Abstract
Chestnut soils developed over mineralized areas of southwestern Spain are characterized by high baseline concentrations of geogenic trace elements, notably Pb (up to 14,562 mg kg−1), As (up to 346 mg kg−1) and Cd (up to 319 mg kg−1), which could pose an unacceptable risk to the health of the hand-harvest workers who are being exposed to surface soil by incidental ingestion and dermal contact. Oral bioaccessibility, as determined by simulating the human digestion process in a test-tube environment (Unified BARGE Method), followed the increasing order of As (3.1%) < Pb (21.5%) < Cd (35.6%) in the gastric phase, and As (3.4%) < Pb (4.5%) < Cd (13.2%) in the gastrointestinal extract. Relative bioavailability (RBA) of As (3.1–2.1%), Pb (17.8–17.5%) and Cd (34.4–23.3%), predicted from in vitro bioaccessibility measurement through linear regression models, seems to be influenced not only by the pH and composition of digestive solutions but also by geochemical partitioning of trace elements among the soil constituents. The integration of RBA data in the risk calculations had a considerable effect on the site-specific risk estimations. After RBA adjustment, the level of carcinogenic risk associated with As exposure (< 1.5E−06) and the hazard index for non-carcinogens (< 0.4) was within the regulatory limits, indicating that occupational risks are not of concern. Hence, it can be concluded that the use of a default value of 100% for bioavailability may dramatically overestimate the chronic exposure to geologically sourced trace elements.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
It is apparent that both the scientific and the regulatory communities have been evolving from an old mentality, where most decisions on risk management were usually based on the total or pseudo-total concentration of the contaminants, to a modern mindset where the bioavailable fraction of the total mass is increasingly being used for refining the risk evaluation (Adriano et al. 2004). Thus, policy guidelines encourage site-specific estimates of oral bioavailability to be incorporated into quantitative human health risk assessment (Mehta et al. 2020; Nathanail and Smith, 2007) to avoid unnecessary and costly soil remediation efforts. In terms of human receptors, oral relative bioavailability (RBA) is the fraction of an ingested dose that crosses the gastrointestinal epithelium and becomes available for distribution to internal tissues and organs relative to an experimentally reported absorption value (USEPA 2007). The fraction of a contaminant that can be dissolved by the digestive fluids in the gastrointestinal tract prior to crossing the intestinal membrane, and therefore is available for absorption, is referred as oral bioaccessibility (Ruby et al. 1999).
Experimental data for assessing RBA of trace elements in soils may come from animal testing (in vivo), including swine and mouse models, or from validated laboratory (in vitro) procedures involving physiologically based extraction tests. RBA assessment using animal bioassays is regarded as the most reliable method for refining metal exposure via soil ingestion (Ng et al. 2015), but the measurements are time-consuming and expensive to obtain. In vitro bioaccessibility (IVBA) tests are gaining widespread acceptance since they offer a simpler and cost-effective alternative for predicting oral RBA (Rodriguez et al. 1999). A variety of IVBA methodologies have been devised to mimic the physiology and chemistry of the human gastrointestinal system (e.g. Diamond et al. 2016; Juhasz et al. 2011, 2014; Li et al. 2015; Oomen et al. 2002), thereby providing a surrogate measurement of RBA based on strong in vitro–in vivo correlations (IVIVC). The use of IVBA assays to predict reliably oral RBA of multiple trace elements in contaminated soils requires robust validation. The Unified BARGE Method (UBM), developed by the Bioaccessibility Research Group of Europe (BARGE), is considered as the IVBA reference method within the European Union and in other countries. It has achieved ISO certification (17,924:2016) and has been validated against in vivo data for As, Cd and Pb (Caboche, 2009; Denys et al. 2012; Wragg et al. 2011), which are ranked among the top ten most toxic trace elements (ATSDR 2019).
Most studies dealing with RBA of trace elements have focused on contaminated soils as direct or indirect result of human activities, such as agricultural, mining, smelting and urban activities. In contrast, RBA data on natural soils from areas with high geochemical baseline concentrations of geologically sourced heavy metals are seldom reported. Moreover, given the high variability of RBA values obtained for As, Cd and Pb (e.g. Bradham et al. 2011; Zhu et al. 2019), there is a need for metal-RBA determinations to refine site-specific risk assessments.
