Abstract
The use of hexachlorocyclohexanes (HCHs) in pesticides has been prohibited for decades in China. Since then, there have been urbanization and transformation of the functional areas of many sites, which were formerly involved in the HCH industry. However, it is possible that, unless properly managed, these sites may still contain HCH residues in the soil and thus pose a threat to the surrounding environment and the quality of groundwater. This study aimed to characterize soil residues in a typical site that was historically involved in HCH production in southern China, by analyzing the α-HCH, β-HCH, and γ-HCH contents of the soil. The results suggested that HCHs persist in the environment and can have long-term effects. It was found that α-HCH and β-HCH were present in many samples in concentrations that were comparable or higher than those specified by China’s Class 1 screening values. The distribution of residues was significantly correlated with the historical HCH production activities in the areas. The characteristic ratios of α-HCH/γ-HCH and β-HCH/(α + γ)-HCH at different soil depths were 1.4–3.7 and 0.21–1.04, respectively, which indicated the presence of significant localized residues of HCHs. The presence of HCHs in the soil suggested a downward migration, with concentrations rapidly decreasing in the upper layer soil (0–5 m), but a gradual increase in the deeper soil (5–14 m). HCHs were detected at depths exceeding 24 m, indicating heavy penetration. The proportions of γ-HCH and β-HCH changed with increasing soil depth, which was related to their relatively volatile and stable molecular structures, respectively. The results strongly suggested that there is widespread contamination of both soil and groundwater by HCHs even after decades. The likelihood of residual HCHs in the soil should therefore be taken into full consideration during urban planning to limit risks to human and environmental health.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
There has historically been large-scale use of organochlorine pesticides (OCPs) in agriculture due to their various benefits, including cost-effectiveness, efficiency, and their broad spectrum of toxicity to insects (Shen et al., 2021; Tang et al., 2023). However, their harm to both soil ecology and human health became increasingly clear due to their high levels of toxicity, persistence, and tendency to bioaccumulate (Chen et al., 2021a; Huang et al., 2021). Consequently, the Stockholm Convention listed certain OCPs, such as hexachlorocyclohexanes (HCHs) and dichlorodiphenyltrichloroethanes (DDTs), as class I carcinogenic organic compounds, and China prohibited their use in 1983 (Li et al., 2020; Sharkey et al., 2020; Yu et al., 2014).
However, recent studies have detected the presence of residues of these restricted pesticides, such as HCHs and DDTs, in various environments, including cities and agricultural areas (Khuman et al., 2022; Tesi et al., 2022; Yu et al., 2019). These findings suggest the persistence of OCPs in the environment, even after their use has been halted for decades (Khuman et al., 2022). Several recent studies have focused on the presence and distribution of OCPs in farmland (Kafaei et al., 2020), watersheds, river and lake sediments (Gandla et al., 2023), and dust and soil in urban areas (Aslam et al., 2021; Skrbic & Marinkovic, 2019). Although most of these studies focused on both traditional and emerging OCPs, the focus in environmental monitoring has remained on HCHs, which often account for 10–50% of OCPs and represent the highest risks to ecosystem and human health (Wang et al., 2023). There is still environmental contamination by OCPs, despite the gradual phasing out of their use (Vudamala et al., 2023; Zhang et al., 2023). Moreover, the distribution of OCPs in the environment is often dependent on the pollution source, which can be broadly categorized as mainly non-point sources generated by agriculture (Chen et al., 2021b; Cui et al., 2017). Furthermore, OCP contamination has been detected in areas very far from the original source of contamination (Tao et al., 2008). While their persistence in the environment may be largely due to their constant migration and transformation, it is possible that there still are neglected sources of OCP contamination in the environment (Siddique et al., 2023a, 2023b).
Historical sites of pesticide production are important sources of OCP contamination (Ma et al., 2020). Analysis of the pesticide residues in these sites is helpful for determining the characteristics of HCH persistence in the environment. Since their closure, many of these historical sites of pesticide production in China were gradually transformed into commercial and residential areas (Wang et al., 2023; Zhang et al., 2022). However, residues of OCPs often persist at these sites. Localized residual soil OCPs can be transferred through volatilization, diffusion, and mass flow processes, leading to pollution of the atmosphere and surface/groundwater, as well as bioaccumulation up the food chain (Derouiche et al., 2023; Siddique et al., 2023a). Therefore, pesticide residues at these historical sites of pesticide production pose risks to both current and future residents. There is a need to improve the understanding of soil pesticide residues in urban land planning and management.
The rapid urbanization of China has emphasized the need to develop land that was historically used for other purposes (Ai et al., 2022; Chen et al., 2022). However, pesticide contamination of land in China places limits on urban development, and not considering residual soil pesticide pollution can pose risks to ecological and human health (Yu et al., 2019, 2020). While pesticide residues tend to concentrate on the surface of the soil, they can also migrate to groundwater or deep soil layers (Vryzas et al., 2018).
Therefore, the aim of the present study was to characterize the occurrence and distribution of HCHs and underlying processes at sites of historical pesticide production in southern China. The objectives of the present study were to (1) characterize the soil HCH residues at sites of historical pesticide production and responses to the functional areas; (2) analyze the distribution, composition, and migration of HCHs to deep soil, surface water and groundwater; and (3) evaluate the presence of HCH residues in the soil of lands now used for urban development.
Materials and method
Study area
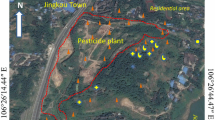
The present study examined a site of historical HCH production in Zhanjiang city in southern China. This site is located in the monsoon marine climate with a mean annual temperature of 22.7–23.5 ℃ and precipitation of 1395.5–1723.1 mm. The pesticide production plant covered an area of ± 40,000 m2 and produced HCHs in powder form from 1966 to 1979. Raw materials used at the plant were mainly benzene and chlorine gas, and the production process mainly included pesticide synthesis, distillation, drying, and packaging. The factory was spatially segregated into areas for pesticide synthesis, packaging, storage of finished-product, sewage-treatment, and offices (Fig. 1 and Fig. S1). The site is characterized by rice-type red soil divided according to formation into quaternary filling and alluvial soil layers (Fig. 2): (1) filling soil (0–1 m); (2) miscellaneous soil (0–2 m); (3) alluvial soil (0–12.0 m); (4) sand silt (0–13.0 m); and (5) clay (0–6.0 m). The site contains shallow groundwater at a depth of 5.35–8.36 m, which is concentrated in the silt layer and flows from northeast to southwest (Figs. 1 and 2). There are plans to develop the site into urban land in the future.
