Abstract
The associations of circulating 25-hydroxyvitamin D [25(OH)D] concentrations with total and site-specific cancer incidence have been examined in several epidemiological studies with overall inconclusive findings. Very little is known about the association of vitamin D with cancer incidence in older populations. We assessed the association of pre-diagnostic serum 25(OH)D levels with incidence of all cancers combined and incidence of lung, colorectal, breast, prostate and lymphoid malignancies among older adults. Pre-diagnostic 25(OH)D concentrations and cancer incidence were available in total for 15,486 older adults (mean age 63, range 50–84 years) participating in two cohort studies: ESTHER (Germany) and TROMSØ (Norway); and a subset of previously published nested-case control data from a another cohort study: EPIC-Elderly (Greece, Denmark, Netherlands, Spain and Sweden) from the CHANCES consortium on health and aging. Cox proportional hazards or logistic regression were used to derive multivariable adjusted hazard and odds ratios, respectively, and their 95 % confidence intervals across 25(OH)D categories. Meta-analyses with random effects models were used to pool study-specific risk estimates. Overall, lower 25(OH)D concentrations were not significantly associated with increased incidence of most of the cancers assessed. However, there was some evidence of increased breast cancer and decreased lymphoma risk with higher 25(OH)D concentrations. Our meta-analyses with individual participant data from three large European population-based cohort studies provide at best limited support for the hypothesis that vitamin D may have a major role in cancer development and prevention among European older adults.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A substantial body of evidence supports the role of vitamin D on skeletal health outcomes such as rickets and osteomalacia [1], and to a lesser extent on non-skeletal health outcomes [2]. The association of low vitamin D status with cancer incidence has recently been assessed in many epidemiological investigations, but overall their results remained inconclusive. In a recent meta-analysis of prospective cohort studies [3], total cancer incidence was inversely associated with serum 25-hydroxyvitamin D (25(OH)D), an integrated measure of vitamin D from sun exposure, diet, and supplements and the best indicator of vitamin D status [4]. However, the results of the meta-analysis are limited by the exclusive reliance on published aggregate data, and the heterogeneity in 25(OH)D categorization and confounder adjustment. Similar limitations apply to recent meta-analyses on the incidence of specific types of cancer, which suggested inverse associations of 25(OH)D concentrations with colorectal [5] and breast cancer [6], but not other forms of cancer [7]. However, given that these studies were carried out in different populations, it is unclear to what extent differences between cancer sites reflect true differences of site-specific associations or result from differences in the populations included. Moreover, suggestions of variation in the association of 25(OH)D concentrations with cancer risk according to sex, age, obesity and smoking status were reported [8–11].
With aging the vitamin D pathway is affected: vitamin D deficiency is more prevalent, its synthesis in the skin is impaired, and renal conversion of 25(OH)D to the active 1,25-dihydroxyvitamin D metabolite and expression of the vitamin D receptor decreases [12]. Furthermore, because cancer is a disease that takes many years to develop, having higher 25(OH)D concentrations at a later age may not be relevant anymore for decreasing cancer risk. The Consortium on Health and Ageing: Network of Cohorts in Europe and the United States (CHANCES) [13] provides a unique opportunity to assess associations of 25(OH)D concentrations with total and site-specific cancer incidence in older adults, in a large, harmonized dataset from the same set of well-defined study populations. Moreover the CHANCES database enables the exploration of variations in these associations according to potential effect modifiers, particularly sex and age.
Subjects and methods
Study design
From all participating studies in the CHANCES consortium, three European cohort studies (EPIC-Elderly, ESTHER and TROMSØ), had available plasma or serum 25(OH)D measurements and complete follow-up for cancer incidence, and were included in this investigation.
Briefly, EPIC is a multicenter, prospective cohort study of healthy volunteers (aged 35–70 years) recruited between 1992 and 2000 from 10 European countries [14]. Four nested case–control studies of colorectal, breast and prostate cancer and lymphoid malignancies cases and matched controls were conducted and have already been published [15–18]. Controls were matched by age, sex, study center, time of the day and fasting status at blood collection and among women further by menopausal status, phase of menstrual cycle and use of hormone replacement therapy at blood collection. From these data, participants aged 60 or over at recruitment were included in the EPIC-Elderly study. However, data from only five countries of EPIC-Elderly were available for the CHANCES consortium (Denmark, Greece, Netherlands, Spain and Sweden).
ESTHER is an ongoing population based-cohort study conducted in Saarland (Germany). Overall 9949 older adults (median age = 63 years) were recruited between 2000 and 2002 during a routine health check-up by their general practitioners and 25(OH)D was measured in the whole cohort [19].
TROMSØ is a repeated population-based cohort study conducted in the municipality of the same name, in Norway. The data included in the present investigation correspond to the 4th survey in which 10,262 participants (median age = 63 years) were recruited. 25(OH)D was measured in the entire cohort but we excluded smokers from the TROMSØ study (n = 2112) because the assay employed resulted in smokers having 15–20 % higher 25(OH)D than non-smokers which was not reproducible with other assays [20].
