Abstract
Urban agriculture should be promoted as long as the food produced is safe for consumption. Located in the metropolitan region of São Paulo-Brazil, Santo André has intense industrial activities and more recently an increasing stimulus to urban gardening. One of the potential risks associated to this activity is the presence of potentially toxic elements (PTEs). In this study, the concentration of PTEs (As, Ba, Cd, Co, Cu, Cr, Ni, Mo, Pb, Sb, Se, V and Zn) was evaluated by soil (n = 85) and soil amendments (n = 19) in urban gardens from this municipality. Only barium was above regulatory limits in agricultural soil ranging from 20 to 112 mg kg−1. Geochemical indexes (Igeo, Cf and Er) revealed moderate to severe pollution for As, Ba, Cr, Cu, Pb Se and Zn, especialy in Capuava petrochemical complex gardens. A multivariate statistical approach discriminated Capuava gardens from the others and correlated As, Cr and V as main factors of pollution. However, carcinogenic and non-carcinogenic risks were below the acceptable range for regulatory purposes of 10–6–10–4 for adults. Soil amendments were identified as a possible source of contamination for Ba, Zn and Pb which ranged from 37 to 4137 mg kg−1, 20 to 701 mg kg−1 and 0.7 to 73 mg kg−1, respectively. The results also indicated the presence of six pathogenic bacteria in these amendments. Besides that, the occurrence of antimicrobial resistance for Shigella, Enterobacter and Citrobacter isolates suggests that soil management practices improvement is necessary.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In Brazil, during the Covid-19 pandemic, food insecurity increased due to social inequalities and reinforcing national nutritional problems, which can have health impacts on individual, community and growth levels at short and long terms. Currently, urban and peri-urban agriculture are considered a sustainable alternative (Salomon et al., 2020). Especially, in developing countries (Nabulo et al., 2012), UA contributes to food security and strengths communities under vulnerability (Roese & Curado, 2004). On a global scale, communities have identified urban agriculture as a viable option to increase their access to health, nutrition, quality of life and low-cost fresh products (Deelstra & Girardet, 2000; Schram-Bijkerk et al., 2018). Approximately 1.1 billion people are involved in some type of urban agriculture in the world (Mougeot, 2015).
However, the introduction of toxic substances (deliberately or not) and inefficient management practices in urban environments can cause the degradation of soil quality, and consequently, affect food security, groundwater quality and human health. Potentially toxic elements (PTEs), such as As, Cd, Cu, Pb and Zn, are often detected in urban soils and in vegetables grown on them (Ćwieląg-Drabek et al., 2020; Laidlaw et al., 2018; McBride et al., 2014; Nabulo et al., 2012; Spliethoff et al., 2016; Wiseman et al., 2013). These elements can cause ecological risks and can accumulate in the human body resulting in several adverse health effects.
Another risk associated with urban agriculture is the contamination by pathogenic microorganisms. Microorganisms can come from the soil itself, from the manure and fertilization system (for example, fresh animal waste or non-composted urban waste that is in direct contact with edible parts of plants), from irrigation water, or even from incorrect handling and hygiene in the post-harvest (Urra et al., 2019). Concern about pathogens in vegetables has increased due to the increased number of disease outbreaks caused by the consumption of fresh, whole foods, cut and minimally processed vegetables (Sant'Anna et al., 2020).
Eighty five percent of the Brazilian population lives in urban areas (IBGE-PNAD, 2015) and 23.3% of the urban families are still suffering from food and nutrition insecurity (IBGE, 2013). The support for urban agriculture became part of the national policy for poverty reduction and food security guarantee from the early 2000s. In 2018, the Ministry of Social Development launched the National Program for urban agriculture (Brazil, 2018), which aims to contribute to the promotion of health and healthy habits and the food and nutritional security. It was estimated that there are more than 600 initiatives of urban and peri‐urban food production in the country for both self‐consumption and commercial purposes (Sant'Anna de Medeiros et al., 2020).
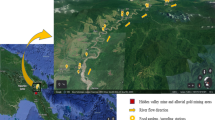
Santo André is a city of metropolitan region of São Paulo (MRSP), Brazil, one of the largest urban conglomerates in the world, with > 20 million inhabitants (UN, 2019). It is one of the cities of an automobile industry cluster (so-called ABC) organized around the Anchieta motorway, which connects São Paulo to the Port of Santos (Fernández-de-Sevilla and dalla Costa, 2019). The city has a fleet of more than 500,000 vehicles (DENATRAN, 2019). PTEs emissions from vehicular source can contaminate urban gardens by atmospheric deposition (Uzu et al., 2014). In the city, there is also an industrial complex, the Capuava complex, with 125 hectares and 14 chemical plants that produce polyethylene, naphtha, cement and fertilizers. The industrialization process began in the 1950’s, with the construction of a petrochemical refinery. This refinery produces about 30% of the fossil fuels consumed in the MRSP (Caumo et al., 2018). Petrochemical plants may be responsible for supplying PTEs to the environment (Manno et al., 2006; Marques et al., 2020).
This region has experienced an intensification of urban agriculture in the last decade (Amato‑Lourenço et al., 2021). It is hypothesized that this long history of industrial activities and environmental pollution may cause the accumulation of some PTEs in soils affecting UA and may pose risk to the urban gardeners. In this scenario, the present study aimed (i) to evaluate the levels of PTEs in soils and soil amendments in the urban gardens of Santo André; (ii) to assess human health effects in these topsoils; (iii) to investigate the possible sources of PTEs; (iv) to assess the presence of pathogenic microorganisms in bed soils and soil amendments. The findings of the present study may provide baseline data needed to plan and improve urban agriculture.
Material and methods
Site selection
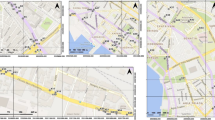
The studied urban gardens were selected at Santo André, Brazil. The city has about 715 thousand inhabitants. The sites were selected into urban perimeter on the surroundings of the industrial area of Capuava (maximum 10 km of distance) (Fig. 1). Sites with the following characteristics were selected: (i) larger than 500 m2; (ii) with commercial purpose; (iii) at least 3 years old of existence; (iv) well known on the surrounding community.
Sampling
Sampling of soil and soil amendments occurred in the dry season of 2019. The number of samples varied widely depending on the number of beds and number of lettuce heads cultivated per bed, as described in Table 1.
