Abstract
A new index is proposed to determine the affinity of heavy metals (HM) to their carrier phases (AHM-fraction), which, in contrast to the traditional index CHM = 100 CHM-fraction/CHM-soil, considers the sum of all metals in the fraction as a share of the bulk content of all HM in the soil. The metal has affinity for the given phase if AHM-fraction > 1; vice versa, the affinity is absent if AHM-fraction < 1. Comparison of the affinity series of metals for a certain phase based on two indices revealed their discrepancy in most cases. The new index can take into consideration the discrepancy in affinity of the given metal for phases extracted by different strength reagents. The effect of the new indicator was tested on several contaminated soils: Haplic Chernozem, Stagnic Phaeozems, and Calcaric Fluvic Arenosol, as well as on two Spolic Technosols. Compared with the index CHM, the results of the new analysis of contaminated soils with the ATM fraction demonstrated that the Zn content in Calcaric Fluvic Arenosol is decreased considerably due to its low buffer capacity. Since the content of organic matter in Calcaric Fluvic Arenosol is insignificant, only organophile elements, such as Cu and Pb, can make up complexes with organic ligands, in contrast to the fixation of Ni and Mn by organic matter in Chernozems. Due to the low buffering capacity of Calcaric Fluvic Arenosol, the mobile forms of Cd and Zn increased, and these forms of Cr decreased. Therefore, the low buffering soil cannot fix Cd and Zn. Increase in contamination in Spolic Technosols (approximate permissible concentration, APC > 5) as compared to the index CHM, the value of the AHM-fraction of metals in the residue (except for cadmium) increased. In addition, the share of Pb and Cu increases in the organic matter. Thus, the use of a new indicator—the affinity of heavy metals to the carrier phases showed their advantage over the traditional index CHM.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metals (HM) contained in air, water, and soil are hazardous for the human organism (Kabata-Pendias, 2011). If the anthropogenic HMs are accumulated in soil, their input to the soil–groundwater system is hampered, on the one hand, and they contaminate the agricultural products consumed by humans and animals, on the other hand (Acosta et al., 2015; Huang et al., 2018). HMs are persistent toxic pollutants that are characterized by latent, long-term, cumulative, and irreversible parameters (Zhao et al., 2019). Therefore, soil scientists pay great attention to the study of HM content in soils (Kabata-Pendias, 2011; Karim et al., 2014; Minkina et al., 2008; Vodyanitskii, 2006; Wang et al., 2021).
Initially, attention was focused on determination of the bulk HM content in soils. It turned out, however, that this bulk content is not an accurate indicator of the hazard level, as it only provides limited information on their chemical behavior and availability (Bacon & Davidson, 2008; Vodyanitskii, 2006). It is also necessary to know the degree of mobility and accessibility of metals for plants, and low buffer capacity of soil to contaminate the soil–groundwater system (Kartal et al., 2006; Reimann & Caritat, 1998). Thus, in addition to the bulk HM content in soils, knowledge about the affinity of metals for the chemical carrier phases of different nature is essential for estimating their hazard level.
Most commonly, the content of HM associated with carrier phases is determined by the sequential chemical extraction from soils (Fedotov et al., 2019; Hasan et al., 2018; Land et al., 1999; Vodyanitskii, 2006). The sequence and type of reagents vary in different techniques, but researchers apply mainly reagents intended for dissolving three or four different carrier phases of metals, for example, exchangeable metals + metals associated with carbonates; metals associated with organic matter; metals associated with differently crystallized Fe–Mn oxides; and metals retained in the insoluble residue (He et al., 2013; Strawn & Baker, 2008). Their role of phases–carriers in the HM fixation depends on the soil type and strength of the bond with the metal (Nevidomskaya et al. 2020). The obtained results—content of HM associated with carrier phases—are processed in such a way that the share of each metal (pCHM-fraction) associated with the given phase is expressed in percentage of its bulk content in soil (Fedotov et al., 2019; Ladonin, 2016; Land et al., 1999).
