Duke 7 clonal rootstock) inoculated under greenhouse conditions (18–26 °C and 56–88 % RH). Above-ground symptoms were evaluated weekly along a period of 81 days after inoculation using a scale of 1–5, where 1 is a healthy, 2 is a plant with the first symptoms of wilt, 3 is a wilted plant, 4 is a wilted plant with the first symptoms of leaf desiccation and 5 is a completely desiccated and dead plant. The data are the means of three independent experiments
In vivo effect of applying five bacteria-Trichoderma combinations on the biological control of avocado white root rot. This experiment was conducted on 24-month-old avocado plants (Hass scion grafted onto Duke 7 clonal rootstock) inoculated under greenhouse conditions (18–26 °C and 56–88 % RH). Above-ground symptoms were evaluated weekly along a period of 81 days after inoculation using a scale of 1–5, where 1 is a healthy plant, 2 is a plant with the first symptoms of wilt, 3 is a wilted plant, 4 is a wilted plant wilted with the first symptoms of leaf desiccation and 5 is a completely desiccated and dead plant. The data are the means of three independent experiments
This fact on the onset of symptoms reveals a delay of disease with an additional effect of biocontrol of the combinations that no were completely effective in nullifying the avocado WRR.
Discussion
Although single antagonists could be active in the soil environments to which they are applied, mixed inoculants could theoretically adapt more readily to a wider range of environmental conditions and may possess a variety of disease-suppression mechanisms (Guetsky et al. 2002; Mendoza García et al. 2003). Thus, the antagonistic activities of biocontrol agents are mediated by different mechanisms, such as antibiotic or enzymatic processes or direct parasitism (Raaijmakers et al. 2002; Romero et al. 2007).
The bacterial and Trichoderma strains used in this study were obtained in previous studies and have demonstrated different antifungal modes of action when applied individually (Cazorla et al. 2006, 2007; Pliego et al. 2007; Ruano-Rosa and López-Herrera 2009). The antibiotics that are produced by bacterial strains include the iturins, fengycins and surfactins produced by B. subtilis PCL1608, phenazines by P. chlororaphis PCL1601 and HPR by P. chlororaphis PCL1606 (Cazorla et al. 2006, 2007). On the other hand, P. pseudoalcaligenes AVO110 is a bacterium that colonises the avocado roots but does not produce antibiotics (Pliego et al. 2007, 2008). T. atroviride CH 101 is a good rhizosphere coloniser but a poor rhizoplane coloniser. In contrast, T. atroviride CH 304.1 is a good coloniser of both the rhizosphere and rhizoplane, and its antagonistic capacity may be attributed to its competency in ecological niches. T. virens CH 303 is less effective in the rhizosphere than the above Trichoderma strains, though it does release some antifungal substances (López-Herrera, personal communication).
Mixing strains with different mechanisms may be a simplest approach for increasing the likelihood of obtaining a versatile and consistently effective BCA against soil-borne pathogens. However, several researchers have indicated that the strains that are combined in biocontrol preparations must be compatible to increase disease suppression when compared individually (Baker 1990; Janisiewicz 1996; Raupach and Kloepper 1998). Accordingly, Woo et al. (2002) suggested that some Trichoderma and Pseudomonas strains could be compatible and thus, this combination could be proposed.
In general, the filtrates of Trichoderma used in this study did not independently have an effect on the in vitro bacterial growth in either of the growth media that were used; there were, however, some exceptions: for example, the Ta CH 101 filtrates inhibited Pp AVO110. In contrast, the bacterial filtrates decreased the growth of the Trichoderma strains in most cases (except when the Bs PCL1608 filtrates were assayed against Tv CH 303). T. virens CH 303 is clearly the Trichoderma strain that is most compatible in vitro against the different bacterial strains assayed. Recent theoretical modelling work has suggested that disease suppression from the combined use of two BCAs was generally very similar to that achieved by the more effective BCA, implying that no synergistic or antagonistic interactions occur. In fact, synergistic effects were evident in only 2 % of the 465 published BCA treatments. Thus, both theoretical and experimental studies suggest that antagonistic interactions among BCAs are more likely to occur than synergistic interactions (Xu et al. 2011). This fact could be associated to the high amount of antifungal compounds produced by these strains, as it has been previously reported (Cazorla et al.. 2007; Calderón et al. 2013), also supported the observed incompatibility against the Trichoderma spp. strains used in this study.
