Abstract
Chronic exposure to environmental cadmium (Cd) and lead (Pb) may have adverse effects on the human health. In this study, we aimed to determine the primary and interactive effects of Cd and Pb exposure on liver function in residents near a mining and smelting area in northwestern China. A total of 451 subjects were recruited, from which blood samples were collected to determine the levels of Cd, Pb, and liver function indices. Additionally, the association between the levels of exposure markers and liver function indices was analysed. Cd and Pb levels were significantly higher in subjects living in the polluted area than in those living in the non-polluted reference area. The liver function levels of subjects in the polluted area were poor compared with those in the reference area. In addition, Cd and Pb levels in the blood were positively associated with gamma glutamyl transpeptidase (GGT) levels and negatively associated with direct bilirubin (DBil) levels. Cd and Pb may be risk factors for abnormal liver function. The risk of abnormal liver function was higher in subjects with moderate Cd and Pb levels, high Cd levels, high Pb levels, and high Cd and Pb levels than in those with low Cd and Pb levels. Our data show that exposure to Cd and/or Pb can cause abnormal liver function. Cd and Pb may have an antagonistic effect on liver function, and high Cd exposure alone has a more profound effect on abnormal liver function compared with co-exposure to Pb and Cd.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cadmium (Cd) and lead (Pb) are toxic trace metals extensively used in industries and are widely existed in the environment. These metals can accumulate in the human body through the food chain or by smoking (Genchi et al., 2020; Satarug & Moore, 2004; Wu et al., 2008). In addition, owing to a lack of an effective excretion mechanism, the half-life of Cd and Pb in the human body can last between 10 and 30 years (Nordberg, 1996; WHO, 1995). Therefore, long-term Cd and Pb accumulation in the human body may have adverse effects on multiple systems throughout the body, causing irreversible damage to different organs, such as the liver, kidney, and bones (Puerto-Parejo et al., 2017; Sanders et al., 2019; Wang & Du, 2013; Werder et al., 2020).
Pb in blood can affect the synthesis of haemoglobin via the inhibition of certain essential enzymes such as δ-aminolevulinic acid dehydratase (ALAD), coproporphyrinogen, and ferrochelatase and Pb can also have direct toxic effects on red blood cells and blood vessels, which can cause damage to the blood system (Mitra et al., 2017; Singh et al., 2018). Similar to Pb, Cd may also interfere with the process, that is, erythropoiesis by reducing iron absorption in the gastrointestinal tract and directly affecting the hematopoietic cells in the bone marrow (Peters et al., 2021). In addition, the kidney can accumulate a large amount of Cd and Pb and become the target organ for chronic heavy metal damage. They mainly damage renal tubular epithelial cells by causing changes such as mitochondrial degeneration, endoplasmic reticulum expansion, and increasing the number of lysosomes, which ultimately lead to kidney damage and renal failure (Orr & Bridges, 2017; Satarug et al., 2020). Evidence suggests that the liver is the second largest target organ for blood circulation and the metabolism and plays a significant role in the accumulation, detoxification, and excretion of heavy metals, including Cd and Pb (Nordberg, 1996). There have been numerous studies that indicate Cd exposure can cause liver oxidative stress and DNA damage, thereby aggravating liver metabolic disorders and genetic toxicity (Gao et al., 2018; Go et al., 2015; Ren et al., 2019). Low-level environmental Cd exposure can lead to elevated serum liver enzyme levels in adults and have been found to be associated with non-alcoholic fatty liver disease (NAFLD) (Kang et al., 2013; Park et al., 2021). Similarly, Pb causes serious damage to the liver function. Prolonged exposure to a low-dose of Pb can induce hepatotoxicity in mice at the physiological, biochemical, and transcriptome levels (Luo et al., 2019). Pb can also aggravate liver damage by inducing hepatocyte apoptosis (Chi et al., 2017; Hou et al., 2019). In addition, elevated Pb levels are also associated with the risk of NAFLD with the acceleration of urbanization, especially among women (Zhai et al., 2017). However, most previous studies have focused on the impacts of single-metal exposure, and to the best of our knowledge, the effects of Cd and Pb co-exposure on liver function have not yet been fully elucidated.
In many circumstances, Cd and Pb pollution can coexist in the environment, and people may be co-exposed to both metals. It has been confirmed that the levels of Pb in blood (BPb) may be positively associated with those of Cd in blood (BCd) (Wang et al., 2020). Most relevant studies have also reported that exposure to Cd and Pb in combination can have negative effects on lung function (Yang et al., 2019), exacerbate kidney function decline (Tsai et al., 2017), and promote the progression of gastrointestinal cancers (Lin et al., 2018). Nevertheless, only a few studies have reported liver injury caused by co-exposure to Cd and Pb. Andjelkovic et al. (2019) also assessed the toxic effects of acute Cd and Pb exposure in rat liver, kidney, and blood. Their data showed that exposure to both Cd and Pb mixtures produced additive adverse effects on the liver than the responses induced after exposure to a single-metal. Chen, Xu et al. (2019) showed that co-exposure to Cd and Pb may increase the liver metabolic burden and induce abnormal liver function. However, in their study, the population comprised hospital patients from an area polluted with e-waste, and samples from the public were not collected. Additionally, although the study reported the harm caused by mixed exposure, it did not compare it with that of single exposure. Cd and Pb pollution poses a serious threat to the environment in China (Chen et al., 2015). However, the effect of co-exposure of Cd and Pb at low environmental levels on the liver function in the general population remains to be further studied.
Thus, we aimed to investigate the associations between Cd and Pb co-exposure and liver function in residents near a mining and smelting area in northwestern (NW) China who were exposed to both metals through the food chain. We aimed to provide a systematic analysis of the association of Cd and Pb co-exposure with human liver function.
