Abstract
Cadmium (Cd) is a ubiquitous environmental pollutant with an exceptionally long biological half-life. The liver is a major organ for Cd metabolism, but the toxicity of Cd is unclear. This study sought to determine whether blood Cd (BCd) level (representing recent exposure [months] to Cd) was associated with liver function in Korean adults, both cross-sectionally and longitudinally. The baseline cross-sectional study involved 2,086 adults (male: 908, female: 1,178) in 2010 − 2011, and 503 of them (male: 207, female: 296) were followed up in 2014 − 2015. BCd was measured by graphite-furnace atomic absorption spectrometry, and liver function indices (aspartate aminotransferase [AST], alanine aminotransferase [ALT], and γ-glutamyltransferase [GGT]) were determined. Liver damage was defined as an abnormal elevation of more than one liver function index. The geometric mean of BCd (1.07 μg/L) was higher in females than in males (1.16 vs. 0.96 μg/L). Liver function indices increased significantly in a dose-dependent manner according to the BCd levels, except for ALT in males, and were higher in males than in females. BCd level was also associated with the risk of liver damage in both sexes. No significant changes in BCd were observed between baseline and follow-up. The liver function indices in 2014 − 2015 were comparable to those in 2010 − 2011 in males, while ALT and GGT were significantly increased in 2014 − 2015 compared to 2010 − 2011 in females with relatively high BCd. These findings suggest that even a low level of environmental Cd exposure, short- and long-term, may affect liver function, and females appear more susceptible than males.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Cadmium (Cd) is a non-essential element that tends to accumulate in the human body, causing toxicity and carcinogenicity (IARC 1993; ATSDR 2012). Cd may also cause various non-carcinogenic diseases, including lung, kidney, bone, liver, cardiovascular system, and reproductive system, as well as increased mortality (Godt et al. 2006; Menke et al. 2009; Tinkov et al. 2018).
Cd is a major environmental pollutant emitted from natural/geogenic sources as well as anthropogenic sources mainly related to the activities of mining and industries, such as smelting, refining, electroplating, battery production, fertilizers, color pigments, and stabilizers (ATSDR 2012; Genchi et al. 2020). It is well known that Cd is persistent in the environment, is not biodegradable, and has an exceptionally long half-life (25 − 30 years) in the body (ATSDR 2012). The absorbed Cd into the body is deposited in the liver initially, then transported to the kidneys, and eventually accumulated there. In our previous estimation of the reference Cd levels in the general Korean population, the mean concentration of Cd in the renal cortex was approximately nine times higher than that in the liver, and the difference between the Cd concentration in the renal cortex and liver increased with biological age up to the 50 s, and plateaued after that, differences were not large prior to age 10 (Park et al. 2000). This finding supported that Cd preferentially accumulates in the kidney and may cause kidney damage due to its long biological half-life (Järup et al. 1998; Bernard 2004). That long-term exposure to Cd can cause kidney damage was first observed in 1912 and identified in 1968 as “Itai-Itai” (“ouch-ouch”) disease by the Japanese Ministry of Health and Welfare (Tsuchiya 1969). Since then, Cd-induced kidney damage has also been reported in the general population under the environmental exposure of low-dose Cd and in workers with occupational exposure to Cd (Järup et al. 1998; Järup and Åkesson 2009; Eom et al. 2017).
The liver is a vital organ, the major site of xenobiotic metabolism, and the second main organ of Cd accumulation in the body (Arroyo et al. 2012). Liver Cd represents recent exposure (months), including chronic exposure, while kidney Cd is more indicative of long-term exposure. Although urinary Cd and blood Cd (BCd) are useful indicators of body Cd exposure, urinary Cd may more closely reflect chronic exposure, such as total body burden, and BCd may reflect more recent exposure (Godt et al. 2006; Arroyo et al. 2012).
Previously, liver damage by Cd was reported in experimental animal studies, both acute and chronic (Dudley et al. 1984, 1985; Habeebu et al. 2000). However, liver toxicity in Cd-exposed workers has been rarely reported (Attia et al. 2009; ATSDR 2012; Tomei et al. 2013), and no evidence for Cd-induced liver damage was found in the general Japanese population (Ikeda et al. 1997, 2000). During the last decade, several epidemiology studies have reported liver damage due to environmental exposure to low levels of Cd in the general population (Hyder et al. 2013; Kang et al. 2013; Chung et al. 2020; Hong et al. 2021), representing a grave concern to human health. However, these previous epidemiology studies of the association between Cd exposure and liver damage were limited to cross-sectional studies. Therefore, further studies are required to substantiate the association between environmental Cd exposure and liver damage in the general population and to evaluate whether current Cd exposure levels may adversely affect liver function in the short- and long-term.
The body burden of Cd in Koreans is higher than that in several Western countries, including the US (Becker et al. 2002; Akerstrom et al. 2013; CDC 2015; Health Canada 2015). Recently, non-alcoholic fatty liver disease (NAFLD) has been increasing worldwide, including in Korea, and liver disease is one of the major causes of death in the Korean population, ranking as the eighth leading cause of death (Lee et al. 2019; Statistics Korea 2021). Therefore, there is an urgent need to evaluate the adverse effects of environmental low-level Cd exposure on liver function in order to take a stand regarding policies in protecting and promoting human health by reducing Cd exposure in the general population.
In this study, we investigated the relation between BCd and liver function using hepatic enzyme activities (aspartate aminotransferase [AST], alanine aminotransferase [ALT], and γ- glutamyltransferase [GGT]) in the general Korean population. Furthermore, we evaluated whether the levels of BCd were predictive of the changes in liver function after 4 − 5 years.
