Abstract
Ulungur Lake is the largest lake in northern Xinjiang and undertakes important aquatic tasks. It is the No. 1 fishing ground in northern Xinjiang, and the problem of persistent organic pollution in the water has received much attention. However, there are few studies on phthalate esters (PAEs) in the water of Ulungur Lake. Understanding the pollution levels, distribution characteristics and sources of PAEs is of great significance for the protection and prevention of water. Fifteen sampling sites are established in Ulungur Lake to collect water samples during flood and dry periods, then seventeen PAEs are extracted from the water samples and purified by liquid–liquid extraction—solid-phase purification. Gas chromatography–mass spectrometry is used to detect the pollution levels and distribution characteristics of the 17 PAEs and analyse their sources. Results show that the concentrations of PAEs in the dry and flood periods are 0.451–9.97 µg/L and 0.0490–6.38 µg/L, respectively. The concentration of ∑PAEs with time is characterised by the dry period > the flood period. The change in flow is the main reason for the diverse concentration distributions of PAEs in different periods. The concentration of ΣPAEs in the dry period is much lower on the side near the lake entrance of the Ulungur River and Irtysh River. In the dry period, PAEs mainly come from chemical production and the use of cosmetics and personal care products; in the flood period, they mainly come from chemical production. River input and atmospheric sedimentation are the main sources of PAEs in the lake.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Phthalate esters (PAEs), also known as phthalates, are amongst the compounds of the product of the esterification reaction between phthalic anhydride and alcohol (Farahani et al., 2017). They are often used as raw materials for pesticide carriers, insect repellents, cosmetics, fragrances, lubricants and detergents (Mohammadi et al., 2022). As plasticisers (Huang et al., 2021), they can transform hard plastics into elastic plastics, so they are widely used in the production of synthetic polyvinyl chloride, polypropylene, polyethylene, polystyrene and other chemicals, which account for about 80% of their total output (Kumar et al., 2022). PAEs are also commonly used as raw materials for the production of personal care products, such as cosmetics and nail polish, and daily necessities, such as laundry detergents, food contact materials and plastic bags, etc. (Huang et al., 2017; Saab et al., 2022; Silano et al., 2019). They are different from other substances when used as plasticisers; when synthesising polymers, PAEs and other polymers combine not chemically but physically, they are combined by molecular force and polymerisation force; they will be detached from plastics with changes in time and the environment such as temperature and humidity, and will be released into the atmosphere, water and surface, causing environmental pollution and even harming human health (Babu-Rajendran et al., 2018; Cao et al., 2022; Lu et al., 2018).
Recent studies have shown that PAEs are widely present in various media, such as atmosphere, water, sediment, soil and dust (Langova et al., 2020; Xing et al., 2022). PAEs in the atmosphere enter the water and ground through atmospheric deposition and precipitation, and PAEs on the ground are washed by rainwater and migrated by water flow (Jebara et al., 2021). PAEs are an environmental endocrine disruptor amongst persistent organic pollutants, and exert the ‘three causes’ effects of teratogenic, carcinogenic and mutagenic. They interfere with endocrine by playing a role similar to oestrogen in humans and animals, and reduction in the amount of semen and sperm in men can even lead to testicular cancer, which is an important factor in male reproductive problems (Wang et al., 2020). In cosmetics, nail polish and the aromatic ingredients of many cosmetics also contain PAEs (Martin-Pozo et al., 2021). This substance in cosmetics will enter the body through the respiratory system and skin (Zhao et al., 2022). If used excessively, it will increase the risk of breast cancer in women and even jeopardise the reproductive systems of their baby boys (Busgang et al., 2022; Ma et al., 2022). PAEs can further affect the human nervous system and cause gastrointestinal reactions (Porras et al., 2020). In view of the EPA, dimethyl phthalate (DMP), diethyl phthalate (DEP), n-dibutyl phthalate (DBP), butyl benzyl phthalate (BBP), diethylhexyl phthalate (DEHP) and di-n-octyl phthalate (DNOP) are listed as priority pollutants, and DMP, DBP and DNOP are included in the blacklist of priority control pollutants in China.