Participants with prevalent cancer at baseline were excluded. A summary description of included studies can be found in Table 1. Further details for the three studies have also been described previously [14, 19, 20]. All included cohorts were approved by local ethics committees, obtained written informed consent from all study participants and were conducted according to the declaration of Helsinki.
Definition of endpoints
Cancer incidence was ascertained by active follow-up and record linkage with national/regional cancer registries [10, 15, 21]. Total cancer incidence was the main endpoint, defined only in the ESTHER and TROMSØ cohorts, and included all malignant neoplasms according to the 10th revision of the International Classification of Disease (ICD-10) codes C00-97 except non-melanoma skin cancers (C44). Lung cancer (C34) incidence was assessed for ESTHER and TROMSØ only due to unavailable 25(OH)D measurements among lung cancer cases in EPIC-Elderly. Colorectal (C18-21), breast (C50) and prostate (C61) cancer incidence were available in all studies. Lymphoma incident cases were identified in ESTHER according to the International Classification of Diseases for Oncology, Second Edition (ICD-O-2) codes: 9590-95, 9650-67, 9670-77, 9680-88, 9690-98, 9700-17, 9731-32, 9760-62, 9764, 9820-28, 9850, 9940 and 9941. Whenever the ICD-O-2 codes were not available, we employed the ICD-10 codes C81-91 to define lymphomas in ESTHER. In EPIC-Elderly lymphomas were originally classified according to the ICD-O-2 codes but were subsequently reclassified according to ICD-O-3 codes as described elsewhere [18]. In EPIC-Elderly and TROMSØ we used the International Classification of Diseases for Oncology, Third Edition (ICD-O-3) codes: 9590-91, 9670-71, 9673, 9675, 9679, 9680, 9684, 9687, 9689, 9690-91, 9695, 9698-99, 9700-02, 9705, 9708-09, 9714, 9716, 9718-19, 9727-29, 9761, 9765, 9823, 9826-27, 9831-37, 9940, 9948, 9650-55, 9659, 9664-67, 9731-32 and 9734. For the analyses, due to small number of cases, no distinctions were made between Hodgkin’s lymphoma, non-Hodgkin’s lymphoma, multiple myeloma, chronic lymphocytic leukemia and other rare or unclassified lymphoid malignancies.
Measurement of circulating 25(OH)D concentration and categorization
All studies employed immunoassay methods as shown in Table 1 and as described in previous publications [15, 16, 20, 22]. Due to seasonal fluctuations in 25(OH)D concentrations (Fig. 1), 25(OH)D concentration was categorized into season-specific quintiles (upper quintile as referent) for all studies since this method is regarded as the most appropriate to reduce bias due to seasonal variation [23]. Season was categorized as winter (December–February), spring (March–May), summer (June–August) and autumn (September–November). Cut-offs for season-specific quintiles can be found in the Supplementary Table 1. Additionally, in order to assess the suitability for cancer risk of the American Institute of Medicine cut-points [24], we also defined the following clinical categories of vitamin D status: vitamin D deficiency (<30 nmol/L), insufficiency (30–50 nmol/L) and sufficiency (>50 nmol/L). For this analysis, season was introduced in the multivariate model as a covariate.
Assessment of covariates
Information on socio-demographic and lifestyle covariates was obtained from self-administered questionnaires passed to participants at baseline and included age, sex, highest level of education (primary or less, more than primary but less than university or college, university or college) and smoking status (never, former and current smoker). Height and weight were measured in EPIC-Elderly and TROMSØ and self-reported in ESTHER. Body mass index (BMI) was calculated by dividing body weight (in kilograms) by the square of height (in meters). Information regarding duration and intensity of physical activity was self-reported in all studies [25–27]. Vigorous physical activity (dichotomous: yes, no) was defined as at least 1 h per week of physical activity intense enough to cause perspiration, out of breath or faster heart beating.
Statistical analyses
Significant differences in baseline characteristics across clinical 25(OH)D categories were tested with Kruskal–Wallis test for continuous and Chi Square test for categorical variables. The association between circulating 25(OH)D concentrations and risk of cancer was assessed with Cox proportional hazards models in the ESTHER and TROMSØ cohorts and with conditional logistic regression models in the EPIC-Elderly nested case–control sub-study. Hazard ratios (HR, from Cox regression) and odds ratios (OR, from logistic regression), numerically approximate each other for short follow-ups, rare diseases, risk estimates near to no effect [28] and under the assumption that the exposure distribution is stable under time [29] which may apply for 25(OH)D [30].