Materials and Methods
Study Subjects
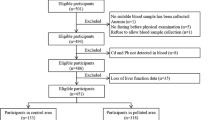
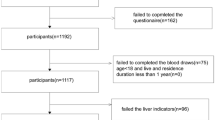
This study used the data from a cross-sectional (baseline) study in 2010 − 2011 and a follow-up study 4 − 5 years later, in 2014 − 2015. The baseline data, drawn from a representative sample of Korean adults (Lim et al. 2015), included 2086 healthy adults (male: 908, female: 1178) aged 19 years or over who had not been exposed to Cd occupationally. The study subjects were sampled nationwide from 102 different sampling sites by a stratified (sex and age) probability method. The number of study subjects in each site was allocated by the square root proportional method based on the population size of each relevant district. In the follow-up study, 4 − 5 years later, 503 subjects (male: 207, female: 296) among the baseline study subjects voluntarily participated in blood sampling. A personal interview was performed using a structured questionnaire by well-trained personnel to collect demographic information, such as age, gender, smoking, alcohol drinking, education level, and occupational history. Smoker was defined as smoking at least 100 cigarettes during their lifetime, and non-drinker was defined as consuming no alcohol during 1 month prior to the interview. Height, weight, and blood pressure were measured, and body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. BMI over 25 kg/m2 was defined as obese or overweight, specifically for Koreans (Seo et al. 2019). The study protocol was approved by the Chung-Ang University Ethical Committee for Medical Research and Other Studies Involving Human Subjects in 2010 − 2011 (CAUEC 2010-06-01) and the Institutional Review Board of Dankook University Hospital in 2014 − 2015 (DKUH 2014-02-016). Written consent was obtained from every participant in both studies.
Blood Sampling
Blood sampling was performed by experienced nurses. Whole blood for BCd determination was sampled with a heparin-treated vacutainer tube. The serum was separated by centrifugation at 1000 X g for 10 min from whole blood collected in a serum separating tube (SST) of polystyrene. Whole blood was stored at − 80 °C for Cd analysis.
Analysis of Cd in Whole Blood
The concentration of Cd in whole blood was determined using two different atomic absorption spectrometers equipped with graphite-furnace (GF-AAS; Thermo, Inc., Cambridge, UK; AAnalyst 600, Perkin-Elmer, Norwalk, CT) in 2010 − 2011 and 2014 − 2015, respectively. In brief, whole blood was diluted using 0.2% Triton X-100 with 1% nitric acid and 0.2% diammonium hydrogen phosphate and then mixed vigorously. A 15 μL aliquot of the diluted sample was injected into a graphite tube, following three steps of dry, ash, and atomization. The analysis of Cd in whole blood was validated in terms of linearity of calibration curve, accuracy and precision using standard reference material (SRM, Bio-Rad Lyphochek Whole Blood Metals Control, Irvine, CA) with an international quality control program (EQUAS, Germany). The calibration curve was linear (r = 0.999), recovery and coefficient of variation of SRM was 98.7 and 2.76%, respectively. The limit of detection for Cd in blood was 0.1 μg/L. As mentioned above, the analytical instrument used in the follow-up study differed from that used in the baseline study. The consistency in analysis of BCd according to measuring period, 2010 − 2011 or 2014 − 2015, was evaluated by repeated measurements of the Cd concentration for 30 samples of whole blood sampled in 2010 − 2011. The difference from the values at baseline was not significant and well-correlated with the re-measured values (r = 0.933). BCd below the detection limit was assigned the value of the detection limit divided by the square root of two.
Measurement of AST, ALT, and GGT
Liver function was evaluated by measuring the activities of AST, ALT, and GGT in the serum using enzymatic methods on a chemistry autoanalyzer (Roche-Hitachi Cobas 8000 c702, Roche Diagnostics, Germany): ASTL reagent (Roche, Germany) to measure AST and ALT levels, and GGT-2 reagent (Roche, Germany) to measure GGT activity. The criteria for abnormal liver function included AST (> 40 IU/L), ALT (> 40 IU/L), and GGT (> 73 IU/L for males and > 48 IU/L for females). Liver damage was defined as meeting more than one criterion for abnormal liver function (AST, ALT, and GGT).
Statistical Analyses
Statistical analyses were performed with SAS version 9.4 (SAS Institute, Cary, NC). The concentration of BCd was log-transformed because the data were log-normally distributed rather than normally distributed. BCd was presented as geometric mean (GM) and geometric standard deviation (GSD), and age, height, weight, BMI, and liver function indices were expressed as arithmetic mean (AM) and standard deviation (SD). Means were compared by performing the Student t-test or analysis of variance (ANOVA), followed by Duncan’s multiple comparison test. The levels of BCd were categorized into tertiles (lowest, intermediate, and highest) based on the baseline study in 2010 − 2011. The generalized linear model (GLM) was performed to compare the adjusted means of liver function indices according to the BCd levels after controlling for various potential confounding factors, such as age, smoking, alcohol drinking, education level, and BMI. The association of BCd levels with liver damage was evaluated by multivariate logistic regression analyses, and the risk of liver damage was presented with the odds ratio (OR) and the 95% confidence interval (CI) according to the BCd levels. In addition, the differences in BCd and liver function indices between the 2010 − 2011 and 2014 − 2015 studies were analyzed by a paired t-test in the repeated measured individuals. The level of statistical significance was set at p < 0.05.