Three subsamples of topsoil samples (0–20 cm) were collected with a stainless-steel manual drill at each point; thus, a composite sample of 2 kg was produced, following a grid of one sample every three beds using a zigzag sampling scheme, according to EMBRAPA (2011) recommendations. Samples were collected, homogenized, removing demolition waste, such as brick, tiles, steel, wood, plastic, glass, rubbers and others. All studied gardens amended their bed soils, however the kind of amendment (Table 1) and the amount applied was very diverse among the sites. Circa of 250 mg of soil amendments samples were collected directly from the piles or storage containers. All samples were packed in plastic bags and immediately taken to laboratory.
Sample preparation and analysis
Soil and soil amendments samples were dried at 40 °C until constant weight and sieved to provide the < 2 mm and < 150 μm size fractions. About 400 mg of soil samples (< 2 mm) from at least 3 samples per site were analyzed for the following parameters: texture, pH, organic matter content, cation exchange capacity, potential soil acidity (H + Al), exchangeable cations (K, Ca, Mg and P), sum of bases (SB) and base saturation (V%) determined by the methodology proposed by Camargo et al. (2009). About 500 mg of soil and soil amendment were pre-digested for 48 h with 10 mL of sub-boiled concentrated HNO3 (Synth, Diadema, Brazil). Then, the samples were heated in a digesting block at 175 °C for 15 min according to USEPA 3051A method (Element, 2007), with some modifications according to Segura et al. (2016) and Suda and Makino (2016). The digested samples were diluted to 50 mL with type 1 water and then, fivefold diluted.
The elements were determined using an inductively coupled with a single quadrupole mass spectrometer (ICP-MS Agilent 7900, Hachioji, Japan). A multi-element stock solution (10 mg L−1) (Perkin Elmer, USA) was used to prepare the calibration curve according to Paniz et al. (2018) and internal standard Ge 10 mg L−1 were also used. Sandy Clay Soil reference material—CRM 049 (Sandy Clay 1, Sigma-Aldrich RTC, Salisbury, UK) and Trace Elements in Multi-Nutrient Fertilizer certified reference material– CRM 695 (NIST, Gaithersburg, MD, USA) were used throughout the analysis for method accuracy. The results of the analysis of the CRMs were statistically consistent with the certified values (Table S1). The detection limit obtained for each element are showed in Table S1.
Microbiological analysis
For microbiological analyses, 40 g of soil and soil amendments samples from beds were collected randomly from different locations in the field. Then, they were immediately put into sterile 50‐ml falcon tubes, transported in an ice chest with ice gel units to the laboratory and stored at 8 °C. A total of 21 samples were collected.
Bed soils and soil amendments samples were cultured in MacConkey agar (Oxoid, Basingstoke, UK) plates which were incubated at temperature of 37 °C during 18–24 h. After bacterial growth, different colonies from each plate were selected and the identification of bacterial genera was performed by biochemical assays with EPM, MILi and Simmons citrate (Ewing, 1986; Toledo et al., 1982a, 1982b) to confirm the presence of Enterobacteriaceae family. The bacterial isolates were stored in Brain Heart Infusion (BHI) (Difco, USA) plus 20% glycerol (Sigma-Aldrich, St-Louis, USA) media at − 80 °C.
The antimicrobial susceptibility of bacterial isolates was determined using the disk-diffusion in agar method, as recommended by the Clinical and Laboratory Standards Institute guidelines (CLSI, 2008; CLSI, 2018). The following antimicrobial agents were used: amoxicillin-clavulanic acid (AMC), aztreonam (ATM), cefepime (FEP), cefotaxime (CTX), ceftazidime (CAZ), cefoxitin (CFO), chloramphenicol (CLO), ciprofloxacin (CIP), fosfomycin (FOS), gentamicin (GEN), imipenem (IMP), nalidixic acid (NAL), nitrofurantoin (NIT), norfloxacin (NOR), tetracycline (TET) and trimethoprim sulfamethoxazole (SXT). All antimicrobial used were from Oxoid. Enrofloxacin (ENO) was also tested because this antimicrobial is commonly used in veterinary clinics. E. coli strain ATCC 25922 was used as a quality control for antimicrobial susceptibility testing.
Contamination indexes
Soil contamination by some PTEs in urban gardens was estimated using the geoaccumulation index (Igeo) (Müller, 1969), according to Eq. 1.
where Cn is the total concentration of an element in the tested soil and Bn is the respective concentration of the element in the reference environment from CETESB (Environmental Protection Agency of the State of São Paulo, Brazil) soil quality values were used (CETESB, 2016). The constant 1.5 was used to compensate minor natural and anthropogenic fluctuations. The calculated results were compared to the seven classes proposed by Müller (1969): Igeo < 0, unpolluted; 0 < Igeo ≤ 1, unpolluted to moderately polluted; 1 < Igeo ≤ 2, moderately polluted; 2 < Igeo ≤ 3, moderately to severely polluted; 3 < Igeo ≤ 4, severely polluted; 4 < Igeo ≤ 5, severely to extremely polluted; Igeo > 5, extremely polluted.
Soil contamination by some PTEs was also assessed by single element contamination factor, Cfi (Eq. 2), single element potential ecological risk, Eri (Eq. 3) and potential ecological risk index. RI (Eq. 4), proposed by Håkanson (1980).
where Cni is the quality reference value from São Paulo State (CESTES, 2016), Ci0–1 is the mean concentration of the PTE in question. Tri is the toxic response factor for the given PTE (Ba = Zn = 1 < Cr = V = 2 < Co = Cu = Ni = Pb = 5 < As = 10 < Cd = 30), only these ten studied elements Tr value are in the literature (Håkanson, 1980). Four qualitative terminologies are used to describe the contamination factor: Cf < 1, low contamination; 1 ≤ Cf < 3, moderate contamination; 3 ≤ Cf < 6, considerable contamination and Cf ≥ 6, very high contamination. For single element potential ecological risk, the evaluation results are divided into 5 grades: Er < 40, low potential ecological risk; 40 ≤ Er < 80, moderate potential ecological risk; 80 ≤ Er < 160, considerable potential ecological risk; 160 ≤ Er < 320, high potential ecological risk; and Er ≥ 320, very high potential ecological risk. The RI: potential ecological risk index; the evaluation results are divided into 5 grades: RI < 150, low ecological risk; 150 ≤ RI < 300, moderate ecological risk; 300 ≤ RI < 600, considerable ecological risk; 600 ≤ RI < 1200, very high ecological risk; and RI ≥ 1200, extremely high ecological risk.