Materials and methods
Study areas
Two areas in NW China were included in this study: the Dongdagou watershed close to mining and smelting areas (polluted area) and Xinglong Mountain National Nature Reserve (non-polluted, reference area). In the polluted area, large amounts of acidic wastewater containing Pb, Cd, and other heavy metals were directly discharged into the soil and groundwater in the area (Chen et al., 2018). Some studies have shown that the content of Cd, Hg, and As in this area is dangerously exceeding the standard, in particular Cd, which is the priority pollutant in soils (Ai et al., 2018; Dai et al., 2012). In addition, the soil Pb concentration was also higher than the local soil background value (Liu et al., 2016, 2017). Due to environmental protection concerns, the Chinese government launched a project for the comprehensive treatment and ecological restoration of heavy metal pollution in 2015, and this area ranked first among 85 cities and regions (Liu et al., 2017). We selected three villages closest to the mineral-polluted locations as the polluted area. Moreover, two villages in the reference area were selected as the non-polluted area. This area is dominated by a Han Chinese population; however, it has many of the same customs, culture, and socioeconomic conditions as the polluted area.
Study population
The study population came from the previously reported Dongdagou–Xinglong (DDGXL) cohort (Zhang et al., 2021). Since its establishment in 2015, this cohort has been dedicated to environmental monitoring of heavy metal polluted areas and the health management of residents. The inclusion and exclusion criteria have also been reported in previous studies.
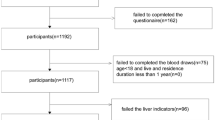
All individuals meeting the inclusion and exclusion criteria were investigated. A total of 503 inhabitants who consumed locally grown food participated in this study. Overall, 52 individuals were not included in the final analysis due to a lack of basic information (n = 2) and suitable blood samples (n = 7), no heavy metal detected in the samples (n = 8), and loss of liver function data (n = 35). Finally, 451 subjects (297 women and 154 men) were included in this study (Fig. 1). The study was approved by the ethics committee of the First Hospital of Lanzhou University (ethical code: LDYYYLL2012-JJ00-04, LDYYYLL2015-0027).
Sample collection and exposure analysis
A previously published protocol for sample collection and determination of blood metal concentrations was followed (Zhang et al., 2021). Peripheral venous blood was collected from each subject by a research nurse. Part of the blood was used for heavy metal determination, and the remaining blood was fully centrifuged, and serum was taken for liver function tests. We measured the concentrations of BCd and BPb using inductively coupled plasma–mass spectrometry (ICP–MS, NexlON 300X PE, USA). We used the calibration standards (PerkinElmer, USA) to control the calibration ranges of blood samples, and in the generally observed concentration ranges, we established the standard curves of six points. We determined a limit concentration of 0.08 µg/L for BCd and 0.11 µg/L for BPb. We checked for possible contamination from proprietary diluents and equipment by laboratory blanks and the matrices effects of blank matrix spikes. After six blank samples spiked, two concentrations (5 and 50 µg/L) were measured, the coefficient of variance (CV) and recovery rate were calculated as well. We found that they varied between the ranges of 1.30–3.16 and 96.3–103.5%, respectively, after subtracting the blank sample test results. We used the reference material from Seronorm™ Trace Elements Whole Blood (SERO AS, Norway) for quality control, to insure the accuracy of the selected detection method (ICP-MS) for each of the 20 samples. All calibration standard checks were satisfactory (Zhang et al., 2021). The measurement unit of BPb and BCd levels was ng/mL.
Data collection
Details of the data collection method have previously been described (Zhang et al., 2021). Each participant completed a detailed questionnaire with trained investigators and provided their informed consent. All participants’ survey information was taken from the database management system, including socio-demographic factor, health status, and environmental exposure information. According to the “Guidelines for the Prevention and Control of Obesity in Overweight Adults in China”, a person with BMI ≥ 28.0 kg/m2 or a waist circumference > 80 cm (for women) or > 85 cm (for men) is considered obese (Cohen et al., 2019). Smokers were defined as smoking 100 cigarettes or more in a lifetime; alcohol drinkers were defined as drinking one drinking unit or more in the past year (Chen et al., 2010). The above indicators can be adjusted as confounding factors in a logistics regression.
Determination of liver function indices
The following indices related to liver function were measured: alanine aminotransferase (ALT), aspartate aminotransferase (AST), cholinesterase (CHE), gamma glutamyl transpeptidase (GGT), alkaline phosphatase (ALP), total bilirubin (TBil), indirect bilirubin (IBil), direct bilirubin (DBil), and total bile acid (TBA). Sample pre-treatment: Five millilitres of the collected anticoagulated venous blood was centrifuged at 3000 × g, and the supernatant was collected after it was left to stand 15 min. We used an automatic chemistry analyser (Beckman AU5800, Japan) to measure the levels of liver function indices mentioned above.
Definition of abnormal liver function
As each hospital uses different equipment and reagents, the recommendations of the International Federation of Clinical Chemistry (IFCC) were not adopted. According to the cut-off value recommended by the laboratory diagnosis centre of the First Hospital of Lanzhou University, the reference value ranges of the ALT, AST, ALP, GGT, CHE, TBil, DBil, IBil, and TBA levels were 1–49 U/L, 1–49 U/L, 20–125 U/L, 3–69 U/L, 5–12 KU/L, 9.1–30.1 umol/L, 0–6.8 umol/L, 0–19 umol/L, and 0.5–10 umol/L, respectively. Abnormal liver function was defined as either more than two liver function indices being elevated above the normal range or one liver function index being at least twice the normal range (Chen, Xu et al., 2019). Therefore, based on the liver function indices, we redefined each person living in the polluted and reference areas as having normal or abnormal liver function.