The above-mentioned trace elements are naturally present at elevated concentrations in chestnut grove soils of the Sierra de Aracena (SW Spain) posing a direct contact hazard to human receptors (Giráldez et al. 2020; Rivera et al. 2016). The traditional land use is sweet chestnut cultivation. The harvest method used in the region is to gather the nuts after they fall naturally from the tree and then pick them up by hand from the ground. Inadvertent ingestion of topsoil particles due to contact between the mouth and contaminated hands is, in fact, a significant pathway of direct trace element exposure. Results from previous work (Rivera et al. 2016) showed that metal availability for plant uptake or leachability into the groundwater was limited by the low activity of dissolved and exchangeable ions in the soil solution. It can be assumed, therefore, that there is limited soil-to-plant transfer of trace elements to chestnut tissue, and accordingly, no detrimental health effects would be expected to occur via consumption of chestnuts. However, further research is needed to assess whether there is an unacceptable risk to worker receptors in the soil ingestion and dermal contact exposure scenarios. Thus, the objectives of this study were threefold: (1) to quantify the bioaccessible pools of As, Cd and Pb that can be solubilized during simulated human digestion; (2) to provide predictive insights on RBA of such contaminants using validated linear regression models; and (3) to assess the occupational risk to the health of the hand-harvest workers from direct exposure to soil particles by incidental ingestion (hand-to-mouth contact) and via dermal contact.
Material and methods
Site description and soil characteristics
The study area is located in the central sector of the Sierra de Aracena y Picos de Aroche Natural Park (Huelva province, SW Spain), between the villages of Castaño del Robledo and Fuenteheridos (Fig. 1), and in the middle of an extensive chestnut forest of economic, scenic and environmental interest that offers the locals a main source of income. It is situated at an elevation between 750 and 960 m.a.s.l. and has a Mediterranean pluviseasonal oceanic bioclimate (Rivas-Martínez et al. 2017), with average annual rainfall of more than 1000 mm and average annual temperature of 15 °C. Geologically, the bedrock consists of volcanic and carbonate rocks of Lower Cambrian age, which were deformed and metamorphosed during the Variscan orogeny. The area is known for the occurrence of stratiform carbonate-hosted base metal deposits, with galena (PbS), sphalerite (ZnS), pyrite (FeS2) being the main ore minerals (Fernández-Caliani et al. 2019a).
Eutric Cambisol is the dominant soil type in the area. The topsoil has a dark brown color (10YR) in wet state, a silty loam texture, oxidizing condition and near-neutral pH values. It is composed mainly of clay minerals (vermiculite, illite, kaolinite, talc) accompanied by quartz, feldspars and minor barite, hematite and goethite. It is noteworthy that the soil developed directly over mineralized bedrock areas contains exceptionally high concentrations of potentially hazardous trace elements, notably Pb, Zn, Cd, As, Sb and Tl, which have been accumulated in the surface soil layer by supergene enrichment and pedogenetic processes (Rivera et al. 2015, 2016).
Composite soil samples were obtained by bulking five point samples of the surface soil (0–20 cm depth) collected with a bucket auger in ten sampling locations over the chestnut grove (Fig. 1). The locations were chosen to represent areas where soil trace element concentrations are well above the regional geochemical baseline values (Galán et al. 2008). The soil samples were air-dried, gently ground, homogenized and then passed through a 2-mm sieve to remove coarse fragments and visible roots. An aliquot of each sieved sample was sieved at 250 μm for bioaccessibility essays, because this is the most likely soil fraction ingested via hand-to-mouth by humans (USEPA 2007).
