Abstract
Chitosan, obtained from chitin by partial N-deacetylation, shows little or no toxicity towards mammalian cells, is biodegradable, and non-allergenic. It is known that chitosan may have antifungal properties, but the effect of defined chitosan or chito-oligosaccharides (CHOS) with different degree of polymerization is not well known. The objective of this study was to produce CHOS with different DPn (average degree of polymerization) and determine the most effective DPn of chitosan and CHOS against Botrytris cinerea Pers. Ex Fr. and Mucor piriformis Fischer. In vitro testing showed that CHOS of DPn 23 and 40 had the highest germination inhibition against the tested pathogens. The original chitosan (DPn 206) and a collection of short CHOS (degree of polymerization of 3–10) were significantly (P < 0.01) less effective than CHOS of DPn 23 and 40. M. piriformis M119J showed the most abnormal swelling in presence of CHOS DPn 40, but all abnormally swollen conidia showed further germ tube elongation. In vivo testing showed that CHOS DPn 23 was the most effective in reducing flower infection by two isolates of B. cinerea. Our results show that CHOS inhibit fungal germination and growth and that the effect depends highly on the level of polymerization of the oligomers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The pathogenic fungus Botrytis cinerea Pers.ex Fr. (anamorph of Botryotinia fuckeliana) causes gray mold in over 200 plant species, mainly dicotyledonous plants, resulting in severe pre- and postharvest losses in agricultural crops like berries, fruits and vegetables (Williamson et al. 2007; Rosslenbroich and Stuebler 2000; Tronsmo 1991). The pathogenic fungus Mucor piriformis Fischer also causes postharvest rots on strawberries as well as on several other fruit crops (Sholberg 1990). Pathogens may develop resistance towards chemical fungicides, creating a need for even more chemicals (Holmes and Eckert 1999). However, as existing chemical fungicides may be harmful for mammals and the environment, there is a need to reduce their use. Attempts have been made to reduce gray mold using biological or cultural control methods (Card et al. 2009), but these often give less consistent results than chemical control. There is a need for antifungal products that cause no harm to the environment and are non-toxic to mammals (Parvu et al. 2010). Chitosan (deacetylated chitin) has long been known to have such properties (Trotel-Aziz et al. 2006).
Chitin is a linear biopolymer consisting of ß 1,4-linked N-acetyl-D-glucosamine (GlcNAc) residues and it is one of the most abundant biopolymers in nature. The fraction of acetylation (FA) of chitin is usually above 0.90, meaning there are few D-glucosamine (GlcN) units present. Chitin is found as a structural polymer in fungi, crustaceans, arthropods, insects, and nematodes (Goody 1990). Chitosan, a linear polymer made by partial deacetylation of chitin, is a heteropolymer consisting of N-acetyl-D-glucosamine (GlcNAc) and D-glucosamine (GlcN) residues (Goody 1990). Chitosan can be hydrolysed into shorter chains to produce chito-oligosaccharides (CHOS). Both chitosan and CHOS show little or no toxicity towards mammalian cells (Jung et al. 1999; Singla and Chawla 2001), are biodegradable, and may protect plants by direct antimicrobial activity or by eliciting plant defence mechanisms (Hadwiger 1979; Hadwiger and Beckman 1980). The antifungal activity of chitosan is influenced by many factors, such as its concentration (Palma-Guerrero et al. 2008), degree of polymerization (DP) or molecular weight (Kendra and Hadwiger 1984), fraction of acetylation (F A) (Stössel and Leuba 1984), pH, and ionic strength of the media (Jung et al. 1999; Wang 1992).
Previous studies on the effect of molecular weight on the antimicrobial activity of chitosan have given diverging results. While some studies found that polymeric chitosan (high molecular weight) had higher antifungal activity than low molecular weight chitosan (Eikenes et al. 2005; Meng et al. 2010), other studies reported the opposite result (Kim and Rajapakse 2005; Xu et al. 2007). However, as most studies involved chitosan with only one or very few different molecular weights that were not obtained from the same source or by the same method, these data may not give comparable results. The aim of this study is therefore to use well-defined chitosan with respect to molecular weight and to determine which show the greatest inhibitory effect against the two common plant pathogens B. cinerea and M. piriformis. Two types of experiments were carried out: an in vitro microtitre plate assay on conidial germination and hyphal growth of B. cinerea and M. piriformis, and an in vivo assay on strawberry flower infection by B. cinerea.