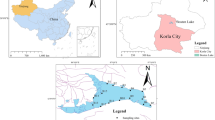
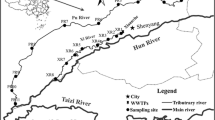
Ulungur Lake (Ji et al., 2022; Ni et al., 2022) is located in the north of the Junggar Basin and the northwest of Fuhai County. It is the largest fishery base in Northern Xinjiang, accounting for more than 1/3 of the total fish production in Xinjiang. The lake area is rich in water and plankton. The north bank is a cliff, only 2.1 km away from the Irtysh River. Ulungur Lake is the only place that must pass between Altay City and Karamay City. The lake primarily consists of Jili Lake (Xiaohaizi) with an area of 165 square km2 and Ulungur Lake (Dahaizi) with an area of 730 km2. The Ulungur River, which originates from the Altai Mountains, is the main water source of Ulungur Lake. To prevent the deterioration of the lake environment, measures of water diversion from the Irtysh River are taken to increase the flow of the lake, and the lake water can be desalinated after its accelerated circulation.
The vast water area of Ulungur Lake provides convenient conditions for local people's water transportation. The good natural conditions and landscapes also provide people with places for tourism, rest and recuperation and necessary places for scientific research and teaching. Nevertheless, frequent human activities and the production of surrounding enterprises will aggravate the deterioration of water environments (Gao et al., 2020). So far, there is no literature on phthalate pollution in Ulungur Lake, so evaluating the pollution characteristics and sources of PAEs in Ulungur Lake is necessary. This study aims to explore the pollution characteristics and composition of PAEs in Ulungur Lake by using the data from September 2021 (flood period) and May 2022 (dry period), investigate the temporal and spatial distribution characteristics and analyse the source of PAEs.
Methods and materials
Sample collection
Fifteen sampling sites were established in Ulungur Lake in September 2021 (flood period) and May 2022 (dry period), (Fig. 1). Relevant information of the sampling sites in Ulungur Lake is shown in Table 1 in Supplementary Material. The sampling sites on the same latitude and longitude did not overlap because the layout of the sampling sites covered the trunk and branches of the lake and combined the densely populated areas and industrial development. A stainless-steel water sample collector was used to collect surface water at a depth of less than 50 cm. Water depth and water temperature were recorded. The sample was placed into brown glass bottles that were washed with ultrapure water and methanol. About 1 L of surface water sample was collected at each site. The sample was transported back to the laboratory, stored in the dark at 4 °C and filtered with a 0.45 μm filter within 48 h to remove particulate matter and other solid impurities in the water. Extraction was completed within 72 h, and detection analysis was completed within 40 days. During sampling, a handheld GPS was used for positioning.
Materials and reagents
PAE standards, including DMP, DEP, DAP, DIBP, DBP, DMEP, BMPP, DEEP, DPP, DHXP, BBP, DBEP, DCHP, DEHP, DPHP, DNOP, DINP and DNP, were obtained from AccuStandard (USA). Benzyl benzoate (BB) was also generated from AccuStandard (USA). Hexane and dichloromethane were GC grade and were acquired from Thermo Fisher (USA). Oasis HLB 6 cc Vac Cartridge (500 mg of sorbent per cartridge, 60 µm, 30/pk) was purchased from Waters. Anhydrous sodium sulphate (analytical grade, Sinopharm Chemical Reagent Co., Ltd.) was baked at 800 °C for 6 h before use.
Sample pretreatment
In brief, 200 mL of water sample was filtered through a 0.45 μm filter to remove impurities and placed in a 250 mL separatory funnel that was rinsed with ultrapure water, hexane and water samples. The water sample was added with 200 μL of BB, followed by 10 mL of hexane: dichloromethane (1:1, V:V), in the separatory funnel for better extraction. The concentration of BB was 1 μg/mL. For liquid–liquid extraction, the sample was shaken for 15 min and stood for stratification. The lower layer extract was collected in a clean conical flask for secondary extraction, and the upper layer extract was placed in a 150 mL chicken heart flask and added with 5 g of sodium sulphate. The aqueous layer was removed with anhydrous sodium sulphate. Extraction was repeated twice, and the extracts were combined. The combined extract was first concentrated to 5 mL with a nitrogen blower, and the HLB cartridge was activated with 5 mL of hexane and 5 mL of dichloromethane by soaking for 5 min and then discarded. The effluent was removed with the flow rate controlled at 2 mL/min. The liquid level should not be lower than the top layer of the packed column. If the liquid level was low, then it needed to be reactivated. The concentrated extract was transferred to the activated cartridge. The concentrate bottle was washed twice with 2–3 mL of hexane and transferred to the activated extraction cartridge. Hexane: dichloromethane (5 mL; 1:1, V:V) was added for elution, and the effluent and eluent were combined. The effluent and eluent were blown to 1 mL on a nitrogen blower at 30 °C; if the volume was less than 1 mL, then hexane was added to obtain the final volume of 1 mL. The mixture was passed through a 0.22 μm filter into a 2 mL brown reagent bottle.