However, we also considered incompatible combinations in vitro because different microorganisms could colonise different rhizospheric niches (Pliego et al. 2008). Therefore, this consideration avoids the theoretical incompatibility that is deduced from in vitro co-cultivation, as can be observed from our results, for instance, Pp AVO110 and Ta CH 304.1 appear as a very effective combination to control avocado WRR, in spite of their observed in vitro incompatibility.
When the bacterial and Trichoderma culture filtrates were assayed against R. necatrix in vitro, a maximum inhibitory effect occurred for the B. subtilis PCL1608 filtrates and consistently all of the different Trichoderma combinations with B. subtilis PCL1608. No significant effects from the other individual bacterial or Trichoderma filtrate assays were observed, however, when mixtures of the bacterial and Trichoderma filtrates were evaluated, the growth of R. necatrix was significantly reduced for certain combinations, such as P. chlororaphis PCL1601 or PCL1606 with T. atroviride CH 304.1. Many studies have shown that the inoculation of plants or soil with beneficial microorganisms has promising potential for improving plant growth (Kloepper et al. 2004).
When the R. necatrix biocontrol experiments were conducted using 24-month-old avocado plants, biocontrol activity has been observed with T. atroviride CH 304.1 treatments when applied alone, as it was previously reported by Ruano-Rosa and López-Herrera (2009). On the other hand, the bacterial strains P. chlororaphis PCL1601 and P. pseudoalcaligenes AVO110 were the less effective when applied individually. Bacterial strains P. chlororaphis PCL1606 and B. subtilis PCL1608 demonstrated satisfactory control of the disease when applied alone, in agreement with previous results (Cazorla et al. 2006, 2007; Calderón et al. 2013). Because these bacterial strains were isolated from avocado roots, their survival in raw soil could be influenced by different factors, such as the watering of the plants, as it has been previously observed that microbial colonization profiles established in the host rhizosphere are modified by the movement of the percolating water (Walker et al. 2002) or difficulties competing with the autochthonous microbial population of the raw soil (Lugtenberg and Kamilova 2009).
However, when the less effective bacterial strains PCL1601 and AVO110 were evaluated in combination with Trichoderma strains, an improvement of their behaviour against R. necatrix during biocontrol experiments was observed. When combined applications of compatible microorganisms were applied, our results coincide with other previous studies (Meyer and Roberts 2002; Raupach and Kloepper 1998) which have reported that using combinations of biocontrol agents increased the suppression of pathogens or disease. The present study showed positive impact on R. necatrix WRR biocontrol through the combination of Trichoderma and some bacteria (relative to their individual effects) considering or not the in vitro compatibility between them. So, the biocontrol of individual strains PCL1601 and AVO110 were increased when they were combined with Trichoderma spp. strains. Several studies regarding combinations of microbial antagonists have demonstrated decreased performance relative to individual applications of these biocontrol agents (Meyer and Roberts 2002).
Although our experiments were performed on adult avocado plants (with the usual difficulties involved when working with woody plants), the results of individual applications agree with those obtained in previous studies in which the inoculations were performed on avocado plants from germinated seeds of cv Topa-Topa (Cazorla et al. 2006; 2007; Ruano-Rosa and López-Herrera 2009) and the results of combined applications are in agreement with other similar previous studies (Mendoza García et al. 2003) were achieved a better control of R. necatrix in cocoa when combinations of Trichodema and Clonostachys rhizophaga. Our results suggest that further investigations are needed to better understand the pathogen-antagonist interactions in complex soil ecosystems. In addition, the compatibility of mycoparasites and the potential interactions between cultural controls, such as liming, the removal of coarse woody debris and biological control under field conditions, need to be addressed.