Materials and methods
Enzymatic production and analysis of chito-oligosaccharides (CHOS)
Chitosan (KitoNor; fraction of acetylation (F A) 0.15; viscosity average molecular weight (MWv) 33.4 kDa and DPn 206, was obtained from Norwegian Chitosan (Gardermoen, Norway). CHOS with varying level of polymerization were produced from chitosan (DPn 206) by enzymatic hydrolysis for different lengths of time, using chitosanase ScCsn46A as described by Heggset and coworkers (Heggset et al. 2010). Briefly, chitosanase ScCsn46A, originally from Streptomyces coelicolor q9rj88, was purified from the culture supernatant of a recombinant Escherichia coli BL21Star (DE3) strain, following the published protocol, without removal of the (His)6-tag after purification. The enzyme was dialyzed against Tris–HCl (20 mM) at pH 8 and stored at 4 °C.
Hydrolysis of chitosan to produce CHOS
Chitosan (DPn 206, 10 mg ml−1) in buffer (0.04 M NaAc, 0.1 M NaCl, pH 5.5) was incubated at 37 °C and shaken at 225 rpm for about 10 min until the chitosan was dissolved. The pH was further adjusted to 5.5 with NaOH (0.5 M). Chitosanase ScCsn46A26 (0.5 μg mg−1 chitosan) was added to the chitosan solution and the mixture was incubated for 10–50 min at 37 °C and 225 rpm. The enzymatic reaction was stopped by decreasing the pH to 2.5 with HCl (0.5 M), followed by immersing the tube in boiling water for at least 10 min to inactivate the enzymes permanently. The resulting CHOS samples were dialyzed against dH2O for 48 h (water was changed every 12 h) using a cellulose membrane (Float-A-Lyzer® MWCO 500 Da, from Spectrum Labs, Texas, USA) to remove buffer salts from the sample. Dialyzed samples were sterile filtrated through Filtropur S 0.2 μm sterile filters (Sarstedt, Germany), lyophilized and stored at 4 °C (Aam et al. 2010).
1H-NMR analysis of CHOS
Lyophilized CHOS (10 mg) were dissolved in deuterium oxide (D2O, 0.5 ml) and the pH was adjusted to 4.2 with sodium deuteroxide (NaOD) and deuterium chloride (DCl) prior to lyophilization. This process was repeated once. Finally the lyophilized CHOS sample was dissolved in D2O (700 μl) and 1H-NMR analysis was performed on a 300 MHz Varian Gemini instrument (Varian, USA) at 85 °C (1H-NMR spectra is shown in Fig. 1). The DPn was calculated with the equation (Dα + Dβ + D + Aα + Aβ + A)/(Dα + Dβ + Aα + Aβ), where Dα, Dβ, Aα and Aβ are the integral of the reducing end signals of the α and β anomers of the deacetylated (D) and acetylated (A) units respectively, D is the integral of the signals from deacetylated units GlcN) in internal and non-reducing end positions, and A is the integral of the signals from acetylated units (GlcNAc) in internal and non-reducing end positions (Sørbotten et al. 2005).
Separation of CHOS DP 3–10 by size exclusion chromatography (SEC)
CHOS DPn 5, made from enzymatic hydrolysis of chitosan (DPn 206) with ScCsn46A, was separated on three SuperdexTM 30 columns (XK columns from GE Healthcare) with an overall dimension of 2.6 × 180 cm. The flow rate of the mobile phase (0.15 M NH4Ac, pH 4.5) was maintained at 0.8 ml min−1 (Sørbotten et al. 2005). A refractive index detector (Gilson model 133, UK) was used to monitor the relative amounts of the CHOS fractions. CHOS (100 mg) sample was applied in each run. CHOS DP 3–10 was collected from several separate runs and pooled followed by dialysis to remove salts from the buffer. The sample was then sterile filtrated through Filtropur S 0.2 μm sterile filters, and lyophilized.
Matrix assisted laser desorption/ ionization time of flight mass spectrometry
MS spectra were acquired using an UltraflexTM TOF/ TOF mass spectrometer (Bruker Daltonik GmbH, Bremen, Germany) with gridless ion optics under control of Flexcontrol 4.1. For sample preparation, 1 μl of isolated CHOS and 2 μl of matrix solution (15 mg ml−1 2,5-dihydroxybenzoic acid) were mixed and 1 μl of the mixed solution was spotted on a target plate (Cederkvist et al. 2008). The spotted samples were dried at room-temperature. The MS experiments were conducted using an accelerating potential of 20 kV in the reflector mode.