Health risk assessment
For results obtained in the soil samples, the risk assessment was divided into non-carcinogenic (hazard quotient (HQ)) and carcinogenic risks (CR), considering three exposure routes: ingestion and inhalation and dermal according to the methodology proposed by Gabarrón et al. (2017) and Hu et al. (2017) based on The US Environmental Protection Agency methodology (USEPA, 2004) and using the selected parameters (Table S2). The details of the methods were provided in the supplementary materials. The carcinogenic risk was calculated only for As, Co, Cr, Ni and Pb and non-carcinogenic risk was estimated for all studied PTEs.
Data analysis method
In order to obtain information about the possible sources of the PTEs, the concentration of the elements was evaluated by principal component analysis (PCA) and Pearson's correlation. Statistical significance levels were expressed as p < 0.05. Principal component analysis (PCA), a multivariate statistical test, was performed to assess multivariate relationships.
PCA has been used to identify natural geochemical origins and anthropogenic sources. The dataset consisted of a matrix with 85 cases (collection points) and 13. Data were normalized and rotated (normalized varimax), grouping parameters. Only eigenvalues > 1 were considered in this study. Group loading values > 0.50 were used with high or strong correlation. Before performing PCA analysis, Kaiser-Meyer-Olkin (KMO) and Bartlett’s tests of sphericity are performed to check the suitability of the datasets. In the current study, the overall KMO value for our data was meritorious (KMO = 0.818) and Bartlett's Test of Sphericity was significant (Chi2 = 1826.433, p < 0.001, df = 78) which rejects the hypothesis the variable matrix is an identity matrix. That give us confidence, the variables are significantly correlated and suitable to factor analysis (Steiner& Grieder, 2020).
Hierarchical Cluster analysis (HCA) was performed in the data set. The values were normalized. Ward's method was used as a procedure of clustering. Euclidean distance was applied to measure similarity and the statistical significance of the clusters formed was checked by the linkage distance (dlink), expressed as the percentage of the range from the maximum to the minimum distance (dmax) in the data set, Dlink/Dmax × 100. A dendrogram illustrates clustering of the similar variables (PTEs) considering the hierarchical structure. All statistical analyzes were performed using Statistica software version 7.0 (Statistica (Tulsa, USA).
Results and discussion
Soil results
Table S3 shows soil (0–20 cm) physical–chemical parameters of six studied gardens. Most samples consisted of neutral soils, with pH ranging from 5.3 to 7.6 and the lowest values (5.9 ± 0.6) were found at Capuava 1 garden. The samples showed content of clay between 224 and 547 g kg−1, with an average of 362 g kg−1, high sand content ranging from 305 to 646 g kg−1 and, in general, a median silt content, with an average of 200 g kg−1. The soil texture varied from loam to clay soil, with a predominance of sand clay loam soil. This type of soil has a low potential for PTEs retention when compared to clay soils, present predominantly in the gardens of Bairro Jardim and Vila Marajoara. The content of organic matter (OM) varied widely from 21 to 68 g kg−1, the highest values were observed at Capuava 1 (average of 61.3 g kg−1).
The cation exchange capacity (CEC), which expresses the capacity of soil to retain cations, presented an average of 152 mmolc dm−3. The base saturation presented was high in all gardens, varying between 88 and 96% and the soil can be classified, according to the current Brazilian soil classification system (SiBCS), as eutrophic soil (V ≥ 50%) (Solos, 2013). The concentrations of exchangeable phosphorus in the vegetable garden soil were all above 120 mg dm−3, which is considered a minimum sufficient value for productive cultivation (Van Raij et al., 2011). The univariate ANOVA statistical test, with 95% confidence interval, was applied to the results presented in Table S3 and revealed that there are significant differences between the parameters in the areas, except for pH and exchangeable P.
The frequency distributions of concentrations of PTEs measured in the garden soil samples are described in Table 2. The data are not normally distributed (Kolmogorov–Smirnov test, p < 0.010) for most elements, except for As, Cr and Ni. Median (50th percentile) were lower than mean concentrations for Ba, Cd, Co, Cu, Mo, Pb, Sb, Se and Zn and higher for V.
The mean concentrations of elements detected in soil samples ranked in the following order: Zn > Ba > V > Cr > Cu > Pb > As > Ni > Co > Mo > Se > Cd > Sb. PTEs results were compared with the local environmental protection agency (Environmental Protection Agency of the State of São Paulo, Brazil) (CETESB, 2016) (Table 2). The quality reference (QRV) represents the natural concentrations of chemical elements in soils without anthropic influence; the prevention value (PV) represents a sort of alert and the intervention value for agricultural (AIV) soils represents the threshold value. The PV and AIV were established based on human health risk (CETESB, 2016). The spatial variation of the PTEs in the studied urban gardens are represented in Fig. 2, except for Co, Mo and Sb because these elements were detected in concentration ranges below quality reference values and presented very narrow variability.
The soils of the studied urban gardens presented content values above the QRV for almost all elements, except for Co, Mo and Sb, whose concentration values are not shown in Fig. 3. In addition, values above the PV were observed for As, Ba, Cr, Cu, Pb, Se and Zn and above the agricultural VI for Ba in the case of the Capuava 1 garden. The areas around the Capuava Petrochemical Complex were those that presented, in general, higher levels of PTEs, especially for Ba, Cu, Pb, Se and Zn (Fig. 2).
Boxplot of the results of geoaccumulation index of Pb, As, Ba, Cr, Cd, Cu, Ni, Se and Zn in soil samples (0–20 cm) from UG, in relation to the Quality Reference values of the State of São Paulo (QRV) (CETESB, 2016)
In a similar study in soils of three urban gardens from the metropolitan region of Belo Horizonte, Dala-Paula et al. (2018) reported values of concentration of Cu (27.9 ± 13.9 mg kg−1), Pb (19.4 ± 7.7 mg kg−1) and Cd (0.16 ± 0.03 mg kg−1) lower than those observed in the mean values of the gardens studied in Santo André, 51 ± 37 mg kg−1; 30 ± 20 mg kg−1 and 0.4 ± 0.2 mg kg−1, respectively.
Studies concerning concentrations of PTEs in soils in the MRSP are scarce. Therefore, the concentration values of As, Ba, Cr and Zn obtained in the soils of this study, collected in the urban region of Santo André, were compared to the study carried out by Figueiredo et al. (2011). In this study, the authors evaluated PTEs contents in superficial soils collected in different public parks in the city of São Paulo, 20 km far from Santo André. These parks in São Paulo are located in different scenarios of urban zoning (central, residential and industrial areas). In 9 of the 12 parks studied by the authors, the levels of Ba concentration exceeded the residential VI of CETESB (2016) and the reported concentration range was 284 to 1022 mg kg−1, which is higher than that observed in the soils of the gardens of Santo André (19–1000 mg kg−1).