Results
Baseline Study
The mean age of the study subjects in the baseline study (2010 − 2011) was 45.7 years and was not significantly different between males and females. Height, weight, and BMI were higher in males than in females. The liver function indices (AST, ALT, and GGT) were significantly higher in males than in females. The GM concentration of BCd was 1.07 μg/L in the total study subjects and was significantly higher in females (1.16 μg/L) than in males (0.96 μg/L). The AM concentration of BCd was 1.24 μg/L (1.12 μg/L in males and 1.34 μg/L in females), and the 95th percentile of BCd was 2.58 μg/L (2.35 μg/L in males and 2.69 μg/L in females, Table 1). Therefore, further analyses were performed according to sex.
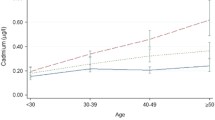
The level of BCd was significantly increased according to the age group in both males and females. BCd was significantly increased by smoking habit in males, being highest in current smokers, followed by ex-smokers and non-smokers. No statistical significance was observed for smoking habits in females. The level of BCd was higher in drinkers than non-drinkers in males, while the opposite pattern was observed in females. The highest BCd was observed in the less educated males and females (Table 2). AST, ALT, and GGT increased by age group and generally increased inversely with the education level, except for ALT in males. GGT was higher in smokers (both males and females) and higher in drinkers in males only. The effects of smoking or alcohol drinking on AST and ALT were not consistent between males and females. The levels of liver function indices (ALT, AST, and GGT) and BCd were significantly higher in obese or overweight people (BMI ≥ 25 kg/m2) than in people with a BMI below 25 kg/m2 (Table 2).
The BCd level was divided into tertiles in males (Q1, low 1/3, < 0.79; Q2, intermediate 1/3, 0.79 − 1.23; Q3, high 1/3, ≥ 1.23) and females (Q1, < 0.97; Q2, 0.97 − 1.48; Q3, ≥ 1.48), respectively, to analyze the associations between BCd and the liver function indices. AST and GGT were significantly increased in a dose-dependent manner in both males and females, respectively, according to the BCd levels. These statistical significances remained after adjustment for age or various confounding variables, such as age, smoking, alcohol drinking, education level, and BMI. ALT also significantly increased according to BCd in females but not males (Table 3). The proportion of liver damage was 23.0% (209/908) in males and 6.8% (80/1,178) in females and increased dose-dependently to 17.2, 20.1, and 31.7% in males and 3.1, 6.4, and 10.9% in females in the lowest, intermediate, and highest tertiles of BCd, respectively. The risk of liver damage was significantly higher by 2.23 and 3.89 times in the highest tertile of BCd compared to the lowest tertile of BCd in males and females, respectively. The significant risk of liver damage by BCd level remained after adjustment against the potential confounding variables, such as age, smoking, alcohol drinking, education level, and BMI (Table 4).
Follow-up Study
The levels of BCd and liver function indices of 503 study subjects who were followed up in 2014 − 2015 among 2,086 study subjects who participated in the baseline study in 2010 − 2011 are shown in Table 5. No significant changes in BCd were observed between the baseline and follow-up studies in both males and females, respectively. The levels of liver function indices were similar between the baseline and follow-up studies in males. In females, although AST was not different between both studies, significant increases in ALT and GGT were observed in 2014 − 2015 compared to 2010 − 2011. Longitudinal changes in liver function indices according to the BCd levels based on the 2010 − 2011 study are presented in Table 6. No significant changes in AST, ALT, and GGT according to the BCd level were observed between the 2010 − 2011 and 2014 − 2015 studies in males. In females, although ALT was not different between the 2010 − 2011 and 2014 − 2015 studies in the lowest tertile of BCd, it was significantly increased in 2014 − 2015 compared to 2010–2011 in the intermediate and highest tertiles of BCd in females. Additionally, GGT was significantly higher in 2014 − 2015 compared to 2010 − 2011 in the intermediate tertile of BCd in females. Namely, increases in some liver function indices were observed in females with relatively high BCd in the 2010 − 2011 study exposed to a low environmental level of Cd in the general population (Table 6).
Discussion
This study analyzed whether environmental low-level Cd exposure affects liver function. The GM concentration of Cd in whole blood was 1.07 μg/L in the general Korean adult population. The mean levels of the liver function indices were 22.0 IU/L in AST, 21.2 IU/L in ALT, and 33.5 IU/L in GGT. Although the levels of BCd and liver function indices were similar to the previous reports on the general population of Korea (Kang et al. 2013; Park et al. 2021), the BCd from this study is still higher than those in the US and Western countries (Becker et al. 2002; Akerstrom et al. 2013; CDC 2015; Health Canada 2015), but it is similar or low compared to other Asian countries (Kurihara et al. 2004; Nie et al. 2016). One possible explanation for the higher level of BCd in Koreans, including other Asian populations, compared to Western populations could be their staple food, rice. Cd tends to accumulate more than other toxic metals in plants, especially rice, because of its relatively high bioavailability, and it can be readily transferred from soil to plants because of its higher mobility compared to other non-essential heavy metals, such as lead and mercury (Satarug et al. 2003; Liu et al. 2005; Cai et al. 2015; Moon et al. 2021). The main exposure source of Cd in the general population is the diet. In our previous study, the daily Cd intake from food in Koreans was estimated at 7.07 μg/day, of which approximately 33% came from grains, followed by seafood (29%), vegetables (20%) and others (Huang et al. 2013). However, the highest concentration of BCd was 5.72 μg/L, and only 3 among 2,086 study subjects had a BCd over 5 μg/L, the biological exposure index proposed by the American Conference of Governmental Industrial Hygienists (ACGIH 2014). This finding indicates that the study subjects had been exposed to low environmental levels of Cd.
