Abstract
This study investigates the pollution of Markman stormwater runoff, which is a tributary to Swartkops River Estuary. Solid-phase and ultrasonic extraction methods were utilized in the extraction of water and sediment samples, respectively. The pH of the sampling sites was above the EU guideline. The ranges of concentration of \(\Sigma_{6}\)PBDE obtained in water and sediment samples for all the seasons were 58.47–1357 ng/L and 175–408 ng/g, respectively. Results also showed that BDE-66 was the dominant congener, specifically in the industrial zone, where its concentrations ranged from 2 to 407 ng/g in sediment. Consequently, the high concentration of BDE- 66 in the sediment of stormwater calls for concern. Penta-BDE suggests potential moderate eco-toxicological risk, as evident in the calculated risk assessment. The result showed possible photodegradation along the contaminant's travel time, as only 7% of the PBDE was detected at the point of entry into the Swartkops River Estuary. Markman stormwater may be contributing heavily to the pollution load of Swartkops River, as evident in the alarming concentrations of PBDEs obtained. The industries at this zone should eliminate the contaminants before discharging their effluents into the canal.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polybrominated diphenyl ethers (PBDEs) are brominated diphenyl ethers used extensively as additives in wide-ranging end-user products in textiles, aircraft, furniture, plastics, electronic appliances, etc. (Bergman et al., 2012; Ohoro et al., 2021). There are 209 different PBDE congeners grouped into ten categories (mono-decabromodiphenyl ethers). Commercial products, however, do not contain compounds with less than four bromine atoms (Darnerud et al., 2001). PBDEs are persistent, bioaccumulative, toxic, and have a long range of transport. These have made three commercial PBDEs mixtures, i.e., penta-BDE, octa-BDE, and deca-BDE, registered as POPs in the Stockholm Convention (United Nations Environment Programme, 2017). The higher congeners like BDE-209 debrominate to lower congeners commonly found in humans (Costa et al., 2008) and even in rats (Huwe & Smith, 2007) by degradation of plant (Jiang et al., 2019; Wang et al., 2012), and their degradation is an essential process that governs their fate in the environment (Rahman et al., 2001). PBDEs are of environmental concern because they are hydrophobic and persistent in the environments (Palm et al., 2002). There is a dearth of experimental field studies on the bioconcentration, bioaccumulation, fate and behavior of PBDEs, regardless of the huge progress in research (Akortia et al., 2016; Kelly et al., 2008; Martin et al., 2004). However, partitioning behavior is mainly dependent on the environment and may vary from region to region (Gouin & Harner, 2003). Penta-BDE tends to dominate in the atmosphere and aqueous matrix, while BDE-209 is found in the soil, sediment, and sludge. Howbeit, the soil is the least studied but thus far the most major sink of PBDE in the environment (Vonderheide et al., 2008). The persistence of PBDEs is envisaged for many years to come owing to new productions and recycling of PBDEs in addition to improper disposal. The fate of PBDEs after being released into the environment is not sure (Guan et al., 2009). Lower brominated diphenyl ethers that enter the atmosphere are likely to be eliminated by photochemical degradation before adsorbing to surface water and soil (Raff & Hites, 2007). The fate of PBDEs has been reported elsewhere (Ohoro et al., 2021). The exponential increase in the alarming rise of PBDE in the Swedish breastmilk samples for over 25 years was influential in supporting EU lawmakers to approve the ban of PBDE (Bush et al., 2001). Likewise, Canadian Environmental Protection Act banned the production and use of new products in 2009 (Abbasi et al., 2015). Deca-BDE marketable products are already barred in Europe and the USA but still utilized in China (Ji et al., 2017). Currently, there is a scarcity of information on the production and use of deca-BDE in South Africa (Daso et al., 2013a). However, Deca-BDE has been detected in Rubik's cube in South Africa (DiGangi et al., 2017), which is suspected to be from the plastic industry (Chokwe et al., 2019).
Discharges from unrestrained electronic waste recycling in developing countries have been the principal source of PBDEs in the global record (Bi et al., 2007; Wong et al., 2007). They are not bonded to materials chemically, that is why they can emanate from polymers into the environment and convey through dust, or in the course of the burning of plastic waste in the domestic furnace or via open burning (Pietron et al., 2019; Romanelli et al., 2017). They can be transported to the marine environment from global discharge sources, like surface runoff of polluted soils, atmospheric deposition, direct industrial and domestic wastewater discharges, and riverine inputs (Li et al., 2018; Wang et al., 2019; Wu et al., 2020). BDE-209 and BDE-183 have been linked to work-related exposure; higher brominated BDEs are frequently eminent in electronics disassembling workers, while BDE-153 is frequently the dominant PBDE congener in non-occupationally exposed populations like in the Netherlands, UK, Sweden, and China (Bi et al., 2007).
Humans’ exposure to PBDEs is through ingestion of food (dietary intake and consumer products), dermal contact, air and dust inhalation (Oloruntoba et al., 2019; Sahlström et al., 2015; Trudel et al., 2011), where their comparative influences to total exposure show a discrepancy by sex, age, geographical region, and congener (Sahlström et al., 2015; Trudel et al., 2011). Some undesirable impacts of POPs and their metabolites in humans include immunosuppression, neurotoxicity, endocrine system damage, carcinogenicity, and reproductive dysfunction (Oloruntoba et al., 2019; Ontiveros-Cuadras et al., 2019; Parry et al., 2018). BDE-47, BDE-99, and BDE-209 apply genotoxic and cytotoxic impacts and play an acute role in the destroying DNA, down-regulation of oxidative stress, and the associated gene expression in bronchial epithelial cells. Inhalation may also cause respiratory diseases in the regions of environmental effluence (Montalbano et al., 2020). Some PBDEs have been revealed to adversely influence the thyroid hormone system regulation, influence liver enzyme activity, influence neurological development at a delicate time of brain growth, and instigate immunotoxicity (Darnerud et al., 2001; De Wit, 2002; Söderström et al., 2004).
Their pollution has drawn attention, especially in marine sediments (Wang et al., 2019). Even in South Africa, many researchers have documented PBDE occurrence (Daso et al., 2012; Olisah et al., 2020), though much work has not been done on the investigation of rivers and estuaries. Eastern Cape Province hosts two significant seaports in Port Elizabeth and East London, responsible for the region's development, urbanization, and heavy industrialization. Consequently, aquatic environments are posed with threat as some of the discharges from these industries find their way to the surface waters. Olisah et al. (2020) reported that the surface waters of Swartkops River in this province are contaminated with PBDE, and the source of this contamination is validated. Markman Canal is a tributary and urban runoff that drains the heavy industrial effluents and then empties into the Swartkops River Estuary. At the upper reaches of the canal is the highly polluted runoff, as reported by Lord and Mackay (1991). However, the vegetation established in the canal bed and the long travel time to the estuary effectively remove most pollutants from the runoff (Lord & Mackay, 1991). Markman stormwater is a significant source of pollution of the Swartkops River (Lord & Mackay, 1991), as sewage water from various industrial zone activities and informal settlements such as Aloes village are discharged without purification (Papu, 2015). The pollution from Markman runoff is a threat to the estuarine ecosystem, which provides important refuge and feeding areas for many species (Nel, 2014) and puts the neighborhood's health (Papu, 2015) threatening food security. There has been pollution from lower heavy metal concentration from the industrial area, fine chemicals from guilty industry members (Nel et al., 2015), and 90% increase in trace metals (Adams, 2019). However, no study has investigated on the occurrence of PBDEs in Markman Canal; therefore, the data generated from this study will assist the managers in monitoring and mitigating the PBDEs contaminants from reaching the Swartkops Estuary. The stormwater under investigation does not empty directly into the Indian Ocean, but it discharges to Swartkops River, which flows into the Indian Ocean (Adams et al., 2019). This study validates the Swartkops River's pollution from Markman runoff and provides the risk evaluation of PBDEs in surface waters of an industrial-based environment.
