Abstract
In the last decades, chitin and its byproducts have got much attraction among the scientific community for their valuable application in the pharmaceutical, agricultural and medical sectors. Availability of chitin is much higher than other biopolymers in nature (among bacteria, fungi, plants and actinomycetes). Chitin-mediated oligosaccharides are widely used in the pharmaceutical sector for their applications in medicine, industry and agriculture. Chitooligosaccharides are low molecular weight, water-soluble chitosans that have proved their biological properties like antibacterial, antifungal, antitumor, immune regulators, etc. against several agents and diseases. The major aim of this chapter is to highlight the antimicrobial property of chitooligosaccharides.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Chitin (Fig. 1) is a natural biopolymer polysaccharide distributed among the shells of sea arthropods. Every year approximately six to eight million tons of shell waste are produced by seafood industry due to its improper discard in environment. However, chitin is extracted at a commercial scale due to its vast applications aiding in reduction of marine waste (Yan and Chen 2015; Singh et al. 2021). The partial deacetylation of chitin generates an N-acetyl-D-glucosamine (GlcNAc) and D-glucosamine (GlcN) copolymer known as chitosan (Šimat et al. 2020) with low solubility in universal solution which makes its application difficult in the food and biomedical sectors. Moreover, high molecular mass, the pattern of acetylation and deacetylation degree are the few variables that acts as barriers for its extensive use in the biomedical field (Li and Zhuang 2020). Few mechanisms such as perturbation of ion transport on the microorganisms have been suggested for the antimicrobial property of chitosan (Yin et al. 2020).
Chemical structure of chitin and its derivatives (Jafari et al. 2020)
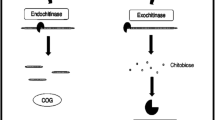
But to overcome these boundaries, hydrolysis of chitosan has been performed yielding small, soluble chitooligosaccharides (COSs) with higher solubility and low viscosity as compared to chitosan along with several biological properties such as antimicrobial activity against a broad spectrum of food-borne microbes acknowledging it as a natural food preservative (Devlieghere et al. 2004; Silva et al. 2021). COSs (Fig. 2) are the chitosans with a degree of polymerization less than 20 and an average molecular weight less than 3.9 kDa. They can also be called as chitosan oligomers or chitooligomers (Lodhi et al. 2014). Due to their hydrophilic nature and low molecular weight, they are of greater demand than their precursor molecule chitosan (Liaqat and Eltem 2018). The shorter chain length and free amino groups in D-glucosamine units in COSs can be considered as a reason for their water solubility and lower viscosity (Bahrke 2008). In contrast to chitin and chitosan, COS has properties like cell membrane penetrability, easy absorption other than water solubility which makes it an extremely valuable product (Liaqat and Eltem 2018). Therefore, the enzyme-mediated production of chitooligosaccharides has been done from chitosan. The glucosamine and acetylated glucosamine rings in COS are connected with each other by β 1,4-glycosidic bond (El-Sayed et al. 2017). It is known that the principle characteristic factors directly affecting the physicochemical and biological activity of COS are the degree of polymerization and molecular weight. COS with a degree of polymerization six or higher and molecular weight low are more biologically active than the COS with low degree of polymerization and high molecular weight (Prashanth and Tharanathan 2007).