In the study subjects, the BCd concentrations and liver function indices generally tended to increase with age, lower education level, and obesity in both males and females. These findings are concurrent with the results of previous studies. Age is a significant contributing factor to the liver enzyme levels and the body Cd level (Ikeda et al. 1997; Kang et al. 2013). Obesity (i.e., increased BMI) is closely linked to metabolic liver dysfunction and its progression to fatty liver (Kojima et al. 2003; Danielsson et al. 2014). In addition, individual lifestyles, such as smoking and alcohol drinking, may also contribute to increased Cd exposure, impaired liver function, or both. Though we have not measured Cd contents in cigarette and alcohol in this study, cigarette smoking is a well-known major source of environmental exposure to Cd equivalent to 0.5 – 1 μg/cigarette (Satarug and Moore 2004). Blood Cd was higher in drinkers than in non-drinkers (Martins et al. 2020) also was significantly higher in relation to alcohol consumption in male smokers (Choi et al. 2020). In addition, excessive smoking and alcohol drinking affect liver function (Klatsky and Armstrong 1992; El-Zayadi 2006; Danielsson et al. 2014; Åberg et al. 2020). Therefore, individual lifestyles including smoking and alcohol drinking could contribute to the potential health issues by Cd exposure. In the current study, the BCd concentrations were increased by smoking habits and alcohol drinking in males but not in females, and no significant effects of smoking and alcohol drinking on AST and ALT were observed in both sexes. However, GGT was affected by smoking in both sexes and alcohol drinking in males only. Some inconsistent effects of smoking and alcohol drinking on liver function compared to previous studies could be ascertained and partially explained by several factors, such as the limited number of smokers among females and the relatively broad definition of alcohol drinkers. Conversely, some epidemiology studies have reported that smoking and alcohol drinking were not associated with hepatic steatosis (Chung et al. 2020), and the effects of smoking or alcohol drinking were inconsistent according to liver function parameters (Danielsson et al. 2014; Hong et al. 2021). However, smoking and alcohol drinking were considered as potential risk factors that could affect liver function in the further analysis of this study, along with age, education level, and obesity.
Cd-induced liver damage has been demonstrated, both after acute and chronic exposure, in several experimental animal studies (Dudley et al. 1984, 1985; Habeebu et al. 2000). The mechanisms of liver damage by Cd, in vitro and in vivo studies, are suggested to involve direct and indirect oxidative stress processes, such as increases in the production of reactive oxygen species, decreases in antioxidative enzymes and non-enzymatic antioxidants, inflammation by activation of Kupffer cells and increased production of interleukin-1-beta (IL-1β), interleukin-8 (IL-8), and C-reactive protein (CRP), and abnormal lipid metabolism by stimulation of c-Jun N-terminal kinase activation (Rikans and Yamano 2000; Go et al. 2015; Mezynska and Brzóska 2018; Werder et al. 2020; Wang et al. 2021) as well as Cd in the liver exceeding the hepatic metallothionein available to sequester Cd (Habeebu et al. 2000). A few studies have reported the effect of Cd exposure on liver damage in industrial workers, but the results did not support that Cd was a causative agent of liver injury in occupationally Cd-exposed workers (Attia et al. 2009; Tomei et al. 2013). Furthermore, Ikeda et al. (1997, 2000) reported that environmental Cd exposure did not affect liver function among general Japanese females, despite relatively high Cd levels in the blood (GM of 2.17 and 1.76 μg/L) and urine (1.98 and 3.94 μg/g of creatinine). Similarly, no association between blood BCd and ALT elevation was observed in the US adult general population (Cave et al. 2010). These findings might have downplayed the concerns of liver function damage induced by low-dose, chronic environmental exposure to Cd in the general population.
Evidence suggests that the BCd level accepted as safe for the general population (5 μg/L) may be too high (ACGIH 2014). An association between urinary Cd and liver dysfunction has been reported in the US based on the National Health and Nutrition Examination Survey (NHANES) in 1988 − 1994 (Hyder et al. 2013) and the 1999 − 2015 NHANES (Hong et al. 2021). Hyder et al. (2013) indicated an increased risk of hepatic necroinflammation in the highest quartile of urinary Cd (≥ 0.65 μg/g of creatinine for males, ≥ 0.83 μg/g of creatinine for females) compared to the lower quartiles in both males (OR 2.21, 95% CI 1.64 − 3.00) and females (OR 1.26, 95% CI 1.01 − 1.57). Furthermore, Hong et al. (2021) presented that liver function parameters, including ALT, AST, and GGT, were positively associated with the urinary Cd level in study subjects with a GM of 0.27 μg/g of creatinine. In both of these previous studies, the levels of urinary Cd were much lower than those in the Korean general population, with a GM of 0.82 and 1.36 μg/g of creatinine in males and females, respectively (Eom et al. 2017). Based on data from the Korea National Health and Nutrition Examination Survey (KNHANES) in 2008 − 2009, the level of BCd under environmental exposure was associated with an elevation of the serum liver enzymes AST, ALT, and alkaline phosphatase (ALP) in the Korean adult population (Kang et al. 2013). Subsequent studies indicated that BCd was associated with an increased risk of hepatic fibrosis in females in 2016 and 2017 (Chung et al. 2020) and with the risk of NAFLD in 2008 − 2013, 2016, and 2017 (Park et al. 2021) based on KNHANES. Those studies concerned environmental exposure to Cd in the general population, and the levels of BCd were similar to those in this study population (GM, 1.07 μg/L), which was performed independently from KNHANES.