Materials and method
Study area
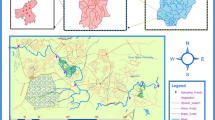
Markman canal is a tributary of Swartkops River sited Markman Industrial area, Port Elizabeth, Eastern Cape Province, South Africa, which also traveled through the Aloes community, a suburb with few residential houses and a mini-relaxation center. This area is a zoned industrial estate situated at 33.7987° S, 25.6332° E, with little animals' grazing. The Markman area population is 59 with 21.43 area km2, as stated in census 2011 (https://census2011.adrianfrith.com/place/299007). Industries situated in this area include textile and automobile industries. Also, fruits and vegetable mall that makes use of plastics is also located in this area. Port Elizabeth has an annual average precipitation of 47 mm (en.climate-data.org). Several points of wastewater discharges were seen running into Markman Canal from these industries. The map (Fig. 1) and the coordinates (Table 1) are shown.
Standards and reagents
Standard mixtures (1.2 mL of 5 µg/L) of six PBDE congeners (BDE-17, 47, 66, 100, 153, 183), purchased from Bymax Pty, South Africa, constituting the most bountiful PBDE congeners in the aquatic environment, were examined in all samples. Surrogate standard PCB-209 was procured from Wellington Laboratories, Ontario, Canada; Phenomenex—strata C18—E (55 µm, 70A, 500 mg, 6 mL), from Separations Pty Ltd, South Africa. Pure sand (50–70 mesh) was purchased from Sigma-Aldrich. At the same time, glass wool and HPLC grade solvents: dichloromethane (DCM), hexane, acetone, ethyl acetate, isooctane, acetonitrile, nonane, and toluene (Sigma-Aldrich, Chemie GmbH, Steinheim, Germany), were supplied by Merck, South Africa. Nitrogen gas (99.999% purity) was ordered from Afrox (Pty.), Pretoria, South Africa. Silica gel (100–200 mesh), Na2SO4, and copper powder (500 g) were bought from SAINS Agencies, South Africa. All organic solvents utilized during the experimental and instrumental processes were of HPLC grade or equivalence.
Sampling and sample pre-treatment
Both water and sediment samples were collected in triplicate from five points in each season across the canal course between August and December 2020. Approximately 40 rainfalls were recorded before commencing the sampling (en.climate-data.org). Each sediment and surface water sample were unique to a sampling point, and discrepancies were avoided using the global positioning system. Water samples (1 L) were collected in amber glass bottles (formerly washed with detergent and rinsed with acetone and subsequently with the sample water). All samples were retained in an icebox and transferred to the laboratory. The filtration of the samples was done with Whatman No. 1 filter paper (140 μm) within 12 h of collection, and extraction was done immediately. Sediment samples (0–40 cm depth) were collected using a 2-L stainless Van Venn grab sampler. The collected samples were separately enfolded in aluminum foil and conveyed to the laboratory. Large stones and pieces of debris were removed from sediment samples before they were air-dried in a dark room, crushed, and homogenized thoroughly with mortar and pestle, and sieved separately with 250-µm mesh sieves and preceded for extraction and instrumental analysis.
Extraction and purification
Water extraction
Extraction was modified and carried out according to Kowalski and Mazur (2014). Phenomenex—strata C18—E (55 µm, 70A, 500 mg, 6 mL) was conditioned with ethyl acetate (6 mL) and distilled water (6 mL) at a flow rate of 1 mL min−1. After that, 500 mL of the filtered water sample was passed over 10 mL min−1 flow rate, after which the column was dried for 3 h, and then, the analyte was eluted with 2 × 2.5 mL of ethyl acetate. The extract was evaporated to dryness under a gentle stream of nitrogen and reconstituted with acetonitrile (1 mL) before GC-µECD analysis.
Sediment extraction
Sediment extraction was done based on a modified ultrasonically assisted extraction method reported by Olukunle et al. (2015), and analytes were measured using a gas chromatography–electron capture detector (GC-µECD). Approximately 10 g sample was weighed into a methodically cleaned amber bottle (100 mL). Afterward, each of the samples was spiked with 200 µl of 18 µg/L of surrogate standard PCB-209 and allowed to soak and equilibrate for 1 h. The samples were washed overnight afterward with 20 mL of n-hexane: acetone (2:1, v/v) in an airtight amber bottle (100 mL), followed by extraction for 30 min at 45 °C in an ultrasonicator (LASEC South Africa Pty Ltd, single frequency 40 Hz, 6 L). After sonication, the sediment sample with extract was left to cool and settle for 60 min at 0 °C before the extract was drawn out of the vial using a Pasteur pipette into another bottle. The extracts were centrifuged at 5000 rpm for 5 min. Extraction was done recurrently three extra times on every single sample with fresh solvents per group, and each of the extracts (about 60 mL) was collected. The crude extracts’ volume containing PBDEs was reduced by rotary evaporator to 1 mL using a Büchi Rotavapor R-210 (vapor: 40 °C, bath temperature: 40 °C, and cooling water temperature: 20 °C). The cleanup of crude extracts was achieved by eluting with n-hexane. The chromatographic glass column (10 mm × 30 cm) used for cleanup was packed with glass wool, 1 g of silica gel, 2 g of copper in layers from the bottom with and topped up with 0.5 g Na2SO4 (anhydrous), separating each layer with glass wool to enhance cleaning. The packed column was pre-saturated with DCM (8 mL) and then n-hexane (8 mL). Approximately 1 mL of the sample extract was put into the column and allowed to run. The adsorbed analyte was eluted with n-hexane (6 mL) at 5 mL min−1 flow rate and transferred carefully into uncontaminated tubes. Nitrogen gas (N2) was fizzed through the eluate and then concentrated to dryness. About 1 mL n-hexane was introduced to reconstitute, and N2 effervesced through to reduce it to a final volume of 1 mL. After that, the analyte extract was injected into the GC-µECD under optimized instrumental conditions.
Instrumental analysis
The PBDEs were analyzed using a 7820A GC coupled to a G239AE -µECD (Agilent Technologies, Santo Clara, CA, USA). An HP-5 (Agilent Technologies, Santo Clara, CA, USA—19091 J-413:1) capillary column (30 m × 320 µm × 0.25 µm i.d. film thickness) was used for congeners separations. The oven temperature program was as follows: 100 °C with 1-min holding time, raised to 190 °C with no holding, a second ramp to 255 °C at 5 °C/min with 0.5-min holding time, and a final ramp to 310 °C at 2 °C/min with 10-min holding time, giving a run time of 61.25 min. The injection and detection temperatures were set to 230 °C and 325 °C, respectively, with carrier gas pressure of 108.43 kPa with an average velocity of 48.868 cms−1. The carrier gas used was helium at a flow rate of 1.0 mL/min. All samples were injected in splitless mode with a volume of 1 μL.
Quality assurance and quality control (QA/QC)
Before use, all glassware was soaked overnight with acid, washed and rinsed with detergent, and lastly rinsed with acetone (analytical grade); and heated at 105 °C for 4 h. Silica gel, copper powder, and anhydrous Na2SO4 were baked at 350 °C before use to eliminate impurities. Silica gel and Na2SO4 were stored in a glass jar pre-cleaned and rinsed with a solvent (n-hexane/acetone). Afterward, it was then sealed to prevent contamination and absorption of moisture. Procedural and solvent blanks were analyzed for every set of five samples to check for interferences and cross-contamination. Surrogate standard PCB-209 were spiked into each sample and analyzed in triplicate for quality control and assurance, and the mean concentrations were reported. The GC syringe was automated to wash twice, each with hexane and acetone, before and after sample injection to prevent cross-contamination. The recoveries for the analytes (45–123%) were within the standard range of recovery (APHA, 2005), which indicated that this method could simultaneously quantify PBDEs in water. The external calibration method was used for quantification, and a standard mixture with seven concentration levels formed the calibration curves at 5–120 µg/L for all congeners of PBDEs diluted with isooctane. LOD and LOQ were calculated using 3.3 (Sy/S) and 10 (Sy/S), respectively (Ibrahim et al., 2018; Olisah et al., 2019). The instrument's precision, estimated as the relative standard deviation (RSD), was estimated from eight replicate injections of a middle-level calibration standard following standard methods (Caruso & Santoro, 2014). BDE- 66 did not give a fair recovery for water, so it was not quantified in the water samples. The recoveries and linearity are represented in Table S1.