Ultrapure water was used as blank control. It was added with 200 μL of mixed standard and 200 μL of BB standard. The concentration of the mixed standard and BB was 1 μg/mL. Subsequent processes were the same as those conducted on the water sample. (all glassware used in the experiment was washed with ultrapure water and methanol in turn after being baked in a muffle furnace at 450 °C for 3 h to avoid potential contamination.)
Gas chromatography–mass spectrometry (GC–MS) analysis
PAEs were detected by GC–MS (Agilent 7890/5975C). The test conditions for chromatography were as follows: column: 5% phenyl-methyl polysiloxane quartz capillary column (30 m × 0.25 mm × 0.25 μm); injection port temperature: 260 °C; programme temperature: an initial column temperature of 60 °C held for 1 min, increased to 220 °C at 20 °C/min and held for 1 min, increased to 250 °C at 5 °C/min and held for 1 min and increased to 290 °C at 20 °C/min and held for 7.5 min; carrier gas: high-purity helium (purity > 99.999%); flow rate: 1.0 mL/min; injection method: splitless injection; injection volume: 1 μL.
The mass spectrometry reference conditions were as follows: ionisation mode: electron bombardment ionisation source; ionisation energy: 70 eV; transmission line temperature: 280 °C; ion source temperature: 230 °C; monitoring method: selected ion scanning (details of PAEs are provided in Table 2 in Supplementary Material); solvent delay: 7 min.
Quality control and quality assurance
The calibration curve consisted of six levels of standard solutions, and the correlation coefficients (R2) of PAEs were above 0.99. The recovery of PAEs in ultrapure water was 71.6–114%. When S/N (SIGNAL–NOISE RATIO) = 3, the concentration is limit of detection (LOD). When S/N = 10, the concentration is limit of quantification (LOQ). LOD was 0.0081–0.29 ng/L, LOQ was 0.027–0.95 ng/L.
Data analysis
Three replicates were performed for each sample, and the results were expressed as the average of the three replicates. Statistical analysis was performed using Microsoft Word 2010, Statistical Product and Service Solutions Software 20.0 (SPSS 20.0), ArcMap 10.2 and Origin 2022. Correlations were analysed via Pearson’s test (two-tailed) by using SPSS 20.0, and P < 0.05 was considered to indicate a significant correlation.
Results and discussion
Concentration and composition of PAEs in water
Samples from the 15 sampling sites in Ulungur Lake were collected in September 2021 (flood period) and May 2022 (dry period). The pollution levels of PAEs in the dry period are shown in Fig. 2a. The concentrations of 17 PAEs in the water of Ulungur Lake during the dry period are DMEP > DCHP > DNOP > DEHP > DBP > DIBP > BBP > DBEP > DPHP > DHXP > DMP > DEP > DAP > DNP > DEEP > BMPP > DPP, indicating that DMEP, DCHP and DNOP are the main pollutants in the dry period. The pollution levels of PAEs in the flood period are depicted in Fig. 2b. According to Fig. 2b, the concentrations of 17 PAEs during the flood period are BBP > DEHP > DCHP > DBEP > DAP > DHXP > DBP > DMEP > DPHP > DNP > DEEP > BMPP > DEP > DMP > DPP > DIBP and DNP, indicating that BBP, DEHP and DCHP are the main pollutants in the flood period.
The characteristics of PAEs pollution in Ulungur Lake during the dry and flood periods are shown in Table 1. The concentrations of PAEs in the water of Ulungur Lake during the dry and flood periods are ND–0.997 μg/L and ND–0.984 μg/L, respectively. The PAE pollution concentration at each sampling site in Ulungur Lake in the dry and flood periods is presented in Table 3 in Supplementary Material. The concentrations of PAEs in rivers and lakes in different regions are shown in Table 2. The concentration ranges of ∑PAEs in Ulungur Lake in the dry and flood periods are 0.451–9.97 µg/L and 0.0490–6.38 µg/L, respectively. Table 2 indicates that the concentration of ∑PAEs in Ulungur Lake is higher than that in Liangzi Lake (He & Shi, 2021) and those in Qiandao Lake (Mi et al., 2022), the Kaveri River of India (Selvaraj et al., 2015) and the Asan Lake of Korea in the dry period (Lee et al., 2019) but lower than that in the Fen River (Han et al., 2017) and that in Qiandao Lake in the flood period (Mi et al., 2022).