Sampling, pretreatment, and analysis
The present study adopted grid distribution and encryption distribution methods and established 79 soil-monitoring sites according to the functional layout of the production area. Of the 475 soil profile samples collected, 132, 242, 29, 43, and 29 were from the areas involved in synthesis, dry-packaging, sewage-treatment, storage of the finished-product, and the office area, respectively, and were collected from different depths. Moreover, the study characterized the hydrogeological features of the site and collected 10 groundwater and 4 surface water samples to explore patterns of HCH migration. Soil and water samples were collected in October 2020. Each 1,000-g soil sample was sealed in a brown glass bottle, frozen, and maintained in the dark during transport to the laboratory, after which it was stored at − 18 ℃ pending analysis. During analysis, soil samples were freeze-dried for 24 h and then ground and filtered through 1-mm filters for the analysis of α-HCH, β-HCH, and γ-HCH. The analysis process followed the protocol of Soil and Sediment-Determination of Organochlorine Pesticides-Gas Chromatography (HJ 921-2017). Briefly, HCHs in the soil were prepared by Soxhlet extraction. Soil samples (0.50 g) were extracted using 100 mL of dichloromethane for 24 h, after which the extract solutions were concentrated to < 2 mL via rotary evaporation at 40 °C, and transferred onto a solid-phase extraction column that had been conditioned by successive passages of 10 mL of dichloromethane. After the loading of the concentrated samples, the HCHs were eluted from the column using 10 mL of dichloromethane. The eluents were concentrated to 1 mL via rotary evaporation at 40 °C and stored at 4 ℃, pending analysis. The 1-L water samples were enriched using an SPE cartridge with the speed of 1 drop/s after which the material was eluted with 10 ml DCM. The eluate fractions were collected and concentrated to 1 mL, pending analysis.
Samples were analyzed by gas chromatography (GC, 7980, Agilent J & W Scientific, Folsom, CA, USA) coupled with ECD detector. One microliter of sample was injected directly onto the column (30 m × 0.32 mm × 0.25 μm, Agilent J & W Scientific) using the splitless mode. (The temperature of the injection port was 220 °C.) The carrier gas was high-purity nitrogen at a flow rate of 2 mL per min. The process was conducted in an oven as follows: the GC oven temperature was initially set to 100℃, increased to 220 °C at a rate of 15 °C/min, maintained for 5 min, and then increased to 260 °C at the same rate, holding for 20 min. The temperature of the detector was 280 °C.
Quality assessment/control (QA/QC)
Prior to sample processing, all glassware was calcined at 450 ℃ for 4 h, and 10% of samples (n = 50) were tested in parallel. The limits of detection (LODs) of α-HCH, β-HCH, and γ-HCH in the soil were 0.07 mg kg−1, 0.06 mg kg−1, and 0.06 mg kg−1, respectively, while the LODs in water were all 0.01 μg L−1. Their recoveries were 59.3–113.5%, 65.2–98.3%, and 78.0–135.7%, respectively. Table 1 summarizes the screening and intervention values of α-HCH, β-HCH, and γ-HCH (GB 36600-2018) in the soil.
Data processing
SPSS statistics 26 and Microsoft Office 2016 were used for data processing, while Origin 2023 and ArcGIS 10.6 were used to plot graphs. Data below the LOD were replaced by half the LOD. Significance was assumed at p < 0.05.
Results and discussion
Occurrence of soil residual HCHs
Table 1 summarizes the detection frequencies (DFs) and concentration of soil HCHs at the site. The DFs of α-HCH, β-HCH, and γ-HCH were 18.1%, 21.3%, and 16.2%, respectively, with concentrations of ND–70.80 mg kg−1 (mean of 0.45 mg kg−1), ND–98.50 mg kg−1 (0.44 mg kg−1), and ND–17.60 mg kg−1 (0.17 mg kg−1), respectively. The mean concentrations of α-HCH and β-HCH exceeded China’s Class 1 screening values (0.09 mg kg−1 for α-HCH and 0.32 mg kg−1 for β-HCH) in the soil (GB36600—2018, Soil environmental quality—risk control standard for soil contamination of development land, Ministry of Ecology and Environment of the People's Republic of China). The ranking according to the degree that they exceeded the screening value was α-HCH (17.26%) > β-HCH (5.68%) > γ-HCH (2.74%). Notably, there were many samples with extreme concentrations throughout the site; for instance, the maximum concentrations of α-HCH, β-HCH, and γ-HCH exceeded the screening values by factors of 787, 308, and 28.4, respectively, suggesting strong contamination. Additionally, the soil ∑3HCHs had a DF and concentration range of 24.6% and ND–186.90 mg kg−1 (mean of 1.05 mg kg−1), respectively, reflecting the persistence of HCHs in the site. Table S1 shows a comparison of the pesticide residue concentrations in elsewhere; the maximum value of HCHs reached 186.90 mg kg−1 in the site, which can be regarded as a moderate residue level. The coefficient of variation (CV) can reflect the spatial heterogeneity of HCHs in the soil, thus indicating possible anthropogenic effects (Khademi et al., 2019), classified as high CV ≥ 1, moderate CV < 1. The CV of HCHs reaching 6.24–11.5 (Table 1) indicated high levels of the effects of anthropogenic factors on their distribution.
Although there is currently reduced attention paid to HCHs in the urban environment, there is, nevertheless, evidence of HCH residues, indicating their continued relevance especially at sites contaminated with HCHs (Ma et al., 2020). The site investigated in the present study is surrounded by urban land, including schools, hospitals, and residences, and there are plans to develop the site itself into residential land. However, the presence of high residual soil HCHs at the site present limits to its development (Balazs et al., 2020). There is, therefore, an urgent need to reduce residual soil HCH prior to land-use planning to facilitate safe development in the transformation of functional areas during urban development (Wang et al., 2016; Yun et al., 2022). Moreover, the high levels of HCHs in the soil at the contamination source usually spread into the surrounding areas through diffusion. Thus, the investigation of the transfer of contamination from soil to water is of significance.