Statistical/data analysis
The following statistics: one-way ANOVA, regression analysis, descriptive statistics, composition pattern of PBDEs in surface water, Pearson correlation, and distribution percentage were calculated using Microsoft Excel 2016. The percentage distribution of PBDEs was calculated across all seasons; sample below LOD was treated as zero all through the statistical analysis. The concentrations were expressed as ng/g dry weight (dw) for sediment and ng/L for water samples.
Health risk assessment
Risk assessment was conducted for water and sediment samples using a model as shown in Eqs. (1) and (2) (Olisah et al., 2020; Yin et al., 2019).
Estimated daily intake (EDI)
where EDI is the estimated daily intake for target PBDEs (ng/kg/day), C is the mean seasonal concentration of PBDE in water (ng/L), BW is the body weight of 60 kg (World Health Organization, 2010), and IV is the ingestion volume of 2 L (Yahaya et al., 2017).
Non-carcinogenic risk
Non-carcinogenic risks for water samples are calculated using Eq. (2):
where HQ is the hazard quotients, and RfD values (100 ng/kg bw/day for BDE-47 and 200 ng/kg bw/day for BDE-100 and 153) are the USEPA values as reported by USEPA 2019 (Wang et al., 2019).
Eco-toxicological risks posed by PBDE in sediment samples were evaluated by employing the assessment as given in Eq. (3) (Canada Environment Protection, 2006; Ge et al., 2018).
where C is the concentration of PBDEs in sediment, PNEC is the predicted no-effect concentration below which no adverse effect is envisaged. The concentrations of 31 and 9100 ng/g dw were used for penta- and octa-BDE, respectively (Chen et al., 2010; Wu et al., 2017). Tri-penta-BDE (17, 47, 66, 100) and hexa-octa-BDE (153, 183) were assumed to be penta- and octa-BDEs, respectively, as classified by USEPA (Wu et al., 2013a).
HQ < 0.1 specifies no eco-toxicological, HQ between 0.1–1 shows low eco-toxicological risk, HQ between 1–10 indicates moderate eco-toxicological risk, and HQ > 10 indicates high eco-toxicological risk (Ge et al., 2018; Yin et al., 2020).
Result and discussion
Impact of physicochemical parameters on concentrations of PBDEs in Markman Canal
The physicochemical parameters of the water and sediment samples are summarized in Table 2. There is no significant difference (p > 0.05) in the properties (Table 2) among the sampling points. There is higher pH in spring than in any other season. The pH values (6.82–9.76) were above EU guidelines (Department of Environmental Affairs (DEA), 2012). However, the chemical features of PBDEs are not affected by pH (Fontana et al., 2009). EC, as well as TDS and TSS, were highest in winter than any other season. EC decreases with increasing altitude (Blais et al., 2006). There was low turbidity in winter but very high in spring (60.5 FNU). Turbidity evaluates the amount of suspended solids in water. High turbidity can prevent debromination (Rügner et al., 2013; Viganò et al., 2011). The highest turbidity of 19.7 FNU was observed in MAK4, which explains why there is a high concentration of PBDE in the winter season. An increase in turbidity always accompanies the persistence of PBDE as it obstructs the penetration of light and lowers photodegradation and debromination (Fondriest Environmental, 2015; Zhang et al., 2008). The highest concentration of BDE-17, BDE-47, BDE-100, BDE-153, and BDE-183 in water and sediments were (723.47, 292.12, 835.11, 246.82 µg/L, and 27.53 ng/L) and (183 356, 407 870, 453 and 300 ng/g), respectively. The temperature was unusually higher in spring (24 °C) than in summer (20 °C). High temperature promotes the degradation of higher BDE to lower BDE. It also helps in transferring PBDE via wet/dry deposition (Shao et al., 2018). There is a paucity of information on the effects of temperature on PBDE. However, an increase in temperature brings about an increase in solubility of PBDEs (Kuramochi et al., 2007). Spring season had the highest temperature, and yet a high concentration of PBDE was detected in summer. The high concentration in summer can be attributed to wet deposition (Shao et al., 2018), as it was a rainy season.
Furthermore, salinity was also low in winter. Kim et al. (2017) reported that the partition sorption process of PBDE could be affected by salinity. Contrastingly, reports showed that sorption is not affected by salinity (Xu et al., 2019). DO for the winter season was not measured due to logistics; however, PBDEs can rapidly weaken DO and, consequently, decrease in oxidation–reduction potential (Chen et al., 2016). Moisture content was not measured in the winter season.
Pearson correlation matrix among water quality parameters
In water samples, BDE-17 correlates moderately with BDE-100 (0.665; p < 0.05) and BDE- 153 (0.577; p < 0.05), pH, EC, TDS, salinity, DO, moisture content, and inversely moderate with temperature and mVORP. BDE- 47 correlates strongly with BDE- 100 (0.756; p < 0.05), moderately with 153 (0.628; p < 0.05), very strongly with 183 (0.939; p < 0.05) and recorded good correlation with all the physicochemical properties. BDE-100 correlates strongly with BDE-183 (0.865; p < 0.05), inversely moderate with turbidity, and moderate with resistivity. BDE-153 strongly correlates with BDE-183 (0.838; p < 0.05) and inversely moderate with ORP. BDE-183 is inversely moderately correlated with turbidity and moderately correlated with resistivity. This implies that an increase in turbidity will decrease the debromination rate of BDE-183.
In sediment samples, BDE-17 correlates very strongly with BDE-47 (0.991; p < 0.05), 66 (0.921; p < 0.05), 183 (0.994; p < 0.05), strongly with BDE-100 (0.885; p < 0.05), and moderately with 153 (0.676; p < 0.05). It correlates inversely moderate with OM, OC and moderately with temperature. BDE-47 correlates very strongly with BDE-100 (0.936; p < 0.05) and BDE-183 (0.975; p < 0.05), strongly with BDE-66 (0.877; p < 0.05), moderately with BDE-153 (0.579; p < 0.05), moderately, inversely moderate with OC and OM. BDE-66 correlates very strongly with BDE-183 (0.957; p < 0.05), strongly with BDE-153 (0.885; p < 0.05), moderately with BDE-100 (0.656; p < 0.05). BDE-66 and 153 did not correlate with all the physicochemical properties. All the physicochemical properties have a good correlation with themselves. The summary of the Pearson correlation is given in Tables S2 and S3.
Spatial distribution and seasonal variation
The PBDE concentrations of both matrices (Table S4) showed no significant difference among the three seasons (p-value > 0.05). The total seasonal occurrence of both water and sediment samples was in the order: Spring > winter > Summer. The higher occurrence of PBDE concentrations in the spring season could be attributed to the heavy rainfall experienced in the spring season by South Africa. Heavy rain can influence the concentrations of PBDE as a result of runoffs from agricultural activities and atmospheric wet deposition (Shao et al., 2018). The low concentration of the contaminants in the summer season (Fig. 2) can be attributed to attenuation caused by heavy rainfall in the catchment area. The highest detected PBDE congener in water samples is BDE- 17, accounting for 48% (72.91–723.47 ng/L) in winter and 0.30–48.63 ng/L in summer. Furthermore, the trend of average congeners detected in water samples in all the seasons is given as BDE- 17 (42%), followed by BDE-100 (37%), BDE-47 (10%), BDE-153 (9%), and BDE-183 (2%). BDE-17 being a lower brominated diphenyl ether is one of the significant debrominated products of anaerobic dehalogenation process of sediment samples (Trinh et al., 2019). Furthermore, BDE-17, BDE-47, and BDE-100 were the dominant congener, especially at MAK1 and MAK 2 (Fig. 3), which are characterized by indiscriminate disposal of waste and vehicular emission.