The highest values of Ba in our study were observed in the Capuava 1 area (Fig. 2), which is located less than 30 m from the petrochemical complex, in high-traffic vehicular routes and next to a vehicle radiator grinder. Arsenic concentrations found in the soil of the gardens ranged from 8.5 to 21 mg kg−1, these values were higher than those reported in the parks of São Paulo (1.2–16 mg kg−1) (Figueiredo et al., 2011). The highest content of As were observed in Bairro Jardim (Fig. 2), which is a neighborhood closer to the central region of the city. It is possible to observe in Fig. 2 that the pattern of distribution of this spatial element was similar to that observed for Cr and V, suggesting that these elements come from the same source.
Chromium values observed in the soils from our studied ranged from 24 to 89 mg kg−1 and were higher than those observed in the soils of urban parks in São Paulo (21–70 mg kg−1). Regarding Zn, the values observed in Santo André vary from 30 ± 383 mg kg−1 and were higher than those observed by Figueiredo et al. (2011), which range from 15 to 179 mg kg−1. Thus, it is possible to infer that despite the municipality of Santo André being less populous and with a much smaller vehicle fleet than a municipality in São Paulo, the concentration levels of As, Cr and Zn elevates in the soils of urban gardens were higher than those reported in São Paulo soils in a region with high vehicular traffic and industrial sources of atmospheric pollution.
Comparing our results with those of other urban gardens soils in the world it emerges that Cu, Cd values are higher than those observed in Melbourne (Laidlaw et al., 2018), Sheffield (Weber et al., 2019) and Toronto (Wiseman et al., 2013) (Table 2). However, Co, Ni, Pb, Sb and Zn values observed in soils from our study are below those reported in the international studies described in Table 2.
The levels of As (23.93–44.33 mg kg−1) and Cr (86.67–160.67 mg kg−1) observed in Sheffield, England, UK, were also higher than those observed in our results, probably due to this city has a long history of industrial activities and environmental pollution (Weber et al., 2019). Therefore, it is possible to conclude with these comparisons that there is a need to locally assess the levels of application of PTEs, because observations made for other regions cannot be generalized.
Contamination indexes
The geoaccumulation index of elements in the soil was calculated for those that have guiding values for soil (CETESB, 2016). However, the results presented in Fig. 3 correspond to the elements that presented minimally some points with Igeo > 0, as following: As, Ba, Cd, Cr, Cu, Ni, Pb, Se and Zn. Considering the median obtained for each element, the soil of the studied gardens can be classified as: unpolluted to moderately polluted by Ba, Cd, Cr, Cu, Cd and Zn for almost all the studied gardens; with the exception of Capuava 1, which was moderately to severely polluted by Ba and moderately polluted by Cu and Pb. Igeo indicated that all gardens presented moderate pollution by As and Se, except for the garden of Capuava 1, which presented moderate to severe pollution by Se.
The single element contamination factor (Table 3) indicated a very high contamination by Ba and Zn and a considerable contamination by Cu in Capuava 1 soils. According to this index moderate to considerable contamination of soil by Ba, Cr and Zn was also observed in almost studied gardens, except in Bela Vista Garden, which Cr contamination was considered low. Moderate contamination by Pb was observed in soils of Capuava 1 and Vila Bastos. No concerning for a single element potential ecological risk was observed, but Capuava 1 soil are in a moderate potential ecological risk (Table 3), followed by Capuava 2. Thus this indexes reinforce that the proximity to the Petrochemical plant represent a potential input of PTEs in gardens soils.
Soil amendments results
The concentrations of PTEs in the soil amendments collected are presented in Table 4. Consistent with the results of collected soils, the median concentrations of elements detected in soil amendments ranked in the following order: Zn > Ba > Cu > V > Cr > Pb > Ni > Co > As > Mo > Se > Cd > Sb. soil amendments with the highest total PTEs concentration ranked in the following order: Spent mushroom substrate (SMS) > Local compost pile (Capuava 1) > Quail manure (Capuava 2) = Cow Manure = Meat bone meal > Chicken manure = Quail manure (Jd. Marajoara) > Castor cake = Lime > Local compost pile (Bela Vista).
Overall, the two soil amendments collected in Capuava 1 (SMS and local compost pile) were those with the highest total concentration of PTEs. SMS refers to the biomass waste generated from mushroom production (Hanafi et al., 2018) and it has been used as fertilizer (Grimm & Wösten, 2018). SMS composition varies according to geographical location and also according to mushroom species (Grimm & Wösten, 2018). Studies reporting Ba content in SMS are scarce. Kalembasa and Wiśniewska (2009) reported a lower content of Ba (52.5 mg kg−1) than ours in Italian SMS. The PTEs content of SMS determined within this study is compared to levels reported in previous studies which it verifies that the Ba and Zn levels are elevated, while Cd and Cu is invariably lower that those reported in other countries (Kalembasa & Wiśniewska, 2009; Jordan et al., 2008; Medina et al., 2012).
When comparing our findings with other studies of soil amendments (Table 4) a notable difference is seen regarding the levels of Ba and Mo in soil amendments samples, the levels of these elements are elevated in soil amendments of Santo André (Alfaro et al., 2017; Han et al., 2013; Margenat et al., 2020; Paradelo et al., 2020).
Brazilian legislation (Brazil, 2016 and 2019) maximum allowed values in soil amendments are 15, 10 and 200 mg kg−1 for As, Cd and Pb, respectively. In meat bone meal sample, As content (31 mg kg−1) was above the Brazilian legislation (15 mg kg−1) (Table 4).
Despite these results of PTEs levels, it is known that some organic compounds can decrease the bioavailability of PTEs in soils for plants through various mechanisms, such as precipitation, complexation, redox reactions, ion exchange and electrostatic interaction (Margenat et al., 2020; Palansooriya et al., 2020). However, the misapplication of these compounds may be contributing to increase the supply of PTEs in the soil of urban gardens in Santo André and also may pose risk for the gardeners’ health.
We noticed a limited knowledge about the best practices for management and application of these amendments in the studied gardens and the gardeners may be unaware about the risks of misapplication. In fact, rates of application, incorporation practices into the soil, maturation status and storing procedures of these compounds were very distinguished across the gardens. In Brazil, the government allows some soil amendments to be used for crop nutrition, with specifications clearly defined by appropriate regulation (Brazil, 2016 and 2019). The partnership between governments, universities and representative institutions plays a strategic role in promoting risk awareness and knowledge about soil testing and fertilization.