Index pCHM is used very widely. Most commonly, pCHM values for different metals in a single fraction are compared, and this parameter is considered an indicator of the affinity of metal for one of the fractions. Values of pCHM are used to determine a series of the affinity of metals for different fractions: the higher the share of the given metal (greater than pCHM), the higher is its affinity for the fraction under consideration and vice versa. A high pCHM value suggests selectivity of the metal contained in the given fraction. The indices pC do not take into account the share of metals in the given chemical fraction, relative to the initial content of the sum of all metals in the initial soil. Therefore, these parameters are unsuitable for estimating the degree of metal enrichment/depletion in the fraction relative to the initial sample. This is an obvious shortcoming of indices pC—they do not yield the critical metal content in each fraction. Therefore, they do not provide the boundary, above (or below) which the fraction is selective (or nonselective) for the given metal.
The aim of this paper is to apply a new indicator of the affinity of metals to their carrier phases (AHM-fraction) and to compare it with the traditional indicator of selectivity (pCHM-fraction, %) using the example of contaminated soils in the south of European Russia.
Objects
Pollution zone near the Novocherkassk Power Plant
We studied soils at monitoring sites subjected to the influence of emissions from the Novocherkassk Power Plant (NPP), one of the largest thermal power stations in southern Russia. During the power station construction, sanitary-protective zone was not outlined, and wind rose was ignored—99% of aerosol emissions from the power station falls into the City of Novocherkassk; more than 50%, into the Rostov region (Minkina et al., 2009). Such significant volume of emissions from the NPP creates a tense ecological situation in the region. The power station consumes annually 4.5–4.7 Mt of the high-ash anthracite briquette, 0.7 Mt of heavy oil, and 380 mln m3 of gas (Linnik et al., 2020). Emissions from NPP consist mainly of ash, sulfurous anhydride, nitrogen oxides, carbon black (over 30 tons/year), hydrogen fluoride (7 kg/year), vanadium pentoxide (about 8 tons/year), iron oxides (over 5 tons/year) and HMs (among which Cu, Zn and Pb dominate), chromic anhydride (about 0.1 tons/year) and others (Ecological, 2020).
In 2000, 12 monitoring sites (nos. 1–12) were deployed at different distances (1–20 km) from the NPP. The sites are assigned to air sampling points outlined for the organization and arrangement of a sanitary-protective zone in the northern industrial cluster of Novocherkassk. Three soil monitoring sites are discussed in the present paper. The soils of the monitoring sites are located in the most typical landscape conditions and represented by various soil types. Monitoring site 10 located near the NPP is represented by the common, heavy loam chernozem (Haplic Chernozem); site 3, by the low-clay, meadow-chernozem soil (Stagnic Phaeozems); and site 2, by sandy alluvial soil (Calcaric Fluvic Arenosol) of the Tuzlov River floodplain. Sites 3 (2.7 km SW from the NPP) and 2 (3.0 km SW from the NPP) are located, respectively, in the near 5-km zone of influence of plant emissions. Monitoring site 10 (located at a distance of 20 km NW from NPP) is located between two highways at 400 m from one of them. Data on the soil type differ significantly in terms of physicochemical properties (Table 1).
The content of silt (< 0.001 mm) in the surficial soil horizon varies significantly from 2.1 to 37.0%; the content of clay (< 0.01 mm), from 7.5 to 68.2%. The highest content of these fractions is recorded in the low-clay, Stagnic Phaeozems variety developed in alluvial sediments (site 3); the lowest content, in Calcaric Fluvic Arenosol (site 2). The latter soil is also marked by low content of organic carbon Corg (0.9%) and low position in the European Waste Classification (15 cmol( +)/kg). Values of pH in soils at the monitoring sites are similar and correspond to the low-alkaline variety with neutral reaction. The content of carbonates in them is 0.5–0.9%.
Pollution zone near Lake Atamanskoe
The lake is located on the floodplain of the Seversky Donets River, the main tributary of the Don River. The lake represents a dried oxbow of the Seversky Donets River with a pulsating water regime. Since the early 1960s until the mid-1990s, Lake Atamanskoe was exploited as waste reservoir for chemical plant "Kamenskvolokno" (Privalenko et al., 2000). The plant is the largest and oldest manufacturer of chemical fibers in the south of Russia. It is the most hazardous textile enterprise that produces semi-synthetic and synthetic fibers, since the effluents from this process contain high concentrations of organic pollutants and metal ions (especially Zn), in excess of the permissible values (Ghosh et al., 2011). Total reserves of Zn in technogenic sediments of Lake Atamanskoe were estimated at 30 kt (Privalenko & Cherkashina, 2012). Hydrological changes in the recent 20–30 years due to the cessation of industrial waste disposal and a more prolonged drought period provoked the disappearance of water in the lake, leading to the beginning of active soil formation (Bauer et al., 2018).