The highest detected concentration in sediments in the winter season was BDE-153 (0.57–453 ng/L), while BDE-100 was the least detected (BDL-8.64 ng/L). This concentration was noticed in MAK 1. In spring, BDE-66 and BDE-100 were the highest detected at 14.23–407 and 0.22–533 ng/L, respectively, followed by BDE-47 (0.22–356 ng/L). High concentration was noticed, especially in MAK 4, which is at the industrial area receiving effluent from the industries. This agrees with the report of Muenhor et al. (2017), in which BDE-66 was higher because of the discharge from the industrial area, as also confirmed by Hwang et al. (2012), who also detected penta-BDE from industrial sludge samples at very high concentrations. BDE-183 was the congener with the highest concentration (0.54–299.86 ng/L) detected in summer at MAK 4. The highest average congener detected in sediment was BDE- 66 (41%), followed by BDE-100 (23%), BDE-153 (19%), BDE-183 (18%), BDE-47 (15%), and BDE-17 (9%) in that order. Higher brominated congeners were detected more in sediment because they are hydrophobic and tend to attach more to sediment than dissolving in water (Jiang et al., 2019). BDE-153 was relatively high, which indicates possible debromination of BDE-183 (Robrock et al., 2008). In the summer season, BDE-17, BDE-47, and BDE-66 were dominant in the sediment matrix, BDE-17, and BDE-47 were dominant in water samples because they are more persistent in the environment (Yao et al., 2020). Though physicochemical parameters played major roles, other factors such as the day of discharge of effluents into the canal may also affect the concentration of the PBDEs in the runoff water. Despite the highest temperature in the spring season, the highest concentrations were noticed at points MAK3, MAK4, MAK5. Temperature and rainfall play a vital function in transporting PBDEs from one environmental matrix to the other. Higher ambient temperature was observed in spring, which positively impacted the transportation of PBDEs from air to water via dry/wet deposition. In addition, surface runoff and wet deposition were the significant factors affecting PBDEs' concentration in water, transferring a higher amount of PBDE from air and soil to aquatic matrix. Higher temperature and strong sunshine may promote photodegradation of higher brominated diphenyl ethers to lower BDE (Shao et al., 2018). The high concentration in the spring season could also probably result from high turbidity (60.5 FNU), which prevents photodegradation (Viganò et al., 2011). Low temperature and rainfall decrease diffusion of air–water flux and volatilization, which in turn suppress the transportation of PBDE from other environmental matrices to the aquatic matrix and consequently give a high concentration of PBDEs. A similar high concentration was observed by Shao et al. (2018). Concentrations in summer were lower than those in winter, possibly due to a a higher degradation rate in summer and atmospheric volatilization/condensation/deposition cycle in different seasons (Ge et al., 2014a). PBDE is hydrophobic and tends to sorb to high organic matter content in particles in waste streams (Ge et al., 2014a).
The seasonal concentrations of the water and sediment spots (Table S4) showed a significant difference among the sampling points (p-value < 0.05). The seasonal differences in the concentrations of PBDEs may not only be attributed to climate change. It could be because of variations in effluent discharge frequency in the industrial zone and non-point source of the contaminants into the canal. Comparing the three seasons, MAK5 was the highest contaminated point in winter and summer in the water samples with \(\Sigma\)BDE at 49% and 42%, respectively. MAK4 had the highest concentration in spring, with \(\Sigma\)BDE at 44%. MAK3 was not affected by the seasons. It was evident that MAK3, MAK4, and MAK5 had the highest contamination. These are the three points with direct effluent discharge from the industrial zone. MAK1 and MAK2 were relatively the lowest in all the seasons, as waste generated here is majorly domestic waste and vehicular emission. From the results, only 7% \(\Sigma_{5}\)BDE of water samples are emptying into Swartkops River. The low concentration shows degradation evidence, corresponding to Swartkops Estuary report (Olisah et al., 2020). In winter, a high concentration in sediment at BDL-452.69 ng/g was observed at MAK1, which is the point of entry of the canal into Swartkops River, followed by MAK2 at 3.80–10.92 ng/g, showing some pollution level from the residential area. MAK4 has the highest contamination in spring at 122.26–533.29 ng/g. MAK4 also had the highest concentration at 31.62–299.86 ng/g. Generally, MAK4 has the highest concentration of \(\Sigma\)BDE at 55%. The high concentration of PBDEs is not out of place as this point hosts many points of discharge from the surrounding industries.
Comparison with other countries
Comparison of the \(\Sigma\)PBDE in this study with the concentrations within South Africa and other countries to evaluate PBDEs pollution status in the canal is given (Table 3). This study recorded higher concentrations in water samples than other reported surface water sites in South Africa (Daso et al., 2013a; Olisah et al., 2020). The highest concentration came from a water sample of MAK4 in the spring season marked with high pollution and turbidity. It was observed that at this point, the concentration in the water sample was higher than in sediment. This can be attributed to the fresh release of effluent from the industry on the sampling day. The concentration of contaminants in the sediment of this study (Table S4) was \(\Sigma_{6}\)278 ng/g, \(\Sigma_{6}\)408 ng/g, and \(\Sigma_{6}\)175 ng/g in winter, and spring, summer, respectively, higher than the concentrations detected (48 ng/g) by Chokwe et al. (2019) in South Africa and 0.5–3 ng/g in the USA (Song et al., 2004). The concentrations of \(\Sigma_{5}\)PBDE in water samples of winter and summer (Table S4) are BDL-724 ng/L (\(\Sigma_{5}\)1061 ng/L) and 2.98–28.56 ng/L (\(\Sigma_{5}\)58.47 ng/L), respectively. This is higher than the concentration range (3–6 ng/L and 1–1.2 µg/L) in winter and summer, respectively, reported in China (Ge et al., 2014b). This could be due to new discharge from the surrounding industries in addition to the runoff from an industrial canal. However, the highest concentration in water samples in spring (\(\Sigma\)1357 ng/L) was lower than the concentration detected in China by Liang et al. (2019) at \(\Sigma_{9}\)PBDE 1.85–7124 ng/L.
Markman canal is tributary stormwater coming from an industrial zone that empties into Swartkops River. Research carried out by Olisah et al. (2020) showed Swartkops River was polluted by PBDE at a concentration of 2.5–169 ng/L, validating that the effluent from this canal contributes heavily to the pollution of the waterbody.
Human risk assessment
The result of human risk assessment for water and sediment samples was based on hazard quotients (HQs), as shown in Tables S6 and S7, respectively. For water samples, the highest HQ was 3.02 × 10–2, which is below 0.1, although tetra-BDE, penta-BDE, hexa-BDE, and hepta-BDE values were higher than the Federal Environmental Quality Guideline (FEQG) (Environment Canada, 2013) when compared as shown (Table S6, supplementary information). However, no eco-toxicological risk is envisaged for water samples. The literature has no RfD values for BDE- 17 and BDE- 183, so the risks were not measured. The sediment concentrations in different seasons were also compared with the FEQG values (Environment Canada, 2013), as grouped in Table S6. It was observed that tetra-BDE and penta-BDE values for all the seasons, including hexa-BDE in the winter season, were higher than the FEQG values. The HQ for penta- and octa-BDE from the sediment sample in Markman Canal were 5.82 and 1.17 × 10–2. Tri-penta-BDE (17, 47, 66, 100) and hexa-octa-BDE (153, 183) were assumed to be penta-BDE and octa-BDEs, respectively, for hazard quotients as classified by USEPA (Wu et al., 2013b). Notwithstanding, the high values of tetra-BDE and penta-BDE were not high enough to cause eco-toxicological risk. However, the penta-BDE indicated potential moderate eco-toxicological risk, while the octa-BDE shows no risk. Although the water samples posed no risk, the runoff stormwater is too turbid to be used for bathing.