Characteristics of residual HCHs in soils in relation to areas with different historical functions
The concentration of residual HCHs in soils in relation to historical function
Figure 3 and Fig. S2 show the spatial distribution and relative proportions of α-HCH, β-HCH, and γ-HCH among the different functional areas. The results indicated that the concentrations of α-HCH exceeded those of β-HCH, and γ-HCH in most of the functional areas. The highest β-HCH residues were detected in the dry-packaging area, while only β-HCH was detected in the office area. The concentration of α-HCH in the finished-product area (1.50 mg kg−1) even exceeded the risk intervention value (0.9 mg kg−1). The maximum concentrations of α-HCH were in the dry-packaging and finished-product areas where they exceeded the screening value by factors of 787 and 504, respectively. The DF of α-HCH reached 37.9% in the sewage-treatment area, exceeding the values of the finished-product area (20.9%), the dry-packaging area (20.7%), the synthesis area (12.1%), and the office area (0%). β-HCH was detected in all functional areas, with its highest mean concentrations in the finished-product area (1.28 mg kg−1) and dry-packaging area (0.58 mg kg−1), and exceeded the screening value (0.32 mg kg−1), but not the controlled value (3.2 mg kg−1). The highest β-HCH concentrations were observed in the dry-packaging and finished-product areas, exceeding the screening value by factors of 307.8 and 145.6, respectively, indicating a high residue risk. In addition, the ranking for β-HCH concentrations exceeding the screening value was the sewage-treatment area (13.8%) > finished-product area (11.6%) > dry-packaging area (6.6%) > synthesis area (1.5%) > office area (0%). However, the ranking of the functional areas according to DFs of β-HCH was the finished-product area (30.2%) > sewage-treatment area (27.6%) > dry-packaging area (24.4%) > synthesis area (12.9%) > office area (6.9%). While γ-HCH was not detected in the office area, its highest mean concentrations in the finished-product area (0.33 mg kg−1) and the dry-packaging area (0.23 mg kg−1) were both within safety limits. Moreover, the ranking of the functional areas according to the DF of γ-HCH was the sewage-treatment area (27.6%) > finished-product area (25.6%) > dry-packaging area (19.0%) > synthesis area (7.6%) > office area (0%). The highest mean concentrations of ∑3HCHs were found in the finished-product area (3.11 mg kg−1) and dry-packaging area (1.33 mg kg−1), both of which exceeded the screening value (1.02 mg kg−1), while the sewage-treatment area (0.50 mg kg−1), synthesis area (0.18 mg kg−1), and office area (0.10 mg kg−1), were at safety levels. The ranking of the functional areas in terms of DF of HCHs was the sewage-treatment area (37.9%) > finished-product area (32.6%) > dry-packaging area (27.3%) > synthesis area (16.7%) > office area (6.9%).
The above results suggest that the soil HCH residues at the study site depended significantly on the source (Liu et al., 2022), seen especially in the high concentrations observed in the finished-product and dry-packaging areas. The maximum soil residual HCH was in a soil sample from a depth of 0–1 m in the dry-packaging area, consistent with the characteristics of most contaminated soils (Liu et al., 2022; Ma et al., 2020). The high concentration of HCHs in this area could possibly be attributed to the spilling and spreading of powdered HCH on the soil surface during the drying and packaging processes, followed by further migration and diffusion of HCH into the soil (Balazs et al., 2021; Ma et al., 2020). However, the higher DFs in the sewage-treatment areas reflected a difference compared with the high-concentration areas. Drainage in the area may show increases in the DFs and decreased concentrations. HCHs can be easily transferred to water systems, resulting in an increase in their DF values. However, the HCHs usually show > 70% removal in wastewater treatment systems after denitrification, resulting in lower concentrations in the sewage-treatment area (Cong et al., 2009).
Detection of HCHs at different depths
The maximum depths of α-HCH, β-HCH, and γ-HCH detection were all observed in the dry-packaging area, reaching 24 m, indicating significant migration and high penetration of the HCH pollution. Moreover, the maximum detection depth of both α-HCH and γ-HCH was found to be 19 m in the synthesis area, much deeper than the depth of 12.5 m observed for β-HCH. In the sewage-treatment area, the values were similar, between 14.5 and 16.5 m. However, the β-HCH concentrations were higher than those of α-HCH in the finished-product storage area but were not higher than those in the aquifer. Only β-HCH was detected in the office area, and its maximum detection depth was only 1 m. The ranking of the maximum detection depths of ∑3HCHs was the dry-packaging area (24 m) > synthesis area (19 m) > sewage-treatment area (16.5 m) > finished-product area (10.5 m) > office area (1 m). It was apparent that the residual HCHs gradually migrated and were retained in the deeper soil following the decommissioning of HCH production four decades ago, implying widespread and long-term contamination. The 24 m depth of detection in the drying-packaging area may have been related to the higher residue level, resulting in penetration. However, the finished-product area also had high HCH concentrations, which did not fully correspond with the detection depth, suggesting that other factors in the soil may have influenced migration, preventing deeper migration into the soil (Sun et al., 2020). Interestingly, while only β-HCH was detected in the office area (at 1 m depth), its concentration did not exceed the screening value. The different functional areas of the factory were very close to each other, and the results indicate that appropriate protection measures can effectively prevent HCH contamination. In the factory, the surface runoff was affected by the terrain, and it was difficult to migrate to the office area. There may have been only limited migration of HCH through dust and other means. After decades, it was apparent that the levels of HCH residues had decreased gradually to below the LOD in most of the sites (Table 2).
HCHs in surface water and groundwater
Table 3 shows the concentrations of HCHs in surface water and groundwater. HCHs were only detected in one of the four surface water samples. This sample was collected from the dry synthesis area (with higher soil residue), showing α-HCH (2.45 μg L−1) and β-HCH (0.73 μg L−1). There was no detection of HCH in the other three samples from the sedimentation and clear tanks, which indicated that HCH contamination may have been present for a long time in the surface water. It is more useful to explore the groundwater characteristics of HCHs. As shown in Table 3, in the groundwater, the DFs of α-HCH, β-HCH, and γ-HCH were 70%, 100%, and 40%, respectively, whereas their concentrations were ND–256 μg L−1 (mean of 29.9 μg L−1), ND–35.8 μg L−1 (5.57 μg L−1), and ND–26.7 μg L−1 (3.18 μg L−1), respectively. The concentrations of ∑3HCHs were 0.285-319 μg L−1 (mean of 38.6 μg L−1). This result confirms the migration of historical HCHs into groundwater and indicates that this process has more serious consequences than those in surface water. HCHs in groundwater may further influence the accumulation of HCHs in deep soil, causing more widespread effects. Furthermore, the high mobility of groundwater could enhance the spread of HCHs, causing technical obstacles and increasing the workload involved in the remediation of HCH-contaminated areas. This is not surprising if we take into account the fact that the levels of OCPs in the water column are subject to very rapid and significant changes over time and that water is not effective for conserving HCHs, given their poor water solubility, but functions rather as a means of transportation (Pardo et al., 2021).