In the present study, the serum activity of liver enzymes increased dose-dependently according to the BCd level in both males and females, except for ALT activity, which increased dose-dependently according to the BCd level in females only. However, the risk of liver damage was significantly higher in the highest tertile of BCd (OR 2.23, 95% CI 1.52 − 3.28) compared to the lowest tertile in males, while it was significantly higher in both the intermediate tertile (OR 2.15, 95% CI 1.07 − 4.34) and the highest tertile of BCd (OR 3.89, 95% CI 2.02 − 7.50) compared to the lowest tertile in females. The significantly increased risks of liver damage in the highest tertile of BCd compared to the lowest tertile of BCd (≥ 1.23 vs. < 0.79 for males; ≥ 1.48 vs. < 0.97 for females) remained in both males and females (risk increased by 2.26- and 2.93-fold, respectively) after controlling for potential confounding variables, such as age, smoking, alcohol drinking, education level, and BMI as well as age. The cutoffs for the highest tertile of BCd in this study could not be compared directly with the previous mean BCd (1.98 ± 0.70 μg/L) in the highest quartile of BCd, which was significantly (p < 0.0001) associated with elevated liver enzyme levels (Kang et al. 2013), but the values were comparable with the cutoffs for BCd of 0.96 and 1.41 μg/L, for suspected NAFLD and hepatic steatosis, respectively, suggested by Park et al. (2021). Meanwhile, the suggested Cd levels associated with abnormal liver function, including those in the current study, are much lower than the recommended biological exposure index (ACGIH 2014). Furthermore, the BCd was not different between the baseline (2010 − 2011) and follow-up (2014 − 2015) studies in the subjects who participated in both studies (503/2086), but a statistically significant elevation of ALT or GGT or both were observed in the tertiles of BCd in females, except for the lowest tertile. These findings suggest that a relatively high BCd exceeding 0.97 μg/L, even after low environmental exposure to Cd, may affect liver function in the short- and long-term only in females.
The susceptibility of human health to Cd according to sex varies among researchers. Namely, Itai-Itai disease was prevalent in females, especially those over the age of 40 and those who had experienced multiple deliveries (Tsuchiya 1969). In Korean adults, females were more susceptible to environmental Cd exposure (Huang et al. 2013), and an association between BCd and hepatic fibrosis was reported in females only (Chung et al. 2020). The mortality risk was more closely associated with an increase in urinary β2-microglobulin in females than in males in a Cd-polluted area of Japan (Nishijo et al. 2004). By contrast, urinary Cd was associated with an increased risk of all-cause, cancer, and cardiovascular disease mortality among male US adults but not among female US adults (Menke et al. 2009), and environmental Cd exposure was more associated with liver dysfunction in males than in females among US adults (Hyder et al. 2013). Susceptibility to Cd by sex might be explained partially by a relatively lower level of essential minerals, such as iron and zinc, activation of receptor-mediated calcium channels by progesterone, and endocrine-like effects in the body as endocrine disruptor (Åkesson et al. 2002; Baker et al. 2003; Ryu et al. 2004; Vahter et al. 2007; Strumylaite et al. 2019; White et al. 2019; Wei and Zhu 2020). However, the mechanism of action is still controversial yet. This study has several limitations. In particular, the interval between the baseline and follow-up studies was not enough to respond to additional Cd exposure, the proportion of baseline study subjects who participated in the follow-up study was relatively low (approximately 24%, 503/2086), and the follow-up was limited to once only. Additionally, we could not provide the data of measuring values, such as levels of inflammatory molecules, reactive oxygen species, MT, and endocrine disruptor-related hormones, to clarify the mechanisms of Cd-induced liver damage and the gender susceptibility to the environmental low-dose Cd exposure in this human population study. Therefore, we consider that more comprehensive further studies are needed to reach a definite conclusion in the future. Nevertheless, our results should be meaningful in the management of environmental pollutants by evaluating whether the current Cd exposure can simultaneously affect liver function in the short- and long-term through cross-sectional and longitudinal observations.
In summary, the GM concentration of BCd was 1.07 μg/L in Korean adults, liver injury and the risk of liver damage increased according to the BCd level in both males and females, and ALT and GGT increased in the relatively high BCd group 4 − 5 years after the baseline study in females but not in males. These findings indicate that environmental Cd exposure can cause liver damage in both the short- and long-term and may be more pronounced in females. Therefore, meticulous control to reduce exposure to Cd by improving individual lifestyles such as smoking, alcohol drinking, and diet habits, and efficient reduction policies of production, release, and use of Cd are necessary to prevent liver-related diseases and to promote public health.