Fungal pathogens
The fungal pathogens used in this experiment were Botrytis cinerea isolate BC 101, isolated from infected strawberry fruit in Grimstad, Norway; B. cinerea isolate BCBD, from a chickpea leaf in Gazipur, Bangladesh and M. piriformis isolate M199J, from an infected strawberry fruit at Hobøl, Norway. For the in vitro and in vivo bioassays, conidia were collected from cultures grown on potato dextrose agar (PDA) (Difco Laboratories, Detroit, MI) under regular laboratory light for two weeks at 23 ± 1 °C. The conidia were suspended in sterile water and were filtered through sterile cotton to remove fragments of mycelia. Concentrations of conidia in aqueous suspensions were determined by haemocytometer counter at 400× magnification (Leica, DM RBE, Germany) and adjusted to the required concentrations.
In vitro bioassay; inhibition of conidia germination and hyphal growth
The effect of chitosan and CHOS on germination of B. cinerea (isolates BC 101 and BCBD) and M. piriformis M199J was tested in a modified synthetic medium (MSM) with pH 5.3 and the following final concentrations: 2.5 mM NH4NO3; 0.28 mM CaCl2 · 2H2O; 0.16 mM MgSO4 · 7H2O; 0.002 mM MnSO4 · 4H2O; 0.002 mM ZnSO4 · 7H2O; 1 mM KH2PO4; 0.06 mM FeC6H5O7 · 5H2O and 55.5 mM glucose. Experiments were set up by adding 100 μl of chitosan or CHOS in 2× MSM to 100 μl conidial suspension (4 × 104 conidia ml−1 in water) in wells in a flat-bottom 96-well microtitre plate (NuncTM, Roskilde, Denmark). There were three replicate wells of each treatment. The microtitre plates were incubated at 23 ± 1 °C for 24 h. The germination percentage 12 and 24 h after inoculation (HAI) was visually estimated at 400× magnification using an invert microscope (Fluovert FU, Ernst Leitz Wetzlar GmbH, Wetzlar, Germany). The conidia were counted as germinated when the germ tube length was at least as long as the diameter of the conidium. The germination of M. piriformis M199J conidia was measured differently since all germinated conidia exposed to chitin or CHOS showed abnormal swelling with amoeba-like structure and one or more protruded portions. When the length of the protruded part was at least as long as the diameter of the swelled conidia the conidia was considered germinated. The germination inhibition percentage was calculated by the following equation:
Where,
- a:
-
germinated conidia in the control (conidia in MSM)
- b:
-
germinated conidia in the presence of chitosan or CHOS in MSM
The pH of the conidia suspension in the microtitre wells was between 5.2 and 5.3 at the start of the experiment, and remained about the same 24 h after inoculation.
Germination and further germ tube elongation were also documented by photographs (10–15, from each treatment with Canon D-400 camera, Japan) taken through the invert microscope at 400× magnification (Fluovert FU, Ernst Leitz Wetzlar GmbH, Wetzlar, Germany) 12 and 44 h after inoculation.
In vivo bioassay: Inhibition of infection of strawberry flowers by Botrytis cinerea
Chitosan (DPn 206) and CHOS (DPn 9, 23, 40 and 48) were tested for their ability to reduce infection by B. cinerea BC 101 and B. cinerea BCBD of newly opened strawberry (Fragaria × ananassa cv. Corona) flowers. The strawberry plants were grown in the greenhouse under controlled temperature (18 °C at day; 12 °C at night), light (16 h, light intensity: 150 μmols m−2 s−1) and relative humidity (65 %) conditions. Eighteen flowers per treatment (six replications of three flowers) were cut off with a 1½–2 cm stem and placed in empty pipette tip racks set in plastic containers filled with 1–2 cm water. Conidia suspension (final concentration: 1 × 106 conidia/ml) was mixed with different test ingredients in sterile water, or with sterile water (control reaction). Flowers were infected by applying 10 μl of the sample at the base of three petals on each flower with a pipette. The treatments were randomized. The plastic containers were covered with aluminium foil and incubated at 23 ± 1 °C. The relative humidity was 90–95 %, measured by a thermohygrometer (Lambrecht, Germany). The experiment was repeated twice. The infection was recorded as visible necrotic regions on the abaxial surface of the flowers under the inoculation point daily for 8 days, and the area under the disease progress curve (AUDPC) was calculated on the basis of the cumulative daily infection by the following equation:
Where, Di = Days of the ith assessment and Si = Proportion of the ith infected inoculation point.