Statistical analysis
We used the Kolmogorov–Smirnov test to evaluate normality. The mean ± standard deviation (SD) was used to show the continuous variables from the quantitative data with a normal distribution. The skewed variables from the non-normal distribution data were expressed as the median (interquartile range, IQR), and qualitative categorical data variables as counts, percentages, and frequencies (rates). According to different data types, we used a Mann–Whitney U test, Student's t test, and Chi-square tests to compare the different data between the polluted and reference control areas. We used the interquartile ranges of BCd and BPb levels to recode the categorical variables of classified data which were grouped as follows: high Cd and Pb group (high Cd & Pb [Cd and Pb > 75th percentile]), high Cd alone group (high Cd [only Cd > 75th percentile]), high Pb alone group (high Pb [only Pb > 75th percentile]), moderate Cd and/or Pb group (moderate Cd & Pb [25th percentile ≤ Cd and/or Pb ≤ 75th percentile]), and low Cd and Pb group (low Cd & Pb [Cd and Pb < 25th percentile]). We used the one-way analysis of variance (ANOVA) and the Kruskal–Wallis rank sum test to analyse the differences between the groups. We assessed the combined effects of Cd and Pb on liver function using a similar method that was described in a previous study by Chen (Chen, Zhu et al., 2019). We also tested a multiplicative combined effect of Cd and Pb by introducing the interaction terms of Cd and Pb (Cd*Pb). The associations between Cd and Pb co-exposure and liver function index levels were analysed using the Spearman rank correlation and multivariable linear regression or logistic regression. We also evaluated the relationship between abnormal liver function and BCd and BPb levels and combined exposure using logistic models. All statistical analyses were based on SPSS 24.0 and Graphpad Prism8. All tests were two-sided, and statistical significance was set at p < 0.05.
Results
Characteristics of study population
The main characteristics of the residents living in the reference and polluted areas are listed in Table 1. The participants living in the reference area were more likely to drink tea than those in the polluted area (p < 0.01). No notable differences were observed for sex, BMI, waist circumference, age, lack of teeth, smoking, and drinking between the two areas. Conversely, the BCd and BPb levels were significantly higher in participants living in the polluted area than in those living in the reference area (p < 0.001).
Liver function indices of subjects living in reference and polluted areas
Levels of liver function indices in both areas are presented in Table 1. AST, ALT, GGT, and CHE levels of individuals in the polluted area were all significantly higher compared with those in the reference area (p < 0.01). The DBil level of individuals in the polluted area was lower than those in the reference area (p < 0.01). In addition, compared with those in the reference area, the median ALP and TBA levels of individuals in the polluted area increased by 5.5 and 0.1 g/L, respectively. No significant differences were observed between the two areas.
BCd, BPb, and liver function index levels in different sexes
The liver function indices and BCd and BPb levels of subjects divided by sex living in both areas are listed in Table 2. ALT, GGT, and CHE levels in both males and females significantly increased in the polluted area (p < 0.05). The median levels of male and female DBil found in the serum in the polluted area were 11.3 and 8.9% lower than those in the reference area, respectively (p < 0.05). Additionally, the AST and ALP levels of individuals living in the polluted area increased in both males and females; however, no significant differences were found between male AST and female ALP levels. BCd and BPb levels in males and females were significantly higher in the polluted area than in the reference area, and the exposure level of males was higher than that of females.
BCd, BPb, and liver function index levels according to age group
The results presented in Table 3 show that subjects of all ages from the polluted area had significantly elevated GGT levels in serum compared to subjects from the reference area (p < 0.05). In addition, AST and CHE levels of individuals in the polluted area were increased in all age groups compared to the reference area; however, no differences were found in AST levels in subjects > 60 years of age or in ALP levels in subjects < 50 years old. BCd and BPb levels in subjects of all ages were significantly higher in the polluted area than in the reference area, and the exposure of subjects aged 50–60 years was more significant than that of subjects of the same age living in the reference area. Subjects aged 50–60 years from the polluted area had blood Cd levels in serum that were 36.06 times higher than those of subjects from the reference area (p < 0.001), and subjects 50–60 years of age from the polluted area had 1.54 times increased levels of blood Pb (p < 0.001).
Association between liver function indices and exposure level
The associations between BCd, BPb, and liver function indices levels are shown in Fig. 2. BCd and BPb levels were both positively correlated with GGT and TBA levels (p < 0.01 or 0.05) and negatively correlated with DBil levels (all p < 0.01). BCd levels were also positively correlated with ALT and ALP levels (p < 0.05), and BPb levels were also positively correlated with CHE levels (p < 0.05). A significant positive correlation was also found between BCd and BPb levels (p < 0.01).
Correlation between exposure and liver function indices levels (n = 451). Cadmium and lead in the blood (BCd, BPb) are positively correlated with gamma glutamyl transpeptidase (GGT) and total bile acid (TBA) levels (p < 0.01 or 0.05), and negatively correlated with direct bilirubin (DBil) levels (all p < 0.01). BCd levels were positively correlated with ALT and ALP levels (p < 0.05), and BPb levels were also positively correlated with CHE levels (p < 0.05). The values are correlation coefficients
Subsequently, we analysed the association between BCd and BPb levels as well as liver function indices using multiple linear regression models (Table 4). BPb levels were negatively associated with DBil levels in both men and women (p < 0.05), and BCd levels were negatively associated with IBil levels in women (p < 0.01). In addition, BCd levels were significantly associated with AST, ALT, and GGT levels in women (p < 0.05). BCd levels were significantly associated with AST, ALT, GGT, and TBA levels in the total population (p < 0.05).