Through this study, we have provided a new potential method for the control of pathogens in pathogen-avocado-soil pathosystems, and the strains evaluated in this study may be used together or separately for the integrated control of WRR in avocado. Abeysinghe (2009) suggested that mixtures of bacteria and Trichoderma should be applied at different times, first by seeds bacterisation and second by the addition of Trichoderma to the soil. These bacteria can be used for the biological control of damping-off in eggplant and pepper, a condition that is caused by Rhizoctonia solani. In this sense, besides separate applications at different times or under different circumstances of avocado culture (seeds bacterisation, Trichoderma soil application or bacteria applied by irrigation) may be implemented. To our knowledge, this is the first time assaying successful combinations of Trichoderma and rhizobacteria in the control of the avocado WRR caused by R. necatrix.
References
Abeysinghe, S. (2009). Effect of combined use of Bacillus subtilis CA32 and Trichoderma harzianum RU01 on biological control of Rhizoctonia solani on Solanum melongena and Capsicum annuum. Plant Pathology Journal, 8, 9–16.
Baker, R. (1990). An overview of current and future strategies and model of biological control. In D. Hornby (Ed.), Biological Control of Soil-borne Plant Pathogens (pp. 375–388). UK: CAB International.
Calderón, C. E., Pérez-García, A., de Vicente, A., & Cazorla, F. M. (2013). The dar genes of Pseudomonas chlororaphis PCL1606 are crucial for biocontrol activity via production of the antifungal compound 2-Hexyl, 5-propyl resorcinol. Molecular Plant-Microbe Interactions, 26, 554–565.
Campbell, C. L., & Madden, L. V. (1990). Temporal analysis of epidemics. I: descriptions and comparisons of disease progress curve. In C. L. Campbell & L. V. Madden (Eds.), Introduction to Plant Disease Epidemiology (pp. 161–202). New York: Wiley.
Cazorla, F. M., Duckett, S. B., Bergström, E. T., Noreen, S., Odijk, R., Lugtenberg, B. J. J., et al. (2006). Biocontrol of avocado Dematophora root rot by antagonistic Pseudomonas fluorescens PCL1606l correlates with the production of 2-hexyl, 5-propyl resorcinol. Molecular Plant-Microbe Interactions, 19, 418–428.
Cazorla, F. M., Romero, D., Pérez-García, A., Lugtenberg, B. J. J., De Vicente, A., & Bloemberg, G. V. (2007). Isolation and characterization of antagonistic Bacillus subtilis strains from the avocado rhizoplane, displaying biocontrol activity. Journal of Applied Microbiology, 103, 1950–1959.
Deacon, J. W. (1994). Rhizosphere constraints affecting biocontrol organisms applied to seeds. In T. Martin (Ed.), Seed Treatment: Progress and Prospects Monograph nº57 (pp. 315–326). UK: BCPC Surrey.
Freeman, S., Sztejnberg, A., & Chet, I. (1986). Evaluation of Trichoderma as a biocontrol agent for Rosellinia necatrix. Plant and Soil, 94, 163–170.
González-Sánchez, M. A., Pérez Jiménez, R. M., Pliego, C., Ramos, C., De Vicente, A., & Cazorla, F. M. (2010). Biocontrol bacteria selected by a direct plant protection strategy against avocado white root rot show antagonism as a prevalent trait. Journal of Applied Microbiology, 109, 65–78.
Guetsky, R., Shtienberg, D., Elad, Y., Fischer, E., & Dinoor, A. (2002). Improving biological control by combining biocontrol agents each with several mechanism of diseased suppression. Phytopathology, 92, 976–985.
Harman, G. E., & Kubicek, C. P. (1998). Trichoderma and Gliocladium Volume 2: Enzymes, Biological Control and commercial applications. UK: Taylor and Francis.
Harman, G. E., Howell, C. R., Viterbo, A., Chet, I., & Lorito, M. (2004). Trichoderma species-oportunistic, avirulent plant symbionts. Nature Review of Microbiology, 2, 43–56.
Janisiewicz, W. (1996). Ecological diversity, niche overlap, and coexistence of antagonists used in developing mixtures for biocontrol of postharvest diseases of apples. Phytopathology, 86, 473–479.
King, E. O., Ward, M. K., & Raney, D. E. (1954). Two simple media for the demonstration of pyocyanin and fluorescin. Journal of Laboratory and Clinical Medicine, 44, 301–307.
Kloepper, J. W. (1991). Development in vitro assays for pre-screening antagonists of Rhizoctonia solani in cotton. Phytopathology, 81, 1006–1013.