References
Åberg F, Färkkilä M, Männistö V (2020) Interaction between alcohol use and metabolic risk factors for liver disease: a critical review of epidemiological studies. Alcohol Clin Exp Res 44:384–403. https://doi.org/10.1111/acer.14271
ACGIH (2014) TLVs and BEIs: based on the documentation of the threshold limit values for chemical substances and physical agents and biological exposure indices. In: American conference of governmental industrial hygienists, Cincinnati, OH
Akerstrom M, Barregard L, Lundh T, Sallsten G (2013) The relationship between cadmium in kidney and cadmium in urine and blood in an environmentally exposed population. Toxicol Appl Pharmacol 268:286–293. https://doi.org/10.1016/j.taap.2013.02.009
Åkesson A, Berglund M, Schütz A, Bjellerup P, Bremme K, Vahter M (2002) Cadmium exposure in pregnancy and lactation in relation to iron status. Am J Public Health 92:284–287. https://doi.org/10.2105/ajph.92.2.284
Arroyo VS, Flores KM, Ortiz LB, Gómez-Quiroz LE, Gutiérrez-Ruiz MC (2012) Liver and cadmium toxicity. J Drug Metab Toxicol S 5:001. https://doi.org/10.4172/2157-7609.S5-001
ATSDR (2012) Toxicological profile for cadmium. Agency for Toxic Substances and Disease Registry, Division of Toxicology, Atlanta, GA
Attia DI, El Mahdy NM, Radwan NM (2009) Evaluation of health hazards among cadmium-exposed workers. Egypt J Occup Med 33:233–251. https://doi.org/10.21608/ejom.2009.681
Baker TK, VanVooren HB, Smith WC, Carfagna MA (2003) Involvement of calcium channels in the sexual dimorphism of cadmium-induced hepatotoxicity. Toxicol Lett 137:185–192. https://doi.org/10.1016/S0378-4274(02)00402-2
Becker K, Kaus S, Krause C, Lepom P, Schulz C, Seiwert M, Seifert B (2002) German environmental survey 1998 (GerES III): environmental pollutants in blood of the German population. Int J Hyg Environ Health 205:297–308. https://doi.org/10.1078/1438-4639-00155
Bernard A (2004) Renal dysfunction induced by cadmium: biomarkers of critical effects. Biometals 17:519–523. https://doi.org/10.1023/B:BIOM.0000045731.75602.b9
Cai LM, Xu ZC, Qi JY, Feng ZZ, Xiang TS (2015) Assessment of exposure to heavy metals and health risks among residents near Tonglushan mine in Hubei, China. Chemosphere 127:127–135. https://doi.org/10.1016/j.chemosphere.2015.01.027
Cave M, Appana S, Patel M, Falkner KC, McClain CJ, Brock G (2010) Polychlorinated biphenyls, lead, and mercury are associated with liver disease in American adults: NHANES 2003–2004. Environ Health Perspect 118:1735–1742. https://doi.org/10.1289/ehp.1002720
CDC (2015) Fourth national report on human exposure to environmental chemicals. Centers for Disease Control and Prevention, Atlanta, GA
Choi JW, Lee MH, Fuii T (2020) Effect of high alcohol intake on heavy metal levels in the blood, urine cotinine metabolism and pulmonary function according to the severity of smoking. Int J Clin Exp Med 13:7700–7708
Chung SM, Moon JS, Yoon JS, Won KC, Lee HW (2020) The sex-specific effects of blood lead, mercury, and cadmium levels on hepatic steatosis and fibrosis: Korean nationwide cross-sectional study. J Trace Elem Med Biol 62:126601. https://doi.org/10.1016/j.jtemb.2020.126601
Danielsson J, Kangastupa P, Laatikainen T, Aalto M, Niemelä O (2014) Impacts of common factors of life style on serum liver enzymes. World J Gastroenterol 20:11743–11752. https://doi.org/10.3748/wjg.v20.i33.11743
Dudley RE, Svoboda DJ, Klaassen CD (1984) Time course of cadmium-induced ultrastructural changes in rat liver. Toxicol Appl Pharmacol 76:150–160. https://doi.org/10.1016/0041-008X(84)90038-3
Dudley RE, Gammal LM, Klaassen CD (1985) Cadmium-induced hepatic and renal injury in chronically exposed rats: Likely role of hepatic cadmium-metallothionein in nephrotoxicity. Toxicol Appl Pharmacol 77:414–426. https://doi.org/10.1016/0041-008X(85)90181-4
El-Zayadi AR (2006) Heavy smoking and liver. World J Gastroenterol 12:6098–6101. https://doi.org/10.3748/wjg.v12.i38.6098
Eom SY, Seo MN, Lee YS, Park KS, Hong YS, Sohn SJ, Kim YD, Choi BS, Lim JA, Kwon HJ, Kim H, Park JD (2017) Low-level environmental cadmium exposure induces kidney tubule damage in the general population of Korean adults. Arch Environ Contam Toxicol 73:401–409. https://doi.org/10.1007/s00244-017-0443-4
Genchi G, Sinicropi MS, Lauria G, Carocci A, Catalano A (2020) The effects of cadmium toxicity. Int J Environ Res Public Health 17:3782. https://doi.org/10.3390/ijerph17113782
Go YM, Sutliff RL, Chandler JD, Khalidur R, Kang BY, Anania FA, Orr M, Hao L, Fowler BA, Jones DP (2015) Low-dose cadmium causes metabolic and genetic dysregulation associated with fatty liver disease in mice. Toxicol Sci 147:524–534. https://doi.org/10.1093/toxsci/kfv149
Godt J, Scheidig F, Grosse-Siestrup C, Esche V, Brandenburg P, Reich A, Groneberg DA (2006) The toxicity of cadmium and resulting hazards for human health. J Occup Med Toxicol 1:22. https://doi.org/10.1186/1745-6673-1-22