Liver function indices and Cd and Pb co-exposure levels
The subjects in the reference area had lower GGT levels than those in the low Cd & Pb, moderate Cd & Pb, high Cd, high Pb, and high Cd & Pb groups (p < 0.01 or 0.05), and the GGT level increased as the exposure level increased (Table 5). ALT and CHE levels in subjects in the reference area were markedly lower than those in the Cd/Pb subgroups in the polluted area (both p < 0.05). However, the highest ALT and CHE levels were found in the low Cd & Pb groups and the moderate Cd & Pb groups, respectively. Similar results were obtained for AST, ALP, and TBA levels; however, there were no significant differences. In addition, the lowest bilirubin levels (including TBil, DBil, and IBil) were observed in subjects in the high Cd group and were significantly lower than those in subjects residing in the reference area (p < 0.01 or 0.05).
Modification effects of BCd and BPb after adjustment for confounders are listed in Table 6 (P interaction < 0.05). The associations between BCd levels and AST, ALT, and CHE levels were strong in subjects with high Cd levels. BPb and bilirubin (including TBil, DBil, and IBil) levels had a strong negative correlation in subjects with high Pb levels. Simultaneously, combined effects of Cd and Pb on GGT and ALP levels were observed.
Risk of abnormal liver function associated with Cd and Pb exposure
BCd and BPb levels were classified into high and low concentration groups according to the median level (P50). The effects of Cd and Pb exposure on abnormal liver function were analysed using a logistic regression analysis, and both BCd and BPb levels were statistically significant in the regression model (p < 0.05) (Table 7). The odds ratio (OR) of subjects with high BCd (≥ 1 µg/L) was 2.529 (95% CI: 1.288–4.963) compared to those with low BCd (< 1 µg/L) after adjusting for confounders.
Next, we analysed the risk of abnormal liver function associated with Cd and Pb co-exposure (Table 7). The total population was divided into low Cd & Pb, moderate Cd & Pb, high Cd, high Pb, and high Cd&Pb groups according to the IQR of BCd and BPb levels. After adjusting for gender and/or other confounders, the ORs of subjects with moderate Cd & Pb, high Cd, high Pb, and high Cd & Pb levels were 11.900 (95% CI: 1.573–90.044), 16.673 (95% CI: 1.944–139.438), 9.163 (95% CI: 1.004–83.616), and 9.447 (95% CI: 1.132–78.870), respectively, compared to those with low Cd & Pb levels.
Discussion
Cd and Pb coexist in the environment. Exposure to Cd and Pb has been shown to cause liver damage (Nakhaee et al., 2019; Qu et al., 2020; Yu et al., 2020). However, the long-term effects of low-level co-exposure on human liver function are debatable, and need to be further clarified (Nakata et al., 2021). In this study, our results indicate that low-levels of Cd and Pb exposure can impair the normal function of the liver and may cause abnormal liver function. Moreover, our data also suggested a non-multiplicative combined effect of Cd and Pb on abnormal liver function. Co-exposure to Cd and Pb pollutants is an important risk factor for abnormal liver function.
Certain liver enzymes (including ALT and AST) have been used as biomarkers of hepatocyte injury in clinical diagnosis (McGill, 2016; Treem et al., 2021). A growing number of researchers consider that Cd and/or Pb exposure can lead to an increase in liver enzymes. Zou et al. (2020) indicated that ALT and AST levels were significantly increased in rats exposed to Cd and/or Pb. Richard et al. (2016) also reported elevated serum ALT and AST contents above the normal ranges in workers exposed to cement. Our results are in line with previous findings. The serum ALT and GGT levels of the population in the polluted area were significantly elevated compared to the reference area, and the GGT levels were the highest in the high Cd and Pb exposure groups. An increasing trend was also observed in serum AST and ALP levels; however, the difference was not statistically significant. Our data showed that Cd and Pb exposure has a damaging effect on liver function and is a risk factor for abnormal liver function. In addition, we propose novel findings compared to previous studies. Choi et al. (2017) showed that co-exposure resulted in increased histopathological and ultrastructural damage to the liver, accompanied by elevated liver enzyme activity, compared to rats exposed to single Cd or Pb. Our data did not show that co-exposure exacerbated hepatocellular damage caused by Cd or Pb alone. On the contrary, single high Cd exposure had more profound effects on liver function than high Cd and Pb exposure. A comprehensive assessment of both metals in populations is still needed to further explore the interaction mechanism between Cd and Pb.
Numerous works have been conducted on the hepatotoxicity mechanisms of mixed exposure to heavy metals. Oxidative stress has long been the key mechanism involved in Cd- and Pb-induced liver damage (Matović et al., 2015; Omotosho, 2019). Although Cd and Pb are non-redox metals, they can induce excessive production of reactive oxygen species (ROS) and decrease in antioxidant enzymes to disrupt the balance between oxidation and antioxidant defence, thus resulting in the accumulation of oxygen free radicals and promoting cell oxidative damage (Kim et al., 2019). By evaluating the relationship between blood oxidative stress parameters in occupational exposure workers and the Cd or Pb levels, it was found that Cd and Pb promoted an increase in GST activity; however, there may be a lack of synergism between the effects of Cd and Pb on the oxidative status in blood (Conterato et al., 2013). This is similar to our results, which showed that residents in the high Cd and Pb exposure group had lower AST and ALT levels than those in the single high Cd or single high Pb groups. Investigations on animals have provided evidence for the role of oxidative stress in the Cd and Pb co-exposure. The accumulation of both metals in the liver of rats exposed to a Cd and Pb mixture was more reduced than those exposed to a single metal, and Cd-only treatment may have a more profound effect on oxidative stress, suggesting that Cd and Pb may play antagonistic roles in inducing oxidative stress in the liver (Pandya et al., 2010). Our study yielded consistent results. The high Cd group had the strongest correlation with AST and ALT levels. In addition, the risk of abnormal liver function in the high Cd group was higher than that in the high Cd and Pb exposure group. Other in vivo experiments have also identified antagonism between Cd and Pb in the liver (Salińska et al., 2012; Pillai et al., 2009). Although the effects of Cd and Pb co-exposure on the kidneys mostly involve the synergism between both metals (which is the opposite of the antagonism in the liver), these differences may be related to the effect of metallothionein in different tissues (Chen, Xu et al., 2019; Wang et al., 2010). Recent studies have shown that Cd and Pb can also lead to liver dysfunction via the induction of excessive autophagy, which destroys mitochondrial function, promotes liver cell apoptosis, and affects DNA repair mechanisms (Liu et al., 2019; Mitkovska et al., 2020; Zou et al., 2020).