Spatial and temporal distribution characteristics of PAEs in water
Temporal distribution characteristics of PAEs in water
PAE contamination levels at various sampling sites during different periods are shown in Fig. 3. In the dry period, DMP, DAP, DIBP, DBP, DMEP, BMPP, DEEP, DPP, DCHP, DEHP, DNOP and DNP have the highest detection rate (100%), followed by DEP and DBEP (93.3%), DPHP (73.3%), BBP (60.0%) and DHXP (40.0%). In the flood period, DBEP, DCHP and DEHP have the highest detection rate (100%), followed by DHXP and BBP (93.3%); the range of detection rate of DIBP, DNOP, DBP, DMEP, DMP, DPP, DAP, DNP, BMPP, DPHP, DEEP and DEP is 0–80.0%. The concentrations of DBP, BMPP, DCHP, DEEP, DEP, DIBP, DMEP, DMP, DNOP, DNP, DPHP and DPP are higher in the dry period than those in the flood period at different sampling sites. The concentration trend of other PAEs is not obvious.
Figure 4a–d shows the pollution level and composition distribution of PAEs at the 15 sampling sites in Ulungur Lake during the dry and flood periods. From Fig. 4a, the concentration of ∑PAEs in Ulungur Lake with time is characterised by the dry period (63.3 µg/L) > the flood period (29.3 µg/L). This is because the pollution source of PAEs in Ulungur Lake is relatively fixed. The rainy period and the melting of ice and snow in the flood period make the flow in the Ulungur Lake area larger, which reduces the content of PAEs in the water. On the contrary, the flow in the dry period is small, which causes the pollution concentration of PAEs in the water to be relatively high.
From Table 1 and Fig. 4b–d, the total concentrations of DMP, DEP, DIBP, DBP, DMEP, BMPP, DEEP, DPP, DCHP, DPHP, DNOP and DNP in the dry period are greater than those in the flood period. The total concentrations of DHXP, BBP, DBEP and DEHP in the dry period are lower than those in the flood period. The proportions of the 17 PAEs in water in the dry period are DMEP > DCHP > DNOP > DBP > DIBP > DEHP > DBEP > BBP > DMP > DPHP > DNP > DEP > DHXP > DAP > DEEP > BMPP > DPP; the proportion of DMEP is the largest, whereas the proportion of DPP is the smallest. The proportions of the 17 PAEs in water during the flood period are as follows: DEHP > BBP > DCHP > DBEP > DAP > DHXP > DBP > DMEP > DPHP > DNP > DEEP > DEP > BMPP > DMP > DPP > DIBP, DNOP; DEHP accounts for the largest proportion, whereas DPP accounts for the smallest proportion. Except for DCHP, the concentration proportion of other PAEs in different sampling periods is consistent with the total concentration. The total concentration of DCHP in the dry period is lower than that in the flood period because some PAEs are not detected in the flood period, which makes the proportion of DCHP high.
Spatial distribution characteristics of PAEs in the water
In this study, the concentration of PAEs in the water at each sampling site during the dry and flood periods of Ulungur Lake is detected and analysed, and its spatial distribution characteristics are evaluated. The spatial distribution characteristics are shown in Fig. 5a–d.
The distribution characteristics of the total concentration of PAEs in different periods are shown in Figs. 5a, b. From Fig. 5a, except that the total concentration of PAEs at W1 in the dry period is lower than that in the flood period, the total concentration of PAEs in other sampling sites is in the order of the dry period > the flood period. Figure 5b demonstrates that in the dry period, the total concentration of PAEs is relatively high in all sites, except at W4, W10 and W14 which indicate minimal deviation. W4 is close to the Ulungur Lake Golden Beach Scenic Spot, and surrounding villages are located here. W10 and W14 are close to densely distributed villages. The increase in the use of plastic products caused by human activities is the main reason for the higher total concentration of PAEs at each sampling site. In particular, at W1, W5 and W7, the total concentrations of PAEs are relatively high during the flood period. There are villages located around W1, and it is close to the provincial highway. The frequent human activities and the emission of automobile exhaust may lead to the high concentration at this sampling site. There are provincial highways, expressways and PetroChina gas stations between W5 and W6. The emission of automobile exhaust is the main reason for the high concentration of PAEs. After the Ulungur River flows into Jili Lake (W6 sampling site), it flows into Ulungur Lake through the Kuyirga River, then flows through the sampling sites W5 and W7, so the total concentrations of PAEs at W5 and W7 are relatively high.
Figure 5c, d shows the spatial distribution of the concentration of ∑PAEs at each sampling site in the dry and flood periods. From Fig. 5c, d, the concentration of ΣPAEs in Ulungur Lake in the dry period is much lower on the side near the lake entrance of the Ulungur River (W6) and Irtysh River (E1, W1). By contrast, the concentration of ΣPAEs in the flood period is higher on the side near the lake entrance of the Ulungur River and Irtysh River (E1, W1–W7, W13, W14).