Feature ratio characteristics
The study analyzed soil HCH residues and their migration and transformation at the study site to assess the risks posed by their presence. In general, industrial HCHs are composed of 60–70% α-HCH, 5–12% β-HCH, and 10–15% γ-HCH (Walker et al., 1999) with a α-HCH/γ-HCH ratio of approximately 4–7 and a β-HCH/ (α + γ)-HCH ratio of approximately 0.06–0.17. In the present study, the proportions of HCHs varied widely in the soil environment. In terms of the functional areas, the α-HCH/γ-HCH ratio (Fig. S3) ranged from 1.98 (sewage-treatment area) to 4.54 (synthesis area), while the β-HCH/(a + γ-HCH) ratio ranged from 0.29 (sewage-treatment area) to 0.77 (dry- packaging area), all of which are higher than 0.17, indicating a dominant historical influence (Li et al., 2015; Meng et al., 2013). In the synthesis area, the high α-HCH/γ-HCH ratio may be related to a continued input from the potential source.
The α-HCH/γ-HCH ratio in the deep soil was 1.4–3.7 (Fig. 4), less than the 4–7 range associated with industrial HCH production, indicating a historical source (Li et al., 2015; Meng et al., 2013). In the 0–1 and 1–2 m soil, the α-HCH/γ-HCH ratios were both 3.7, which is close to the characteristics of industrial HCHs, suggesting a historical source of HCHs in the topsoil. In addition, in the 3–7 and 9–24 m soil, the ratios were less than 1.6, reflecting a difference from an industrial source and suggesting the presence of different osmotic effects in the soil. Notably, the ranking of the different HCH monomers according to their water solubility was γ-HCH (7.30 mg L−1) > α-HCH (2.00 mg L−1) > β-HCH (0.24 mg L−1). The 5–9 m soil covers the aquifer, and in the lower layer of the aquifer (7–9 m), the ratio was found to be significantly increased, possibly due to difference in the water solubility of HCHs, causing changes in the migration patterns of HCHs. Moreover, characteristic ratio of β-HCH/(α + γ)-HCH (0.21–1.04) in all layers was much higher than 0.17, indicating that residual HCH was the dominant source of HCH at the site. The situation was significantly occurred in the 0–1 and 1–2 m soil layer (1.04 and 0.86). Willett et al. (1998) reported that the presence of HCH residues may be related to their historical sources as well as differences in volatility, water solubility, and biodegradability, affecting their breakdown and migration in the soil environment (Wang et al., 2006). Since the different isoforms of HCH have different stabilities and toxicities, they differ in terms of their ability to undergo microbial degradation in the soil environment (Garg et al., 2016). The four decades since the discontinuation of HCH production at the site would result in different degrees of attenuation and degradation, together with changes in the HCH compositions (Gao et al., 2008; Wang et al., 2015). The ranking of the different HCH monomers in terms of de-chlorination associated with degradation was γ-HCH > α-HCH > β-HCH. γ-HCH is the most susceptible to degradation by soil microorganisms and can be transformed to α-HCH by photochemical reactions, while α-HCH can be metabolically converted to β-HCH. The proportion of α-HCH (specific) in the site soil was significantly reduced compared with those of industrial HCHs (60–70%), which occurred concurrently with an increase in β-HCH. This result indicated that α-HCH was converted to β -HCH, with the latter characterized by lower biodegradability, water solubility, and volatility in the complex soil environment (Mrema et al., 2013; Yadav et al., 2015).
Migration and transformation of soil HCHs
Concentrations of soil HCHs in relation to depth
HCHs can not only degrade but also migrate to deeper soil layers. Figure 5 shows the vertical distribution of HCHs at the different soil depths at the historical HCH production site. The concentration of total soil HCHs as well as those of the three isomers rapid decreased with 0–5 m, with the lowest observation at 3–5 m, indicating significant downward penetration from the surface soil layers. In contrast, the concentrations of HCHs gradually increased with increasing soil depth between 5 and 14 m and decreased further at 14–24 m, although these changes were insignificant (p > 0.05). Surface backfill of construction waste occurred at the site, which could have contributed to the downward migration of soil HCHs through hardened surface cracks (Wycisk et al., 2013). Moreover, it is possible that reconstruction and demolition of equipment may have promoted the accumulation of soil HCHs, mainly in the 0–5 m soil layer. The depth of the aquifer at the study site is 5.35–8.36 m and is characterized by silty clay and a weak permeable floor layer. Therefore, HCHs would have accumulated in the bottom layer of the aquifer, resulting in a minor increase in the concentrations of HCHs in the soil layer below 7–9 m.
The results indicated an initial decrease in the total concentration of soil HCHs with increasing soil depth, followed by a gradual increase. The mean concentrations of α-HCH, β-HCH, γ-HCH, and ∑3HCHs in the 0–1 m soil layer reached 1.46, 1.92, 0.40, and 3.78 mg kg−1, respectively, significantly exceeding that below 3 m (< 0.4 mg kg−1). The significant downward migration of HCHs observed at the site may have been possibly due to the wet weather in southern China. The soil structure is an important factor that controls the combination of soil organic matter and water infiltration with HCHs, allowing co-migration into the deeper soil (Lupi et al., 2019; Zhang et al., 2009). The typical geological profile characteristics of the site from top to bottom are miscellaneous fill or plain fill (0–2 m); sandy silt, silt, or sand layer (2–12 m), and clay layer (> 12 m). The water permeability of soil tends to gradually decrease with increasing soil depth. Moreover, shallow groundwater at the site is mainly present in the silt soil layer, with groundwater at a depth of 5.35–8.36 m. The migration of HCHs to the groundwater resulted in lateral migration with groundwater and consequent increased concentrations of HCHs in the gas zone and aquifer of the local block.
Compositions of soil HCHs in relation to depth
Figure 5 shows the vertical distribution of soil HCHs at different depths. The ranking of the HCH monomers according to concentration at a soil depth of 0–24 m was α-HCH (42.5%; mean of 34.9–58.3%) > β-HCH (41.7%; mean 17.6–50.9%) > γ-HCH: (15.8%; mean 10.6–33.7%). The proportions of the different HCH isoforms changed greatly compared to their original proportions, during which the percentage of β-HCH significantly increased from 5–12% to a 17.6–50.9% level. This result indicated that residual soil HCHs underwent significant environmental degradation and transformation (Ding et al., 2021; Liu et al., 2022). The concentrations of α-HCH, β-HCH, and γ-HCH decreased rapidly from the depth of 0–5 m, while increasing gradually with increasing soil depth up until 14 m, following which they gradually decreased at soil depths of 14–24 m. The HCHs showed a similar trend in terms of concentration while differing in their proportions. The higher permeability of γ-HCH and α-HCH compared to that of β-HCH may have led to the accumulation of β-HCH in the 0–7 m soil layer (> 29%). In contrast, the concentrations of α-HCH and γ-HCH in deep soil significantly exceeded that of β-HCH due to their stronger permeability and higher water solubility (Heeb et al., 2012). The differences between the upper and the deeper soil layers were also related to the permeability and degradability of HCHs (Ding et al., 2021). These differences in water permeability in addition to the ready conversion of γ-HCH to α-HCH under the action of microorganisms contributed to the accumulation of α-HCH in the 5–14 m soil layer (composed of silty clay and the aquifer) and its gradual decline at depths exceeding 14 m (Kranzioch-Seipel et al., 2016). The permeability of γ-HCH increased with increasing soil depth, and as microorganisms are not active under anoxic conditions (Langenhoff et al., 2013), the degradation of γ-HCH was significantly reduced as the soil depth increased. Thus, the proportion of γ-HCH increased gradually increased in the deep soil, accounting for 30–34% of HCHs in the 9–24 m soil layer compared with that of β-HCH.