The protection index (in %) was calculated by using the AUDPC values in the following equation (Bardin et al. 2008):
Where AUDPCcontrol represent flowers inoculated with B. cinerea BC 101 or BCBD conidia alone and AUDPCtreatment represents flowers inoculated with B. cinerea BC 101 or BCBD conidia premixed with chitosan or CHOS.
Data analysis
In the microtitre plate assay, the percentage of germination inhibition of pathogens by chitosan and CHOS were transformed by arcsine transformation and tested by one way ANOVA (only non transformed data are presented). In the strawberry flower assay, the AUDPC was calculated based on the cumulative daily infections from one to eight days, and tested by one way ANOVA. When appropriate, means were separated by Tukey’s Honestly Significant Difference method. All statistical analyses were done by Microsoft Office Excel 2007 and Minitab 16 (MINITAB, USA).
Results
Production and characterization of CHOS
CHOS fractions were produced by degrading chitosan (DPn 206 and F A of 0.15) with ScCsn46A, as described in the Materials and methods section. By varying the incubation time, CHOS fractions with DPn values between 75 and 5 were produced. DPn was used as an indicator of molecular weights for all CHOS samples since this is straightforwardly determined by 1H NMR analysis.27 The 1H-NMR spectra of the CHOS fractions showed that all reducing ends were completely deacetylated (signals at 5.43 ppm for -Dα and 4.92 ppm for -Dβ) (Fig. 1). There were no signals for new acetylated reducing ends (signal for -A α at 5.19 ppm and for DA β at 4.74). A CHOS fraction of DPn of 5 was further purified by size exclusion chromatography (SEC). CHOS of DP 3–10 were collected and pooled together to serve as the fraction with lowest molecular weights.
Effects of chitosan and CHOS on the ability to inhibit germination and germ tube elongation in vitro
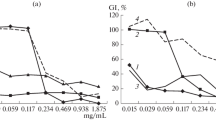
The inhibitory effects on conidial germination of chitosan and CHOS with different DPn (80 μg ml−1) against B. cinerea and M. piriformis are shown in Table 1. All three isolates experienced a higher degree of inhibition at 12 vs. 24 h after inoculation (HAI). M. piriformis was sensitive (in terms of germination inhibition) to a wider range of CHOS (DPn 9–75) while B. cinerea BC 101 was only markedly inhibited by CHOS DPn 23 and 40. The results clearly show a size dependency for the antifungal effect of CHOS.
The antifungal activity was also tested for the original chitosan (F A 0.15, DPn 206) and CHOS DP3-10 as controls for high and low molecular weight fractions. At 80 μg ml−1, there was no significant difference between DP 3–10 and chitosan in terms of germination inhibition, but at a high concentration (1,300 μg ml−1) DP 3–10 showed significantly higher germination inhibition than chitosan of all tested pathogens 24 h after inoculation (Table 2).
The effects of DPn on the ability of chitosan and CHOS to inhibit conidial germination and further germ tube elongation were observed visually (Figs. 2 and 3). Chitosan (DPn 206) and CHOS (DPn 40) caused abnormal swelling of M. piriformis (Fig. 2), but no such effect was observed on the two B. cinerea isolates. However, CHOS DPn 40 caused granular inclusions in the cytoplasm of all tested pathogens. In the presence of CHOS DPn 40, germ tubes from both B. cinerea isolates ceased to grow after germination and no further growth was observed 44 h after inoculation (Fig. 3). The M. piriformis M199J conidia treated with CHOS DPn 40 were abnormally swollen 12 h after inoculation (Fig. 2) but continued to grow; however, many non-germinated and non-swollen conidia were also present. Chitosan (DPn 206) and CHOS DPn 9 did not affect germ tube elongation of the tested pathogens (Fig. 3).