Bilirubin is often used as a clinical indicator of liver metabolism (Fevery, 2008), whereby bilirubin levels often increase when liver function is abnormal. Elevated TBil and DBil levels were also found in people who were co-exposed to Cd and Pb (Jurdziak et al., 2018; Richard et al., 2016). However, our study showed reduced DBil levels in the polluted areas, and the decrease in TBil, DBil, and IBil was most significant in the high Cd exposure group. This is consistent with the results of Pollack et al. (2015) and shows that Cd may be closely associated with decreased bilirubin levels. Chen, Xu et al. (2019) showed decreased TBil levels in patients from an area polluted with e-waste. We also found elevated CHE levels in polluted areas in the present study, which may be related to increased Pb levels. Our findings suggest that heavy metals exposure (including Cd and Pb) may be related to bilirubin and CHE. Further studies are required to confirm these findings.
Cd and Pb exposure levels were also affected by many factors, such as age and gender. Our study found that men had higher blood Cd and Pb levels than women, which is consistent with previous findings, and that lifestyle factors (such as smoking and drinking) may have contributed to gender differences (Liao et al., 2019; Wennberg et al., 2017). In addition, it is generally believed that blood heavy metal levels increase with age, which is associated with the long half-lives of Cd and Pb in the body. However, research on heavy metal levels in Taiwan’s population has shown that youths have higher Pb exposure levels than adults (Liao et al., 2019), and Wang et al. (2020) showed that youth (< 60 years) was associated with increased blood Cd concentrations. We found that blood Cd and Pb levels were lowest in young subjects (< 50 years old); however, the exposure levels were highest in people aged 50–60 years, which was possibly related to their occupation.
This study has several strengths. Above all, we examined the relationships between BCd and BPb levels and abnormal liver function in Chinese residents near a heavy metal pollution area, instead of focusing on animals, patients, or workers. Second, we stratified age and sex to exclude these confounders. Third, we compared the effects of Cd and Pb co-exposure and single exposure on liver function. Our results show that both Cd and Pb are hazardous and induce abnormal liver function; however, single high Cd exposure had a more profound effect on liver function than Cd and Pb co-exposure.
This study also has some limitations. Causal relationships between heavy metal levels and abnormal liver function were inferred. Prospective cohort studies are still needed to further confirm that co-exposure to Cd and Pb is a risk factor for abnormal liver function. Additionally, only the BCd and BPb levels were detected in this study, but heavy metals are usually present as mixtures in the blood; therefore, more heavy metals should be detected in future studies.
Conclusion
High BCd and BPb levels affect the liver health of local residents. Although countries around the world have taken a series of measures to regulate heavy metal pollution, more studies are required to assess the long-term effects of Cd and Pb exposure on the health of the general population. Our study concludes that Cd and Pb exposure is associated with impaired liver function. Furthermore, our data showed that Cd and Pb may have an antagonistic effect on liver function, high exposure to Cd alone could be an important risk factor for abnormal liver function, and Pb may partially offset Cd toxicity in the liver. Further studies on the interaction of Cd and Pb in the liver and the association between Cd and Pb co-exposure and liver function are needed.
References
Ai, S., Liu, B., Yang, Y., Ding, J., Yang, W., Bai, X., Naeem, S., & Zhang, Y. (2018). Temporal variations and spatial distributions of heavy metals in a wastewater-irrigated soil-eggplant system and associated influencing factors. Ecotoxicology and Environmental Safety, 153, 204–214.
Andjelkovic, M., Buha Djordjevic, A., Antonijevic, E., Antonijevic, B., Stanic, M., Kotur-Stevuljevic, J., Spasojevic-Kalimanovska, V., Jovanovic, M., Boricic, N., Wallace, D., Bulat, Z. (2019). Toxic effect of acute cadmium and lead exposure in rat blood, liver, and kidney, International Journal of Environmental Research and Public Health, 16.
Chen, R., Ma, S., Fan, C., & Tian, H. (2010). Logistic regression analysis on alcohol-related injury risk behaviour of college students and the influencing factors. Chinese Center for Disease Control and Prevention, 14(949), 52.
Chen, H., Teng, Y., Lu, S., Wang, Y., & Wang, J. (2015). Contamination features and health risk of soil heavy metals in China. Science of the Total Environment, 512–513, 143–153.
Chen, Y., Jiang, Y., Huang, H., Mou, L., Ru, J., Zhao, J., & Xiao, S. (2018). Long-term and high-concentration heavy-metal contamination strongly influences the microbiome and functional genes in Yellow River sediments. Science of the Total Environment, 637–638, 1400–1412.