Source identification and relationships between PTEs
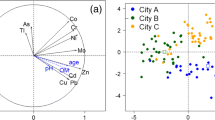
To define clearly dominant or discrete sources of PTEs in urban gardens soils is difficult, due to the complex temporal scale and spatial distribution of distinct inputs of PTEs. Data set was evaluated by multivariate statistics. The first factor represents 64.54% (Fig. 4.a) of the data variation and indicated a directly strong (> 0.7) proportional association of Ba, Cd, Co, Cu, Mo, Ni, Pb, Sb, Se and Zn in the 1st and 4th quadrants. Therefore, this suggests that these elements are from the same source, probably from Capuava Petrochemical complex particulate matter and from soil amendments. This assumption was reinforced by the PCA representation by cases (Fig. 4.b) where samples from Capuava urban garden were discriminated in the same quadrants (1st and 4th quadrants).
At same time, factor 2 discriminated samples by its contents of As, Cr and V and represents 18.53% (Fig. 4.a). Then, Marajoara, Bairro Jardim and Vila Bastos urban gardens were placed in the 1st and 2nd quadrants due to the higher levels of these elements (Fig. 4.b). As mentioned before, Santo André is in a region of many automobiles and auto-parts industries, such as General Motors, Volkswagen, Mercedes-Benz, Scania and others. Thus, the region has a long history of foundries and metallurgical process companies which contributes to As, Cr and V (alloys elements) inputs in the environment (Freire et al., 2021; Lange et al., 2017).
The same associations observed in PCA were identified in the hierarchical dendrograms of each urban garden (Fig. 4c–h). For example, in Capuava 1 garden (Fig. 4c) two main groups were identified. The first group by Cd, Co, Cu, Cr, Mo, Ni, Pb, Sb, Se and Zn, which can be associated to the petrochemical complex and vehicular sources. In the second group, a remarkable association between Ba and Zn can be attributed to the inputs caused by spent mushroom substrate (Table 4). For the majority of urban gardens, cluster analysis showed a common association between As, Cr and V, as discussed above for PCA findings. Interesting, for Vila Bastos garden (Fig. 4f), Pb, Cd and Zn were discriminated from other elements, a very common association in soils polluted by fine particular matter, in heavy industrialized environments (Smieja-Król et al., 2022). The cluster obtained for Bela Vista (Fig. 4h) showed a district pattern compared to other gardens, since As, V, Cr, Zn, Pb an Sb were correlated in one cluster and Ba, Se, Cd, Cu, Mo, Ni and Co in another cluster. This garden showed the lowest concentrations levels of PTEs and is the furthest from Capuava petrochemical complex and nearest garden from Anchieta motorway, suggesting that these associations are more probably related to atmospheric vehicular pollution.
Table S4 presents the results of Pearson's correlations between the PTEs concentrations and the soil characterization parameters. Acidity is considered a key parameter in the mobility of elements in soils, however in this study pH did not significantly correlate with PTEs and this may be related to the small variation of the pH value between the samples. The elements Ba, Cd, Cu, Mo, Ni, Se, Sb and Zn correlated with OM. The elements Ba, Co, Cu, Ni, Se, Sb and Zn correlated significantly with CEC. Therefore, organic compounds commonly used by farmers added to soil may also result in an increase of CEC. The sand fraction correlated inversely with As content, and directly with Cd and Pb content, indicating that the latter two may be associated with a natural origin or, most probably from fine particulate matter. Arsenic correlated directly with clay contents, indicating that the areas that presented higher clay contents were able to accumulate this element the most.
Risk associated with the presence of PTEs in the soil
Table 5 shows the concentration of PTEs in the soil of urban gardens in Santo André corresponding to the 95th percentile (Csoil), the toxicity values (RfD and FC), the chronic daily doses (DDC), the hazard quotients (HQ), hazard indices (HI), carcinogenic risks (CR), total carcinogenic risk probabilities (TCR) obtained for adults, that is, the risk to which urban gardeners are exposed.
For most PTEs, the results obtained for the non-carcinogenic risk quotient indicated that the route of exposure that contributed most to the total risk was the oral route, followed by dermal absorption and finally the route by inhalation of soil particles, except for Ba and Cd, for which the inhalation route contributed more than the dermal route. The oral route of exposure to PTEs in urban soils is the most common (Gabarrón et al., 2017).
The non-carcinogenic hazard index (HI) results obtained for all PTEs, considering adults, were less than 1.0, therefore, health risks are not expected to occur (USEPA, 1989). The order of HI values for the PTEs observed was As > Cr > Ba > Pb > Cu > Ni > Zn > Sb > Co > V > Cd. The total carcinogenic risk values obtained for all PTEs were below the acceptable range for regulatory purposes of 10–6–10–4 (Ross, 2009) for adults. The decreasing order of TCR for PTEs observed was Cr > As > Ni > Pb > Co.
Microorganisms in bed soils and soil amendments
The microbial results revealed the identification of 6 bacterial genera (Table 6) and 8 isolates were unidentified. Around 30% of non-animal amendments samples were contaminated either with Serratia or Klebsiella genera; however, no antimicrobial resistance was observed. Animal amendments that were positive for at least one microorganism accounted for 37.5% of this type of sample, and four bacterial genera were isolated. Among them, resistance to TET and NIT was identified for Citrobacter and Enterobacter genera was resistant to CFO. Antimicrobial presence in animal manures was reported previously and may be related to the resistance observed in our findings (Bloem et al., 2017).
Fifty percent of the bed soil samples showed at least one bacterial genera. Shigella isolate demonstrated the broadest spectrum of antimicrobial resistance, being resistant to three antimicrobials (SXT, FOS and NAL). Citrobacter isolate was resistant to CFO, while Klebsiella isolate did not present any resistance.
Conclusion
Our findings showed an enrichment of some PTEs in the soils of some gardens, but at concentration levels below the agricultural intervention values established by the local environmental protection agency, except for Ba. The multivariate analysis of the data revealed that some PTEs are important discriminators of urban pollution inputs and may provide valuable information for mitigation strategies. Results also revealed that the organic matter directly influences the geochemistry of PTEs in the soils of these gardens. Some organic compounds used in these gardens showed high levels of As, Ba, Pb and Zn. The risk assessment revealed that gardeners are not subject to health damage from exposure to PTEs. However, pathogenic and resistant microorganisms were identified in soil beds and amendments.
To our knowledge, these are the first data from PTEs urban soils in this region; however, our study does not include isotopic analytical determination to assure the origin of PTEs in these urban gardens.