In vitro bioaccessibility measurement
Oral bioaccessibility of As, Cd, and Pb was determined by in vitro simulation of the human digestion process following the UBM method, which produces two extracts per sample for analysis (gastric phase and gastrointestinal phase). Four synthetic analogues of digestive fluids were used: saliva (pH = 6.5 ± 0.5), gastric fluid (pH = 1.1 ± 0.1), duodenal fluid (pH = 7.4 ± 0.2) and bile (pH = 8.0 ± 0.2). Detailed components of the saliva and digestive juices can be found in Wragg et al. (2009, 2011). All soil samples were mixed by end-over-end agitation at the physiological temperature of the human body (37 °C). The gastric phase of the UBM assay (UBM-G) is a digest extract collected after 1-h agitation with saliva and gastric fluid, and the gastrointestinal (UBM-GI) phase is the extract collected after 1-h agitation with saliva and gastric fluid followed by 4-h agitation with duodenal fluid and bile. Therefore, the UBM-GI phase includes both sequential stomach and intestine steps. A schematic diagram giving details of the gastric and gastrointestinal extraction procedures is shown in Figure S1.
The extract solutions were analyzed by ICP-MS on an Agilent 7700 ICP-MS instrument to determine the concentrations of As and Cd and using ICP-OES (Agilent 5100/5110 ICP-OES) for the analytical determination of Pb. Mean repeatability, expressed as relative deviation standard (RDS), was less than 4% for the UBM-G phase and less than 2% for the UBM-GI phase. The accuracy of the extraction protocol was estimated by analyzing the standard reference material NIST 2710a, finding good agreement with values reported in the literature (e.g. Caboche, 2009; Wragg et al. 2011). Mean recoveries ranged from 86 to 124% for As, from 92 to 116% for Cd, and from 78 to 82% for Pb. Quality control included the use of reagent blanks of gastric and intestinal fluids.
In vitro bioaccessibility (IVBA) was determined by dividing the amount of each trace element of concern extracted from soil following gastric and gastrointestinal phase extraction, by its total concentration in the ingested soil before digestion, and expressed on a percentage basis, as follows:
The total concentrations of As, Cd and Pb in soil were determined by ICP-MS after a multi-acid (HF-HClO4-HNO3-HCl) digestion. Quality control included the analysis of reagent blank samples, certified reference materials (SDC-1, SCO-1 and GXR series) and replicate samples to check accuracy and precision of the analytical data. The RSD for all these elements was less than 5%.
Exposure assessment and risk evaluation
A conceptual site model was adopted in the risk evaluation process, in which potential sources, pathways and receptors involved in the occupational exposure scenario are identified. Direct exposure to surface soil contaminants was assessed through two pathways: (1) inadvertent ingestion of soil particles via hand-to-mouth transfer and (2) dermal absorption from direct skin contact. Based on site-specific exposure factor data collected through a questionnaire survey, the occupational receptor is assumed to be an adult female weighing around 60 kg, who is exposed 9 h a day for two months of the year (exposure frequency), for a period of 40 years of work activity (duration of exposure). These seasonal hand-harvest workers were assumed to ingest 50 mg/day of soil, which represents a reasonable worst-case default value (USEPA 2011).
The average daily dose (ADDi) from incidental soil ingestion, expressed as mass of contaminant per unit body weight over time (mg kg−1-day), was calculated using the exposure equation [2] (USEPA 1989):
where C is the concentration of trace element in soil (mg kg−1), IR is the ingestion rate of soil (mg/day), EF is the exposure frequency (days/year), ED is the exposure duration (years), BW is the body weight (kg) of the exposed individual, and AT is the averaging time period (for non-carcinogenic contaminants AT = 365*ED days, and for carcinogenic contaminants a 78-year lifetime is considered), and CF is a unit conversion factor.
For dermal exposure assessment, the conservative default values recommended by USEPA (2011) were used as input data in the algorithm. It was assumed an exposed skin surface area of 3100 cm2 (including both hands, forearms and head), a soil-to-skin adherence factor of 0.3 mg cm−2, and the following dermal absorption factors: 0.03 for As, 0.01 for Cd and 0.1 for Pb.
Under these assumptions, the average daily dose (ADDd) for each trace element from dermal absorption was derived by the equation [3]:
where SA is the exposed skin surface (cm2), AF is the skin-soil adhesion factor (mg cm−2), ABS is the dermal absorption factor (unitless), and C, EF, ED, BW, AT, and CF are as defined in equation [2].