Chen, X., Zhu, G., Wang, Z., Zhou, H., He, P., Liu, Y., & Jin, T. (2019a). The association between lead and cadmium co-exposure and renal dysfunction. Ecotoxicology and Environmental Safety, 173, 429–435.
Chen, Y., Xu, X., Zeng, Z., Lin, X., Qin, Q., & Huo, X. (2019b). Blood lead and cadmium levels associated with hematological and hepatic functions in patients from an e-waste-polluted area. Chemosphere, 220, 531–538.
Chi, Q., Liu, T., Sun, Z., Tan, S., Li, S., & Li, S. (2017). Involvement of mitochondrial pathway in environmental metal pollutant lead-induced apoptosis of chicken liver: Perspectives from oxidative stress and energy metabolism. Environmental Science and Pollution Research International, 24, 28121–28131.
Choi, J., Bae, S., Lim, H., Lim, J. A., Lee, Y. H., Ha, M., & Kwon, H. J. (2017). Mercury exposure in association with decrease of liver function in adults: A longitudinal study. Journal of Preventive Medicine and Public Health, 50, 377–385.
Cohen, O., Strizich, G. M., Ramos, A. R., Zee, P. C., Reid, K. J., Mani, V., Rapoport, D. M., Redline, S., Kaplan, R. C., & Shah, N. A. (2019). Sex differences in the association between smoking and sleep-disordered breathing in the hispanic community health study/study of Latinos. Chest, 156, 944–953.
Conterato, G. M., Bulcão, R. P., Sobieski, R., Moro, A. M., Charão, M. F., de Freitas, F. A., de Almeida, F. L., Moreira, A. P., Roehrs, M., Tonello, R., Batista, B. L., Grotto, D., Barbosa, F., Jr., Garcia, S. C., & Emanuelli, T. (2013). Blood thioredoxin reductase activity, oxidative stress and hematological parameters in painters and battery workers: Relationship with lead and cadmium levels in blood. Journal of Applied Toxicology, 33, 142–150.
Dai, X., Feng, L., Ma, X., & Zhang, Y. (2012). Concentration level of heavy metals in wheat grains and the health risk assessment to local inhabitants from Baiyin. Gansu, China, Advanced Materials Research, 518–523, 951–956.
Fevery, J. (2008). Bilirubin in clinical practice: A review. Liver International, 28, 592–605.
Gao, M., Yang, Y., Lv, M., Song, W., & Song, Z. (2018). Oxidative stress and DNA damage in zebrafish liver due to hydroxyapatite nanoparticles-loaded cadmium. Chemosphere, 202, 498–505.
Genchi, G., Sinicropi, M. S., Lauria, G., Carocci, A., & Catalano, A. (2020). The effects of cadmium toxicity. International Journal of Environmental Research and Public Health, 17(11), 3782.
Go, Y. M., Sutliff, R. L., Chandler, J. D., Khalidur, R., Kang, B. Y., Anania, F. A., Orr, M., Hao, L., Fowler, B. A., & Jones, D. P. (2015). Low-dose cadmium causes metabolic and genetic dysregulation associated with fatty liver disease in mice. Toxicological Sciences, 147, 524–534.
Hou, G., Surhio, M. M., Ye, H., Gao, X., Ye, Z., Li, J., & Ye, M. (2019). Protective effects of a Lachnum polysaccharide against liver and kidney injury induced by lead exposure in mice. International Journal of Biological Macromolecules, 124, 716–723.
Jurdziak, M., Gać, P., Poręba, M., Szymańska-Chabowska, A., Mazur, G., & Poręba, R. (2018). Concentration of thyrotropic hormone in persons occupationally exposed to lead, cadmium and arsenic. Biological Trace Element Research, 182, 196–203.
Kang, M. Y., Cho, S. H., Lim, Y. H., Seo, J. C., & Hong, Y. C. (2013). Effects of environmental cadmium exposure on liver function in adults. Occupational and Environmental Medicine, 70, 268–273.
Kim, J. J., Kim, Y. S., & Kumar, V. (2019). Heavy metal toxicity: An update of chelating therapeutic strategies. Journal of Trace Elements in Medicine and Biology, 54, 226–231.
Liao, K. W., Pan, W. H., Liou, S. H., Sun, C. W., Huang, P. C., & Wang, S. L. (2019). Levels and temporal variations of urinary lead, cadmium, cobalt, and copper exposure in the general population of Taiwan. Environmental Science and Pollution Research International, 26, 6048–6064.
Lin, X., Peng, L., Xu, X., Chen, Y., Zhang, Y., & Huo, X. (2018). Connecting gastrointestinal cancer risk to cadmium and lead exposure in the Chaoshan population of Southeast China. Environmental Science and Pollution Research International, 25, 17611–17619.
Liu, B., Ma, X., Ai, S., Zhu, S., Zhang, W., & Zhang, Y. (2016). Spatial distribution and source identification of heavy metals in soils under different land uses in a sewage irrigation region, northwest China. Journal of Soils and Sediments, 16, 1547–1556.
Liu, B., Ai, S., Zhang, W., Huang, D., & Zhang, Y. (2017). Assessment of the bioavailability, bioaccessibility and transfer of heavy metals in the soil-grain-human systems near a mining and smelting area in NW China. Science of the Total Environment, 609, 822–829.
Liu, X., Chen, Q., Ali, N., Zhang, J., Wang, M., & Wang, Z. (2019). Single and joint oxidative stress-related toxicity of sediment-associated cadmium and lead on Bellamya aeruginosa. Environmental Science and Pollution Research International, 26, 24695–24706.