Soils at the monitoring sites characterized by a high degree of technogenic pollution are qualified as Spolic Technosols, according to the World Reference Base of Soil Resources (IUSS Working Group WRB, 2015). The monitoring sites were grouped according to the excess of the approxible permissible concentration (APC) of HMs in soils (HN 2.1.7.2511-09, 2009) and corresponded to high (2–5 APC of metal) and very high levels of contamination (5–10 APC of metal). Based on the analysis of physicochemical properties of soils, the content of organic carbon in them is sufficiently homogeneous (1.7–1.8%); the pH value is 7.0–7.4%, suggesting a neutral and low-alkaline reaction of the medium; the absorbing complex is dominated by Ca2+; the grain size composition is mainly loamy; and the content of silt particles (< 0.001 mm) varies from 16.4 to 20.0% (average 16.7%) (Table 2).
Methods
Soil properties
The soil particle size distribution analysis was performed according to the pipette method with pyrophosphate procedure of soil preparation (ISO 13317-2, 2001) to obtain the content of clay soil size fraction (particles < 0.001 mm) and physical clay (particles < 0.01 mm). Soil properties were determined using standard methods: pH of soil suspension in water by ISO 10,390 (2005); the organic matter (OM) content by sulfochromic (ISO 14235, 1998); carbonates content by a Scheibler apparatus, ISO 10693 (1995), the cation exchange capacity (CEC) and exchangeable cations by hexamminecobalt trichloride solution, ISO 23470 (2011). All laboratory tests were performed in triplicate.
Sequential extraction procedures
In soils of the NPP area, we analyzed seven metals: Cr, Ni, Mn, Cd, Zn, Cu, and Pd; in soils of the Lake Atamanskoe area, five metals: Cr, Cd, Zn, Cu, and Pd. The composition of HM compounds in soils of these areas was analyzed with the sequential fractionation method proposed by Tessier et al., (1979). This method is widely known and applied often in Russia and Europe for evaluating the fractional HM composition in soils (Evans et al., 2019; Orecchio & Polizzotto, 2013; Rosado et al., 2016). The method guarantees the extraction of five fractions of HM compounds: exchangeable fraction (1 M MgCl2); fraction associated with carbonates (1 M CH3COONa); fraction associated with Fe–Mn (hydr)oxides (0.04 M NH2OH·HCl in 25% CH3COOH); fraction associated with organic matter (at first 0.02 M HNO3 + 30% H2O2 with pH = 2, then 3.2 M CH3COONH4 in 20% HNO3); and residual fraction (at first HF + HClO4, then HNO3conc. and evaporation).
New index of the affinity of HM for carrier phases
During the chemical fractionation, the initial soil is separated into several (five or six) fractions, in accordance with the formula (Land et al., 1999; Silveira et al., 2006):
where CHM is the share of metal associated with the given phase, %; CHM-phase is the metal content in the given phase, mg/kg; and CHM-soil is the bulk metal content in soil, mg/kg.
If index pC is used for comparing the metal content in each fraction with the initial soil, this parameter does not show the critical value. Therefore, we cannot determine the boundary, above (or below) which the fraction is selective (or nonselective) for the given metal, relative to the initial soil. To negotiate this issue, it is necessary, first of all, to adjust metals with different Clarke values based on the normalizing index as in formula (2).
Let us transform formula (2). Leaving all elements in the normalized form and taking into consideration the contents of all elements, let us write indicator of the affinity of metals A relative to the sum of all normalized metals. Then, indicator of the affinity of HM for the given soil phase AHM-phase is obtained from formula:
where CHM-phase is the normalized HM content in the given fraction; ΣCHM-phase is the sum of all HMs in the given fraction; CHM-soil is the normalized content of the given HM in the initial soil; and ΣCHM-soil is the sum of all HMs in the initial soil.