Source analysis of PAEs
Agricultural plastic films, fertilisers and pesticides containing PAEs are important sources of PAEs in soil (Cheng et al., 2018). PAEs are the most abundant semivolatile organic compounds in chemical fertilisers; consequently, most of the PAEs directly enter the soil when using fertilisers. The more fertiliser is applied, the more PAEs the soil contains. When meteorological conditions change, PAEs in soil will cause water or air pollution through evaporation, leaching, deposition and drainage. PAEs entering the atmosphere are easily adsorbed on particulate matter. Owing to the high boiling point, high-molecular-weight PAEs (DBEP, DEHP, DNP, etc.) are easily adsorbed by particles. PAEs in PM2.5 mainly come from the production of plastic products and coatings. For example, paint, fertiliser, plastic and other plants will emit PAEs into the atmosphere during production. Meteorological factors are important factors affecting the atmospheric PAE concentration (Kang et al., 2017; Liu et al., 2019).
PAEs migrate from the gas phase to particulate matter with the decrease in temperature and can transfer from particulate matter to the gas phase with the increase in temperature. The difference in temperature, humidity, wind speed, wind direction and other meteorological conditions in various regions at different time periods will have a great impact on the diffusion and dilution of PM2.5 (Huang et al., 2022). PAEs in the atmosphere will enter the soil or water through precipitation or free sedimentation. The photolysis of PAEs in the water environment mainly occurs through absorption of 290–400 nm ultraviolet light. The ultraviolet light content in the sunlight is very low, and the energy of long wavelength is insufficient to break the covalent bond. Therefore, in natural water, PAEs have a long half-life and low mineralisation (Wang et al., 2021a, 2021b).
To determine the correlation between PAEs and explore their possible pollution sources, this study uses SPSS 20.0 to conduct correlation analysis and principal component analysis on the content of 17 PAEs in the dry and flood periods. The correlation between the dry and flood periods is shown in Table 3. DEEP and DBP; DMP and DAP; DNP and DBP; DIBP, DPP and DEEP are extremely significantly correlated in the dry period, whilst DAP and DIBP; DIBP and DNP; DCHP and DBEP are significantly correlated, indicating that DEEP, DBP, DMP, DIBP, DPP, DAP and DNP have similar sources and DCHP and DBEP have similar pollution sources. During the flood period, DMP and DBP; DMEP, DNP, DEP and DAP; DCHP, DEHP, DAP and DPHP; DBP and DMEP; DHXP and BBP; DBEP, BBP and DBEP; DCHP and DEHP are extremely significantly correlated, whilst DMP and DEP are significantly correlated, indicating that these PAEs have similar contamination sources.
The principal component analysis of the dry and flood periods is shown in Fig. 6a, b. In the dry period, two principal components with a total contribution rate of 40.1% are screened out by principal component analysis, of which the contribution rate of the first principal component is 21.1% and the contribution rate of the second principal component is 19.0%. Amongst the first principal components, DNOP, DAP, DIBP, DBP and DNP have higher loads, indicating that they have similar pollution sources. Studies have shown that DBP and DIBP are mainly used in cosmetics and personal skin care products, whilst DAP and DNOP are mainly employed as plasticisers in chemical production. Hence, the first principal components mainly reflect the pollution originating from cosmetics, personal care products and chemical production containing plasticisers (Liu et al., 2021; Wang et al., 2021a, 2021b). Amongst the second principal components, BMPP, DMP, DEEP and DPP show higher loads, indicating that they have similar pollution sources. Studies have demonstrated that DMP and DPP are mainly used as plasticisers in chemical production, so the second principal components mainly reflect the pollution originating from chemical production.
During the flood period, two principal components with a total contribution rate of 54.2% are screened out by principal component analysis, of which the contribution rate of the first principal component is 33.8% and the contribution rate of the second principal component is 20.4%. Amongst the first principal components, BMPP, DMEP, DMP, DBP and DNP show higher loads, indicating that they have similar pollution sources. Studies have shown that DMP, DNP and DMEP are mainly used as plasticisers in chemical production (Li et al., 2021), so the first principal components mainly represent the pollution that comes from chemical production. Amongst the second principal components, DPHP, BBP, DHXP, DAP, DBEP, DCHP, DEHP and DEP have higher loads, indicating that they may have similar pollution sources. Studies have presented that BBP is mainly used in cosmetics and personal care products. DAP, DPHP, DHXP, DEHP, DBEP and DCHP are mainly utilised as plasticisers in chemical production, so the second main components mainly represent the pollution from cosmetics, personal care products and chemical production containing plasticisers (He et al., 2019).