Effect of chitosan DPn 206 and CHOS DPn 9 and 40 (all 80 μg ml−1 in MSM) on conidia germination and further germ tube elongation of Botrytis cinerea (BC 101 and BCBD), and Mucor piriformis M199J (12 HAI, 23 ± 1 °C). BC 101: B. cinerea BC 101; BCBD: B. cinerea BCBD, M199J: M. piriformis M199J. Control is conidia in MSM
Effect of chitosan (DPn 206) and CHOS (DPn 9 and 40) (80 μg ml−1 in MSM) on germ tube elongation of Botrytis cinerea (BC 101 and BCBD), and Mucor piriformis M199J (44 h after inoculation at 23 ± 1 °C). BC 101: B. cinerea BC 101; BCBD: B. cinerea BCBD, M199J: M. piriformis M199J. Control is conidia in MSM
Strawberry flower bioassay
CHOS DPn 23 followed by CHOS DPn 40 were the most effective chitooligosaccharides in reducing flower infection by B. cinerea (BC 101 and BCBD) (Table 3and Fig. 4). In vivo, both B. cinerea isolates caused 100 % infection of strawberry flowers 6 days after inoculation in the control and when treated with chitosan DPn 206 and CHOS DPn 9 (500 μg ml−1) (Fig. 4). CHOS DPn 23 and CHOS DPn 40 reduced the B. cinerea BC 101 infection to 60 % and 30 % respectively, and the B. cinerea BCBD infection to 43 and 20 % respectively at 6 days after inoculation (data not shown).
Antifungal effect of chitosan and CHOS. The photos illustrate the inhibitory effect of chitosan (DPn 206) and CHOS DPn 9, 23, 40 and 48 (500 μg ml−1) on disease caused by Botrytis cinerea (BC 101 and BCBD) applied to detached strawberry flowers six days after inoculation. Control was conidia in sterile water. The flowers were considered 100 % infected when all three inoculation points displayed necrotic signs. All treatments included 18 flowers, but only nine flowers are shown here
Discussion
Molecular weight, and consequently the degree of polymerization, as well as the degree of acetylation, are important factors affecting the antifungal activity of chitosan (Aam et al. 2010; Kendra and Hadwiger 1984; Oliveira Junior et al. 2012; Rahman 2013). Our previous in vitro and in vivo studies on chitosan with different F A showed that chitosan with low F A (0.11 and 0.18) was more inhibitory than chitosan with higher F A (0.39) (Rahman 2013). Chitosan with F A 0.15 was therefore selected for the present study, in which well-defined chitosan and CHOS fractions of different DP/DPn obtained from the same chitosan were used to test for antifungal activity against Botrytis cinerea and Mucor piriformis in vitro and in vivo. The antifungal activity of chitosan and CHOS varied with the DPn in both bioassays, and CHOS 23 and 40 were the most effective of the tested CHOS in inhibiting germination and further germ tube elongation of the tested pathogens in vitro. Antifungal activity of chitosan has been previously reported to depend on DP, where 6 kDa (DPn around 40) was the most effective on a range from 5 to 27 kDa in inhibiting Candida krusei (Gerasimenko et al. 2004). In our study, chitosan with high polymerization (DPn 206, MWv 34.4 kDa) and low polymerization (CHOS DP 3–10) were only effective in inhibiting germination and further germ tube elongation of B. cinerea and M. piriformis at a high concentration (1,300 μg ml−1) and CHOS DP 3–10 was more effective than chitosan (DPn 206). This corresponds to a previous study stating that CHOS with low DP (DP 3–9) were more effective in inhibiting B. cinerea than chitosan with high DP (molecular weight 300–500 kDa) (Xu et al. 2007).
The effectiveness of chitosan and CHOS also depends on the cell wall composition of the tested pathogen (Allan and Hadwiger 1979). The cell walls of the ascomycetous fungi (B. cinerea) contain chitin, whereas the zygomycetous fungi (M. piriformis) contain both chitin and chitosan (Ruiz-Herrera 1992). Our study shows that M. piriformis is more sensitive to the tested chitosans than B.cinerea. However, previous research showed contradictory results regarding the correlation between chitosan’s antifungal activity and fungal cell wall composition. Allan and Hadwiger documented that fungi with cell walls containing chitosan (Mucor spp.) were not sensitive to chitosan (1,000 μg ml−1) (Allan and Hadwiger 1979). However, using the same chitosan concentration, El-Ghaouth showed germination and growth inhibition of other zygomycetous fungi (Rhizopus stolinifer and M. racemosus) with chitosan in their cell wall (El-Ghaouth et al. 1992). Our results showed that the sensitivity of fungal pathogens to chitosan varied with the DP. M. piriformis M199J was sensitive (more than 50 % inhibition of germination 24 h after inoculation) to CHOS with a wide range of DP (DPn 15–48), B. cinerea BCBD was sensitive to CHOS with a narrower range of DP (DPn 15–40) and B. cinerea BC 101 was only sensitive to DPn 23 and 40.