Luo, T., Shen, M., Zhou, J., Wang, X., Xia, J., Fu, Z., & Jin, Y. (2019). Chronic exposure to low doses of Pb induces hepatotoxicity at the physiological, biochemical, and transcriptomic levels of mice. Environmental Toxicology, 34, 521–529.
Matović, V., Buha, A., Ðukić-Ćosić, D., & Bulat, Z. (2015). Insight into the oxidative stress induced by lead and/or cadmium in blood, liver and kidneys. Food and Chemical Toxicology, 78, 130–140.
McGill, M. R. (2016). The past and present of serum aminotransferases and the future of liver injury biomarkers. EXCLI Journal, 15, 817–828.
Mitkovska, V. I., Dimitrov, H. A., Chassovnikarova, T. G., 2020. Chronic exposure to lead and cadmium pollution results in genomic instability in a model biomonitor species (Apodemus flavicollis Melchior, 1834), Ecotoxicology and Environmental Safety, 194, 10413.
Mitra, P., Sharma, S., Purohit, P., & Sharma, P. (2017). Clinical and molecular aspects of lead toxicity: An update. Critical Reviews in Clinical Laboratory Sciences, 54(7–8), 506–528.
Nakata, H., Nakayama, S., Yabe, J., Muzandu, K., Toyomaki, H., Yohannes, Y. B., Kataba, A., Zyambo, G., Ikenaka, Y., Choongo, K., Ishizuka, M., 2021. Clinical biochemical parameters associated with the exposure to multiple environmental metals in residents from Kabwe, Zambia, Chemosphere, 262: 127788.
Nakhaee, S., Amirabadizadeh, A., Brent, J., & Mehrpour, O. (2019). Impact of chronic lead exposure on liver and kidney function and haematologic parameters. Basic & Clinical Pharmacology & Toxicology, 124, 621–628.
Nordberg, G. F. (1996). Current issues in low-dose cadmium toxicology: Nephrotoxicity and carcinogenicity. Environmental Sciences, 4, 133–147.
Omotosho, I. O. (2019). Oxidative stress indices as markers of lead and cadmium exposure toxicity in auto technicians in Ibadan, Nigeria. Oxidative Medicine and Cellular Longevity, 2019, 3030614.
Orr, S. E., & Bridges, C. C. (2017). Chronic kidney disease and exposure to nephrotoxic metals. International Journal of Molecular Sciences, 18(5), 1039.
Pandya, C. D., Pillai, P. P., & Gupta, S. S. (2010). Lead and cadmium co-exposure mediated toxic insults on hepatic steroid metabolism and antioxidant system of adult male rats. Biological Trace Element Research, 134, 307–317.
Park, E., Kim, J., Kim, B., Park, E. Y. (2021). Association between environmental exposure to cadmium and risk of suspected non-alcoholic fatty liver disease, Chemosphere, 266, 128947.
Peters, J. L., Perry, M. J., McNeely, E., Wright, R. O., Heiger-Bernays, W., Weuve, J. (2021). The association of cadmium and lead exposures with red cell distribution width, PloS one, 16 (1), e0245173.
Pillai, P., Patel, R., Pandya, C., & Gupta, S. (2009). Sex-specific effects of gestational and lactational coexposure to lead and cadmium on hepatic phase I and phase II xenobiotic/steroid-metabolizing enzymes and antioxidant status. Journal of Biochemical and Molecular Toxicology, 23, 419–431.
Pollack, A. Z., Mumford, S. L., Mendola, P., Perkins, N. J., Rotman, Y., Wactawski-Wende, J., & Schisterman, E. F. (2015). Kidney biomarkers associated with blood lead, mercury, and cadmium in premenopausal women: A prospective cohort study. Journal of Toxicology and Environmental Health. Part A, 78, 119–131.
Puerto-Parejo, L. M., Aliaga, I., Canal-Macias, M. L., Leal-Hernandez, O., Roncero-Martín, R., Rico-Martín, S., Moran, J. M. (2017). Evaluation of the dietary intake of cadmium, lead and mercury and its relationship with bone health among postmenopausal women in Spain, International Journal of Environmental Research and Public Health, 14.
Qu, Y., Cai, J., Chen, X., Zheng, L., Huang, L., Yang, J., Ye, X., Wang, Q., Si, G., & Cao, Z. (2020). Association of cadmium pollution with liver function of population in mineral polluted areas of Guangxi. Zhonghua Yu Fang Yi Xue Za Zhi, 54, 839–843.
Ren, L., Qi, K., Zhang, L., Bai, Z., Ren, C., Xu, X., Zhang, Z., & Li, X. (2019). Glutathione might attenuate Cadmium-induced liver oxidative stress and hepatic stellate cell activation. Biological Trace Element Research, 191, 443–452.
Richard, E. E., Augusta Chinyere, N. A., Jeremaiah, O. S., Opara, U. C., Henrieta, E. M., & Ifunanya, E. D. (2016). Cement dust exposure and perturbations in some elements and lung and liver functions of cement factory workers. J Toxicol, 2016, 6104719.
Salińska, A., Włostowski, T., & Zambrzycka, E. (2012). Effect of dietary cadmium and/or lead on histopathological changes in the kidneys and liver of bank voles Myodes glareolus kept in different group densities. Ecotoxicology, 21, 2235–2243.
Sanders, A. P., Mazzella, M. J., Malin, A. J., Hair, G. M., Busgang, S. A., Saland, J. M., Curtin, P. (2019). Combined exposure to lead, cadmium, mercury, and arsenic and kidney health in adolescents age 12–19 in NHANES 2009–2014, Environment International, 131, 104993.
Satarug, S., & Moore, M. R. (2004). Adverse health effects of chronic exposure to low-level cadmium in foodstuffs and cigarette smoke. Environmental Health Perspectives, 112, 1099–1103.