The risk analysis was conducted by calculating the carcinogenic risk (CR), which is a function of exposure to As, and the hazard quotient (HQ) for toxic effects of individual elements, which is proportional to the concentrations of As, Cd and Pb in soil, using the relationships [4] and [5] in accordance with the standard guidelines (USEPA 1989):
where CR is the cancer risk, ADD is the daily exposure dose (mg kg−1-day), SF is the slope factor, HQ is the hazard quotient, and RfD is the reference dose or tolerable daily intake values of As (3.0E−04 mg kg−1-day for oral and dermal exposures) and Cd (1.0E−03 and 2.5E−05 mg kg−1-day for oral and dermal routes, respectively) listed in the IRIS (Integrated Risk Information System) database of the USEPA, and reported by RIVM (the Dutch National Institute for Public Health and the Environment) in the case of Pb (3.5E−03 mg kg−1-day for both pathways). The SF value currently listed in the USEPA IRIS database for As is 1.5 (mg kg−1-day)−1 for oral and dermal exposures.
Because the trace elements may cause cumulative toxic effects, the individual hazard quotients (HQs) were summed to yield an overall hazard index (HI), as follows:
The quantitative risk analysis was performed using the RBCA Tool Kit for Chemical Releases version 2.6, a comprehensive modelling and risk characterization software package designed to support risk-based corrective action (Connor et al. 2007).
The assessment was refined to provide more realistic estimates of exposure by incorporating site-specific RBA adjustments into risk equations. The cancer risk resulting from oral exposure was adjusted (CRadjusted) with the predicted RBA adjustment factor into the risk assessment of potential carcinogenic effects of As exposure (USEPA 2007), as follows:
Similarly, for non-cancer effects, the individual hazard quotient values for As, Cd and Pb were adjusted (HQadjusted) with the RBA data, according to the following equation:
The IVBA values were used as the surrogate for the RBA adjustment. In this study, the predictive IVIVC models of Caboche (2009) and Wragg et al. (2011) were selected to estimate the oral RBA of As, Cd and Pb from the UBM bioaccessibility data, as they meet the benchmark criteria for assessing the fitness for the purpose of the IVBA measurements (Denys et al. 2012; Wragg et al. 2011). The RBA values were estimated by substituting the IVBA values of the UBM-G phase into a simple linear regression model:
where a is the y-intercept of the regression line and b is the slope. The equations relating RBA and IVBA of each model are given in Table 1.
Results and discussion
The chestnut soil contains high total concentrations of As, Cd, and especially Pb, ranging widely depending on the sampling location (Table 2). The maximum contents of As (346 mg kg−1), Cd (319 mg kg−1) and Pb (14,562 mg kg−1) were found in soil derived from sulfide-bearing carbonate rocks and their ferruginous products of oxidation (gossan) and consistently point to strong geogenic contamination. Under the present soil conditions, characterized by near-neutral pH values and moderately oxidizing regime (Rivera et al. 2016), the elements of interest are mostly strongly bound to silicate minerals and iron oxy-hydroxides, and significant proportions of Cd and Pb are associated with carbonates, based on the metal fractionation pattern obtained by sequential extraction methods (Giráldez et al. 2020) from soil samples used in this study.
Bioaccessible concentrations
Average concentrations of As, Cd and Pb extracted from the soil samples (< 250 µm size fraction) by the UBM-G and UBM-GI fluids under simulated gastric and gastrointestinal conditions, respectively, are listed in Table 2. The extent of trace element extraction varied greatly between the two digestive compartments and among soil samples. With a single exception (sample 11), Pb was by far the most abundant metal in all the digest extracts, reaching bioaccessible concentrations as high as 4124 mg kg−1 in the UBM-G phase and 1058 mg kg−1 in the UBM-GI phase of the sample 14 (soil overlying gossanized bedrock). The amount of As extracted from the ingested soil appeared to be rather similar in both compartments, with mean values ranging from 2.0 to 9.7 mg kg−1. The bioaccessible content of Cd generally was below 12 mg kg−1, except in the samples 11 and 12, where the UBM-G level was up to 189 and 39.9 mg kg−1, respectively.