Microscopic observations confirmed that the DP of CHOS was important for its ability to inhibit germination and growth (further germ tube elongation) of the tested pathogens, since CHOS DPn 40 showed more cytoplasmic disorder and abnormal swelling of conidia than other tested DPn. B. cinerea conidia developed granular substances in the cytoplasm in the presence of CHOS DPn 40 at low concentration (80 μg ml−1) while higher concentrations of CHOS DP 3–10 (1,300 μg ml−1) and chitosan DPn 206 (≥2,500 μg ml−1) were required for the same effect. Similar morphological changes have previously been found in B. cinerea treated with chitosan (Ait et al. 2004). Still, B. cinerea conidia, even at a high chitosan concentration (5,000 μg ml−1), did not show abnormal swelling, which has also been observed in a previous study (El-Ghaouth et al. 1992). In contrast, all our tested DP of chitosan and CHOS, except the lowest DP (CHOS DPn 9 and DP 3–10), caused abnormal swelling of M. piriformis conidia. The swelling varied with the concentration and DPn of chitosan and CHOS where the most abnormal swelling (amoeba-like structure) occurred in M. piriformis treated with CHOS DPn 40. Also, other studies have confirmed that the DP of chitosan influenced the extent of abnormal swelling of fungal conidia. Chitosan with high DP (molecular weight 30.7 kDa, DP ≈ 150) caused more abnormal swelling of Rhizopus stolonifer conidia than chitosan with low DP (molecular weight 17.4 kDa, DP ≈ 90) (Hernández-Lauzardo et al. 2008) and low DP (exact molecular weight not mentioned) at a high concentration (15,000 μg ml−1) changed the shape of Rhizoctonia solani conidia (Bautista-Baños et al. 2004).
No lyses of abnormally swollen M. piriformis conidia were observed and all swollen conidia continued to grow (further elongation of germ tube after germination). Also, El-Ghaouth et al. reported excessive branching and abnormal swelling of R. stolonifer treated with chitosan (1,500 μg ml−1, molecular weight not mentioned), and no alternation nor lysis of the cells (El-Ghaouth et al. 1992). Other reasons for further growth of the abnormally swelled M. piriformis conidia could be that the cells with abnormal swelling belonged to a more resistant subpopulation or survived as the chitosan concentration was reduced through binding to other cells (Rhoades and Roller 2000). The reason why conidia of M. piriformis, but not B. cinerea, showed abnormal swelling could be due to the inherent different cell wall compositions. It could be that the application of chitosan affected new cell wall synthesis of M. piriformis and resulted in abnormal swelling of conidia.
In the strawberry flower assay, CHOS DPn 23 was the most effective in reducing flower infection by B. cinerea in line with what was observed in vitro. In addition to the direct antifungal effects of chitosan seen in the in vitro bioassay, chitosan may protect flowers indirectly by eliciting plant defence mechanisms against pathogens (Palma-Guerrero et al. 2008). Previous studies showed that chitosan acts as an elicitor of multiple defence responses of higher plants (Aziz et al. 2006; Vander et al. 1998), and that the eliciting effect depends on the DP of chitosan (Lin et al. 2005). Thus, in our study CHOS DPn 23 may have been more effective in inducing defence responses in strawberry than chitosan (DPn 206).
In conclusion, the objective of our study was to find the most effective DP of chitosan and CHOS against B. cinerea and M. piriformis. Our study demonstrated that the DPn of chito-oligosaccharides (CHOS) affected their antifungal activity both in vitro and in vivo. CHOS were more effective in inhibiting plant pathogens than chitosan (DPn 206), and the most effective DP of CHOS was in the range of DPn 15–40. CHOS in this range may be a potential environmentally friendly product that can be used in biodynamic and organic agri- and horticulture.
References
Aam, B. B., Heggset, E. B., Norberg, A. L., Sørlie, M., Vårum, K. M., & Eijsink, V. G. H. (2010). Production of chitooligosaccharides and their potential applications in medicine. Marine Drugs, 8, 1482–1517.