Percentage distribution of PBDEs in the surface water and sediment
The percentage distribution of the congeners in water samples is summarized in Fig. 4a. Generally, the highest contamination of PBDEs in water samples emanated from MAK4 (34%). This point was associated with high turbidity, which could have contributed to the slow degradation of PBDEs. The highest concentrations of BDE- 17 were obtained from MAK4 (35%). The highest concentrations of BDE-47 were from MAK5 (56%). The highest concentrations of BDE-100 were obtained from MAK5 (52%). The highest concentrations of BDE-153 were obtained from MAK5 (49%). MAK5 gave the highest concentration of BDE-183 (39%).
In sediment samples (Fig. 4b), the highest detected concentration of BDE-17 is at MAK4 (55%), followed by MAK1 (30%). The highest concentrations of BDE- 47 were from MAK4 (66%), followed by MAK1 (26%). The highest detected concentrations of BDE-66 were from MAK4 (45%) and then MAK1 (42%). The highest BDE-100 concentration detected was from MAK4 (88%); then, each of MAK 1, MAK2, MAK5 was 4%. The highest concentrations of BDE-153 found were from MAK1 (61%). The highest concentrations of BDE-183 detected were from MAK4 (57%). Most of the contaminations come from MAK4 and MAK1.
Contamination pattern and source apportionment of pollution in Markman Canal using a dendrogram
Dendrogram establishing the categorized cluster breakdown (Hellar-Kihampa et al., 2013) of PBDEs of water and sediment samples from five sampling points at the Markman Canal is illustrated (Fig. 5). Locations in the same clusters display related pollution. The mean water samples (MAK1, MAK2, MAK3, MAK4, MAK5) and sediment samples (MAK1, MAK2, MAK3, MAK4, MAK5) across the sites were represented. The cluster indicates that the study site is grouped into three clusters. The first cluster, which has the highest concentration, is divided into two (MAK4 and MAK1). The second cluster comprises MAK4, while the third cluster, which is related to similar concentrations, comprises (MAK1, MAK2, MAK3, MAK4, MAK5, MAK2, MAK5, MAK3).
Conclusion
Investigation of PBDEs (BDE-17, BDE-47, BDE-66, BDE-100, BDE-153, BDE-183) was carried out using SPE and USE for water and sediment extraction. Analysis was carried out with GC-µECD. The highest detected of all the congeners in sediment was BDE-153. MAK5 was the highest contaminated in water for summer, while MAK4 has the highest contamination in spring. The three points of the industrial zone gave the most increased pollution. The pollution state of the stormwater runoff, based on this study's findings, poses a health risk to both its dependents and the receiving water bodies. Consequently, the industries emptying their effluents in the Markman canal should continue to work on the elimination process to see that these congeners are mitigated to a reasonable value so that the receiving river (Swartkops River) is safe to the public.
References
Abbasi, G., Buser, A. M., Soehl, A., Murray, M. W., & Diamond, M. L. (2015). Stocks and flows of PBDEs in products from use to waste in the U.S. and canada from 1970 to 2020. Environmental Science and Technology, 49, 1521–1528. https://doi.org/10.1021/es504007v
Adams, J. B., Pretorius, L., & Snow, G. C. (2019). Deterioration in the water quality of an urbanised estuary with recommendations for improvement. Water SA, 45, 86–96. https://doi.org/10.4314/wsa.v45i1.10
Akortia, E., Okonkwo, J. O., Lupankwa, M., Osae, S. D., Daso, A. P., Olukunle, O. I., & Chaudhary, A. (2016). A review of sources, levels, and toxicity of polybrominated diphenyl ethers (PBDEs) and their transformation and transport in various environmental compartments. Environmental Reviews. https://doi.org/10.1139/er-2015-0081
American Public Health Association (APHA). (2005). Standard methods for the examination of water and wastewater 2.
Bergman, Å., Rydén, A., Law, R. J., de Boer, J., Covaci, A., Alaee, M., Birnbaum, L., Petreas, M., Rose, M., Sakai, S., Van den Eede, N., & van der Veen, I. (2012). A novel abbreviation standard for organobromine, organochlorine and organophosphorus flame retardants and some characteristics of the chemicals. Environment International, 49, 57–82. https://doi.org/10.1016/j.envint.2012.08.003
Bi, X., Thomas, G. O., Jones, K. C., Qu, W., Sheng, G., Martin, F. L., & Fu, J. (2007). Exposure of electronics dismantling workers to polybrominated diphenyl ethers, polychlorinated biphenyls, and qrganochlorine pesticides in South China. Environmental Science and Technology, 41, 5647–5653. https://doi.org/10.1021/es070346a
Blais, J. M., Charpentié, S., Pick, F., Kimpe, L. E., Amand, A. S., & Regnault-Roger, C. (2006). Mercury, polybrominated diphenyl ether, organochlorine pesticide, and polychlorinated biphenyl concentrations in fish from lakes along an elevation transect in the French Pyrénées. Ecotoxicology and Environmental Safety, 63, 91–99. https://doi.org/10.1016/j.ecoenv.2005.08.008
Bush, P.G.W., April, S., Epa, T., Survey, G., (2001). Environmental M News pp. 50–59.
Cai, M. G., Hong, Q. Q., Wang, Y., Luo, X. J., Chen, S. J., Cai, M. H., Qiu, C. R., Huang, S. Y., & Mai, B. X. (2012). Distribution of polybrominated diphenyl ethers and decabromodiphenylethane in surface sediments from the Bering Sea, Chukchi Sea, and Canada Basin. Deep. Res. Part II Top. Stud. Oceanogr., 81–84, 95–101. https://doi.org/10.1016/j.dsr2.2011.05.006
Canada Environment Protection. (2006). Canadian Environmental Protection Act, 1999. Ecological Screening Assessment Report on Polybrominated Diphenyl Ethers (PBDEs), Canadian Environmental.
Caruso, A., Santoro, M., (2014). Detection of Organochlorine Pesticides by GC-ECD Following U.S. EPA Method 8081. Detect. Organochlor. Pestic. by GC-ECD Follow. U.S. EPA Method 8081 4.
Chen, D., Hale, R.C., Watts, B.D., La Guardia, M.J., Harvey, E., Mojica, E.K., (2010). Species-specific accumulation of polybrominated diphenyl ether flame retardants in birds of prey from the Chesapeake Bay region, USA. Environ. Pollut. https://doi.org/10.1016/j.envpol.2009.10.042
Chen, J., Zhou, H. C., Pan, Y., Shyla, F. S., & Tam, N. F. Y. (2016). Effects of polybrominated diphenyl ethers and plant species on nitrification, denitrification and anammox in mangrove soils. Science of the Total Environment, 553, 60–70. https://doi.org/10.1016/j.scitotenv.2016.02.052
Chokwe, T. B., Magubane, M. N., Abafe, O. A., Okonkwo, J. O., & Sibiya, I. V. (2019). Levels, distributions, and ecological risk assessments of polybrominated diphenyl ethers and alternative flame retardants in river sediments from Vaal River, South Africa. Environmental Science and Pollution Research, 26, 7156–7163. https://doi.org/10.1007/s11356-018-04063-4
Costa, L. G., Giordano, G., Tagliaferri, S., Caglieri, A., & Mutti, A. (2008). Polybrominated diphenyl ether (PBDE) flame retardants: Environmental contamination, human body burden and potential adverse health effects. Acta Biomedica L’ateneo Parmense, 79, 172–183.