Habeebu SS, Liu J, Liu Y, Klaassen CD (2000) Metallothionein-null mice are more sensitive than wild-type mice to liver injury induced by repeated exposure to cadmium. Toxicol Sci 55:223–232. https://doi.org/10.1093/toxsci/55.1.223
Health Canada (2015) Third report on human biomonitoring of environmental chemicals in Canada: results of the Canadian health measures survey cycle 3 (2012–2013). Minister of Health, Ottawa, ON
Hong D, Min JY, Min KB (2021) Association between cadmium exposure and liver function in adults in the United States: a cross-sectional study. J Prev Med Public Health 54:471–480. https://doi.org/10.3961/jpmph.21.435
Huang M, Choi SJ, Kim DW, Kim NY, Bae HS, Yu SD, Kim DS, Kim H, Choi BS, Yu IJ, Park JD (2013) Evaluation of factors associated with cadmium exposure and kidney function in the general population. Environ Toxicol 28:563–570. https://doi.org/10.1002/tox.20750
Hyder O, Chung M, Cosgrove D, Herman JM, Li Z, Firoozmand A, Gurakar A, Koteish A, Pawlik TM (2013) Cadmium exposure and liver disease among US adults. J Gastrointest Surg 17:1265–1273. https://doi.org/10.1007/s11605-013-2210-9
IARC (1993) Cadmium and cadmium compounds. IARC Monogr Eval Carcinog Risks Hum 58:119–237
Ikeda M, Watanabe T, Zhang ZW, Moon CS, Shimbo S (1997) The integrity of the liver among people environmentally exposed to cadmium at various levels. Int Arch Occup Environ Health 69:379–385. https://doi.org/10.1007/s004200050164
Ikeda M, Zhang ZW, Moon CS, Shimbo S, Watanabe T, Nakatsuka H, Matsuda-Inoguchi N, Higashikawa K (2000) Normal liver function in women in the general Japanese population subjected to environmental exposure to cadmium at various levels. Int Arch Occup Environ Health 73:86–90. https://doi.org/10.1007/PL00007943
Järup L, Åkesson A (2009) Current status of cadmium as an environmental health problem. Toxicol Appl Pharmacol 238:201–208. https://doi.org/10.1016/j.taap.2009.04.020
Järup L, Berglund M, Elinder CG, Nordberg G, Vahter M (1998) Health effects of cadmium exposure – a review of the literature and a risk estimate. Scand J Work Environ Health 24:1–51
Kang MY, Cho SH, Lim YH, Seo JC, Hong YC (2013) Effects of environmental cadmium exposure on liver function in adults. Occup Environ Med 70:268–273. https://doi.org/10.1136/oemed-2012-101063
Klatsky AL, Armstrong MA (1992) Alcohol, smoking, coffee, and cirrhosis. Am J Epidemiol 136:1248–1257. https://doi.org/10.1093/oxfordjournals.aje.a116433
Kojima S, Watanabe N, Numata M, Ogawa T, Matsuzaki S (2003) Increase in the prevalence of fatty liver in Japan over the past 12 years: analysis of clinical background. J Gastroenterol 38:954–961. https://doi.org/10.1007/s00535-003-1178-8
Kurihara I, Kobayashi E, Suwazono Y, Uetani M, Inaba T, Oishiz M, Kido T, Nakagawa H, Nogawa K (2004) Association between exposure to cadmium and blood pressure in Japanese peoples. Arch Environ Health 59:711–716. https://doi.org/10.1080/00039890409602957
Lee Y, Cho Y, Lee BW, Park CY, Lee DH, Cha BS, Rhee EJ (2019) Nonalcoholic fatty liver disease in diabetes. Part I: epidemiology and diagnosis. Diabetes Metab J 43:31–45. https://doi.org/10.4093/dmj.2019.0011
Lim JA, Kwon HJ, Ha M, Kim H, Oh SY, Kim JS, Lee SA, Park JD, Hong YS, Sohn SJ, Pyo H, Park KS, Lee KG, Kim YD, Jun S, Hwang MS (2015) Korean research project on the integrated exposure assessment of hazardous substances for food safety. Environ Health Toxicol 30:e2015004. https://doi.org/10.5620/eht.e2015004
Liu H, Probst A, Liao B (2005) Metal contamination of soils and crops affected by the Chenzhou lead/zinc mine spill (Hunan, China). Sci Total Environ 339:153–166. https://doi.org/10.1016/j.scitotenv.2004.07.030
Martins AC, Urbano MR, Almeida Lopes ACB, Carvalho MFH, Buzzo ML, Docea AO, Mesas AE, Aschner M, Silva AMR, Silbergeld EK, Paoliello MMB (2020) Blood cadmium levels and sources of exposure in an adult urban population in southern Brazil. Environ Res 187:109618. https://doi.org/10.1016/j.envres.2020.109618
Menke A, Muntner P, Silbergeld EK, Platz EA, Guallar E (2009) Cadmium levels in urine and mortality among U.S. adults. Environ Health Perspect 117:190–196. https://doi.org/10.1289/ehp.11236
Mezynska M, Brzóska MM (2018) Environmental exposure to cadmium—a risk for health of the general population in industrialized countries and preventive strategies. Environ Sci Pollut Res Int 25:3211–3232. https://doi.org/10.1007/s11356-017-0827-z
Moon JY, Eom SY, Seo JW, Lee JE, Choi BS, Kim H, Hong YS, Chang JY, Jeon MJ, Park WJ, Sakong J, Park JD (2021) Effects of exposure to lead and cadmium on health of inhabitants of abandoned metal mine area in Korea. Arch Environ Contam Toxicol 80:490–498. https://doi.org/10.1007/s00244-021-00813-7