Ait, B. E., Eullaffroy, P., Clément, C., & Vernet, G. (2004). Chitosan improves development, and protects Vitis vinifera L. against Botrytis cinerea. Plant Cell Reports, 22, 608–614.
Allan, C. R., & Hadwiger, L. A. (1979). The fungicidal effect of chitosan on fungi of varying cell wall composition. Experimental Mycology, 3, 285–287.
Aziz, A., Trotel-Aziz, P., Dhuicq, L., Jeandet, P., Couderchet, M., & Vernet, G. (2006). Chitosan oligomers and copper sulfate induce grapevine defense reactions and resistance to gray mold and downy mildew. Phytopathology, 96, 1188–1194.
Bardin, M., Fargues, J., & Nicot, P. (2008). Compatibility between biopesticides used to control grey mould, powdery mildew and whitefly on tomato. Biological Control, 46, 476–483.
Bautista-Baños, S., Hernández-López, M., & Bosquez-Molina, E. (2004). Growth inhibition of select fungi by chitosan and plant extracts. Mexican Journal of Phytopathology, 22, 178–186.
Card, S. D., Walter, M., Jaspers, M. V., Sztejnberg, A., & Stewart, A. (2009). Targeted selection of antagonistic microorganisms for control of Botrytis cinerea of strawberry in New Zealand. Australasian Plant Pathology, 38, 183–192.
Cederkvist, F. H., Parmer, M. P., Vårum, K. M., Eijsink, V. G. H., & Sørlie, M. (2008). Inhibition of a family 18 chitinase by chitooligosaccharides. Carbohydrate Polymers, 74, 41–49.
Eikenes, M., Alfredsen, G., Christensen, B. E., Militz, H., & Solheim, H. (2005). Comparison of chitosans with different molecular weights as possible wood preservatives. Journal of Wood Science, 51, 387–394.
El-Ghaouth, A., Arul, J., Grenier, J., & Asselin, A. (1992). Antifungal activity of chitosan on two post harvest pathogens of strawberry fruits. Phytopathology, 82, 398–402.
Gerasimenko, D. V., Avdienko, I. D., Bannikova, G. E., Zueva, O. Y., & Varlamov, V. P. (2004). Antibacterial effects of water-soluble low-molecular-weight chitosans on different microorganisms. Applied Biochemistry and Microbiolology, 40, 253–257.
Goody, G. W. (1990). Physiology of microbial degradation of chitin and chitosan. Biodegradation, 1, 177–190.
Hadwiger, L. A. (1979). Chitosan formation in Fusarium solani macroconidia on pea tissue. Plant Physiology, 63, 133.
Hadwiger, L. A., & Beckman, J. M. (1980). Chitosan as a component of pea-Fusarium solani interactions. Plant Physiology, 66, 205–211.
Heggset, E. B., Dybvik, A. I., Hoell, I. A., Norberg, A. L., Sørlie, M., Eijsink, V. G. H., & Vårum, K. M. (2010). Degradation of chitosans with a family 46 chitosanase from Streptomyces coelicolor A3(2). Biomacromolecules, 11, 2487–2497.
Hernández-Lauzardo, A. N., Bautista-Baños, S., Velázquez-delValle, M. G., Méndez-Montealvo, M. G., Sánchez-Rivera, M. M., & Bello-Pérez, L. A. (2008). Antifungal effects of chitosan with different molecular weights on in vitro development of Rhizopus stolonifer (Ehrenb.:Fr.) Vuill. Carbohydrate Polymers, 73, 541–547.
Holmes, G. J., & Eckert, J. W. (1999). Sensitivity of Penicillium digitatum and P. italicum to postharvest citrus fungicides in California. Phytopathology, 89, 716–721.
Jung, B., Kim, C., Choi, K., Lee, Y. M., & Kim, J. (1999). Preparation of amphilic chitosan and their antimicrobial activities. Journal of Applied Polymer Science, 72, 1713–1719.
Kendra, D. F., & Hadwiger, L. A. (1984). Characterization of the smallest chitosan oligomers that is maximally antifungal to Fusarium solani and elicit Pisatin formation in Pisum sativum. Experimental Mycology, 8, 276–281.
Kim, S., & Rajapakse, N. (2005). Enzymatic production and biological activities of chitosan oligosaccharides (COS): a review. Carbohydrate Polymers, 62, 357–368.