Thus, the new formula based on index A takes into consideration the content of all elements in computations of the metal content. This index can determine the affinity of different metals for the given carrier phase. The new index can substitute the traditional indicator of affinity pCHM based on formula (1).
First, we accept Clarke values of each metal in the European soil, according to A. Kabata-Pendias (2011), as normalizing values for the chemical analysis. Clarke of chemical elements for European soils can be used in comparison with the studied soils in the European part of Russia. Then, all further operations are accomplished with metals in the nondimensional mode. When analyzing contents of different metals in the given phase, index AHM-fraction > 1 suggests that the phase is selective for the given metal; vice versa, if AHM-fraction < 1, the phase is nonselective for this metal. When analyzing contents of the given metal in different phases, AHM-fraction > 1 suggests that the metal is more selective than other metals for the given phase; vice versa, if AHM-fraction < 1, the metal is “repulsed” more strongly than the remaining metals. To exclude values of AHM-fraction ~ 1, we shall accept that values of AHM-fraction > 1.2 and < 0.6 are significant.
Reliability of index A of metal shows direct correlation with the number of metals used in the calculations—the higher their number, the more reliable is the calculation of index A. Therefore, the new method is not suitable for rare cases of soil contamination with one metal.
Results and discussion
Pollution zone near the NPP
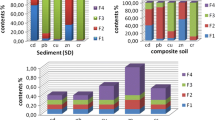
Tables 3–5 present the data for calculating index of affinity of HM to the carrier phases. Based on index pCHM, affinity of HM (e.g., Cr) for phases increases in the following succession: exchangeable metals (0.93) < carbonates (3.18) < organic matter (11.96) < iron minerals (18.88) < residue (62.71) (Table 4). Note that the number in parentheses does not indicate boundary values. Based on index AHM-phase, however, affinity of Cr for phases increases in a different succession: carbonates (0.53) < organic matter (0.87) < residue (0.98) < exchangeable metals (1.24) < iron minerals (1.3) (Table 5). Position of Cr relative to the residue is markedly different: level 1 versus level 62.7 according to index pCHM. Similar results were also obtained for other metals: Table 5 shows that correlation coefficient between indices pC and A is marked by an insignificant variation from 0.843 to 0.864.
Use of index A makes it possible to: (1) obtain numeral characteristics that discriminate the affinity of metals for the given phase or indicate their deficit; (2) record position changes in the affinity of metals for carrier phases: transition of the affinity of metals from the last position (“residue”) based on index pCHM to a higher position and vice versa.
Thus, we believe that index pCHM does not reflect the affinity of metals for fractions in most cases. Calculations of the affinity of metals for different fractions based on the new index AHM-phase are described below. Table 5 presents comparisons of index A in Haplic Chernozem, Stagnic Phaeozems and Calcaric Fluvic Arenosol with a low buffering capacity and Corg content. In contrast to Chernozems, Calcaric Fluvic Arenosol are marked by the following metal fixation patterns. Note that the Zn content decreases appreciably (to 1.04) in the residual fraction of Calcaric Fluvic Arenosol.
Because of a low content of organic matter in Calcaric Fluvic Arenosol, only organophiles, such as Cu (A = 1.36) and Pb (A = 2.12), can make up complexes with organic ligands, in contrast to the fixation of Ni (to 1.58) and Mn (to 1.32) by organic matter in Chernozems. Lead discussed in many works is considered a highly hazardous element, because it is consumed with food and water (Bol’shakov et al., 2004). However, thanks to the buffer capacity of soils, the hazard level of Pb is decreased strongly. The most representative data were obtained with the synchrotron technique (Manceau et al., 1996, 2002; Morin et al., 1999; Ostergren et al., 1999). Most commonly, a significant share of Pb is associated with organic matter: the EXAFS spectrum indicates a mixture of 80% Pb-humate and 20% Pb adsorbed on goethite (Morin et al., 1999). The predominance of Pb-humates is consistent with the high content (77%) of Pb extracted by Na4P2O7 from the silt fraction. Evidently, organic matter seals the mineral particle surface and hampers the sorption of Pb from the forest humus soil (Morin et al., 1999).