Satarug, S., Gobe, G. C., Vesey, D. A., & Phelps, K. R. (2020). Cadmium and lead exposure. Nephrotoxicity, and Mortality, Toxics, 8(4), 86.
Singh, N., Kumar, A., Gupta, V. K., & Sharma, B. (2018). Biochemical and molecular bases of lead-induced toxicity in mammalian systems and possible mitigations. Chemical Research in Toxicology, 31(10), 1009–1021.
Treem, W. R., Palmer, M., Lonjon-Domanec, I., Seekins, D., Dimick-Santos, L., Avigan, M. I., Marcinak, J. F., Dash, A., Regev, A., Maller, E., Patwardhan, M., Lewis, J. H., Rockey, D. C., Di Bisceglie, A. M., Freston, J. W., Andrade, R. J., & Chalasani, N. (2021). Consensus guidelines: best practices for detection, assessment and management of suspected acute drug-induced liver injury during clinical trials in adults with chronic viral hepatitis and adults with cirrhosis secondary to hepatitis B, C and nonalcoholic steatohepatitis. Drug Safety, 44, 133–165.
Tsai, T. L., Kuo, C. C., Pan, W. H., Chung, Y. T., Chen, C. Y., Wu, T. N., & Wang, S. L. (2017). The decline in kidney function with chromium exposure is exacerbated with co-exposure to lead and cadmium. Kidney International, 92, 710–720.
Wang, L., Li, J., Li, J., & Liu, Z. (2010). Effects of lead and/or cadmium on the oxidative damage of rat kidney cortex mitochondria. Biological Trace Element Research, 137, 69–78.
Wang, B., Du, Y. (2013). Cadmium and its neurotoxic effects, Oxid Med Cell Longev, 898034.
Wang, D. H., Xu, H., Zheng, Y. H., Gu, D. S., Zhu, Y. J., Ren, Y., Wang, S. C., Yang, L., Xu, L. W. (2020). Environmental exposure to lead and cadmium and hearing loss in Chinese adults: A case-control study, PLoS One, 15, e0233165.
Wennberg, M., Lundh, T., Sommar, J. N., & Bergdahl, I. A. (2017). Time trends and exposure determinants of lead and cadmium in the adult population of northern Sweden 1990–2014. Environmental Research, 159, 111–117.
Werder, E. J., Beier, J. I., Sandler, D. P., Falkner, K. C., Gripshover, T., Wahlang, B., Engel, L. S., Cave, M. C. (2020). Blood BTEXS and heavy metal levels are associated with liver injury and systemic inflammation in Gulf states residents. Food and Chemical Toxicology, 139, 111242.
WHO. (1995). Environmental Health Criteria 165. inorganic lead, World Health Organization.
Wu, X., Liang, Y., Jin, T., Ye, T., Kong, Q., Wang, Z., Lei, L., Bergdahl, I. A., & Nordberg, G. F. (2008). Renal effects evolution in a Chinese population after reduction of cadmium exposure in rice. Environmental Research, 108, 233–238.
Yang, G., Sun, T., Han, Y. Y., Rosser, F., Forno, E., Chen, W., Celedón, J. C., 2019. Serum Cadmium and Lead, Current Wheeze, and Lung Function in a Nationwide Study of Adults in the United States. The Journal of Allergy and Clinical Immunology: In Practice, 7, 2653–60 e3.
Yu, D., Zhang, L., Yu, G., Nong, C., Lei, M., Tang, J., Chen, Q., Cai, J., Chen, S., Wei, Y., Xu, X., Tang, X., Zou, Y., & Qin, J. (2020). Association of liver and kidney functions with Klotho gene methylation in a population environment exposed to cadmium in China. International Journal of Environmental Health Research, 30, 38–48.
Zhai, H., Chen, C., Wang, N., Chen, Y., Nie, X., Han, B., Li, Q., Xia, F., & Lu, Y. (2017). Blood lead level is associated with non-alcoholic fatty liver disease in the Yangtze River Delta region of China in the context of rapid urbanization. Environmental Health, 16, 93.
Zhang, H., Yan, J., Niu, J., Wang, H., Li, X. (2021). Association between lead and cadmium co-exposure and systemic immune inflammation in residents living near a mining and smelting area in NW China, Chemosphere, 287 (3),132190.
Zou, H., Sun, J., Wu, B., Yuan, Y., Gu, J., Bian, J., Liu, X., & Liu, Z. (2020). Effects of cadmium and/or lead on autophagy and liver injury in rats. Biological Trace Element Research, 198, 206–215.
Acknowledgements
We express thanks to all the participants, postgraduates of the Lanzhou University, research nurses in the First Hospital of Lanzhou University, and all members of the study team.
Funding
This work was supported by the National Natural Science Foundation of China (No. 32060289, No. 81872578), Natural Science Foundation of Gansu Province (No. 20JR10RA699), and Science and Technology Foundation of Gansu Province (No. 18JR2TA018).
Author information
Authors and Affiliations
Contributions
JY contributed to conceptualization, investigation, writing—review and editing, and funding acquisition. HZ contributed to data curation, validation, investigation, and writing—original draft. JN contributed to methodology and funding acquisition. Bin Luo contributed to supervision and investigation. HW contributed to formal analysis and writing—review and editing. MT curated the data. XL contributed to supervision, project administration, resources, and funding acquisition.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yan, J., Zhang, H., Niu, J. et al. Effects of lead and cadmium co-exposure on liver function in residents near a mining and smelting area in northwestern China. Environ Geochem Health 44, 4173–4189 (2022). https://doi.org/10.1007/s10653-021-01177-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-021-01177-6