Despite of the hostile environmental situation of the studied region, the results herein showed an optimistic scenario in the soils of urban gardens of Santo André. Future studies to investigate PTEs in the food produced in these areas are strongly recommended. The studies about the atmospheric deposition of these elements can also contribute to the mass balance and source identification.
References
Alfaro, M. R., Do Nascimento, C. W. A., Ugarte, O. M., Álvarez, A. M., de Aguiar Accioly, A. M., Martín, B. C., & Aguilar, M. G. (2017). First national-wide survey of trace elements in Cuban urban agriculture. Agronomy for Sustainable Development, 37(4), 1–7. https://doi.org/10.1007/s13593-017-0437-7
Amato-Lourenço, L. F., Buralli, R. J., Ranieri, G. R., Hearn, A. H., Williams, C., & Mauad, T. (2021). Building knowledge in urban agriculture: The challenges of local food production in Sao Paulo and Melbourne. Environment, Development and Sustainability, 23(2), 2785–2796. https://doi.org/10.1007/s10668-020-00636-x
Bloem, E., Albihn, A., Elving, J., Hermann, L., Lehmann, L., Sarvi, M., & Ylivainio, K. (2017). Contamination of organic nutrient sources with potentially toxic elements, antibiotics and pathogen microorganisms in relation to P fertilizer potential and treatment options for the production of sustainable fertilizers: A review. Science of the Total Environment, 607, 225–242. https://doi.org/10.1016/j.scitotenv.2017.06.274
Brazil. PORTARIA Nº 467, DE 7 DE FEVEREIRO DE 2018 - Institui o Programa Nacional de Agricultura Urbana e Periurbana. https://www.jusbrasil.com.br/diarios/177346593/dou-secao-1-09-02-2018-pg-64 Accessed 25 Feb 2022.
Brazil Ministry of agriculture, livestock and supply. Normative instruction n. 5 of 10 March 2016, Distrito federal http://www.agricultura.gov.br/assuntos/insumos-agropecuarios/insumos-agricolas/fertilizantes/legislacao/in-5-de-10-3-16-remineralizadores-e-substratos-para-plantas.pdf
Camargo, O. A., Moniz, A. C., Jorge, J. A., & Valadares, J. M. A. S. (2009). Métodos de análise química, mineralógica e física de solos do Instituto Agronômico de Campinas [Methods of Chemical, Mineralogical and Soil Physics Analysis of the Agronomic Institute of Campinas]. Technical Bulletin, 106, 94.
Caumo, S., Vicente, A., Custódio, D., Alves, C., & Vasconcellos, P. (2018). Organic compounds in particulate and gaseous phase collected in the neighbourhood of an industrial complex in São Paulo (Brazil). Air Quality, Atmosphere and Health, 11(3), 271–283. https://doi.org/10.1007/s11869-017-0531-7
CETESB Environmental Company of the State of São Paulo. Guiding Values for soils and water underground in the State of São Paulo (2016). https://cetesb.sp.gov.br/aguas-subterraneas/wpcontent/uploads/sites13/2013/11/tabela_vos_2016_site.pdf. Accessed 09 June 2022
CLSI - Clinical and Laboratory Standards Institute (2008). Performance standards for antimicrobial disk and dilution susceptibility tests for bacteria isolated from animals, approved standard, CLSI document M31-A3. 3rd ed. CLSI, Wayne, PA.
CLSI - Clinical and Laboratory Standards Institute, 2018. Performance Standards for Antimicrobial Susceptibility Testing: Twenty-third Informational Supplement M100-S23. CLSI, Wayne, PA.
Ćwieląg-Drabek, M., Piekut, A., Gut, K., & Grabowski, M. (2020). Risk of cadmium, lead and zinc exposure from consumption of vegetables produced in areas with mining and smelting past. Scientific Reports, 10(1), 1–9. https://doi.org/10.1038/s41598-020-60386-8
Dala-Paula, B. M., Custódio, F. B., Knupp, E. A., Palmieri, H. E., Silva, J. B. B., & Glória, M. B. A. (2018). Cadmium, copper and lead levels in different cultivars of lettuce and soil from urban agriculture. Environmental Pollution, 242, 383–389. https://doi.org/10.1016/j.envpol.2018.04.101
Deelstra, T., & Girardet, H. (2000). Urban agriculture and sustainable cities. Bakker N., Dubbeling M., Gündel S., Sabel-Koshella U., de Zeeuw H. Growing cities, growing food. Urban agriculture on the policy agenda. Feldafing, Germany: Zentralstelle für Ernährung und Landwirtschaft (ZEL), 43–66.
DENATRAN – DEPARTAMENTO NACIONAL DE TRÂNSITO. Estatísticas, 2019. Available in: http://www.denatran.gov.br/frota. Accessed 14 Mar 2020.
Element, C. A. S. (2007). Method 3051A microwave assisted acid digestion of sediments, sludges, soils, and oils. Z. Für Anal. Chem, 111, 362–366.
EMBRAPA. Manual de métodos de análise de solo/Centro Nacional de Pesquisa de Solos (2a. ed), Empresa Brasileira de Pesquisa Agropecuária. Centro Nacional de Pesquisa de Solos, rev. revista. – Rio de Janeiro, 225p, 2011.
Ewing, W. H. (1986). Edwards and Ewing's identification of Enterobacteriaceae. Edwards and Ewing's Identification of Enterobacteriaceae., (Edition 4).
Fernández-de-Sevilla, T., & dalla Costa, A. J. (2019). Catching the ladder: The formation and growth of the São Paulo automotive industry cluster 1. In Regional Economic Development and History (pp. 119–144). Routledge.
Figueiredo, A. M. G., Tocchini, M., & dos Santos, T. F. (2011). Metals in playground soils of Sao Paulo city, Brazil. Procedia Environmental Sciences, 4, 303–309.