The share of mobile Cd speciations is increased in the light soil fraction. Thus, soil with weak buffer capacity cannot fix heavy metals, and they are transported to the Tuzlov River.
Pollution zone in the Lake Atamanskoe area
The calculation of the HM affinity index to carrier phases is given in Tables 6–8. Let us examine based on pCHM values the affinity of Ni for soil phases in sites with the contamination level corresponding to 2–5 approximate permissible concentration (APC). Affinity of metals increases in the following succession: exchangeable metals (4.02) < carbonates (18.7) < organic matter (13.9) < iron minerals (25.2) < residue (41.2) (Table 7). Note that the number in parentheses does not indicate boundary values. Based on index AHM-phase, however, affinity of Ni for phases increases in a different succession: organic matter (0.57) = exchangeable metals (0.58) < iron minerals (0.97) < residue (1.05) < carbonates (1.70) (Table 8). Relative to the residue, Ni occupies a markedly different position: level 1 versus 51.1 based on index pCHM. Relative to organic matter, Ni also occupies a different position (level 0.57). Similar results were also obtained for other metals.
When calculating index A for soils in the NPP area, reliability of values based on a small number (n = 5) of metals is worse than those based on a greater number (n = 7). This is reflected in higher values of the correlation coefficient between indices pC and A. For soils with different levels of contamination in the Lake Atamanskoe area, this parameter varies from 0.227 to 0.941 (Table 8), and the majority of R values are unreliable.
Let us examine the results of strong contamination (5–10 APC), relative to a lower level of contamination (< 5 APC) (Table 8). Owing to intensification of contamination, the residue is enriched in the majority of metals (except Cd). The increase is prominent for Pb: from 0.80 to 1.23. For Ni, Zn, Cu, and Pb, the share of reagent-insoluble compounds increases dramatically. For Pb, the share of organic matter increases from 1.79 to 2.17; for Cu, from 1.28 to 1.41.
The proposed method is marked by a low influence of hydroxylamine hydrochloride on metal extraction. As shown in (Ladonin, 2016), treatment with hydroxylamine hydrochloride is weakest for iron minerals: application of Tamm’s reagent (under the ultraviolet radiation) accepted in the McLaren-Crawford method (1973) yielded a much greater amount of Cu, Zn, and Pb than the hydroxylamine hydrochloride (Ladonin, 2016).
The share of Cd increases to 1.44–1.47 in the exchangeable form of Spolic Technosols (Table 8), which indicates its weak absorption by the soil.
Conclusions
Estimation of the hazard level of HMs in soil is based on not only their bulk content but also mobility, which is governed by the affinity for HM-carrier phases. Index of the affinity of heavy metal pCHM for the given phase is based on the share of metal (% of its bulk content in soil). High pCHM value suggests the affinity of this metal for the phase under consideration; vice versa, low value suggests the lack of affinity. Index CHM, however, has the following shortcoming: it does not suggest the critical value CHM(crit), above (or below) which the affinity is present (or absent). Therefore, the degree of metal extraction (CHM) shows a maximum dependence on the strength of reagent, but not on the affinity of metal for the extracted phases.
The new indicator of the affinity of metals for their carrier phases (AHM-phase) is distinguished from the traditional index pCHM by an additional constant that takes into account the sum of all HMs in the given phase as a share from the total bulk content of all HMs in the soil. The metal has affinity for the given phase if AHM-phase > 1; vice versa, the affinity is lacking if AHM-phase < 1. The new index can take into consideration discrepancy in the affinity of the given metal for phases extracted by different strength reagents. The degree of metal analysis shows a positive correlation with the number of metals involved in the procedure––the higher the number, the more reliable is the calculation of index A. This method cannot be used for soils contaminated with one metal.
Data obtained for contaminated soils in the Rostov region (Russia) revealed an appreciable decrease in the residual and organic matter-associated fractions of Zn in Calcaric Fluvic Arenosol. Because of a low content of organic matter, only organophiles, such as Cu and Pb, can react with organic ligands, in contrast to the fixation of Ni and Mn by organic matter in Chernozems. Light soils are marked by increase in the share of mobile Cd and Zn speciations and decrease in the share of Cr. Thus, soils with low buffer capacity cannot withhold metals (e.g., Cd and Zn).