Kloepper, J. W., Ryu, C. M., & Zhang, S. (2004). Induced systemic resistance and promotion of plant growth by Bacillus. Phytopathology, 94, 1259–1266.
Knudsen, G. R., Eschen, D. J., Dandurand, L. M., & Bin, L. (1991). Potential for biocontrol of Sclerotinia sclerotiorum through colonization of sclerotia by Trichoderma harzianum. Plant Disease, 75, 466–470.
López-Herrera, C. J. (1998). Hongos de suelo en el cultivo del aguacate (Persea americana Mill.) del litoral andaluz. In Consejería de Agricultura y Pesca (Ed.), V Jornadas andaluzas de frutos tropicales, Congresos y Jornadas 47/98. (pp. 139-152). Spain: Junta de Andalucía.
López-Herrera, C. J., & Zea Bonilla, T. (2007). Effects of benomyl, carbendazim, fluazinam and thiophanate methyl on white root rot of avocado. Crop Protection, 26, 1186–1192.
López-Herrera, C. J., Pérez Jiménez, R. M., Basallote Ureba, M. J., Zea Bonilla, T., & Melero Vara, J. M. (1998). Soil solarisation in established avocado trees for Dematophora necatrix. Plant Disease, 82, 1088–1092.
López-Herrera, C. J., Pérez Jiménez, R. M., Basallote Ureba, M. J., Zea Bonilla, T., & Melero Vara, J. M. (1999). Loss of viability of Dematophora necatrix in solarized soils. European Journal of Plant Pathology, 105, 571–576.
Lugtenberg, B. J. J., & Kamilova, F. (2009). Plant-Growth promoting rhizobacteria. Annual Review of Microbiology, 63, 541–556.
Mendoza García, R. A., Ten Hoopen, G. M., Kass, D. C. J., Sánchez Garita, V. A., & Krauss, U. (2003). Evaluation of mycoparasites as biocontrol agents of Rosellinia root rot in cocoa. Biological Control, 27, 210–227.
Meyer, S. L. F., & Roberts, D. P. (2002). Combinations of biocontrol agents for management of plant-parasitic nematodes and soilborne plant-pathogenic fungi. Journal of Nematology, 34, 1–8.
Okon, Y., Heti, C., & Henis, Y. (1973). Effect of lactose, ethanol and cycloheximide on translocation pattern of radioactive compounds and sclerotium formation in Sclerotium rolfsii. Journal of General Microbiology, 74, 251–258.
Pliego, C., Cazorla, F. M., González Sánchez, M. A., Pérez Jiménez, R. M., De Vicente, A., & Ramos, C. (2007). Selection for biocontrol bacteria antagonistic toward Rosellinia necatrix by enrichment of competitive avocado root tip colonizers. Research in Microbiology, 158, 463–470.
Pliego, C., De Weert, S., Lamers, G., De Vicente, A., Bloemberg, G., Cazorla, F. M., et al. (2008). Two similar enhanced root-colonizing Pseudomonas strains differ largely in their colonization strategies of avocado roots and Rosellinia necatrix hyphae. Environmental Microbiology, 10, 3295–3304.
Pliego, C., Ramos, C., De Vicente, A., & Cazorla, F. M. (2011). Screening for candidate bacterial biocontrol agents against soilborne fungal plant pathogens. Plant and Soil, 340, 505–520.
Pliego, C., López-Herrera, C., Ramos, C., & Cazorla, F. (2012). Developing tools to unravel the biological secrets of Rosellinia necatrix, an emergent threat to woody crops. Molecular Plant Pathology, 13, 226–239.
Raaijmakers, J. M., Vlami, M., & De Souza, J. T. (2002). Antibiotic production by bacterial biocontrol agents. Antonie Van Leeuwenhoek, 81, 537–547.
Raupach, G. S., & Kloepper, J. W. (1998). Mixtures of plant growth promoting rhizobacteria enhance biological control of multiple cucumber pathogens. Phytopathology, 88, 1158–1164.
Romero, D., De Vicente, A., Rakotoaly, R. V., Dufour, S. E., Veening, J. W., Arrebola, E., et al. (2007). The iturin and fengycin families of lipopeptides are key factors in antagonism of Bacillus subtilis towards Podosphaera fusca. Molecular Plant-Microbe Interactions, 20, 430–440.