2 Antimicrobial Activity of Chitooligosaccharides
In 1979, the broad-spectrum antimicrobial activity of chitosan and its derivatives was reported by Allan and Hadwiger for the first-time (1979). Since then, chitosan and COS have been used by industries for their antimicrobial properties. To define the antimicrobial potential of COS and its utilization in agriculture, medical and food sectors, the use of well-characterized COS is necessary for in vitro as well as in vivo studies (Liaqat and Eltem 2018). Due to the antimicrobial resistance in various microbes, there is a need for new compounds with enhanced activity presenting options for the treatment of several bacterial, yeast and parasitic diseases (El-Sayed et al. 2017; Bloom and Cadarette 2019). The degree of polymerization, degree of N-acetylation and physicochemical characteristics, microorganism type are few factors responsible for the antimicrobial activities of COS (Chung et al. 2004). Moreover, the existence of the primary amino groups in the structure of COS is also supposed to be the reason for its antimicrobial property for now, since the molecular reason is not known yet. The alteration in cell membrane permeability by COS causes the death of the microbial cell due to the release of cell constituents and uncontrolled entry of materials into the cell from outer environment (Choi et al. 2001; Mei et al. 2015). Through the use of UF membrane in association with enzymatic reactor, three different chitooligosaccharides fractions were prepared based on their molecular weight (Jeon and Kim 2000). These were used to determine the antimicrobial activity against Gram-positive, negative and lactic acid bacteria in order to explore the effects of molecular mass on the microbial population (Silva et al. 2021). COS was observed to be highly effective for S. typhi that was inhibited 90% (approximately) at 0.1% concentration with 0.12% MIC (Minimum Inhibitory Concentration). For E. coli and E. coli O-157, high molecular weight COS possessed the highest antibacterial effect with MIC 0.06 and 0.12%, respectively. All COSs indicated a suppressed growth for Pseudomonas aeruginosa with antimicrobial activity 50% or lower at 0.1% concentration. The COS with higher molecular weight (>10 KDa) showed effective activity against pathogens rather than non-pathogens (Jeon et al. 2001). An enzymatically produced, well-characterized COS has been reported for its in vivo anti-dermatophytic activity against Trichophyton rubrum in a guinea pig model indicating clinical application of COS due to its safety, biocompatibility and biodegradability (Mei et al. 2015). Antifungal effect of COS was also determined against the Botrytris cinerea in vitro and in vivo condition with a combination of synthetic fungicide and COS. Results rebelled that combination of both was highly effective against the Botrytris cinerea (Rahman et al. 2015).
El-Sayed and co-workers reported the in vitro antimicrobial activity of four sets of chitooligosaccharides (I, II, III and IV) were prepared using immobilized pepper chitosanase with a molecular weight greater than 100, 100–10, 10–1 and less than 1 KDa, respectively. For antimicrobial activity determination, disc diffusion plates method (50 µg/ml) was used to detect in vitro antimicrobial activity against the pathogens namely B. cereus, B. subtilis, Staphylococcus aureus, Pseudomonas aeruginosa, Candida albicans and Saccharomyces chevalieri in comparison to chitosan. Results showed that immobilized pepper chitosanase can be helpful for the industrial production of antimicrobial chitooligosaccharides from chitosan (El-Sayed et al. 2017). The combination of COS in chitosan films was used to improve the antimicrobial activity against the pathogens such as Lactobacillus plantarum, S. aureus, B. cereus Serratia liquefaciens and E. coli (Fernández-de Castro et al. 2016). This serves as a proof of the fruitful use of COS in food packaging films due to their antimicrobial activities. The white-leg shrimp shells have also been used for the extraction of chitosan and COS through chemical treatment to determine their antibacterial and antifungal activities against several bacterial and fungal species (spoilage and pathogenic microbes). COS was highly soluble in water than chitosan due to its low molecular weight and high degree of deacetylation (13 kDa and 54.83%). The marked inhibitory activity was shown by both COS and chitosan against food-borne pathogenic, spoilage bacterial and fungal strains during antimicrobial assays. Higher inhibitory effect or antimicrobial effect have been observed against Bacillus cereus and Rhizopus sp. in bread upon substitution of flour by COS (1/100 g total weight basis) as compared to use of chitosan; along with delay in the fruity odor generally present in bread (Rakkhumkaew and Pengsuk 2018). A study was conducted to determine the antimicrobial activity of COS in compression of broad-spectrum antibiotic flomox and kluacid. Four groups of COS (1, 2, 3 and 4) were fractionated on the basis of their molecular weights by ultrafiltration. MIC was determined against B. cereus, P. aeruginosa and C. albicans. The inhibitory activities of COS 1 and 3 were highly effective like flomox and kluacid against B. Cereus. COS 4 (0.11 mg/ml concentration, less than 1.0 KDa) completely inhibited the precise growth rate of C. albicans and was stronger than kluacid (0.42 mg/ml). COS 3 (1–10 KDa) had 1.67 mg/ml MIC against P. aeruginosa. These outcomes suggest that COS can play a key role in antimicrobial activity (El-Sayed et al. 2019).