Both risk estimates were calculated with their respective 95 % confidence intervals for two models with different level of confounder adjustment. Model 1 was adjusted for age (continuous) and sex (categorical) and, in the analysis with clinical 25(OH)D categories, was additionally adjusted for season of blood draw (categorical). Model 2 was additionally adjusted for vigorous physical activity (categorical: yes, no), highest level of education (ordinal: primary or less, between primary and university, university), BMI (continuous) and smoking status (categorical: never, former, current). Moreover, in the analyses with EPIC-Elderly data, adjustment for country was performed. Further confounder variables such as fish, red meat, vegetables and fruits intakes were considered, but only those variables that were common to all studies were chosen in order to reduce heterogeneity between studies. Subjects with missing values for the confounders adjusted for in model 2 [BMI (0.3 %), highest level of education (1.8 %), vigorous physical activity (3.0 %) and smoking status (1.8 %)] were excluded. Risk estimates according to clinical categories of vitamin D status are shown as main results (the results employing season-specific 25(OH)D quintiles can be found in the Appendix). Dose–response graphs were created by plotting risk estimates across season-specific quintiles of 25(OH)D concentrations. In a sensitivity analysis for total cancer incidence, we excluded subjects developing cancer during the initial 1, 2, 3 and 4 years of follow-up. The rationale for this sensitivity analysis was to exclude potential bias by reverse causality from cancers that might have been already present but not yet clinically manifest at the time of recruitment. We further modeled 25(OH)D concentration continuously in order to test a potential linear association. Effect modification by sex, age (< or ≥65 years), BMI (< or ≥30 kg/m2) and vigorous physical activity (yes, no) was tested for statistical significance by creating product terms of the continuous 25(OH)D variable by the potential effect modifier of interest, and then adding them to the multivariate model 2 for total cancer incidence only.
All statistical tests were two sided with an alpha level of 0.05. Cohort-specific analyses were conducted with SAS, version 9.3 (Cary, North Carolina, USA). Cohort-specific risk ratios were pooled with meta-analysis using random-effects models in a conservative approach in order to account for the variation of the true effects between studies [31]. Heterogeneity was tested for significance with Cochran’s Q test [32]. Meta-analyses, tests of heterogeneity, forest plots and dose–response graphs were derived in Microsoft Excel 2010 (Redmond, Washington, USA) using the formulas described by Borenstein et al. [33]. This report was prepared in accordance to standard guidelines for reporting of observational studies [34] (see checklist in the Appendix).
Results
Baseline characteristics for the three participating studies according to clinical categories of 25(OH)D concentration are presented in Table 2 (and according to season-specific 25(OH)D quintiles in the Supplementary Table 2). The prevalence of vitamin D deficiency (25(OH)D < 30 nmol/L) was significantly higher in ESTHER (15.1 %) as compared to EPIC-Elderly (7.8 % in controls) and particularly TROMSØ (5.3 %). Suboptimal 25(OH)D concentrations (i.e. below 50 nmol/L) were observed in 40.7 % of controls in EPIC-Elderly, and in 58.9 and 40.9 % of the ESTHER and TROMSØ participants, respectively. In all studies, vitamin D deficient participants were more often women, significantly older, had significantly higher BMI, and in ESTHER and TROMSØ significantly less often performed vigorous physical activity and were more likely to be never smokers. Higher concentrations of 25(OH)D were associated with higher levels of education in ESTHER and TROMSØ, but not in EPIC-Elderly.
During an average of 12 years of follow-up, a total of 1082 and 806 total cancer cases were identified in the ESTHER and TROMSØ studies, respectively. The association of clinical categories of 25(OH)D concentrations with cancer risk adjusted for age, sex, season of blood draw, highest level of education, smoking status, BMI, and vigorous physical activity is shown in Table 3 (the results according to season-specific 25(OH)D quintiles can be found in the Supplementary Table 3). The results for the age, sex, and season adjusted model were very similar and can be found in the supplementary figures in the Appendix.
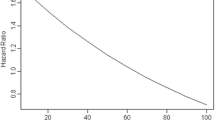
Overall, clinical categories of 25(OH)D concentrations were not significantly associated with total cancer risk (Table 3). Plotted season-specific 25(OH)D quintiles with respect to total cancer risk suggested an U-shaped association with a statistically significant decreased total cancer risk in the second quintile (HR 0.81, 95 % CI 0.69;0.95) and higher total cancer risk in the extreme quintiles (Fig. 2a). No significant heterogeneity was observed in all meta-analyses for total cancer incidence with both categorizations. No significant variation in the risk estimates across quintiles was observed when excluding cancer diagnoses occurring during the initial 1, 2, 3 and 4 years (data not shown). No significant effect modification (Table 4) was observed when stratifying by sex (Pinteraction = 0.62), age (Pinteraction = 0.56), BMI (Pinteraction = 0.66) and vigorous physical activity (Pinteraction = 0.55).
Pooled risk ratios and 95 % confidence intervals for risk of a total cancer, b lung cancer, c colorectal cancer, d breast cancer, e prostate cancer and f lymphoma across 25(OH)D study- and season-specific quintiles with the top quintile [higher 25(OH)D] as reference. For total and lung cancer incidence only data from the ESTHER and TROMSØ study were combined
During follow-up, a total number of 192 lung cancers, 616 colorectal cancers, 378 breast cancers, 392 prostate cancers and 279 lymphomas were observed. In ESTHER and TROMSØ cohorts, individually and in combination, low 25(OH)D concentrations were associated with a statistically non-significant increased lung cancer risk (Table 3). There was no significant evidence of a linear increase in lung cancer risk with higher 25(OH)D concentration (p = 0.32).