The relative proportions of the different HCH monomers have been found to change according to the soil depth (Concha-Grana et al., 2006; Yang et al., 2005). The proportion of stable β-HCH generally decreased with increasing soil depth, whereas that of active γ-HCH gradually increased, while α-HCH showed relatively little change (Ma et al., 2020). This result implied that the different HCH monomers showed different capacities for migration due to the influences of soil characteristics in different soil layers (Liu et al., 2021). While all three HCHs monomers showed downward migration, they migrated at different rates (Hites & Venier, 2023). The faster downward migration of γ-HCH reflected its relatively rapid increase, whereas the gradual decline in β-HCH in deep soil could be attributed to its stability and resistance to migration with dissolution and penetration by water (Ashesh et al., 2022; Srivastava et al., 2022). Overall, the permeabilities of γ -HCH and α-HCH exceeded that of β-HCH, resulting in increasing and decreasing proportions of β-HCH with increasing soil depth, respectively.
Environmental impact and management
Re-use of historical sites is a common practice in urban land management. Contaminants at these sites can continue to pose risks to human and ecological health due to the lack of prolonged management and the historical production processes conducted at a site. Therefore, there is an urgent need for an assessment of risks posed by soil contaminants at sites before re-development during urbanization. Despite the passage of four decades since the cessation of pesticide production, there remains a need to investigate and analyze historical activities at a site. The waste generated by pesticide industries remains an important source of urban OCP pollution. The presence of soil OCP residues can limit the land that can be used for urbanization. The results of the present study indicated the presence of significant HCH soil residues at the site that were closely related to historical activities. Although the HCH concentrations decreased rapidly with increasing soil depth (0-5 m), they were found to gradually increase in the deeper soil layers, with high detection observed at a depth of 24 m, indicating serious pollution of the deep soils. Moreover, the groundwater showed higher levels of HCH pollution than surface water, augmenting the pollution risk of the deeper soil layers. Therefore, the possible risks posed by residue HCHs to the soil ecology and human health should be considered during urbanization. Since pesticide residues have wide-ranging effects on groundwater, sites with HCH contamination should undergo comprehensive evaluation and remediation.
Conclusions
The results of the present study indicate that the soil at the study site remains HCH contamination. Additionally, variations in HCH concentrations across different functional areas of the site suggest that the contamination originated from historical sources. The ranking of the different soil HCH species according to mean concentration was α-HCH (0.45 mg kg−1) > β-HCH (0.44 mg kg−1) > γ-HCH (0.17 mg kg−1), with α-HCH and β-HCH exceeding the Chinese Class 1 screening levels, indicating the presence of serious residual pollution. The residual soil HCH concentrations of the site differed according to the functional areas of the site, with the highest residues observed in the finished-product and dry-packaging areas. We confirmed the presence of obvious migration and transformation trends of the HCHs in the soil at the site, even four decades after the cessation of HCH production. The γ-HCH and α-HCH forms were transformed into the more stable β-HCH and were found at higher levels in the surface soil rather than the deep soil. HCHs in the surface soil readily migrated into the aquifer, even as far as a depth of 24 m, indicative of widespread and serious pollution. However, in different layers of soil, the HCH isoforms were found to be distributed according to their specific composition, mainly due to their different water solubilities and stabilities. The study revealed that HCH production sites are potential sources of soil HCH pollution even after four decades and that HCH can migrate to deeper soils and groundwaters, with reduced degradation with increasing depth, thus causing a more widespread harm. Thus, despite the present reduced attention on HCHs in urban environments, these chemicals still pose a significant risk when historical production sites are transformed and redeveloped during urbanization.
References
Ai, Q., Zhang, P., Gao, L., Zhou, X., Liu, Y., Huang, D., Qiao, L., Weng, J., & Zheng, M. (2022). Air-soil exchange of and risks posed by short- and medium-chain chlorinated paraffins: Case study in a contaminated area in China. Chemosphere, 297, 134230. https://doi.org/10.1016/j.chemosphere.2022.134230
Ashesh, A., Singh, S., Devi, N. L., & Yadav, I. C. (2022). Organochlorine pesticides in multi-environmental matrices of India: A comprehensive review on characteristics, occurrence, and analytical methods. Microchemical Journal, 177, 107306. https://doi.org/10.1016/j.microc.2022.107306
Aslam, I., Mumtaz, M., Qadir, A., Jamil, N., Baqar, M., Mahmood, A., Ahmad, S. R., & Zhang, G. (2021). Organochlorine pesticides (OCPs) in air-conditioner filter dust of indoor urban setting: Implication for health risk in a developing country. Indoor Air, 31, 807–817. https://doi.org/10.1111/ina.12772
Balazs, H. E., Schmid, C. A. O., Cruzeiro, C., Podar, D., Szatmari, P.-M., Buegger, F., Hufnagel, G., Radl, V., & Schroeder, P. (2021). Post-reclamation microbial diversity and functions in hexachlorocyclohexane (HCH) contaminated soil in relation to spontaneous HCH tolerant vegetation. Science of the Total Environment, 767, 144653. https://doi.org/10.1016/j.scitotenv.2020.144653
Balazs, H. E., Schmid, C. A. O., Podar, D., Hufnagel, G., Radl, V., & Schroeder, P. (2020). Development of microbial communities in organochlorine pesticide contaminated soil: A post-reclamation perspective. Applied Soil Ecology, 150, 103467. https://doi.org/10.1016/j.apsoil.2019.103467
Chen, L., Qian, Y., Jia, Q., Weng, R., Zhang, X., Li, Y., & Qiu, J. (2022). A large geographic-scale characterization of organochlorine pesticides (OCPs) in surface sediments and multiple aquatic foods of inland freshwater aquaculture ponds in China: Co-occurrence, source and risk assessment. Environmental Pollution, 308, 119716. https://doi.org/10.1016/j.envpol.2022.119716
Chen, W., Zeng, F., Liu, W., Bu, J., Hu, G., Xie, S., Yao, H., Zhou, H., Qi, S., & Huang, H. (2021a). Organochlorine pesticides in karst soil: Levels, distribution, and source diagnosis. International Journal of Environmental Research and Public Health. https://doi.org/10.3390/ijerph182111589
Chen, Y. P., Zhao, Y., Zhao, M. M., Wu, J. H., & Wang, K. B. (2021b). Potential health risk assessment of HFRs, PCBs, and OCPs in the Yellow River basin. Environmental Pollution, 275, 116648. https://doi.org/10.1016/j.envpol.2021.116648
Concha-Grana, E., Turnes-Carou, M. I., Muniategui-Lorenzo, S., Lopez-Mahia, P., Prada-Rodriguez, D., & Fernandez-Fernandez, E. (2006). Evaluation of HCH isomers and metabolites in soils, leachates, river water and sediments of a highly contaminated area. Chemosphere, 64, 588–595. https://doi.org/10.1016/j.chemosphere.2005.11.011
Cong, X., Zhu, S., Xue, N., & Li, F. (2009). Vertical distribution of pollutants in soils of a former organochlorine pesticide manufacturing field. Research of Environmental Sciences, 22, 351–355. (Chinese).