The squid processing industry generally discards squid (Loligo formosana) pen that is a great source of β-chitin. This β-chitin can be easily deacetylated to chitosan due to its higher reactivity toward various solvents and loose structure, further generating COS upon hydrolysis using non-specific enzymes (Singh et al. 2019a). In presence of reactive groups like amino, hydroxyl groups and smaller size, COS has more antioxidant and antimicrobial activity than chitosan (Lodhi et al. 2014). In another study, COS–EGCG (epigallocatechin-3-gallate) conjugates were prepared to determine the antimicrobial potential against E. coli and Listeria monocytogenes. The MIC values for L. monocytogenes (Gram-positive) were observed to be higher for both COS and C1-E0.5-conjugate as compared to E. coli due to its thick cell wall, therefore more tolerant against antimicrobial agents (Singh et al. 2020). The antibacterial potential of substances can be determined by MBC/MIC ratio (Minimum Bactericidal Concentration/Minimum Inhibitory Concentration) (Singh et al. 2019b). Antimicrobial agent with MBC/MIC ratio =/<2 is bacteriostatic and MBC/MIC ratio >2 is bactericidal. For COS, the MBC/MIC ratios against L. monocytogenes and E. coli were 1 and 2, respectively. For C1-E0.5-conjugate, MBC/MIC ratios were 1 and 5 toward L. monocytogenes and E. coli, respectively, suggesting COS activity as a bactericidal agent for both bacteria, whereas C1-E0.5-conjugate as bactericidal and bacteriostatic agent for E. coil and L. monocytogenes, respectively (Singh et al. 2020). COSs antimicrobial activity against several bacteria and yeasts such as E. coli, P. aeruginosa, K. pneumonia, S. aureus, S. epidermidis, E. faecalis and C. albicans was determined using microdilution method in Mueller Hinton broth. A higher antimicrobial effect upon Gram-positive bacteria and Candida yeast was exhibited by COS. The MIC for S. epidermidis and C. albicans were less than 0.25 and 0.5 mg/ml, respectively (Silva et al. 2021). Therefore, the broader biological activity of COSs represents them as active agents for use in various industrial sectors.
3 Future Prospects
Presently, there is a need for novel compounds in the pharmaceutical industry showing potent biological activities. Antimicrobial resistance has created an emergency of novel antibiotics; therefore, COS-mediated derivatives can be an alternate of novel antimicrobial agents against various microbial-mediated diseases. The research on biological activities of COS will provide a new emerging area for emerging biotechnological industries. Moreover, entire knowledge regarding the molecular study of biological activities for COS is not available. Therefore, there is a great need for prospective research centered on the molecular functioning so that complete biochemical functioning of COS could be known.
4 Conclusion
COS has a vast range of biological properties and significant potential for numerous industrial applications. Upon citing the previous literature on the antimicrobial activity of chitooligosaccharide, it’s utilization as a potential antimicrobial agent against several pathogens like bacteria, yeast and fungi have been summarized. There is a great need for well characterization of COS for utilizing it as antimicrobial agent in food, medical and agriculture sectors.
References
Allan CR, Hadwiger LA (1979) The fungicidal effect of chitosan on fungi of varying cell wall composition. Exp Mycol 3(3):285–287
Bahrke S (2008) Mass spectrometric analysis of chitooligosaccharides and their interaction with proteins. Doctoral dissertation, PhD thesis, Universität Potsdam, Potsdam, Germany
Bloom DE, Cadarette D (2019) Infectious disease threats in the twenty-first century: strengthening the global response. Front Immunol 10:549
Choi BK, Kim KY, Yoo YJ, Oh SJ, Choi JH, Kim CY (2001) In vitro antimicrobial activity of a chitooligosaccharide mixture against Actinobacillus actinomycetemcomitans and Streptococcus mutans. Int J Antimicrob Agents 18(6):553–557
Chung YC, Su YP, Chen CC, Jia G, Wang HL, Wu JG, Lin JG (2004) Relationship between antibacterial activity of chitosan and surface characteristics of cell wall. Acta Pharmacol Sin 25(7):932–936
Devlieghere F, Vermeulen A, Debevere J (2004) Chitosan: antimicrobial activity, interactions with food components and applicability as a coating on fruit and vegetables. Food Microbiol 21(6):703–714