Royse, D. J., & Ries, S. M. (1978). The influence of soil fungi isolated from peach twigs on the pathogenicity of Cytospora cincta. Phytopathology, 68, 603–607.
Ruano-Rosa, D., & López-Herrera, C. J. (2009). Evaluation of Trichoderma spp. as biocontrol agents against avocado white root rot. Biological Control, 51, 66–71.
Ruano-Rosa D., Del Moral-Navarrete L., & López-Herrera C. J. (2003a). Study of in vitro growth temperatures of Trichoderma spp. and Rosellinia necatrix. Evaluation of antagonism through dual cultures. In Consejería de Agricultura y Pesca, Junta de Andalucía (Ed.), Proceedings of V World Avocado Congress, October 19–24, 2003, Torremolinos, Malaga, Spain, pp. 525–529.
Ruano-Rosa D., Del Moral-Navarrete L., & López-Herrera C. J. (2003b). Experiments of biological control of avocado White Root Rot. In Consejería de Agricultura y Pesca, Junta de Andalucía (Ed.), Proceedings of V World Avocado Congress, October 19-24, 2003, Torremolinos, Malaga, Spain, pp. 519–523.
Ruano-Rosa, D., Del Moral-Navarrete, L., & López-Herrera, C. J. (2010). Selection of Trichoderma spp. isolates antagonistic to Rosellinia necatrix. Spanish Journal of Agricultural Research, 8, 1084–1097.
Sallam, N., Abd Elrazik, A. A., Hassan, M., & Koch, E. (2009). Powder formulations of Bacillus subtilis, Trichoderma spp and Coniothyrium minitans for biocontrol of Onion White Rot. Archives of Phytopathology and Plant Protection, 42, 142–147.
Steel, R. G. D., & Torrie, J. H. (1985). Bioestadística: Principios y Procedimientos. México: McGraw-Hill.
Sztejnberg, A., & Madar, Z. (1980). Host range of Dematophora necatrix, the cause of white root rot disease in fruit trees. Plant Disease, 64, 662–664.
Ten Hoopen, G. M., & Krauss, U. (2006). Biology and control of Rosellinia bunodes, Rosellinia necatrix and Rosellinia pepo: a review. Crop Protection, 25, 89–107.
Vinale, F., Arjona-Girona, I., Nigro, M., Mazzei, P., Piccolo, A., Ruocco, M., et al. (2012). Cerinolactone, a hydroxy-lactone derivate from Trichoderma cerinum. Journal of Natural Products, 75, 103–106.
Walker, R., Rossall, S., & Asher, M. J. C. (2002). Colonization of the developing rhizosphere of sugar beet seedlings by potential biocontrol agent applied as seed treatments. Journal of Applied Microbiology, 92, 228–237.
Woo, S., Fogliano, V., Scala, F., & Lorito, M. (2002). Synergism between fungal enzymes and bacterial antibiotics may enhance biocontrol. Antonie Van Leeuwenhoek, 81, 353–356.
Xu, X. M., Jeffries, P., Pautasso, M., & Jeger, M. J. (2011). Combined use of biocontrol agents to manage plant diseases in theory and practice. Phytopathology, 101, 1024–1030.
Acknowledgments
This research was supported by the “Consejería de Innovación Ciencia y Empresa”-Junta de Andalucía grant (Grupos PAIDI AGR-169, AGR-235) and by the Plan Nacional I+D+I from Ministerio de Ciencia e Innovación (AGL 2008-05453-C02-01, AGL 2008-05453-C02-02, AGL 2011-030354-CO2-01 and AGL 2011-030354-CO2-02). In addition, this research was co-financed by FEDER funds (EU). The authors acknowledge the support of Dr. Araceli Barceló and the “Instituto de Investigación y Formación Agraria y Pesquera” (IFAPA)-Churriana facilities (Junta de Andalucía, Málaga).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ruano-Rosa, D., Cazorla, F.M., Bonilla, N. et al. Biological control of avocado white root rot with combined applications of Trichoderma spp. and rhizobacteria. Eur J Plant Pathol 138, 751–762 (2014). https://doi.org/10.1007/s10658-013-0347-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-013-0347-8