The above comparison illustrates that the main pollution sources of PAEs in the dry and flood periods are cosmetics, personal care products and chemical production containing plasticisers. The influence of cosmetics and personal care products in the dry period is relatively stronger than that in the flood period. River input and atmospheric sedimentation are the main sources of PAEs in the lake.
Conclusion
-
(1)
The concentration ranges of PAEs in the water of Ulungur Lake in the dry and flood periods are ND–0.997 µg/L and ND–0.984 µg/L, respectively. DMEP, DCHP and DNOP are the main pollutants in the dry period; BBP, DEHP and DCHP are the main pollutants in the flood period.
-
(2)
The concentration of ∑PAEs with time is characterised by the dry period > the flood period. The change in flow is the main reason for the different concentration distributions of PAEs in various periods.
-
(3)
The concentration of ΣPAEs in the dry period is lower on the side near the lake entrance of the Ulungur River and Irtysh River. By contrast, the concentration of ΣPAEs in the flood period is higher on the side near the lake entrance of the Ulungur River and Irtysh River.
-
(4)
The main pollution sources of PAEs in the dry and flood periods are cosmetics, personal care products and chemical production containing plasticisers, and the main pollution sources of PAEs in the flood periods are chemical production containing plasticisers. River input and atmospheric sedimentation are the main sources of PAEs in the lake.
References
Babu-Rajendran, R., Preethi, G., Poopal, R. K., Nikhil, N. P., Vimalkumar, K., Subramanian, A., & Krishna-Kumar, S. (2018). GC-MS determination of phthalate esters in human urine: A potential biomarker for phthalate bio-monitoring. Journal of Chromatography B-Analytical Technologies in the Biomedical and Life Sciences, 1079, 15–24.
Busgang, S. A., Spear, E. A., Andra, S. S., Narasimhan, S., Bragg, J. B., Renzetti, S., Curtin, P., Bates, M., Arora, M., Gennings, C., & Stroustrup, A. (2022). Application of growth modeling to assess the impact of hospital-based phthalate exposure on preterm infant growth parameters during the neonatal intensive care unit hospitalization. Science of the Total Environment, 850, 157830.
Cao, Y. R., Lin, H. J., Zhang, K., Xu, S. P., Yan, M., Leung, K. M. Y., & Lam, P. K. S. (2022). Microplastics: A major source of phthalate esters in aquatic environments. Journal of Hazardous Materials, 432, 128731.
Cheng, J. J., Liu, Y. A., Wan, Q., Yuan, L., & Yu, X. Y. (2018). Degradation of dibutyl phthalate in two contrasting agricultural soils and its long-term effects on soil microbial community. Science of the Total Environment, 640, 821–829.
Farahani, A., Ramezani, M., Hassan, J., & Niazi, A. (2017). In tube ultrasonic and air assisted liquid-liquid microextraction-gas chromatography-mass spectrometry determination: A novel method for the determination of phthalate esters in aqueous samples. Journal of the Brazilian Chemical Society, 28(6), 967–974.
Gao, F., He, B., Yan, Z. L., Xue, S. S., & Li, Y. Z. (2020). Analysis of the changes and driving force of the water area in the Ulungur Lake over the past 40 years. Journal of Water Supply Research and Technology-Aqua, 69(5), 500–511.
Han, W. H., Zhao, Y., Dang, J. H., & Xiong, L. (2017). Distribution and ecological risk evaluation of phthalate esters in Fen River Basin. Environmental Chemistry, 36(6), 1377–1387. (in Chinese).
He, X. M., & Shi, M. F. (2021). Distribution characteristics and ecological and health risk assessment of phthalic acid esters in surface water and sediment of Liangzi lake China. Environmental Monitoring in China, 37(2), 115–127. (in Chinese).
He, Y., Wang, Q. M., He, W., & Xu, F. L. (2019). Phthalate esters (PAEs) in atmospheric particles around a large shallow natural lake (Lake Chaohu, China). Science of the Total Environment, 687, 297–308.
Huang, Y. F., Pan, W. C., Tsai, Y. A., Chang, C. H., Chen, P. J., Shao, Y. S., Tsai, M. S., Hou, J. W., Lu, C. S., & Chen, M. L. (2017). Concurrent exposures to nonylphenol, bisphenol A, phthalates, and organophosphate pesticides on birth outcomes: A cohort study in Taipei Taiwan. Science of the Total Environment, 607, 1126–1135.
Huang, L., Zhu, X. Z., Zhou, S. X., Cheng, Z. R., Shi, K., Zhang, C., & Shao, H. (2021). Phthalic acid esters: Natural sources and biological activities. Toxins, 13(7), 495.