There was a strong positive linear relationship between total content and bioaccessible concentrations of As, Cd and Pb in both extraction phases (Fig. 2), with r-square values greater than 0.92. Accordingly, the quantity of these geogenic trace elements that can be solubilized by digestive fluids in the gastrointestinal tract, and therefore available for absorption at the intestinal membrane barrier, increased significantly (p < 0.05) with increasing total metal concentrations in soil.
Percent bioaccessibility results are depicted graphically in Fig. 3. The IVBA measurements indicated that Cd was most bioaccessible than Pb and As, as previously reported in the literature for a range of contaminated soils (Mehta et al. 2020; Pascaud et al. 2014; Zhu et al. 2019). According to IVBA mean values, metal bioaccessibility in the UBM-G phase followed the increasing order of: As (3.1%) < Pb (21.5%) < Cd (35.6%), while in the UBM-GI phase the order was: As (3.4%) < Pb (4.5%) < Cd (13.2%). The bioaccessibility values of these geogenic trace elements are relatively low in comparison with those recorded in soils contaminated with heavy metals from anthropogenic sources (Mehta et al. 2020; Pelfrêne et al. 2011; Xia et al. 2016).
Chemical fractionation of Cd and Pb between the UBM-G and UBM-GI phases of the sequential in vitro extraction seems to be controlled by the pH and composition of the digestive fluids. Indeed, Cd and Pb were preferentially released from the ingested soil into the gastric fluid, as shown in Fig. 3, because the highly acidic environment of the gastric compartment enhanced the solubility of these strongly pH-dependent heavy metals. When the ingested soil moved from the stomach to the intestinal tract during the simulated digestion, a sharp decrease in Cd and Pb bioaccessibility was observed, accounting for up to 35% of the total concentration of each element. This reduction in metal bioaccessibility may be due to chemical precipitation from solution under the slightly alkaline conditions prevailing in the small intestine, although other plausible explanations cannot be ruled out for the decreased bioaccessibility, like metal re-adsorption onto remaining soil particles or complexation by pepsin (Ellickson et al. 2001; Pelfrêne et al. 2011). Arsenic was nearly equally distributed between the UBM-G and UBM-GI phases, with bioaccessibility values of 3.1 ± 0.5% in the stomach and 3.4 ± 0.3% in the intestinal tract, indicating that the solubility of this oxyanion-forming element was similarly low in both compartments.
Predicted oral bioavailability
The UBM protocol was validated against in vivo models for predicting RBA of As, Cd and Pb in soil from IVBA measurements across a diverse set of soils, reflecting various sources and soil characteristics. Although until now most studies dealing with RBA have focused on soils contaminated by mining and smelting activities, IVIVC study results have been reported also from naturally contaminated soil samples for accurate RBA estimates (e.g. Juhasz et al. 2011, Wragg et al. 2011, Denys et al. 2012).
Based on the low bioaccessibility of the studied trace elements, notably As but also Pb, a large proportion of them may be tightly bound to soil matrix and so may not be available for intestinal absorption following incidental soil ingestion. The IVBA value of the UBM-G phase was taken as the metal fraction that an individual is exposed to soil ingestion, as the gastric compartment alone is a reasonable analogue of IVBA (Pelfrêne et al. 2011) and then used as a surrogate for estimating oral RBA. The regression models of Caboche (2009) and Wragg et al. (2011) were selected for predicting the RBA from UBM bioaccessibility data because these predictive models meet the benchmark criteria suggested for IVIVC (Denys et al. 2012; Wragg et al. 2011), on repeatability (RSD ≤ 10%), reproducibility (RSD ≤ 20%) and regression statistics (strong correlation coefficient, r2 > 0.6; slope of the best-fit curve 0.8–1.2 and intercepts close to 0).
The RBA values predicted from UBM-G results by using the linear regression models of Caboche (2009) and Wragg et al. (2011) are given in Table 3. The RBA mean value of Cd was 34.4 ± 11.8% or 23.3 ± 7.1% depending on the used model. This disparity arises from differences in slopes and y-intercepts of the regression lines that can be ascribed to variability associated with IVIVC when using different methodologies (Juhasz et al. 2014). The RBA mean values of Pb (17.8–17.5%) and As (3.1–2.1%) were rather similar regardless of which regression model was chosen.