Cui, L., Wei, L., & Wang, J. (2017). Residues of organochlorine pesticides in surface water of a megacity in central China: Seasonal-spatial distribution and fate in Wuhan. Environmental Science and Pollution Research, 24, 1975–1986. https://doi.org/10.1007/s11356-016-7956-7
Derouiche, A., Achour, A., & Driss, M. R. (2023). Organochlorine pesticides and polychlorinated biphenyls in raw bovine milk from various dairy farms in Beja, Tunisia: Contamination status, dietary intake, and health risk assessment for the consumers. Environmental Science and Pollution Research International, 30, 65427–65439. https://doi.org/10.1007/s11356-023-26976-5
Ding, Y., Li, L., Wania, F., Huang, H., Zhang, Y., Peng, B., Chen, Y., & Qi, S. (2021). Do dissipation and transformation of gamma-HCH and p, p’-DDT in soil respond to a proxy for climate change? Insights from a field study on the eastern Tibetan Plateau. Environmental Pollution, 278, 116824. https://doi.org/10.1016/j.envpol.2021.116824
Gandla, V., Chiluka, M., Gupta, H., Sinha, S. N., & Chakraborty, P. (2023). Sediment-water partitioning and risk assessment of organochlorine pesticides along the urban, peri-urban and rural transects of Krishna River Basin, Peninsular India. Science of the Total Environment, 874, 162360. https://doi.org/10.1016/j.scitotenv.2023.162360
Gao, F., Jia, J., & Wang, X. (2008). Occurrence and ordination of dichlorodiphenyltrichloroethane and hexachlorocyclohexane in agricultural soils from Guangzhou, China. Archives of Environmental Contamination and Toxicology, 54, 155–166. https://doi.org/10.1007/s00244-007-9023-3
Garg, N., Lata, P., Jit, S., Sangwan, N., Singh, A. K., Dwivedi, V., Niharika, N., Kaur, J., Saxena, A., Dua, A., Nayyar, N., Kohli, P., Geueke, B., Kunz, P., Rentsch, D., Holliger, C., Kohler, H.-P.E., & Lal, R. (2016). Laboratory and field scale bioremediation of hexachlorocyclohexane (HCH) contaminated soils by means of bioaugmentation and biostimulation. Biodegradation, 27, 179–193. https://doi.org/10.1007/s10532-016-9765-6
Heeb, N. V., Zindel, D., Geueke, B., Kohler, H.-P.E., & Lienemann, P. (2012). Biotransformation of hexabromocyclododecanes (HBCDs) with LinB-An HCH-converting bacterial enzyme. Environmental Science & Technology, 46, 6566–6574. https://doi.org/10.1021/es2046487
Hites, R. A., & Venier, M. (2023). Good news: Some insecticides have been virtually eliminated in air near the great lakes. Environmental Science & Technology. https://doi.org/10.1021/acs.est.2c08318
Huang, H., Liu, H., Xiong, S., Zeng, F., Bu, J., Zhang, B., Liu, W., Zhou, H., Qi, S., Xu, L., & Chen, W. (2021). Rapid transport of organochlorine pesticides (OCPs) in multimedia environment from karst area. Science of the Total Environment, 775, 145698. https://doi.org/10.1016/j.scitotenv.2021.145698
Kafaei, R., Arfaeinia, H., Savari, A., Mahmoodi, M., Rezaei, M., Rayani, M., Sorial, G. A., Fattahi, N., & Ramavandi, B. (2020). Organochlorine pesticides contamination in agricultural soils of southern Iran. Chemosphere, 240, 124983. https://doi.org/10.1016/j.chemosphere.2019.124983
Khademi, H., Gabarrón, M., Abbaspour, A., Martinez-Martínez, S., Faz, A., & Acosta, J. A. (2019). Environmental impact assessment of industrial activities on heavy metals distribution in street dust and soil. Chemosphere, 217, 695–705. https://doi.org/10.1016/j.chemosphere.2018.11.045
Khuman, S. N., Park, M. K., Kim, H. J., Hwang, S. M., Lee, C. H., & Choi, S. D. (2022). Organochlorine pesticides in the urban, suburban, agricultural, and industrial soil in South Korea after three decades of ban: Spatial distribution, sources, time trend, and implicated risks. Environmental Pollution, 311, 119938. https://doi.org/10.1016/j.envpol.2022.119938
Kranzioch-Seipel, I., Beckert, U., Shen, C., Yin, D., & Tiehm, A. (2016). Microbial dechlorination of HCB, PCP, PCB180, HCH and PCE in a Yangtze three gorges reservoir enrichment culture China. Environmental Earth Sciences. https://doi.org/10.1007/s12665-016-5653-y
Langenhoff, A. A. M., Staps, S. J. M., Pijls, C., & Rijnaarts, H. H. M. (2013). Stimulation of Hexachlorocyclohexane (HCH) biodegradation in a full scale in situ bioscreen. Environmental Science & Technology, 47, 11182–11188. https://doi.org/10.1021/es4024833
Li, L., Zhang, Y., Wang, J., Lu, S., Cao, Y., Tang, C., Yan, Z., & Zheng, L. (2020). History traces of HCHs and DDTs by groundwater dating and their behaviours and ecological risk in northeast China. Chemosphere, 257, 127212. https://doi.org/10.1016/j.chemosphere.2020.127212
Li, Y., Zhang, J., Miao, W., Wang, H., & Wei, M. (2015). Disposal of historically contaminated soil in the cement industry and the evaluation of environmental performance. Chemosphere, 134, 279–285. https://doi.org/10.1016/j.chemosphere.2015.04.048
Liu, X., Bonhomme, J., Merbach, I., Kuemmel, S., & Richnow, H. H. (2021). Uptake of alpha-HCH by wheat from the gas phase and translocation to soil analyzed by a stable carbon isotope labeling experiment. Chemosphere, 264, 128489. https://doi.org/10.1016/j.chemosphere.2020.128489