El-Sayed ST, Ali AM, El-Sayed EM, Shousha WG, Omar NI (2017) Characterization and potential antimicrobial effect of novel chitooligosaccharides against pathogenic microorganisms. J Appl Pharm Sci 7:6–12
El-Sayed ST, Ali AM, Omar NI (2019) A comparative evaluation of antimicrobial activity of chitooligosaccharides with broad spectrum antibiotics on growth of some pathogenic microorganisms. Biocatal Agric Biotechnol 22:101382
Fernández-de Castro L, Mengíbar M, Sánchez Á, Arroyo L, Villarán MC, de Apodaca ED, Heras Á (2016) Films of chitosan and chitosan-oligosaccharide neutralized and thermally treated: effects on its antibacterial and other activities. LWT 73:368–374
Jafari H, Bernaerts KV, Dodi G, Shavandi A (2020) Chitooligosaccharides for wound healing biomaterials engineering. Mater Sci Eng C 111266
Jeon YJ, Kim SK (2000) Continuous production of chitooligosaccharides using a dual reactor system. Process Biochem 35(6):623–632
Jeon YJ, Park PJ, Kim SK (2001) Antimicrobial effect of chitooligosaccharides produced by bioreactor. Carbohydr Polym 44(1):71–76
Li J, Zhuang S (2020) Antibacterial activity of chitosan and its derivatives and their interaction mechanism with bacteria: current state and perspectives. Eur Polym J 138:109984
Liaqat F, Eltem R (2018) Chitooligosaccharides and their biological activities: a comprehensive review. Carbohydr Polym 184:243–259
Lodhi G, Kim YS, Hwang JW, Kim SK, Jeon YJ, Je JY, Ahn CB, Moon SH, Jeon BT, Park PJ (2014) Chitooligosaccharide and its derivatives: preparation and biological applications. Biomed Res Int
Mei YX, Dai XY, Yang W, Xu XW, Liang YX (2015) Antifungal activity of chitooligosaccharides against the dermatophyte Trichophyton rubrum. Int J Biol Macromol 77:330–335
Prashanth KH, Tharanathan RN (2007) Chitin/chitosan: modifications and their unlimited application potential—an overview. Trends Food Sci Technol 18(3):117–131
Rahman MH, Hjeljord LG, Aam BB, Sørlie M, Tronsmo A (2015) Antifungal effect of chito-oligosaccharides with different degrees of polymerization. Eur J Plant Pathol 141(1):147–158
Rakkhumkaew N, Pengsuk C (2018) Chitosan and chitooligosaccharides from shrimp shell waste: characterization, antimicrobial and shelf life extension in bread. Food Sci Biotechnol 27(4):1201–1208
Silva NS, Araújo NK, Daniele-Silva A, Oliveira JW, Medeiros JM, Araújo RM, Ferreira LD, Rocha HA, Silva-Junior AA, Silva MS, Fernandes-Pedrosa MD (2021) Antimicrobial activity of chitosan oligosaccharides with special attention to antiparasitic potential. Mar Drugs 19(2):110
Šimat V, Elabed N, Kulawik P, Ceylan Z, Jamroz E, Yazgan H, Čagalj M, Regenstein JM, Özogul F (2020) Recent advances in marine-based nutraceuticals and their health benefits. Mar Drugs 18(12):627
Singh A, Benjakul S, Prodpran T (2019a) Chitooligosaccharides from squid pen prepared using different enzymes: characteristics and the effect on quality of surimi gel during refrigerated storage. Food Prod Process Nutr 1(1):1
Singh A, Benjakul S, Prodpran T (2019b) Ultrasound-assisted extraction of chitosan from squid pen: molecular characterization and fat binding capacity. J Food Sci 84(2):224–234
Singh A, Benjakul S, Huda N, Xu C, Wu P (2020) Preparation and characterization of squid pen chitooligosaccharide–epigallocatechin gallate conjugates and their antioxidant and antimicrobial activities. RSC Adv 10(55):33196–33204
Singh RV, Sambyal K, Negi A, Sonwani S, Mahajan R (2021) Chitinases production: a robust enzyme and its industrial applications. Biocatal Biotransform 4;39(3):161–89
Yan N, Chen X (2015) Sustainability: don’t waste seafood waste. Nat News 524(7564):155
Yin M, Wang Y, Zhang Y, Ren X, Qiu Y, Huang TS (2020) Novel quaternarized N-halamine chitosan and polyvinyl alcohol nanofibrous membranes as hemostatic materials with excellent antibacterial properties. Carbohydr Polym 232:115823
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Sambyal, K., Sharma, P., Singh, R.V. (2022). Antimicrobial Activity of Chitooligosaccharides. In: Kim, SK. (eds) Chitooligosaccharides. Springer, Cham. https://doi.org/10.1007/978-3-030-92806-3_18
Download citation
DOI: https://doi.org/10.1007/978-3-030-92806-3_18
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-92805-6
Online ISBN: 978-3-030-92806-3
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)