Tentatively increased colorectal cancer risk with low 25(OH)D concentrations was also observed in EPIC-Elderly and TROMSØ, but not in ESTHER. The meta-analysis yielded no significant association of 25(OH)D concentrations with colorectal cancer risk, which was also visible in the dose–response graph (Fig. 2c). There was no evidence of a linear increase in colorectal cancer risk with higher 25(OH)D concentration (p = 0.39).
A significant breast cancer risk reduction was observed for 25(OH)D concentrations between 30 and 50 nmol/L (HR 0.67, 95 % CI 0.52;0.87). Such decrease in breast cancer risk with lower 25(OH)D concentrations compared to the highest quintile was also apparent across season-specific quintiles, with the exception of the third quintile (Fig. 2d). There was significant evidence of a linear increase in breast cancer risk with higher 25(OH)D (p < 0.01).
Overall, no statistically significant association of 25(OH)D with prostate cancer risk was observed, even though a tentatively reduced risk was seen for 25(OH)D concentrations between 30 and 50 nmol/L (HR 0.81, 95 % CI 0.63;1.04). No sign of linearity was observed.
Statistically significantly higher lymphoma risk was observed with 25(OH)D concentrations <30 nmol/L (HR 1.76, 95 % CI 1.00;3.11). The dose–response relationship showed a tendency towards decreasing lymphoma risk with higher 25(OH)D season-specific quintiles, but the confidence intervals were wide and included the null value for each category (Fig. 2f). Furthermore, increases in 25(OH)D concentrations were not significantly linearly associated with decreased lymphoma risk (p = 0.10).
For virtually all meta-analyses in all cancer sites, the tests for heterogeneity were not statistically significant.
Discussion
The CHANCES consortium provides the largest study population up to date examining the association of measured 25(OH)D concentrations and total cancer risk among older adults. Additionally, we provide an assessment of the association of low 25(OH)D concentrations with the incidence of major site-specific cancer endpoints. Overall, we observed no significant association of lower 25(OH)D concentrations with increased total or site-specific cancer incidence. In fact, 25(OH)D concentrations in the range of the recommendations by the Institute of Medicine were associated with the lowest risk of total cancer and breast cancer.
A recent meta-analysis summarizing the findings from 5 previously published prospective studies assessing the relationship between 25(OH)D concentrations and total cancer incidence suggested a 11 % reduction of total cancer incidence for a 50 nmol/L increase in 25(OH)D [3]. Shortcomings of this meta-analysis were the study populations (consisting mainly of men and specific occupational groups) and the restricted data provided by the original publications. Whereas inverse associations of vitamin D with total cancer risk had been more often observed in male than in female study populations [8, 10, 35], no significant differences were observed between males and females in our meta-analysis with individual participant data. We also did not observe any age differences regarding total cancer incidence, but the data for the age stratification was limited to only two cohorts. Additionally, our analyses were based on a population of older adults (mean age 63 years) which could suggest that vitamin D may not be relevant for cancer development at old age. It is worth noting that the strongest effect of vitamin D on cancer risk was observed by Giovannucci and colleagues, who employed a statistical algorithm for predicting 25(OH)D rather than measuring it in the complete population [35]. Even though our study, despite its large overall size, had limited power to detect potential weak inverse associations between 25(OH)D and total cancer risk, moderate or strong inverse associations would appear highly unlikely in the light of the confidence intervals estimated for vitamin D insufficiency and deficiency.
Previous reports of associations between 25(OH)D and lung cancer risk have observed comparable magnitudes of association i.e., approximately 20 % lower risk with increasing (or 20 % higher risk with decreasing) 25(OH)D concentrations [8–10, 35, 36]. Although these reported associations were not statistically significant, they are in line with the not statistically significant higher lung cancer risk for vitamin D deficient participants observed in our study. In this investigation we did not adjust for other measures of smoking such as the duration or intensity of smoking, but as pointed out in previous studies that corrected for these variables, the influence of residual confounding is expected be minimal [8, 36]. Therefore, we can neither confirm nor reject a possible role of vitamin D in lung cancer development. Given the low number of observational studies conducted and the potential mechanisms of vitamin D in lung tumorigenesis suggested by in vivo and in vitro studies [37], further investigations are warranted.
An earlier meta-analysis of published epidemiologic studies had suggested an inverse association of 25(OH)D concentrations with colorectal cancer risk [5] even though few of the included studies, most of which were conducted in the US, individually show statistically significant results. In line with these findings we observed non-significantly higher colorectal cancer incidence at low 25(OH)D concentrations. However, the magnitude of the association observed in our study was smaller than that of previous studies, which could be due to our population being of older age. These findings suggest that a possible protective role of vitamin D against colorectal cancer among older adults may be rather small. Nevertheless, a number of biological mechanisms by which vitamin D can induce cell differentiation, cell cycle arrest and apoptosis in colorectal cancer tumors have been suggested [38]. Additionally, several studies have consistently suggested that vitamin D status may also be relevant for the survival of colorectal cancer patients [39].