Intensification of Spolic Technosols pollution (up to 5–10 APC) increases the share of metals (except Cd) in residue. This is particularly prominent for Pb. The share of Pb in the organic matter increases from 1.79 to 2.17; the share of Cu, from 1.28 to 1.41. The proposed method is distinguished by a weak influence of hydroxylamine hydrochloride on metal extraction. Thus, the use of a new indicator—the affinity of HMs to their carrier phases AHM-phase showed its advantage over the traditional Index CHM.
References
Acosta, J. A., Gabarron, M., Faz, A., Martınez-Martınez, S., Zornoza, R., & Arocena, J. M. (2015). Influence of population density on the concentration and speciation of metals in the soil and street dust from urban areas. Chemosphere, 134, 328–337. https://doi.org/10.1016/j.chemosphere.2015.04.038
Bacon, J. R., & Davidson, C. M. (2008). Is there a future for sequential chemical extraction? The Analyst, 133, 25–46. https://doi.org/10.1039/b711896a
Bauer, T. V., Linnik, V. G., Minkina, T. M., Mandzhieva, S. S., & Nevidomskaya, D. G. (2018). Ecological-geochemical studies of technogenic soils in the flood plain landscapes of the Seversky Donets. Lower Don Basin. Geochemistry International, 56(10), 992–1002. https://doi.org/10.1134/S001670291810004X
Bol’shakov V. A., Belobrov, V. P., Shishov, L. L., (2004). Glossary. Terms, their short definitions, reference materials on soil ecology, geography and soil classification. Moscow: Dokuchaev soil science institute, (p. 138) (in Russian).
Ecological bulletin of the don: On the state of the environment and natural resources of the Rostov region in 2019. (2020). Rostov-on-Don (in Russian).
Evans, Z. C., Ryswyk, H. V., Huertos, M. L., & Srebotnjak, T. (2019). Robust spatial analysis of sequestered metals in a Southern California Bioswale. Science of The Total Environment, 650(1), 155–162. https://doi.org/10.1016/j.scitotenv.2018.08.441
Fedotov, P. S., Rogova, O. B., Dzhenloda, RKh., & Karandashov, V. K. (2019). Metal-organic complexes in soils as a major sink for rare earth elements. Environmental Chemistry, 16(5), 323–332. https://doi.org/10.1071/EN18275
Ghosh, P., Samanta, A. N., & Ray, S. (2011). Reduction of COD and removal of Zn2+ from rayon industry wastewater by combined electro-Fenton treatment and chemical precipitation. Desalination, 266(1–3), 213–217. https://doi.org/10.1016/j.desal.2010.08.029
HN 2.1.7.2511-09. (2009). Approxible permissible concentrations (APC) of chemical matters in soil. Moscow: Federal center for hygiene and epidemiology of rospotrebnadzor (in Russian).
Hasan, M., Kausar, D., Akhter, G., & Shah, M. H. (2018). Evaluation of the mobility and pollution index of selected essential/toxic metals in paddy soil by sequential extraction method. Ecotoxicology and Environmental Safety, 147, 283–291. https://doi.org/10.1016/j.ecoenv.2017.08.054
He, Q., Ren, Y., Mohamed, I., Ali, M., Hassan, W., & Zeng, F. (2013). Assessment of trace and heavy metal distribution by four sequential extraction procedures in a contaminated soil. Soil and Water Research, 8, 71–76. https://doi.org/10.17221/20/2012-swr
Huang, Y., Chen, Q., Deng, M., Japenga, J., Li, T., Yang, X., & He, Z. (2018). Heavy metal pollution and health risk assessment of agricultural soils in a typical peri-urban area in southeast China. Journal of Environmental Management, 207, 159–168. https://doi.org/10.1016/j.jenvman.2017.10.072
ISO 10693 (1995). Soil quality—Determination of carbonate content—volumetric method.
ISO 14235 (1998). Soil quality—Determination of organic carbon by sulfochromic oxidation.
ISO 13317-2 (2001). Determination of particle size distribution by gravitational liquid sedimentation methods—Part 2: Fixed pipette method.
ISO 10390 (2005). Soil quality—Determination of pH.