Darnerud, P. O., Eriksen, G. S., Jóhannesson, T., Larsen, P. B., & Viluksela, M. (2001). Polybrominated diphenyl ethers: Occurence, dietary exposure, and toxicology. Environmental Health Perspectives, 109, 49–68. https://doi.org/10.2307/3434846
Daso, A. P., Fatoki, O. S., & Odendaal, J. P. (2013a). Occurrence of polybrominated diphenyl ethers (PBDEs) and 2,2’,4,4’,5,5’-hexabromobiphenyl (BB-153) in water samples from the Diep River, Cape Town, South Africa. Environmental Science and Pollution Research. https://doi.org/10.1007/s11356-013-1503-6
Daso, A. P., Fatoki, O. S., Odendaal, J. P., & Olujimi, O. O. (2012). Occurrence of selected polybrominated diphenyl ethers and 2,2’,4,4’,5,5’-hexabromobiphenyl (BB-153) in sewage sludge and effluent samples of a wastewater-treatment plant in Cape Town, South Africa. Archives of Environmental Contamination and Toxicology, 62, 391–402. https://doi.org/10.1007/s00244-011-9720-9
Daso, A. P., Fatoki, O. S., Odendaal, J. P., & Olujimi, O. O. (2013b). Polybrominated diphenyl ethers (PBDEs) and 2,2′,4,4′,5, 5′-hexabromobiphenyl (BB-153) in landfill leachate in Cape Town, South Africa. Environmental Monitoring and Assessment, 185, 431–439. https://doi.org/10.1007/s10661-012-2565-5
De Boer, J., Wester, P. G., Van Der Horst, A., & Leonards, P. E. G. (2003). Polybrominated diphenyl ethers in influents, suspended particulate matter, sediments, sewage treatment plant and effluents and biota from the Netherlands. Environmental Pollution, 122, 63–74. https://doi.org/10.1016/S0269-7491(02)00280-4
De Wit, C. A. (2002). An overview of brominated flame retardants in the environment. Chemosphere. https://doi.org/10.1016/S0045-6535(01)00225-9
Department of Environmental Affairs (DEA). (2012). Chapter 9: Oceans and Coasts: Ocean and coasts ecosystem services are important as they directly and indirectly impact on human livelihoods, food security and agriculture.
Di Gangi, J., Strakova, J., Bell, L. (2017). POPs recycling contaminates children’s toys with toxic flame retardants 19.
Env.Canada. (2013). Federal Environmental Quality Guidelines Cobalt.
Environment Canada. (2013). Federal Environmental Quality Guidelines Cobalt.
Fondriest Environmental, I. (2015). Turbidity, Total Suspended Solids & Water Clarity - Environmental Measurement Systems. http://www.fondriest.com.
Fontana, A. R., Silva, M. F., Martínez, L. D., Wuilloud, R. G., & Altamirano, J. C. (2009). Determination of polybrominated diphenyl ethers in water and soil samples by cloud point extraction-ultrasound-assisted back-extraction-gas chromatography-mass spectrometry. Journal of Chromatography A, 1216, 4339–4346. https://doi.org/10.1016/j.chroma.2009.03.029
Ge, J., Liu, M., Yun, X., Yang, Y., Zhang, M., Li, Q. X., & Wang, J. (2014a). Occurrence, distribution and seasonal variations of polychlorinated biphenyls and polybrominated diphenyl ethers in surface waters of the East Lake, China. Chemosphere. https://doi.org/10.1016/j.chemosphere.2013.12.014
Ge, J., Liu, M., Yun, X., Yang, Y., Zhang, M., Li, Q. X., & Wang, J. (2014b). Occurrence, distribution and seasonal variations of polychlorinated biphenyls and polybrominated diphenyl ethers in surface waters of the East Lake, China. Chemosphere, 103, 256–262. https://doi.org/10.1016/j.chemosphere.2013.12.014
Ge, W., Mou, Y., Chai, C., Zhang, Y., Wang, J., Ju, T., Jiang, T., & Xia, B. (2018). Polybrominated diphenyl ethers in the dissolved and suspended phases of seawater from Sanggou Bay, east China. Chemosphere, 203, 253–262. https://doi.org/10.1016/j.chemosphere.2018.03.184
Gouin, T., & Harner, T. (2003). Modelling the environmental fate of the polybrominated diphenyl ethers. Environment International, 29, 717–724. https://doi.org/10.1016/S0160-4120(03)00116-8
Guan, Y. F., Sojinu, O. S. S., Li, S. M., & Zeng, E. Y. (2009). Fate of polybrominated diphenyl ethers in the environment of the Pearl River Estuary, South China. Environmental Pollution, 157, 2166–2172. https://doi.org/10.1016/j.envpol.2009.02.006
Hellar-Kihampa, H., De Wael, K., Lugwisha, E., Malarvannan, G., Covaci, A., & Van Grieken, R. (2013). Spatial monitoring of organohalogen compounds in surface water and sediments of a rural-urban river basin in Tanzania. Science of the Total Environment, 447, 186–197. https://doi.org/10.1016/j.scitotenv.2012.12.083
Huwe, J. K., & Smith, D. J. (2007). Erratum: Accumulation, whole-body depletion, and debromination of decabromodiphenyl ether in male Sprague-Dewley rats following dietary exposure (Environmental Science and Technology (2007) 41, (2371–2377)). Environmental Science and Technology, 41, 4486. https://doi.org/10.1021/es070875e
Hwang, I. K., Kang, H. H., Lee, I. S., & Oh, J. E. (2012). Assessment of characteristic distribution of PCDD/Fs and BFRs in sludge generated at municipal and industrial wastewater treatment plants. Chemosphere, 88, 888–894. https://doi.org/10.1016/j.chemosphere.2012.03.098
Ibrahim, R. S., Khairy, A., Zaatout, H. H., Hammoda, H. M., & Metwally, A. M. (2018). Digitally-optimized HPTLC coupled with image analysis for pursuing polyphenolic and antioxidant profile during alfalfa sprouting. Journal of Chromatography B Analytical Technologies in the Biomedical and Life Sciences, 1099, 92–96. https://doi.org/10.1016/j.jchromb.2018.09.021
Ji, X., Ding, J., Xie, X., Cheng, Y., Huang, Y., Qin, L., & Han, C. (2017). Pollution status and human exposure of decabromodiphenyl ether (BDE-209) in China. ACS Omega, 2, 3333–3348. https://doi.org/10.1021/acsomega.7b00559
Jiang, Y., Yuan, L., Lin, Q., Ma, S., & Yu, Y. (2019). Polybrominated diphenyl ethers in the environment and human external and internal exposure in China: A review. Science of the Total Environment. https://doi.org/10.1016/j.scitotenv.2019.133902
Kelly, B. C., Ikonomou, M. G., Blair, J. D., & Gobas, F. A. P. C. (2008). Bioaccumulation behaviour of polybrominated diphenyl ethers (PBDEs) in a Canadian Arctic marine food web. Science of the Total Environment. https://doi.org/10.1016/j.scitotenv.2008.03.045
Kim, M., Li, L. Y., Gorgy, T., & Grace, J. R. (2017). Review of contamination of sewage sludge and amended soils by polybrominated diphenyl ethers based on meta-analysis. Environmental Pollution, 220, 753–765. https://doi.org/10.1016/j.envpol.2016.10.053
Kowalski, B., & Mazur, M. (2014). The simultaneous determination of six flame retardants in water samples using SPE pre-concentration and UHPLC-UV method. Water, Air, and Soil Pollution. https://doi.org/10.1007/s11270-014-1866-4
Kuramochi, H., Maeda, K., & Kawamoto, K. (2007). Physicochemical properties of selected polybrominated diphenyl ethers and extension of the UNIFAC model to brominated aromatic compounds. Chemosphere, 67, 1858–1865. https://doi.org/10.1016/j.chemosphere.2006.05.076
Li, T. Y., Zhou, J. F., Wu, C. C., Bao, L. J., Shi, L., & Zeng, E. Y. (2018). Characteristics of polybrominated diphenyl ethers released from thermal treatment and open burning of E-waste. Environmental Science and Technology, 52, 4650–4657. https://doi.org/10.1021/acs.est.8b00780
Liang, X., Junaid, M., Wang, Z., Li, T., & Xu, N. (2019). Spatiotemporal distribution, source apportionment and ecological risk assessment of PBDEs and PAHs in the Guanlan River from rapidly urbanizing areas of Shenzhen, China. Environmental Pollution, 250, 695–707. https://doi.org/10.1016/j.envpol.2019.04.107
Lord, D., Mackay, H. (1991). The effect of urban runoff on the water quality of the swartkops estuary Report to the. Rep. TO WATER Res. Comm. WRC Rep. No 324/1/93 1–94.