For breast cancer, a previous meta-analysis suggested the possibility of reverse causality since the associations of vitamin D with breast cancer risk were observed mainly in case–control studies with measurements of 25(OH)D after diagnosis [40]. Yet, an updated summary including only prospective studies still showed higher 25(OH)D concentrations measured at baseline to be associated with reduced breast cancer incidence during follow-up [6]. Unexpectedly, our data showed an increased breast cancer risk with higher 25(OH)D concentrations. Still, similar trends have been observed in a number of epidemiologic studies [41–43]. Different findings between our study and the latest meta-analysis could be explained by the different settings, study populations involved, and level of adjustment. Most of the studies included in the previous meta-analysis were nested case–control studies conducted in the US, with limited and heterogeneous adjustment for confounders. In contrast, our analyses included cohort data from European older adult populations only, and employed consistent adjustment for the most important confounder variables common to all included studies. Nevertheless, it is also possible that the anticancer properties of vitamin D in the breast tissue may pertain more to the progression of the disease since various studies have observed increases in survival of breast cancer patients with high vitamin D status [39].
Previous epidemiologic studies on the association of 25(OH)D with prostate cancer risk have suggested either no association, increased risk with higher 25(OH)D [44], but also U-shaped associations, especially for high-grade disease [45]. In agreement with most single studies conducted hitherto, our data showed no evidence of an association. It has been hypothesized that circulating 1,25(OH)2D concentrations may be more relevant since the enzymatic conversion of 25(OH)D to 1,25(OH)2D is impaired in prostate cancer cells [46]. In fact, an association of low 1,25(OH)2D with aggressive prostate cancer risk has been suggested [44]. Measurements of 1,25(OH)2D were not available in our study, and aggressive prostate cancer incidence could not be assessed. Given the findings from laboratory studies describing a link of vitamin D with prostate cancer [46], the possibility that the physiologically active vitamin D metabolite may be more relevant for aggressive prostate cancer deserves further study.
Considerably less studies have assessed the risk of developing lymphoma according to 25(OH)D concentrations, overall showing no effect [18, 35, 47, 48]. Our data showed an increased lymphoma risk for vitamin D deficiency. In comparison to a larger pooled analysis [48], 25(OH)D concentrations were overall lower in our study population, which could explain why we were able to discern an increased lymphoma risk at very low 25(OH)D concentrations. In our study we combined all lymphoid malignancies due to the low number of cases for some of them. However, previous studies have observed variations in risk associated to high 25(OH)D concentrations for some of these malignancies, such as increased risk for multiple myeloma [35] or reduced risk for Non-Hodgkin’s lymphoma [35, 47] and chronic lymphocytic leukemia [18]. These discrepancies in the findings for different lymphoid malignancies may deserve further study, particularly in older populations.
To date no randomized controlled trial (RCT) provided sufficient evidence of an association of vitamin D supplementation with total, colorectal or breast cancer incidence [49]. Instead, a meta-analysis of published RCTs showed that vitamin D supplementation consistently decreased total cancer mortality but not incidence [50]. This suggests that vitamin D may be more relevant for survival, a finding that is in agreement with the results of observational studies [13, 39]. However, most RCTs were limited mainly by not being originally designed for assessing cancer risk, and sometimes also by the low doses provided, low compliance with the intervention and the use of supplements by subjects in the placebo group [49]. A re-analysis of the Women’s Health Initiative conducted among women not taking supplements at randomization, yielded reductions in total, breast and colorectal cancer risk (not statistically significant for the latter) for the intervention group [51]. Even though promising results for colorectal and breast cancer have been reported in previous observational studies, and there is also substantial evidence from basic and preclinical studies on potential protective effects of vitamin D [52], our results cannot confirm neither exclude that vitamin D may be beneficial for cancer prevention. Owing to these disagreeing findings and limited data available, further investigations, preferably well-designed RCTs are desirable.
This is the largest investigation with 25(OH)D measurements assessing total and site-specific cancer risk in a population of older adults. This meta-analysis with individual participant data also ranks among the largest investigations on the association of 25(OH)D with incidence of lung, colorectal, prostate, breast, and lymphoid cancers in older adults. Furthermore, the availability of individual participant data, harmonization of covariates and common multivariable models, allowed us to minimize the heterogeneity between studies and avoid misclassification of exposure, outcome and covariates, a common issue in meta-analysis employing data reported by individual studies. In fact, the tests for heterogeneity were not statistically significant for all of the meta-analyses performed, thus suggesting a consistency of the effects estimated by the different studies involved. Alternatively, it could also be that the test for heterogeneity was underpowered due to the low number of studies.