Huang, L. H., Qiao, Y. Q., Deng, S. X., Wang, X. K., Zhao, W. P., & Yue, Y. (2022). Phthalates in house dust in Chinese urban residences: Concentrations, partition, origin and determinants. Chemosphere, 286, 131703.
Jebara, A., Albergamo, A., Rando, R., Potorti, A. G., Lo Turco, V., Ben Mansour, H., & Di Bella, G. (2021). Phthalates and non-phthalate plasticizers in Tunisian marine samples: Occurrence, spatial distribution and seasonal variation. Marine Pollution Bulletin, 163, 111967.
Ji, F. F., Ma, X. F., Qiu, L. H., Kang, Z. P., & Shen, J. Z. (2022). Quantifying the effects of introduced Bighead Carp (Cyprinidae; Aristichthys nobilis) stocking on dominant fish species in the Ulungur Lake. China. Biological Invasions, 24(5), 1253–1265.
Kang, M. J., Yang, F., Ren, H., Zhao, W. Y., Zhao, Y., Li, L. J., Yan, Y., Zhang, Y. J., Lai, S. C., Zhang, Y. Y., Yang, Y., Wang, Z. F., Sun, Y. L., & Fu, P. Q. (2017). Influence of continental organic aerosols to the marine atmosphere over the East China Sea: Insights from lipids, PAHs and phthalates. Science of the Total Environment, 607, 339–350.
Kumar, R., Adhikari, S., Driver, E., Zevitz, J., & Halden, R. U. (2022). Application of wastewater-based epidemiology for estimating population-wide human exposure to phthalate esters, bisphenols, and terephthalic acid. Science of the Total Environment, 847, 157616.
Langova, R., Jarosova, A., Polakova, S., & Kos, I. (2020). Analysis of phthalic acid esters in agricultural soils. Environmental Monitoring and Assessment, 192(2), 92.
Lee, Y. M., Lee, J. E., Choe, W., Kim, T., Lee, J. Y., Kho, Y., Choi, K., & Zoh, K. D. (2019). Distribution of phthalate esters in air, water, sediments, and fish in the Asan Lake of Korea. Environment International, 126, 635–643.
Li, X., Han, X. X., Vogt, R. D., Zhou, J. Y., Zheng, B. Y., Song, Y. T., & Lu, X. Q. (2021). Distributions, temporal trends and ecological risks of polyethylene terephthalate (PET) and di-(2-ethylhexyl) phthalate (DEHP) in sediments of Jiaozhou Bay China. Marine Pollution Bulletin, 165, 112176.
Liu, X. T., Peng, C. F., Shi, Y. M., Tan, H. L., Tang, S. Q., & Chen, D. (2019). Beyond phthalate diesters: Existence of phthalate monoesters in south china house dust and implications for human exposure. Environmental Science & Technology, 53(20), 11675–11683.
Liu, B. X., Jiang, T., Li, Z. Y., Ge, W., Wu, J., Song, N. N., & Chai, C. (2021). Phthalate esters in surface sediments from fishing ports in Circum-Bohai-Sea region China. Marine Pollution Bulletin, 171, 112782.
Lu, X. L., Xu, X. J., Lin, Y. C., Zhang, Y., & Huo, X. (2018). Phthalate exposure as a risk factor for hypertension. Environmental Science and Pollution Research, 25(21), 20550–20561.
Ma, S. T., Hu, X., Tang, J., Cui, J. T., Lin, M. Q., Wang, F., Yang, Y., & Yu, Y. X. (2022). Urinary metabolites and handwipe phthalate levels among adults and children in southern China: Implication for dermal exposure. Journal of Hazardous Materials, 439, 129639.
Martin-Pozo, L., Gomez-Regalado, M. D., Moscoso-Ruiz, I., & Zafra-Gomez, A. (2021). Analytical methods for the determination of endocrine disrupting chemicals in cosmetics and personal care products: A review. Talanta, 234, 122642.
Mi, Q. X., Guo, X. C., Lu, S. Y., Deng, Y. X., Lu, H. B., Li, X., Liu, X. H., & Chen, J. M. (2022). Distribution characteristics and health risk assessment of phthalates (PAEs) in Qiandao Lake. Environmental Science, 43(4), 1966–1975. (in Chinese).
Mohammadi, A., Malakootian, M., Dobaradaran, S., Hashemi, M., & Jaafarzadeh, N. (2022). Occurrence, seasonal distribution, and ecological risk assessment of microplastics and phthalate esters in leachates of a landfill site located near the marine environment: Bushehr port, Iran as a case. Science of the Total Environment, 842, 156838.