Martin, M., Lam, P. K. S., & Richardson, B. J. (2004). An Asian quandary: Where have all of the PBDEs gone? Marine Pollution Bulletin. https://doi.org/10.1016/j.marpolbul.2004.05.015
Montalbano, A. M., Albano, G. D., Anzalone, G., Moscato, M., Gagliardo, R., Di Sano, C., Bonanno, A., Ruggieri, S., Cibella, F., & Profita, M. (2020). Cytotoxic and genotoxic effects of the flame retardants (PBDE-47, PBDE-99 and PBDE-209) in human bronchial epithelial cells. Chemosphere. https://doi.org/10.1016/j.chemosphere.2019.125600
Muenhor, D., Moon, H. B., Lee, S., & Goosey, E. (2017). Polybrominated diphenyl ethers (PBDEs) in floor and road dust from a manual e-waste dismantling facility and adjacent communities in Thailand. Journal of Environmental Science and Health: Part A Toxic/hazardous Substances and Environmental Engineering. https://doi.org/10.1080/10934529.2017.1357405
Nel, L. (2014). Presence, levels and distribution of pollutants in the estuarine food web- Swartkops River Estuary, South Africa. Nelson Mand. Univ. Masters Diss.
Ohoro, C. R., Adeniji, A. O., Okoh, A. I., & Okoh, O. O. (2021). Polybrominated diphenyl ethers in the environmental systems: A review. Journal of Environmental Health Science and Engineering. https://doi.org/10.1007/s40201-021-00656-3
Olisah, C., Adeniji, A. O., Okoh, O. O., & Okoh, A. I. (2019). Occurrence and risk evaluation of organochlorine contaminants in surface water along the course of Swartkops and Sundays River Estuaries, Eastern Cape Province South Africa. Environmantal Geochemistry Health, 41, 2777–2801. https://doi.org/10.1007/s10653-019-00336-0
Olisah, C., Okoh, O. O., & Okoh, A. I. (2020). Polybrominated diphenyl ethers (PBDEs) in surface water and fish tissues from Sundays and Swartkops Estuaries, Eastern Cape Province, South Africa: Levels, spatial distribution, seasonal variation and health implications. Regional Studies in Marine Science. https://doi.org/10.1016/j.rsma.2020.101319
Oloruntoba, K., Sindiku, O., Osibanjo, O., Balan, S., & Weber, R. (2019). Polybrominated diphenyl ethers (PBDEs) in chicken eggs and cow milk around municipal dumpsites in Abuja, Nigeria. Ecotoxicology and Environmental Safety, 179, 282–289. https://doi.org/10.1016/j.ecoenv.2019.04.045
Olukunle, O., Okonkwo, J., Kefeni, K., & Lupankwa, M. (2012). Concentrations of polybrominated diphenyl ethers in sediments from Jukskei River, Gauteng, South Africa. Bulletin of Environment Contamination and Toxicology, 88, 461–466. https://doi.org/10.1007/s00128-011-0481-y
Olukunle, O. I., Okonkwo, O. J., Wase, A. G., & Sha’ato, R. (2015). Polybrominated diphenyl ethers in car dust in Nigeria: Concentrations and implications for non-dietary human exposure. Microchemical Journal. https://doi.org/10.1016/j.microc.2015.05.023
Ontiveros-Cuadras, J. F., Ruiz-Fernández, A. C., Sanchez-Cabeza, J. A., Sericano, J., Pérez-Bernal, L. H., Páez-Osuna, F., Dunbar, R. B., & Mucciarone, D. A. (2019). Recent history of persistent organic pollutants (PAHs, PCBs, PBDEs) in sediments from a large tropical lake. Journal of Hazardous Materials, 368, 264–273. https://doi.org/10.1016/j.jhazmat.2018.11.010
Oros, D. R., Hoover, D., Rodigari, F., Crane, D., & Sericano, J. (2005). Levels and distribution of polybrominated diphenyl ethers in water, surface sediments, and bivalves from the San Francisco. Environmental Science and Technology, 39, 33–41. https://doi.org/10.1021/es048905q
Palm, A., Cousins, I. T., Mackay, D., Tysklind, M., Metcalfe, C., & Alaee, M. (2002). Assessing the environmental fate of chemicals of emerging concern: A case study of the polybrominated diphenyl ethers. Environmental Pollution, 117, 195–213. https://doi.org/10.1016/S0269-7491(01)00276-7
Papu, L. (2015). Public health impacts of storm communities.
Parry, E., Zota, A. R., Park, J. S., & Woodruff, T. J. (2018). Polybrominated diphenyl ethers (PBDEs) and hydroxylated PBDE metabolites (OH-PBDEs): A six-year temporal trend in Northern California pregnant women. Chemosphere, 195, 777–783. https://doi.org/10.1016/j.chemosphere.2017.12.065
Pietron, W., Pajurek, M., Mikolajczyk, S., Maszewski, S., Warenik-Bany, M., & Piskorska-Pliszczynska, J. (2019). Exposure to PBDEs associated with farm animal meat consumption. Chemosphere, 224, 58–64. https://doi.org/10.1016/j.chemosphere.2019.02.067
Raff, J. D., & Hites, R. A. (2007). Deposition versus photochemical removal of PBDEs from lake superior air. Environmental Science and Technology. https://doi.org/10.1021/es070789e
Rahman, F., Langford, K. H., Scrimshaw, M. D., & Lester, J. N. (2001). Polybrominated diphenyl ether (PBDE) flame retardants. Science of the Total Environment, 275, 1–17. https://doi.org/10.1016/S0048-9697(01)00852-X
Ramu, K., Isobe, T., Takahashi, S., Kim, E. Y., Min, B. Y., We, S. U., & Tanabe, S. (2010). Spatial distribution of polybrominated diphenyl ethers and hexabromocyclododecanes in sediments from coastal waters of Korea. Chemosphere, 79, 713–719. https://doi.org/10.1016/j.chemosphere.2010.02.048
Robrock, K. R., Korytár, P., & Alvarez-Cohen, L. (2008). Pathways for the anaerobic microbial denomination of polybrominated diphenyl ethers. Environmental Science and Technology, 42, 2845–2852. https://doi.org/10.1021/es0720917
Romanelli, S., Bettazzi, F., Martellini, T., Shelver, W. L., Cincinelli, A., Galarini, R., & Palchetti, I. (2017). Evaluation of a QuEChERS-like extraction approach for the determination of PBDEs in mussels by immuno-assay-based screening methods. Talanta, 170, 540–545. https://doi.org/10.1016/j.talanta.2017.04.027
Rügner, H., Schwientek, M., Beckingham, B., Kuch, B., & Grathwohl, P. (2013). Turbidity as a proxy for total suspended solids (TSS) and particle facilitated pollutant transport in catchments. Environment and Earth Science, 69, 373–380. https://doi.org/10.1007/s12665-013-2307-1
Sahlström, L. M. O., Sellström, U., de Wit, C. A., Lignell, S., & Darnerud, P. O. (2015). Estimated intakes of brominated flame retardants via diet and dust compared to internal concentrations in a Swedish mother-toddler cohort. International Journal of Hygiene and Environmental Health, 218, 422–432. https://doi.org/10.1016/j.ijheh.2015.03.011
Shao, Y., Han, S., Ma, L., Luo, M., Yang, G., Liu, W., & Xu, D. (2018). Polybrominated diphenyl ethers in surface waters around Beijing: Occurrence, distribution and sources. Applied Geochemistry, 98, 58–64. https://doi.org/10.1016/j.apgeochem.2018.09.011
Söderström, G., Sellström, U., De Wit, C. A., & Tysklind, M. (2004). Photolytic Debromination of Decabromodiphenyl Ether (BDE 209). Environmental Science and Technology, 38, 127–132. https://doi.org/10.1021/es034682c
Song, W., Ford, J. C., Li, A., Mills, W. J., Buckley, D. R., & Rockne, K. J. (2004). Polybrominated diphlenyl ethers in the sediments of the great lakes. 1 Lake Superior. Environmental Science and Technology, 38, 3286–3293. https://doi.org/10.1021/es035297q
Trinh, M. M., Tsai, C. L., & Chang, M. B. (2019). Characterization of polybrominated diphenyl ethers (PBDEs) in various aqueous samples in Taiwan. Science of the Total Environment. https://doi.org/10.1016/j.scitotenv.2018.08.204
Trudel, D., Scheringer, M., Von Goetz, N., & Hungerbühler, K. (2011). Total consumer exposure to polybrominated diphenyl ethers in north america and europe. Environmental Science and Technology, 45, 2391–2397. https://doi.org/10.1021/es1035046
United Nations Environment Programme, n.d. Listing of POPs in the Stockholm Convention.