Several limitations also need to be addressed. First, although our study is one of the largest on the topic, it may still have not been sufficient to detect a significant small effect of vitamin D on cancer development. Second, current smokers were excluded from the TROMSØ study because the assay employed resulted in smokers having 15–20 % higher 25(OH)D than non-smokers which was not reproducible with other assays [20]. This could have attenuated the pooled effect estimates observed since in the ESTHER data a stronger association with total cancer was observed among current smokers [10]. Third, data from the EPIC-Elderly study were not available for total and lung cancer incidence so that estimates for these two outcomes were based on two cohorts only. Fourth, 25(OH)D was measured only at baseline and thus it may not adequately represent a long term vitamin D status. Nevertheless, the correlations between the measurements at baseline and at follow-up are rather high [30]. Fifth, 25(OH)D was measured with immunoassay methods in all studies (although in the ESTHER study a standardization to the LC–MS/MS was performed), while LC–MS/MS is regarded as the most reliable but also the most costly method. Finally, the findings of our study are restricted to European older adults, while associations of 25(OH)D concentrations with cancer risk have often been observed in observational studies conducted among American younger adults.
To conclude, in this large consortium of prospective studies including European older adults, we observed that overall higher 25(OH)D concentrations were not associated with reduced total and site-specific cancer incidence. Our results do not support the hypothesis that vitamin D has a large impact on cancer development and we suggest waiting for the results of RCTs before formulating recommendations regarding vitamin D supplementation for cancer prevention.
References
Cashman KD, Kiely M. Towards prevention of vitamin D deficiency and beyond: knowledge gaps and research needs in vitamin D nutrition and public health. Br J Nutr. 2011;106(11):1617–27.
Dobnig H. A review of the health consequences of the vitamin D deficiency pandemic. J Neurol Sci. 2011;311(1–2):15–8.
Yin L, Ordóñez-Mena JM, Chen T, Schottker B, Arndt V, Brenner H. Circulating 25-hydroxyvitamin D serum concentration and total cancer incidence and mortality: a systematic review and meta-analysis. Prev Med. 2013;57(6):753–64.
Zerwekh JE. Blood biomarkers of vitamin D status. Am J Clin Nutr. 2008;87(4):1087S–91S.
Lee JE, Li H, Chan AT, Hollis BW, Lee IM, Stampfer MJ, et al. Circulating levels of vitamin D and colon and rectal cancer: the Physicians’ Health Study and a meta-analysis of prospective studies. Cancer Prev Res (Phila). 2011;4(5):735–43.
Wang D, Velez de-la-Paz OI, Zhai JX, Liu DW. Serum 25-hydroxyvitamin D and breast cancer risk: a meta-analysis of prospective studies. Tumour Biol. 2013;34(6):3509–17.
Theodoratou E, Tzoulaki I, Zgaga L, Ioannidis JP. Vitamin D and multiple health outcomes: umbrella review of systematic reviews and meta-analyses of observational studies and randomised trials. BMJ. 2014;348:g2035.
Afzal S, Bojesen SE, Nordestgaard BG. Low plasma 25-hydroxyvitamin D and risk of tobacco-related cancer. Clin Chem. 2013;59(5):771–80.
Kilkkinen A, Knekt P, Heliovaara M, Rissanen H, Marniemi J, Hakulinen T, et al. Vitamin D status and the risk of lung cancer: a cohort study in Finland. Cancer Epidemiol Biomarkers Prev. 2008;17(11):3274–8.
Ordóñez-Mena JM, Schottker B, Haug U, Muller H, Kohrle J, Schomburg L, et al. Serum 25-hydroxyvitamin D and cancer risk in older adults. Results from a large German prospective cohort study. Cancer Epidemiol Biomarkers Prev. 2013;22(5):905–16.
Eaton CB, Young A, Allison MA, Robinson J, Martin LW, Kuller LH, et al. Prospective association of vitamin D concentrations with mortality in postmenopausal women: results from the Women’s Health Initiative (WHI). Am J Clin Nutr. 2011;94(6):1471–8.
Gallagher JC. Vitamin D and aging. Endocrinol Metab Clin North Am. 2013;42(2):319–32.
Schottker B, Jorde R, Peasey A, Thorand B, Jansen EH, Groot L, et al. Vitamin D and mortality: meta-analysis of individual participant data from a large consortium of cohort studies from Europe and the United States. BMJ. 2014;348:g3656.
Riboli E, Hunt K, Slimani N, Ferrari P, Norat T, Fahey M, et al. European Prospective Investigation into Cancer and Nutrition (EPIC): study populations and data collection. Public Health Nutr. 2002;5(6b):1113–24.
Jenab M, Bueno-de-Mesquita HB, Ferrari P, van Duijnhoven FJ, Norat T, Pischon T, et al. Association between pre-diagnostic circulating vitamin D concentration and risk of colorectal cancer in European populations: a nested case-control study. BMJ. 2010;340:b5500.