Nie X, Wang N, Chen Y, Chen C, Han B, Zhu C, Chen Y, Xia F, Cang Z, Lu M, Meng Y, Jiang B, Jensen MD, Lu Y (2016) Blood cadmium in Chinese adults and its relationships with diabetes and obesity. Environ Sci Pollut Res Int 23:18714–18723. https://doi.org/10.1007/s11356-016-7078-2
Nishijo M, Nakagawa H, Morikawa Y, Kuriwaki J, Miura K, Kido T, Nogawa K (2004) Mortality in a cadmium polluted area in Japan. Biometals 17:535–538. https://doi.org/10.1023/B:BIOM.0000045734.44764.ab
Park JD, Choi BS, Kweon IH, Hong YP, Chang IW (2000) Reference values of cadmium in kidney and liver in Korean. Korean J Occup Environ Med 12:346–355. https://doi.org/10.35371/kjoem.2000.12.3.346
Park E, Kim J, Kim B, Park EY (2021) Association between environmental exposure to cadmium and risk of suspected non-alcoholic fatty liver disease. Chemosphere 266:128947. https://doi.org/10.1016/j.chemosphere.2020.128947
Rikans LE, Yamano T (2000) Mechanisms of cadmium-mediated acute hepatotoxicity. J Biochem Mol Toxicol 14:110–117. https://doi.org/10.1002/(sici)1099-0461(2000)14:2%3c110::aid-jbt7%3e3.0.co;2-j
Ryu DY, Lee SJ, Park DW, Choi BS, Klaassen CD, Park JD (2004) Dietary iron regulates intestinal cadmium absorption through iron transporters in rats. Toxicol Lett 152:19–25. https://doi.org/10.1016/j.toxlet.2004.03.015
Satarug S, Moore MR (2004) Adverse health effects of chronic exposure to low-level cadmium in foodstuffs and cigarette smoke. Environ Health Perspect 112:1099–1103. https://doi.org/10.1289/ehp.6751
Satarug S, Baker JR, Urbenjapol S, Haswell-Elkins M, Reilly PEB, Williams DJ, Moore MR (2003) A global perspective on cadmium pollution and toxicity in non-occupationally exposed population. Toxicol Lett 137:65–83. https://doi.org/10.1016/S0378-4274(02)00381-8
Seo MH, Lee WY, Kim SS, Kang JH, Kang JH, Kim K, Kim BY, Kim YH, Kim WJ, Kim EM, Kim HS, Shin YA, Shin HJ, Lee KR, Lee KY, Lee SY, Lee SK, Lee JH, Lee CB, Chung S, Cho YH, Choi KM, Han JS, Yoo SJ (2019) 2018 Korean society for the study of obesity guideline for the management of obesity in Korea. J Obes Metab Syndr 28:40–45. https://doi.org/10.7570/jomes.2019.28.1.40
Statistics Korea (2021) Cause of death statistics in 2020. http://kostat.go.kr/portal/eng/pressReleases/8/10/index.board
Strumylaite L, Kregzdyte R, Bogusevicius A, Poskiene L, Baranauskiene D, Pranys D (2019) Cadmium exposure and risk of breast cancer by histological and tumor receptor subtype in white caucasian women: a hospital-based case-control study. Int J Mol Sci 20:3029. https://doi.org/10.3390/ijms20123029
Tinkov AA, Filippini T, Ajsuvakova OP, Skalnaya MG, Aaseth J, Bjørklund G, Gatiatulina ER, Popova EV, Nemereshina ON, Huang PT, Vinceti M, Skalny AV (2018) Cadmium and atherosclerosis: a review of toxicological mechanisms and a meta-analysis of epidemiologic studies. Environ Res 162:240–260. https://doi.org/10.1016/j.envres.2018.01.008
Tomei F, Ciarrocca M, Rosati MV, Casale T, Di Pastena C, Nieto HA, Antuono V, Iannattone G, Tomei G, Caciari T (2013) Relationship between occupational exposure to cadmium, transaminases and γ-GT in workers exposed to urban stressors. Ann Ig 25:353–363. https://doi.org/10.7416/ai.2013.1937
Tsuchiya K (1969) Causation of ouch-ouch disease (Itai-Itai Byō)—an introductory review—II epidemiology and evaluation. Keio J Med 18:195–211. https://doi.org/10.2302/kjm.18.195
Vahter M, Åkesson A, Lidén C, Ceccatelli S, Berglund M (2007) Gender differences in the disposition and toxicity of metals. Environ Res 104:85–95. https://doi.org/10.1016/j.envres.2006.08.003
Wang X, Wang B, Zhou M, Xiao L, Xu T, Yang S, Nie X, Xie L, Yu L, Mu G, Ma J, Chen W (2021) Systemic inflammation mediates the association of heavy metal exposures with liver injury: a study in general Chinese urban adults. J Hazard Mater 419:126497. https://doi.org/10.1016/j.jhazmat.2021.126497
Wei Y, Zhu J (2020) Blood levels of endocrine-disrupting metals and prevalent breast cancer among US women. Med Oncol 37:1. https://doi.org/10.1007/s12032-019-1328-3
Werder EJ, Beier JI, Sandler DP, Falkner KC, Gripshover T, Wahlang B, Engel LS, Cave MC (2020) Blood BTEXS and heavy metal levels are associated with liver injury and systemic inflammation in Gulf states residents. Food Chem Toxicol 139:111242. https://doi.org/10.1016/j.fct.2020.111242
White AJ, O’Brien KM, Niehoff NM, Carroll R, Sandler DP (2019) Metallic air pollutants and breast cancer risk in a nationwide cohort study. Epidemiology 30:20–28. https://doi.org/10.1097/EDE.0000000000000917
Acknowledgements
This study was supported by the Korea Food & Drug Administration (KFDA) (Grant numbers 10162KFDA994 in 2010−2011 and 13162MFDS778 in 2014).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Seo, MN., Eom, SY., Lim, JA. et al. Effects of Environmental Cadmium Exposure on the Liver in Korean Adults: Cross-Sectional and Longitudinal Studies. Arch Environ Contam Toxicol 84, 237–247 (2023). https://doi.org/10.1007/s00244-023-00982-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-023-00982-7