Freire, B. M., Gonzaga, R. G., Pedron, T., Monteiro, L. R., Lange, C. N., Pedreira Filho, W. D. R., & Batista, B. L. (2021). Occupational exposure to potentially toxic elements in the foundry industry: An integrated environmental and biological monitoring. Environmental Science and Pollution Research, 28(26), 34630–34641. https://doi.org/10.1007/s11356-021-13099-y
Gabarrón, M., Faz, A., & Acosta, J. A. (2017). Soil or dust for health risk assessment studies in urban environment. Archives of Environmental Contamination and Toxicology, 73(3), 442–455. https://doi.org/10.1007/s00244-017-0413-x
Grimm, D., & Wösten, H. A. (2018). Mushroom cultivation in the circular economy. Applied Microbiology and Biotechnology, 102(18), 7795–7803. https://doi.org/10.1007/s00253-018-9226-8
Hakanson, L. (1980). An ecological risk index for aquatic pollution control: A Sedimentological Approach. Water Research, 14(8), 975–1001. https://doi.org/10.1016/0043-1354(80)90143-8
Han, D., Luo, D., Chen, Y., & Wang, G. (2013). Transfer of Cd, Pb, and Zn to water spinach from a polluted soil amended with lime and organic materials. Journal of Soils and Sediments, 13(8), 1360–1368. https://doi.org/10.1007/s11368-013-0711-5
Hu, T., Zhang, J., Ye, C., Zhang, L., Xing, X., Zhang, Y., & Zhang, Q. (2017). Status, source and health risk assessment of polycyclic aromatic hydrocarbons (PAHs) in soil from the water-level-fluctuation zone of the Three Gorges Reservoir, China. Journal of Geochemical Exploration, 172, 20–28. https://doi.org/10.1016/j.ecoenv.2014.04.031
IBGE – Instituto Brasileiro de Geografia e Estatística (2013). Pesquisa Nacional por Amostra de Domicílios: Segurança alimentar 2013, Rio de Janeiro. https://biblioteca.ibge.gov.br/visualizacao/livros/
IBGE – Instituto Brasileiros de Geografia e Estatística (2015). Pesquisa Nacional por Amostra de Domicílios. Brasília: IBGE, 2015.
Jordan, S. N., Mullen, G. J., & Murphy, M. C. (2008). Composition variability of spent mushroom compost in Ireland. Bioresource Technology, 99(2), 411–418. https://doi.org/10.1016/j.biortech.2006.12.012
Kalembasa, D., & Wiśniewska, B. (2009). Influence of mushroom substrate on lithium, barium and strontium contents at Italian ryegrass. Ecological Chemistry and Engineering A, 16(4), 357–363.
Laidlaw, M. A., Alankarage, D. H., Reichman, S. M., Taylor, M. P., & Ball, A. S. (2018). Assessment of soil metal concentrations in residential and community vegetable gardens in Melbourne, Australia. Chemosphere, 199, 303–311. https://doi.org/10.1016/j.chemosphere.2018.02.044
Lange, C. N., Figueiredo, A. M. G., Enzweiler, J., & Castro, L. (2017). Trace elements status in the terrain of an impounded vehicle scrapyard. Journal of Radioanalytical and Nuclear Chemistry, 311(2), 1323–1332. https://doi.org/10.1007/s10967-016-5078-9
Manno, E., Varrica, D., & Dongarrà, G. (2006). Metal distribution in road dust samples collected in an urban area close to a petrochemical plant at Gela. Sicily. Atmospheric Environment, 40(30), 5929–5941. https://doi.org/10.1016/j.atmosenv.2006.05.020
Margenat, A., You, R., Cañameras, N., Carazo, N., Díez, S., Bayona, J. M., & Matamoros, V. (2020). Occurrence and human health risk assessment of antibiotics and trace elements in Lactuca sativa amended with different organic fertilizers. Environmental Research, 190, 109946. https://doi.org/10.1016/j.jhazmat.2020.123424
Marquès, M., Domingo, J. L., Nadal, M., & Schuhmacher, M. (2020). Health risks for the population living near petrochemical industrial complexes: 2—Adverse health outcomes other than cancer. Science of the Total Environment, 730, 139122. https://doi.org/10.1016/j.scitotenv.2020.139122
McBride, M. B., Shayler, H. A., Spliethoff, H. M., Mitchell, R. G., Marquez-Bravo, L. G., Ferenz, G. S., & Bachman, S. (2014). Concentrations of lead, cadmium and barium in urban garden-grown vegetables: The impact of soil variables. Environmental Pollution, 194, 254–261. https://doi.org/10.1016/j.envpol.2014.07.036
Medina, E., Paredes, C., Bustamante, M. A., Moral, R., & Moreno-Caselles, J. (2012). Relationships between soil physico-chemical, chemical and biological properties in a soil amended with spent mushroom substrate. Geoderma, 173, 152–161. https://doi.org/10.1016/j.geoderma.2011.12.011
Mohd Hanafi, F. H., Rezania, S., Mat Taib, S., Md Din, M. F., Yamauchi, M., Sakamoto, M., & Ebrahimi, S. S. (2018). Environmentally sustainable applications of agro-based spent mushroom substrate (SMS): An overview. Journal of Material Cycles and Waste Management, 20(3), 1383–1396. https://doi.org/10.1007/s10163-018-0739-0
Mougeot, L. (2015). Urban agriculture in cities of the Global South: four logics of integration. Food and the city: Histories of culture and cultivation, 163–193.
Müller, G. (1969). Index of geoaccumulation in sediments of the Rhine River. GeoJournal, 2, 108–118.
Nabulo, G., Black, C. R., Craigon, J., & Young, S. D. (2012). Does consumption of leafy vegetables grown in peri-urban agriculture pose a risk to human health? Environmental Pollution, 162, 389–398. https://doi.org/10.1016/j.envpol.2011.11.040
Palansooriya, K. N., Shaheen, S. M., Chen, S. S., Tsang, D. C., Hashimoto, Y., Hou, D., & Ok, Y. S. (2020). Soil amendments for immobilization of potentially toxic elements in contaminated soils: A critical review. Environment International, 134, 105046. https://doi.org/10.1016/j.envint.2019.105046
Paniz, F. P., Pedron, T., Freire, B. M., Torres, D. P., Silva, F. F., & Batista, B. L. (2018). Effective procedures for the determination of As, Cd, Cu, Fe, Hg, Mg, Mn, Ni, Pb, Se, Th, Zn, U and rare earth elements in plants and foodstuffs. Analytical Methods, 10(33), 4094–4103. https://doi.org/10.1039/C8AY01295D
Paradelo, R., Villada, A., & Barral, M. T. (2020). Heavy metal uptake of lettuce and ryegrass from urban waste composts. International Journal of Environmental Research and Public Health, 17(8), 2887. https://doi.org/10.3390/ijerph17082887
Van Raij, B. (2011). Melhorando o ambiente radicular em subsuperfície. Informações Agronômicas, 135. https://www.ipni.net/publication/ia-brasil.nsf/0/07522979282F212C83257A8F005D7C7D/$FILE/Page8-18-135.pdf
Roese, A. D., & Curado, F. F. (2004). A contribuição da agricultura urbana na segurança alimentar comunitária em Corumbá e Ladário, MS. IV Simpósio sobre recursos naturais e socioeconômicos do pantanal. Corumbá/MS-23–26 nov.