Ni, Z. Y., Zhang, E. L., Sun, W. W., Meng, X. Q., Ning, D. L., Jiang, Q. F., & Zheng, W. X. (2022). Response of the chironomid community to late Holocene climate change and anthropogenic impacts at Lake Ulungur, arid Central Asia. Quaternary International, 613, 91–100.
Porras, S. P., Hartonen, M., Koponen, J., Ylinen, K., Louhelainen, K., Tornaeus, J., Kiviranta, H., & Santonen, T. (2020). Occupational exposure of plastics workers to diisononyl phthalate (DiNP) and di(2-propylheptyl) phthalate (DPHP) in Finland. International Journal of Environmental Research and Public Health, 17(6), 2035.
Saab, Y., Oueis, E., Mehanna, S., Nakad, Z., Stephan, R., & Khnayzer, R. S. (2022). Risk assessment of phthalates and their metabolites in hospitalized patients: a focus on di- and mono-(2-ethylhexyl) phthalates exposure from intravenous plastic bags. Toxics, 10(7), 357.
Selvaraj, K. K., Sundaramoorthy, G., Ravichandran, P. K., Girijan, G. K., Sampath, S., & Ramaswamy, B. R. (2015). Phthalate esters in water and sediments of the Kaveri River, India: Environmental levels and ecotoxicological evaluations. Environmental Geochemistry and Health, 37(1), 83–96.
Silano, V., Baviera, J. M. B., Bolognesi, C., Chesson, A., Cocconcelli, P. S., Crebelli, R., Gott, D. M., Grob, K., Lampi, E., Mortensen, A., Riviere, G., Steffensen, I. L., Tlustos, C., Van Loveren, H., Vernis, L., Zorn, H., Cravedi, J. P., Fortes, C., Pocas, M. D. T., … Enzyme, E. P. F. C. M. (2019). Update of the risk assessment of di-butylphthalate (DBP), butyl-benzyl-phthalate (BBP), bis(2-ethylhexyl)phthalate (DEHP), di-isononylphthalate (DINP) and di-isodecylphthalate (DIDP) for use in food contact materials. EFSA Journal, 17(12), 5838.
Wang, B., Qin, X. L., Xiao, N., Yao, Y. M., Duan, Y. S., Cui, X. F., Zhang, S., Luo, H. N., & Sun, H. W. (2020). Phthalate exposure and semen quality in infertile male population from Tianjin, China: Associations and potential mediation by reproductive hormones. Science of the Total Environment, 744, 140673.
Wang, C. C., Huang, P. P., Qiu, C. S., Li, J., Hu, S. L., Sun, L. P., Bai, Y. H., Gao, F., Li, C. C., Liu, N. N., Wang, D., & Wang, S. P. (2021a). Occurrence, migration and health risk of phthalates in tap water, barreled water and bottled water in Tianjin China. Journal of Hazardous Materials, 408, 124891.
Wang, D., Xi, Y., Shi, X. Y., Han, Y. N., Zhang, N., Ahmad, F., & Li, F. M. (2021b). Reduction effects of solar radiation, mechanical tension, and soil burial on phthalate esters concentrations in plastic film and soils. Science of the Total Environment, 778, 146341.
Xing, H. H., Yu, X. L., Huang, J. H., Du, X. D., Wang, M. T., Sun, J. T., Lu, G. N., & Tao, X. Q. (2022). Characteristics and health risks of phthalate ester contamination in soil and plants in coastal areas of South China. International Journal of Environmental Research and Public Health, 19(15), 9516.
Zhao, A. Q., Wang, L. X., Pang, X. Y., & Liu, F. (2022). Phthalates in skin wipes: Distribution, sources, and exposure via dermal absorption. Environmental Research, 204, 112041.
Acknowledgements
This work is supported by the National Natural Science Foundation of China (No. 21667026). Thanks to Yining Customs Technology Centre.
Funding
This work was funded by the National Natural Science Foundation of China (No. 21667026).
Author information
Authors and Affiliations
Contributions
MW contributed to visualisation, formal analysis, data curation and writing-original draft preparation. JL contributed to investigation, validation, writing-reviewing and editing. LZ contributed to investigation and supervision. YS contributed to conceptualization and supervision. H contributed to formal analysis and investigation. ML contributed to formal analysis and validation. XY contributed to formal analysis.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interest or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, M., Lu, J., Zhou, L. et al. Residual status and source analysis of phthalate esters in Ulungur Lake, China. Environ Geochem Health 45, 5991–6007 (2023). https://doi.org/10.1007/s10653-023-01586-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-023-01586-9