Travis RC, Crowe FL, Allen NE, Appleby PN, Roddam AW, Tjonneland A, et al. Serum vitamin D and risk of prostate cancer in a case–control analysis nested within the European Prospective Investigation into Cancer and Nutrition (EPIC). Am J Epidemiol. 2009;169(10):1223–32.
Kuhn T, Kaaks R, Becker S, Eomois PP, Clavel-Chapelon F, Kvaskoff M, et al. Plasma 25(OH)vitamin D and the risk of breast cancer in the european prospective investigation into cancer and nutrition (EPIC): a nested case-control study. Int J Cancer. 2013;133(7):1689–700.
Luczynska A, Kaaks R, Rohrmann S, Becker S, Linseisen J, Buijsse B, et al. Plasma 25-hydroxyvitamin D concentration and lymphoma risk: results of the European Prospective Investigation into Cancer and Nutrition. Am J Clin Nutr. 2013;98(3):827–38.
Schöttker B, Haug U, Schomburg L, Kohrle J, Perna L, Muller H, et al. Strong associations of 25-hydroxyvitamin D concentrations with all-cause, cardiovascular, cancer, and respiratory disease mortality in a large cohort study. Am J Clin Nutr. 2013;97(4):782–93.
Grimnes G, Almaas B, Eggen AE, Emaus N, Figenschau Y, Hopstock LA, et al. Effect of smoking on the serum levels of 25-hydroxyvitamin D depends on the assay employed. Eur J Endocrinol. 2010;163(2):339–48.
Jorde R, Schirmer H, Wilsgaard T, Joakimsen RM, Mathiesen EB, Njolstad I, et al. Polymorphisms related to the serum 25-hydroxyvitamin D level and risk of myocardial infarction, diabetes, cancer and mortality. The Tromso Study. PLoS ONE. 2012;7(5):e37295.
Schöttker B, Jansen EH, Haug U, Schomburg L, Köhrle J, Brenner H. Standardization of misleading immunoassay based 25-hydroxyvitamin D levels with liquid chromatography tandem-mass spectrometry in a large cohort study. PLoS ONE. 2012;7(11):e48774.
Wang Y, Jacobs EJ, McCullough ML, Rodriguez C, Thun MJ, Calle EE, et al. Comparing methods for accounting for seasonal variability in a biomarker when only a single sample is available: insights from simulations based on serum 25-hydroxyvitamin d. Am J Epidemiol. 2009;170(1):88–94.
IOM (Institute of Medicine). Dietary reference intakes for calcium and vitamin D. Washington, DC: The National Academies Press; 2011.
Morseth B, Ahmed LA, Bjornerem A, Emaus N, Jacobsen BK, Joakimsen R, et al. Leisure time physical activity and risk of non-vertebral fracture in men and women aged 55 years and older: the Tromso Study. Eur J Epidemiol. 2012;27(6):463–71.
Haftenberger M, Schuit AJ, Tormo MJ, Boeing H, Wareham N, Bueno-de-Mesquita HB, et al. Physical activity of subjects aged 50–64 years involved in the European Prospective Investigation into Cancer and Nutrition (EPIC). Public Health Nutr. 2002;5(6B):1163–76.
Raum E, Rothenbacher D, Ziegler H, Brenner H. Heavy physical activity: risk or protective factor for cardiovascular disease? A life course perspective. Ann Epidemiol. 2007;17(6):417–24.
Symons M, Moore D. Hazard rate ratio and prospective epidemiological studies. J Clin Epidemiol. 2002;55(9):893–9.
Knol MJ, Vandenbroucke JP, Scott P, Egger M. What do case-control studies estimate? Survey of methods and assumptions in published case-control research. Am J Epidemiol. 2008;168(9):1073–81.
Sonderman JS, Munro HM, Blot WJ, Signorello LB. Reproducibility of serum 25-hydroxyvitamin d and vitamin D-binding protein levels over time in a prospective cohort study of black and white adults. Am J Epidemiol. 2012;176(7):615–21.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88.
Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–58.
Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods. 2010;1(2):97–111.
Von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Prev Med. 2007;45(4):247–51.
Giovannucci E, Liu Y, Rimm EB, Hollis BW, Fuchs CS, Stampfer MJ, et al. Prospective study of predictors of vitamin D status and cancer incidence and mortality in men. J Natl Cancer Inst. 2006;98(7):451–9.
Weinstein SJ, Yu K, Horst RL, Parisi D, Virtamo J, Albanes D. Serum 25-hydroxyvitamin D and risk of lung cancer in male smokers: a nested case-control study. PLoS ONE. 2011;6(6):e20796.
Foong RE, Zosky GR. Vitamin D deficiency and the lung: disease initiator or disease modifier? Nutrients. 2013;5(8):2880–900.
Lamprecht SA, Lipkin M. Chemoprevention of colon cancer by calcium, vitamin D and folate: molecular mechanisms. Nat Rev Cancer. 2003;3(8):601–14.