Ross, M. A. (2009). Integrated science assessment for particulate matter. US Environmental Protection Agency: Washington DC, USA, 61-161.
Salomon, M. J., Watts-Williams, S. J., McLaughlin, M. J., & Cavagnaro, T. R. (2020). Urban soil health: A city-wide survey of chemical and biological properties of urban agriculture soils. Journal of Cleaner Production, 275, 122900.
Sant’Anna, P. B., de Melo Franco, B. D., & Maffei, D. F. (2020). Microbiological safety of ready-to-eat minimally processed vegetables in Brazil: An overview. Journal of the Science of Food and Agriculture, 100(13), 4664–4670. https://doi.org/10.1002/jsfa.10438
Sant’anna-de-Medeiros, N., do-Carmo, D. L., Priore, S. E., & Santos, R. H. S. (2020). Diverse food in urban gardens in the promotion of food and nutrition security in Brazil: a review. Journal of the Science of Food and Agriculture, 100(4), 1383–1391. https://doi.org/10.1002/jsfa.10127
Schram-Bijkerk, D., Otte, P., Dirven, L., & Breure, A. M. (2018). Indicators to support healthy urban gardening in urban management. Science of the Total Environment, 621, 863–871. https://doi.org/10.1016/j.scitotenv.2017.11.160
Segura, F. R., Nunes, E. A., Paniz, F. P., Paulelli, A. C. C., Rodrigues, G. B., Braga, G. Ú. L., & Batista, B. L. (2016). Potential risks of the residue from Samarco’s mine dam burst (Bento Rodrigues, Brazil). Environmental Pollution, 218, 813–825. https://doi.org/10.1016/j.envpol.2016.08.005
Smieja-Król, B., Pawlyta, M., & Gałka, M. (2022). Ultrafine multi-metal (Zn, Cd, Pb) sulfide aggregates formation in periodically water-logged organic soil. Science of the Total Environment, 820, 153308. https://doi.org/10.1016/j.scitotenv.2022.153308
Solos, E. (2013). Sistema brasileiro de classificação de solos. Centro Nacional de Pesquisa de Solos.
Spliethoff, H. M., Mitchell, R. G., Shayler, H., Marquez-Bravo, L. G., Russell-Anelli, J., Ferenz, G., & McBride, M. (2016). Estimated lead (Pb) exposures for a population of urban community gardeners. Environmental Geochemistry and Health, 38(4), 955–971.
Steiner, M. D., & Grieder, S. G. (2020). EFAtools: An R package with fast and flexible implementations of exploratory factor analysis tools. Journal of Open Source Software, 5(53), 2521. https://doi.org/10.21105/joss.02521
Suda, A., & Makino, T. (2016). Functional effects of manganese and iron oxides on the dynamics of trace elements in soils with a special focus on arsenic and cadmium: A review. Geoderma, 270, 68–75. https://doi.org/10.1016/j.geoderma.2015.12.017
Toledo, M. R. F. D., Fontes, C. F., & Trabulsi, L. R. (1982a). EPM-A modification of Rugai and Araujo medium for simultaneous test of gas production from glucose, H2S, urease and tryptophan deaminase. Revista De Microbiologia, 13, 309–315.
Toledo, M. R. F., Fontes, C. F., & Trabulsi, L. R. (1982b). MI: A medium for detection of motilily, 468 indole, and lysine decarboxylase. Revista De Microbiologia, 13, 230–235.
United Nations, Department of Economic and Social Affairs, Population Division (2019). World Population Prospects 2019: Data Booklet (ST/ESA/SER.A/424).
Urra, J., Alkorta, I., & Garbisu, C. (2019). Potential benefits and risks for soil health derived from the use of organic amendments in agriculture. Agronomy, 9(9), 542. https://doi.org/10.3390/agronomy9090542
USEPA (1989). Risk assessment guidance for superfund volume I human health evaluation manual (Part A). Office of Emergency and Remedial Response. U.S. Environmental Protection Agency Washington, 20450. EPA/540/1-89/002.
USEPA (2004). Risk assessment guidance for superfund volume I: human health evaluation manual (Part E, Supplemental Guidance for Dermal Risk Assessment). Office of Superfund Remediation and Technology Innovation U.S. Environmental Protection Agency Washington, EPA/540/R/99/005.
Uzu, G., Schreck, E., Xiong, T., Macouin, M., Lévêque, T., Fayomi, B., & Dumat, C. (2014). Urban market gardening in Africa: Foliar uptake of metal (loid) s and their bioaccessibility in vegetables; implications in terms of health risks. Water, Air, & Soil Pollution, 225(11), 1–13. https://doi.org/10.1007/s11270-014-2185-5
Weber, A. M., Mawodza, T., Sarkar, B., & Menon, M. (2019). Assessment of potentially toxic trace element contamination in urban allotment soils and their uptake by onions: A preliminary case study from Sheffield, England. Ecotoxicology and Environmental Safety, 170, 156–165. https://doi.org/10.1016/j.ecoenv.2018.11.090
Wiseman, C. L., Zereini, F., & Püttmann, W. (2013). Traffic-related trace element fate and uptake by plants cultivated in roadside soils in Toronto, Canada. Science of the Total Environment, 442, 86–95. https://doi.org/10.1016/j.scitotenv.2012.10.051
Funding
The authors kindly thank Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, grant numbers 2014/05151-0, 2016/10060-9, 2020/00284-2, 2022/10619-7, 2022/00208-0, 2022/08618-2) and Conselho Nacional de Pesquisa grant 153204/2018-4. This study was funded in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brazil (CAPES)—Finance Code 001.
Author information
Authors and Affiliations
Contributions
Camila Neves Lange done analysis and manuscript writing; Bruna Moreira Freire done soil analysis and manuscript writing; Lucilena Rebelo Monteiro helped in statistical analysis and manuscript writing; Tatiane Araújo de Jesus done soil sampling, Roberta Albino dos Reis helped in soil amendments analysis; Gerson Nakazato and Renata Katsuko Takayama Kobayashi done microbiological analysis; Bruno Lemos Batista helped in manuscript writing. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lange, C.N., Freire, B.M., Monteiro, L.R. et al. Multiple potentially toxic elements in urban gardens from a Brazilian industrialized city. Environ Geochem Health 46, 36 (2024). https://doi.org/10.1007/s10653-023-01808-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10653-023-01808-0