Liu, X., Yang, A., Kuemmel, S., & Richnow, H. H. (2022). Uptake and Metabolization of HCH isomers in trees examined over an annual growth period by compound-specific isotope analysis and enantiomer fractionation. Environmental Science & Technology. https://doi.org/10.1021/acs.est.2c02697
Lupi, L., Bedmar, F., Alberto Wunderlin, D., & Beatriz Miglioranza, K. S. (2019). Levels of organochlorine pesticides in soils, mesofauna and streamwater from an agricultural watershed in Argentina. Environmental Earth Sciences. https://doi.org/10.1007/s12665-019-8579-3
Ma, Y., Yun, X., Ruan, Z., Lu, C., Shi, Y., Qin, Q., Men, Z., Zou, D., Du, X., Xing, B., & Xie, Y. (2020). Review of hexachlorocyclohexane (HCH) and dichlorodiphenyltrichloroethane (DDT) contamination in Chinese soils. Science of the Total Environment, 749, 141212. https://doi.org/10.1016/j.scitotenv.2020.141212
Meng, J., Wang, T., Wang, P., Giesy, J. P., & Lu, Y. (2013). Perfluorinated compounds and organochlorine pesticides in soils around Huaihe river: A heavily contaminated watershed in Central China. Environmental Science and Pollution Research, 20, 3965–3974. https://doi.org/10.1007/s11356-012-1338-6
Mrema, E. J., Rubino, F. M., Brambilla, G., Moretto, A., Tsatsakis, A. M., & Colosio, C. (2013). Persistent organochlorinated pesticides and mechanisms of their toxicity. Toxicology, 307, 74–88. https://doi.org/10.1016/j.tox.2012.11.015
Pardo, A., Rodríguez-Casals, C., Santolaria, Z., Arruebo, T., Urieta, J. S., & Lanaja, F. J. (2021). HCH isomers change in Pyrenean freshwater ecosystems triggered by the transfer operation of Bailín landfill: The case of Sabocos tarn. Science of the Total Environment, 787, 147655. https://doi.org/10.1016/j.scitotenv.2021.147655
Sharkey, M., Harrad, S., Abdallah, M. A. E., Drage, D. S., & Berresheim, H. (2020). Phasing-out of legacy brominated flame retardants: The UNEP Stockholm convention and other legislative action worldwide. Environment International, 144, 106041. https://doi.org/10.1016/j.envint.2020.106041
Shen, B., Wu, J., Zhan, S., & Jin, M. (2021). Residues of organochlorine pesticides (OCPs) and polycyclic aromatic hydrocarbons (PAHs) in waters of the Ili-Balkhash Basin, arid central Asia: Concentrations and risk assessment. Chemosphere, 273, 129705. https://doi.org/10.1016/j.chemosphere.2021.129705
Siddique, S., Chaudhry, M. N., Ahmad, S. R., Javed, R., Nazir, R., Mubarak, S., Alghamdi, H. A., & Mahmood, A. (2023a). Comprehensive GIS based risk surveillance of organochlorine pesticides (OCPs) in edible fish species of River Chenab. Pakistan. Science of the Total Environment, 871, 162084. https://doi.org/10.1016/j.scitotenv.2023.162084
Siddique, S., Chaudhry, M. N., Ahmad, S. R., Nazir, R., Zhao, Z., Javed, R., Alghamdi, H. A., & Mahmood, A. (2023b). Ecological and human health hazards; integrated risk assessment of organochlorine pesticides (OCPs) from the Chenab River, Pakistan. The Science of the Total Environment, 882, 163504. https://doi.org/10.1016/j.scitotenv.2023.163504
Skrbic, B. D., & Marinkovic, V. (2019). Occurrence, seasonal variety of organochlorine compounds in street dust of Novi Sad, Serbia, and its implication for risk assessment. Science of the Total Environment, 662, 895–902. https://doi.org/10.1016/j.scitotenv.2019.01.133
Srivastava, V., Puri, M., Srivastava, T., Nidheesh, P. V., & Kumar, M. S. (2022). Integrated soil washing and bioreactor systems for the treatment of hexachlorocyclohexane contaminated soil: A review on enhanced degradation mechanisms, and factors affecting soil washing and bioreactor performances. Environmental Research, 208, 112752. https://doi.org/10.1016/j.envres.2022.112752
Sun, Y., Chang, X., Zhao, L., Zhou, B., Weng, L., & Li, Y. (2020). Comparative study on the pollution status of organochlorine pesticides (OCPs) and bacterial community diversity and structure between plastic shed and open-field soils from northern China. Science of the Total Environment, 741, 13962010.
Tang, C., Chen, Z., Huang, Y., Solyanikova, I. P., Mohan, S. V., Chen, H., & Wu, Y. (2023). Occurrence and potential harms of organochlorine pesticides (OCPs) in environment and their removal by periphyton. Critical Reviews in Environmental Science and Technology. https://doi.org/10.1080/10643389.2023.2196226
Tao, S., Liu, W., Li, Y., Yang, Y., Zuo, Q., Li, B., & Cao, J. (2008). Organochlorine pesticides contaminated surface soil as reemission source in the Haihe Plain, China. Environmental Science & Technology, 42, 8395–8400. https://doi.org/10.1021/es8019676
Tesi, G. O., Obi-Iyeke, G. E., Ossai, J. C., Ogbuta, A. A., Ogbara, E. F., Olorunfemi, D. I., & Agbozu, I. E. (2022). Human exposure to organochlorine pesticides in vegetables from major cities in south-south Nigeria. Chemosphere, 303, 135296. https://doi.org/10.1016/j.chemosphere.2022.135296
Vryzas, Z. (2018). Pesticide fate in soil-sediment-water environment in relation to contamination preventing actions. Current Opinion in Environmental Science & Health, 4, 5–9. https://doi.org/10.1016/j.coesh.2018.03.001
Vudamala, K., Chakraborty, P., Chatragadda, R., Tiwari, A. K., & Qureshi, A. (2023). Distribution of organochlorine pesticides in surface and deep waters of the Southern Indian ocean and coastal Antarctic waters. Environmental Pollution, 321, 12120610.