Maalmi H, Ordóñez-Mena JM, Schöttker B, Brenner H. Serum 25-hydroxyvitamin D levels and survival in colorectal and breast cancer patients: systematic review and meta-analysis of prospective cohort studies. Eur J Cancer. 2014;50(8):1510–21.
Yin L, Grandi N, Raum E, Haug U, Arndt V, Brenner H. Meta-analysis: serum vitamin D and breast cancer risk. Eur J Cancer. 2010;46(12):2196–205.
Freedman DM, Chang SC, Falk RT, Purdue MP, Huang WY, McCarty CA, et al. Serum levels of vitamin D metabolites and breast cancer risk in the prostate, lung, colorectal, and ovarian cancer screening trial. Cancer Epidemiol Biomarkers Prev. 2008;17(4):889–94.
McCullough ML, Stevens VL, Patel R, Jacobs EJ, Bain EB, Horst RL, et al. Serum 25-hydroxyvitamin D concentrations and postmenopausal breast cancer risk: a nested case control study in the Cancer Prevention Study-II Nutrition Cohort. Breast Cancer Res. 2009;11(4):R64.
Eliassen AH, Spiegelman D, Hollis BW, Horst RL, Willett WC, Hankinson SE. Plasma 25-hydroxyvitamin D and risk of breast cancer in the Nurses’ Health Study II. Breast Cancer Res. 2011;13(3):R50.
Gilbert R, Martin RM, Beynon R, Harris R, Savovic J, Zuccolo L, et al. Associations of circulating and dietary vitamin D with prostate cancer risk: a systematic review and dose-response meta-analysis. Cancer Causes Control. 2011;22(3):319–40.
Kristal AR, Till CA, Song X, Tangen CM, Goodman PJ, Neuhouser ML, et al. Plasma Vitamin D and Prostate Cancer Risk; Results from the Selenium and Vitamin E Cancer Prevention Trial. Cancer Epidemiol Biomarkers Prev. 2014;23(8):1494–504.
Giovannucci E. The epidemiology of vitamin D and cancer incidence and mortality: a review (United States). Cancer Causes Control. 2005;16(2):83–95.
Lim U, Freedman DM, Hollis BW, Horst RL, Purdue MP, Chatterjee N, et al. A prospective investigation of serum 25-hydroxyvitamin D and risk of lymphoid cancers. Int J Cancer. 2009;124(4):979–86.
Purdue MP, Freedman DM, Gapstur SM, Helzlsouer KJ, Laden F, Lim U, et al. Circulating 25-hydroxyvitamin D and risk of non-hodgkin lymphoma: Cohort Consortium Vitamin D Pooling Project of Rarer Cancers. Am J Epidemiol. 2010;172(1):58–69.
Lazzeroni M, Serrano D, Pilz S, Gandini S. Vitamin d supplementation and cancer: review of randomized controlled trials. Anticancer Agents Med Chem. 2013;13(1):118–25.
Keum N, Giovannucci E. Vitamin D supplements and cancer incidence and mortality: a meta-analysis. Br J Cancer. 2014;111(5):976–80.
Bolland MJ, Grey A, Gamble GD, Reid IR. Calcium and vitamin D supplements and health outcomes: a reanalysis of the Women’s Health Initiative (WHI) limited-access data set. Am J Clin Nutr. 2011;94(4):1144–9.
Feldman D, Krishnan AV, Swami S, Giovannucci E, Feldman BJ. The role of vitamin D in reducing cancer risk and progression. Nat Rev Cancer. 2014;14(5):342–57.
Acknowledgments
This analysis was part of the CHANCES project funded in the FP7 (www.chancesfp7.eu) framework program of DG-RESEARCH in the European Commission (Grant No. 242244). The CHANCES project is coordinated by the Hellenic Health Foundation, Greece. The ESTHER study was funded by the Baden-Württemberg state Ministry of Science, Research and Arts (Stuttgart, Germany), the Federal Ministry of Education and Research (Berlin, Germany) and the Federal Ministry of Family Affairs, Senior Citizens, Women and Youth (Berlin, Germany). Measurements of 25(OH)D in men were conducted in the context of the German Cancer Aid project number 108250 and 108426. The TROMSØ Study was funded by the Norwegian Research Council and performed by the University of Tromsø in cooperation with the National Health Screening Service. In EPIC-Elderly, 25(OH)D measurements in colorectal cancer cases and controls were funded by the World Cancer Research Fund (WCRF, London, UK; Grant Number 2005/12). The EPIC-Elderly study was funded by the “Europe Against Cancer” programme of the European Commission. Additionally, each center received local financial support (http://epic.iarc.fr/funding.php). All authors read and approved the final manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ordóñez-Mena, J.M., Schöttker, B., Fedirko, V. et al. Pre-diagnostic vitamin D concentrations and cancer risks in older individuals: an analysis of cohorts participating in the CHANCES consortium. Eur J Epidemiol 31, 311–323 (2016). https://doi.org/10.1007/s10654-015-0040-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10654-015-0040-7