ISO NF EN 23470 (2011). Soil quality—Determination of effective cation exchange capacity (CEC) and exchangeable cations.
IUSS Working Group WRB (2015) World reference base for soil resources 2014, update 2015. International soil classification system for naming soils and creating legends for soil maps. World soil resources reports, FAO, Rome, No. 106.
Kabata-Pendias, A. (2011). Trace elements in soils and plants N.Y. CRC Press. London: Boca Raton, (p. 450).
Karim, Z., Qureshi, B. A., Mumtaz, M., & Qureshi, S. (2014). Heavy metal content in urban soils as an indicator of anthropogenic and natural influences on landscape of Karachi—A multivariate spatio-temporal analysis. Ecological Indicators, 42, 20–31. https://doi.org/10.1016/j.ecolind.2013.07.020
Kartal, S., Aydın, Z., & Tokalioglu, S. (2006). Fractionation of metals in street sediment samples by using the BCR sequential extraction procedure and multivariate statistical elucidation of the data. Journal of Hazardous Materials, 132, 80–89. https://doi.org/10.1016/j.jhazmat.2005.11.091
Ladonin, D.V., Forms of heavy metals compounds in technogenically contaminated soils. Dr. of biological sciences thesis abstract, Moscow, (p. 42) (in Russian).
Land, M., Ohlander, B., Ingri, J., & Thunberg, J. (1999). Soil speciation and fractionation of rare earth elements in a spodosol profile from northern Sweden as revealed by sequential extraction. Chemical Geology, 160, 121–138. https://doi.org/10.1016/S0009-2541(99)00064-9
Linnik, V. G., Minkina, T. M., Bauer, T. V., Saveliev, A. A., & Mandzhieva, S. S. (2020). Geochemical assessment and spatial analysis of heavy metals pollution around coal-fired power station. Environmental Geochemistry and Health, 42(12), 4087–4100. https://doi.org/10.1007/s10653-019-00361-z
Manceau, A., Boisset, M. C., Sarret, G., Hazemann, J. L., Mench, M., Cambier, P., & Prost, R. (1996). Direct determination of lead speciation in contaminated soils by EXAFS spectroscopy. Environmental Science & Technology, 30, 1540–1552
Manceau, A., Marcus, M. A., & Tamura, N. (2002). Quantitative speciation of heavy metals in soils and sediments by synchrotron X-ray techniques. Reviews in Mineralogy and Geochemistry, 49(1), 341–428. https://doi.org/10.2138/gsrmg.49.1.341
Minkina, T. M., Motuzova, G. V., Nazarenko, O. G., Kryshchenko, V. S., & Mandzhieva, S. S. (2008). Forms of heavy metal compounds in soils of the steppe zone. Eurasian Soil Science, 41(7), 708–716. https://doi.org/10.1134/S1064229308070053
Minkina, T. M., Nazarenko, O. G., Motuzova, G. V., & Mandzhieva, S. S. (2009). Group composition of heavy metal compounds in the soils contaminated by emissions from the Novocherkassk power station. Eurasian Soil Science, 42(13), 1533–1542. https://doi.org/10.1134/S1064229309130158
Morin, G., Ostergren, J. D., Juillot, F., Ildefonse, P., Calas, G., & Brown, J. E. (1999). XAFS determination of the chemical form of lead in smelter-contaminated soils and mine tailings: Importance of adsorption process. American Mineralogist, 84, 420–434. https://doi.org/10.2138/am-1999-0327
Nevidomskaya, D. G., Minkina, T. M., Soldatov, A. V., Bauer, T. V., Shuvaeva, V. A., Zubavichus, Y. V., Trigub, A. L., Mandzhieva, S. S., Dorovatovskii, P. V., & Popov, Y. V. (2020). Speciation of Zn and Cu in Technosol and evaluation of a sequential extraction procedure using XAS, XRD and SEM–EDX analyses. Environmental Geochemistry and Health. https://doi.org/10.1007/s10653-020-00693-1
Orecchio, S., & Polizzotto, G. (2013). Fractionation of mercury in sediments during draining of Augusta (Italy) coastal area by modified Tessier method. Microchemical Journal, 110, 452–457. https://doi.org/10.1016/j.microc.2013.05.015
Ostergren, J. D., Brown, G. E., Parks, G. A., & Tingle, T. N. (1999). Quantitative speciation of lead in selected mine tailing from Leadvill, CO. Environmental Science & Technology, 33(10), 1627–1636. https://doi.org/10.1021/es980660s
Privalenko, V. V., & Cherkashina, I. F. (2012). Rekul’tivacija shlamonakopitelej himicheskih zavodov v Rostovskoj oblasti [Recultivation of Rostov region chemical industry waste fields]. In T. S. Chibrik (Ed.), Biologicheskaja rekul’tivacija i monitoring narushennyh zemel’ [Biological recultivation and monitoring of disturbed lands]. (pp. 205–209). Publishing House of the Ural University.