Lin, W., Hu, X., Zhang, W., Rogers, W. J., & Cai, W. (2005). Hydrogen peroxide mediates defence responses induced by chitosans of different molecular weights in rice. Journal of Plant Physiology, 162, 937–944.
Meng, X., Yang, L., Kennedy, J. F., & Tian, S. (2010). Effects of chitosan and oligochitosan on growth of two fungal pathogens and physiological properties in pear fruit. Carbohydrate Polymers, 81, 70–75.
Oliveira Junior, E. N., Gueddari, N. E. E., Moerschbacher, B. M., & Franco, T. T. (2012). Growth rate inhibition of phytopathogenic fungi by characterized chitosans. Brazilian Journal of Microbiology, 43, 800–809.
Palma-Guerrero, J., Jansson, H. B., Salinas, J., & Lopez-Llorca, L. V. (2008). Effect of chitosan on hyphal growth and spore germination of plant pathogenic and biocontrol fungi. Journal of Applied Microbiology, 104, 541–553.
Parvu, M., Parvu, A. E., Craciun, C., Barbu-Tudoran, L., Vlase, L., Tamas, M., et al. (2010). Changes in Botrytis cinerea conidia caused by Berberis vulgaris extract. Notulae Botanicae Horti Agrobotanici Cluj-Napoca, 38, 15–20.
Rahman, M. H. (2013). Antifungal activity of chitosan/chitooligosaccharides alone and in combination with chemical fungicides against fungal pathogens. PhD thesis number 2013–12. Norwegian University of Life Sciences. ISBN 978-82-575-1115-9.
Rhoades, J., & Roller, S. (2000). Antimicrobial actions of degraded and native chitosan against spoilage organisms in laboratory media and foods. Applied and Environmental Microbiology, 66, 80–86.
Rosslenbroich, H., & Stuebler, D. (2000). Botrytis cinerea- history of chemical control and novel fungicides for its management. Crop Protection, 19, 557–561.
Ruiz-Herrera, J. (1992). Fungal cell wall: Structure, synthesis, and assembly. London: CRS Press.
Sholberg, P. L. (1990). A new postharvest rot of peaches in Canada caused by Mucor piriformis. Canadian Journal of Plant Pathology, 12, 219–221.
Singla, A. K., & Chawla, M. (2001). Chitosan: some pharmaceutical and biological aspects -an update. Journal of Pharmacy and Pharmacology, 53, 1047–1067.
Sørbotten, A., Horn, S. J., Eijsink, V. G. H., & Vårum, K. M. (2005). Degradation of chitosans with chitinase B from Serratia marcescens production of chito-oligosaccharides and insight into enzyme processivity. FEBS Journal, 272, 538–549.
Stössel, P., & Leuba, J. L. (1984). Effect of chitosan, chitin and some aminosugar on growth of various soil born phytopathogenic fungi. Journal of Phytopathology, 111, 82–90.
Tronsmo, A. (1991). Biological and integrated controls of Botrytis cinerea on apple with Trichoderma harzianum. Biological Control, 1, 59–62.
Trotel-Aziz, P., Couderchet, M., Vernet, G., & Aziz, A. (2006). Chitosan stimulates defense reactions in grapevine leaves and inhibits developmet of Botrytis cinerea. European Journal of Plant Pathology, 114, 405–413.
Vander, P., Vårum, K. M., Domard, A., Gueddari, N. E. E., & Moerschbacher, B. M. (1998). Comparison of the ability of partially N-acetylated chitosans and chitooligosaccharides to elicit resistance reaction in wheat leaves. Plant Physiology, 118, 1353–1359.
Wang, G. H. (1992). Inhibition and inactivation of five species of foodborne pathogens by chitosan. Journal of Food Protection, 55, 916–919.
Williamson, B., Tudzynski, B., Tudzynski, P. L., & Vankan, J. A. L. (2007). Botrytis cinerea: the cause of grey mould disease. Molecular Plant Pathology, 8, 561–580.
Xu, J., Zhao, X., Han, X., & Du, Y. (2007). Antifungal activity of oligochitosan against Phytophthora capsici and other plant pathogenic fungi in vitro. Pesticide Biochemistry and Physiology, 87, 220–228.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rahman, M.H., Hjeljord, L.G., Aam, B.B. et al. Antifungal effect of chito-oligosaccharides with different degrees of polymerization. Eur J Plant Pathol 141, 147–158 (2015). https://doi.org/10.1007/s10658-014-0533-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-014-0533-3