Walker, K., Vallero, D. A., & Lewis, R. G. (1999). Factors influencing the distribution of lindane and other hexachlorocyclohexanes in the environment. Environmental Science & Technology, 33, 4373–4378. https://doi.org/10.1021/es990647n
Wang, B., Wu, C., Liu, W., Teng, Y., Luo, Y., Christie, P., & Guo, D. (2016). Levels and patterns of organochlorine pesticides in agricultural soils in an area of extensive historical cotton cultivation in Henan province. China. Environmental Science and Pollution Research, 23(7), 6680–6689. https://doi.org/10.1007/s11356-015-5864-x
Wang, L., Zhang, Z. F., Liu, L. Y., Zhu, F. J., & Ma, W. L. (2023). National-scale monitoring of historic used organochlorine pesticides (OCPs) and current used pesticides (CUPs) in Chinese surface soil: Old topic and new story. Journal of Hazardous Materials, 443, 13028510.
Wang, X., Piao, X., Chen, J., Hu, J., Xu, F., & Tao, S. (2006). Organochlorine pesticides in soil profiles from Tianjin, China. Chemosphere, 64, 1514–1520. https://doi.org/10.1016/j.chemosphere.2005.12.052
Wang, Y., Li, Q., Wang, S., Wang, Y., Luo, C., Li, J., & Zhang, G. (2015). Seasonal and diurnal variations of atmospheric PAHs and OCPs in a suburban paddy field, South China: Impacts of meteorological parameters and sources. Atmospheric Environment, 112, 208–215. https://doi.org/10.1016/j.atmosenv.2015.04.047
Willett, K. L., Ulrich, E. M., & Hites, R. A. (1998). Differential toxicity and environmental fates of hexachlorocyclohexane isomers. Environmental Science & Technology, 32, 2197–2207. https://doi.org/10.1021/es9708530
Wycisk, P., Stollberg, R., Neumann, C., Gossel, W., Weiss, H., & Weber, R. (2013). Integrated methodology for assessing the HCH groundwater pollution at the multi-source contaminated mega-site Bitterfeld/Wolfen. Environmental Science and Pollution Research, 20(4), 1907–1917. https://doi.org/10.1007/s11356-012-0963-4
Yadav, I. C., Devi, N. L., Syed, J. H., Cheng, Z., Li, J., Zhang, G., & Jones, K. C. (2015). Current status of persistent organic pesticides residues in air, water, and soil, and their possible effect on neighboring countries: A comprehensive review of India. Science of the Total Environment, 511, 123–137. https://doi.org/10.1016/j.scitotenv.2014.12.041
Yang, R. Q., Jiang, G. B., Zhou, Q. F., Yuan, C. G., & Shi, J. B. (2005). Occurrence and distribution of organochlorine pesticides (HCH and DDT) in sediments collected from East China Sea. Environment International, 31, 799–804. https://doi.org/10.1016/j.envint.2005.05.027
Yu, H., Liu, Y., Shu, X., Ma, L., & Pan, Y. (2020). Assessment of the spatial distribution of organochlorine pesticides (OCPs) and polychlorinated biphenyls (PCBs) in urban soil of China. Chemosphere, 243, 12539210.
Yu, S. Y., Liu, W. J., Xu, Y. S., Zhao, Y. Z., Cai, C. Y., Liu, Y., Wang, X., Xiong, G. N., Tao, S., & Liu, W. X. (2019). Organochlorine pesticides in ambient air from the littoral cities of northern China: Spatial distribution, seasonal variation, source apportionment and cancer risk assessment. Science of the Total Environment, 652, 163–176. https://doi.org/10.1016/j.scitotenv.2018.10.230
Yu, Y., Li, Y., Shen, Z., Yang, Z., Mo, L., Kong, Y., & Lou, I. (2014). Occurrence and possible sources of organochlorine pesticides (OCPs) and polychlorinated biphenyls (PCBs) along the Chao River, China. Chemosphere, 114, 136–143. https://doi.org/10.1016/j.chemosphere.2014.03.095
Yun, S. M., Yoon, J.-K., Kim, J.-I., Kim, I. J., Kim, H. K., Chung, H. M., Kim, D. J., & Noh, H.-J. (2022). Evaluation of residual level and distribution characteristics of organochlorine pesticides in agricultural soils in South Korea. Environmental Science and Pollution Research, 29, 46003–46017. https://doi.org/10.1007/s11356-022-18858-z
Zhang, H., Luo, Y., & Li, Q. (2009). Burden and depth distribution of organochlorine pesticides in the soil profiles of Yangtze river delta region, China: Implication for sources and vertical transportation. Geoderma, 153, 69–75. https://doi.org/10.1016/j.geoderma.2009.07.016
Zhang, M., Wang, L., Li, X., Song, L., Luo, D., Li, Q., Wang, Y., Wan, Z., & Mei, S. (2023). Individual and mixtures of polychlorinated biphenyls and organochlorine pesticides exposure in relation to metabolic syndrome among Chinese adults. Science of the Total Environment, 877, 16293510.
Zhang, S., Zhao, W., Yang, C., Li, Y., Liu, M., Meng, X. Z., & Cai, M. (2022). Assessment of currently used organochlorine pesticides in surface water and sediments in Xiangjiang river, a drinking water source in China: Occurrence and distribution characteristics under flood events. Environmental Pollution, 304, 11913310.
Acknowledgements
This research was supported by the National Natural Science Foundation of China through Grants 41877516.
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Contributions
CX did conceptualization, methodology, software, data curation, writing—original draft preparation, formal analysis, and validation; YC done software, writing—review & editing; RW performed formal analysis and validation; GY and JW were involved in investigation, experiment, data curation; YL, DL, YD, YZ and QZ investigated and experimented the study; LW contributed to data curation, review & editing, fund acquisition; SZ did conceptualization, methodology, writing—original draft preparation, writing—review & editing, supervision, formal analysis, and validation. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xu, C., Cai, Y., Wang, R. et al. Reduced attention on restricted organochlorine pesticides, whereas still noteworthy of the impact on the deep soil and groundwater: a historical site study in southern China. Environ Geochem Health 45, 8787–8802 (2023). https://doi.org/10.1007/s10653-023-01761-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-023-01761-y