Privalenko, V. V., Mazurenko, V. T., Panaskov, V. I., Moshkin, V. M., Mukhin, N. V., & Senin, B. K. (2000). Ecological problems in the city of Kamensk-Shakhtinsk. Tsvetnaya pechat’.
Reimann, C., & de Caritat, P. (1998). Chemical elements in the environment—factsheets for the geochemist and environmental scientist. Springer-Verlag.
Rosado, D., Usero, J., & Morillo, J. (2016). Ability of 3 extraction methods (BCR, Tessier and protease K) to estimate bioavailable metals in sediments from Huelva estuary (Southwestern Spain). Marine Pollution Bulletin, 102, 65–71. https://doi.org/10.1016/j.marpolbul.2015.11.057
Silveira, M. L., Alleoni, L. R. F., O’Connor, G. A., & Chang, A. C. (2006). Heavy metal sequential extraction methods—A modification for tropical soils. Chemosphere, 64, 1929–1938. https://doi.org/10.1016/j.chemosphere.2006.01.018
Strawn, D. G., & Baker, L. L. (2008). Speciation of Cu in a contaminated agricultural soil measured by XAFS, μ-XAFS, and μ-XRF. Environmental Science and Technology, 42(1), 37–42. https://doi.org/10.1021/es071605z
Tessier, A., Campbell, P. G. O., & Bisson, M. (1979). Sequential extraction procedure for the speciation of the particulate trace metals. Analytical Chemistry, 51, 844–851
Vodyanitskii, Yu. N. (2006). Methods of sequential extraction of heavy metals from soils: New approaches and the mineralogical control (a review). Eurasian Soil Science, 39(10), 1074–1083. https://doi.org/10.1134/S106422930610005X
Wang, Yu., Xu, W., Li, J., Song, Y., Hua, M., Li, W., Wen, Yu., Li, T., & He, X. (2021). Assessing the fractionation and bioavailability of heavy metals in soil–rice system and the associated health risk. Environmental Geochemistry and Health. https://doi.org/10.1007/s10653-021-00876-4
Zhao, X., Huang, J., Lu, J., & Sun, Yu. (2019). Study on the influence of soil microbial community on the long-term heavy metal pollution of different land use types and depth layers in mine. Ecotoxicology and Environmental Safety, 170, 218–226. https://doi.org/10.1016/j.ecoenv.2018.11.136
Acknowledgments
The research was financially supported by the Russian Foundation for Basic Research (no. 19-34-60041) and the Grant of the President of the Russian Federation, MK-6137.2021.1.5.
Funding
The research was financially supported by the Russian Foundation for Basic Research (no. 19-34-60041) and the Grant of the President of the Russian Federation, MK-6137.2021.1.5.
Author information
Authors and Affiliations
Contributions
YNV and TB were responsible for data curation. YNV was responsible for methodology, conceptualization and writing—original draft preparation—review and editing. YNV and TM were responsible for supervision. TM was responsible for writing—review and editing. TB was responsible for investigation, formal analysis and writing.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Availability of data and material
The raw/processed data required to reproduce these findings cannot be shared at this time as the data also forms part of an ongoing study.
Ethical Approval
Not applicable since the manuscript has not been involved the use of any animal or human data or tissue.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Vodyanitskii, Y., Minkina, T. & Bauer, T. Methods to determine the affinity of heavy metals for the chemically extracted carrier phases in soils. Environ Geochem Health 44, 1387–1398 (2022). https://doi.org/10.1007/s10653-021-00955-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-021-00955-6