Viganò, L., Roscioli, C., & Guzzella, L. (2011). Decabromodiphenyl ether (BDE-209) enters the food web of the River Po and is metabolically debrominated in resident cyprinid fishes. Science of the Total Environment, 409, 4966–4972. https://doi.org/10.1016/j.scitotenv.2011.07.062
Vonderheide, A. P., Mueller, K. E., Meija, J., & Welsh, G. L. (2008). Polybrominated diphenyl ethers: Causes for concern and knowledge gaps regarding environmental distribution, fate and toxicity. Science of the Total Environment, 400, 425–436. https://doi.org/10.1016/j.scitotenv.2008.05.003
Wang, Q., Li, X., Liu, S., Zhang, D., & Duan, X. (2019). The effect of hydrodynamic forcing on the transport and deposition of polybrominated diphenyl ethers (PBDEs) in Hangzhou Bay. Ecotoxicology and Environmental Safety, 179, 111–118. https://doi.org/10.1016/j.ecoenv.2019.04.026
Wang, S., Zhang, S., Huang, H., Lu, A., & Ping, H. (2012). Debrominated, hydroxylated and methoxylated metabolism in maize (Zea mays L.) exposed to lesser polybrominated diphenyl ethers (PBDEs). Chemosphere, 89, 1295–1301. https://doi.org/10.1016/j.chemosphere.2012.05.026
Wong, M. H., Wu, S. C., Deng, W. J., Yu, X. Z., Luo, Q., Leung, A. O. W., Wong, C. S. C., Luksemburg, W. J., & Wong, A. S. (2007). Export of toxic chemicals - A review of the case of uncontrolled electronic-waste recycling. Environmental Pollution, 149, 131–140. https://doi.org/10.1016/j.envpol.2007.01.044
World Health Organization, 2010. WHO human health risk assessment toolkit: chemical hazards. IPCS Harmon. Proj. Doc.
Wu, J. P., Wu, S. K., Tao, L., She, Y. Z., Chen, X. Y., Feng, W. L., Zeng, Y. H., Luo, X. J., & Mai, B. X. (2020). Bioaccumulation characteristics of PBDEs and alternative brominated flame retardants in a wild frog-eating snake. Environmental Pollution. https://doi.org/10.1016/j.envpol.2019.113661
Wu, M. H., Tang, L., Xu, G., Ma, J., Liu, N., Wang, L., & Lei, J. Q. (2013a). Polybrominated diphenyl ethers in surface sediments from principal watersheds of Shanghai, China: Levels, distribution, influencing factors, and risk assessment. Environmental Science and Pollution Research, 20, 2651–2660. https://doi.org/10.1007/s11356-012-1163-y
Wu, M. H., Tang, L., Xu, G., Ma, J., Liu, N., Wang, L., & Lei, J. Q. (2013b). Polybrominated diphenyl ethers in surface sediments from principal watersheds of Shanghai, China: Levels, distribution, influencing factors, and risk assessment. Environmental Science and Pollution Research. https://doi.org/10.1007/s11356-012-1163-y
Wu, M. H., Xu, B. T., Xu, G., Wang, M. N., Ma, J., Pan, C. Y., Sun, R., Han, T., & Tang, L. (2017). Occurrence and profiles of polybrominated diphenyl ethers (PBDEs) in riverine sediments of Shanghai: A combinative study with human serum from the locals. Environmental Geochemistry and Health. https://doi.org/10.1007/s10653-016-9843-z
Wurl, O., Lam, P. K. S., & Obbard, J. P. (2006). Occurrence and distribution of polybrominated diphenyl ethers (PBDEs) in the dissolved and suspended phases of the sea-surface microlayer and seawater in Hong Kong, China. Chemosphere, 65, 1660–1666. https://doi.org/10.1016/j.chemosphere.2006.02.024
Xu, P., Ge, W., Chai, C., Zhang, Y., Jiang, T., & Xia, B. (2019). Sorption of polybrominated diphenyl ethers by microplastics. Marine Pollution Bulletin, 145, 260–269. https://doi.org/10.1016/j.marpolbul.2019.05.050
Yahaya, A., Okoh, O. O., Okoh, A. I., & Adeniji, A. O. (2017). Occurrences of organochlorine pesticides along the course of the Buffalo river in the eastern cape of South Africa and its health implications. International Journal of Environmental Research and Public Health. https://doi.org/10.3390/ijerph14111372
Yang, Y., Xie, Q., Liu, X., & Wang, J. (2015). Occurrence, distribution and risk assessment of polychlorinated biphenyls and polybrominated diphenyl ethers in nine water sources. Ecotoxicology and Environmental Safety, 115, 55–61. https://doi.org/10.1016/j.ecoenv.2015.02.006
Yao, Y., Zhou, Y., Wang, W., Zhou, D., Wang, L., Corvini, P. F. X., & Ji, R. (2020). Fate of lower-brominated diphenyl ethers (LBDEs) in a red soil – Application of 14C-labelling. Science of the Total Environment. https://doi.org/10.1016/j.scitotenv.2020.137735
Yin, H., Tang, Z., Meng, T., & Zhang, M. (2020). Concentration profile, spatial distributions and temporal trends of polybrominated diphenyl ethers in sediments across China: Implications for risk assessment. Ecotoxicology and Environmental Safety. https://doi.org/10.1016/j.ecoenv.2020.111205
Yin, S., Guo, F., Aamir, M., Liu, Y., Tang, M., & Liu, W. (2019). Multicenter biomonitoring of polybrominated diphenyl ethers (PBDEs) in colostrum from China: Body burden profile and risk assessment. Environmental Research, 179, 108828. https://doi.org/10.1016/j.envres.2019.108828
Zhang, Y., Geißen, S. U., & Gal, C. (2008). Carbamazepine and diclofenac: Removal in wastewater treatment plants and occurrence in water bodies. Chemosphere. https://doi.org/10.1016/j.chemosphere.2008.07.086
Acknowledgements
The authors are grateful to the South African Medical Research Council (SAMRC) for funding support.
Funding
The research was funded by the South African Medical Research Council (Grant No. UFH/SAMRC/P790).
Author information
Authors and Affiliations
Contributions
CRO performed conceptualization, data collection and analyses, and preparation of the manuscript; AOA, OOO, LS, and AIO contributed to data analyses and review and updating of the manuscript ; OOO and AIO done supervision and funding of the research.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ohoro, C.R., Adeniji, A.O., Okoh, A.I. et al. Spatial monitoring and health risk assessment of polybrominated diphenyl ethers in environmental matrices from an industrialized impacted canal in South Africa. Environ Geochem Health 44, 3409–3424 (2022). https://doi.org/10.1007/s10653-021-01114-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-021-01114-7