These predictive findings are in good agreement with the metal fractionation results gained by using sequential extraction methods (Giráldez et al. 2020), suggesting a link between bioavailability and operational speciation or mode of occurrence of trace elements in soil. The relatively high RBA of Cd can be explained by the fact that a relevant proportion of Cd (22%) is found in exchangeable forms and precipitated or coprecipitated with carbonates. It was shown also by sequential chemical extraction that As appears largely partitioned between the strongly reducible fraction (53%), specifically associated with crystalline Fe oxides, and the residual fraction (42%), tightly bound to silicate minerals. Consistently, the low RBA values predicted from the IVBA essay are related to the negligible fraction of labile As in soil. Similarly, more than half of the total Pb concentration appears to be strongly chemisorbed onto Fe oxides and oxyhydroxides by inner-sphere surface complexation (Giráldez et al. 2020), which are not easily dissolved by the simulated digestive solutions used in the UBM extraction (Denys et al. 2012). However, there is a labile pool of Pb mostly bound to carbonates that explain the relatively high RBA values (up to 35%) obtained in some sampling sites. This interpretation is supported by the occurrence of cerussite (PbCO3) in such soil samples (Giráldez et al. 2020). It is apparent, therefore, that bioavailability of ingested contaminants is affected not only by the pH and composition of digestive solutions but also, to some extent, it is influenced by chemical partitioning of trace elements among the soil components.
Chronic daily intake and risk assessment
The average daily doses of As, Cd and Pb through oral ingestion (ADDi) and dermal contact (ADDd) are presented in Table 4, together with the HQ of each trace element, and the HI and the CR values for occupational exposure. In agreement with the Spanish regulatory guidelines (Tarazona et al. 2005), which are integrated within the European legal framework, the target health risk value considered for the assessment is 1.0E−05 for genotoxic carcinogens, i.e. one excess case of cancer in 100,000 individuals exposed over a 78-year lifetime, and the unity value for potentially toxic heavy metals.
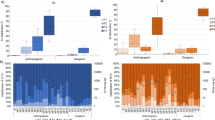
It should be noted that total concentrations of As were found in levels above which carcinogenic risk would be unacceptable. The carcinogenic dose received by the hand-harvest workers ranged from 3.4E−06 to 2.4E−05 mg kg−1-day for incidental soil ingestion and from 1.9E−06 to 1.4E−05 mg kg−1-day for dermal contact, resulting occupational CR values higher than the regulatory value of 1.0E−05 in most sampling sites (Fig. 4a). For non-carcinogens, the HI value of the sample 14 slightly exceeded the limit of admissible risk (Fig. 4b), with Pb being the primary contributor to the overall hazard, with an individual HQ value of 1.63. However, no adverse health effects would be expected to occur through Cd exposure since the HQ value for this metal fell within the acceptable level for toxic effects in all samples.
Bioavailability adjustment and implications to risk reduction
It is noteworthy that the above risk analysis results are expressed in terms of ingested dose rather than absorbed dose. For human health risk assessment purposes, it is important to consider the extent to which the ingested dose of contaminants can be absorbed into the systemic circulation (i.e. its bioavailability). Studies have demonstrated that when bioavailability is assumed to be 100% (worst-case scenario), the human health risk arising from contaminated soil exposure is overestimated (Bradham et al. 2011; Fernández-Caliani et al. 2019b; Juhasz et al. 2007; Li et al. 2014). Hence the need to consider site-specific RBA, and not just IVBA, for refining the human health risk assessment through the use of RBA-adjusted toxicity values, thus giving more realistic indications for the soil ingestion exposure.
Interestingly, when quantifying trace element exposure adjusted for RBA, the occupational risk to the health of the local hand-harvest workers from direct exposure to surface soil was considerably lower than that previously estimated assuming a default RBA value of 100%. This was most noticeable for As and Pb. After RBA adjustment, the level of carcinogenic risk associated with As exposure was acceptable because the CRadjusted values fell within the regulatory threshold value in all the sampling localities (Fig. 5a). The HI recalculated after RBA adjustment of individual HQ values was also below the allowable limit, with HIadjusted values ranging to a maximum of 0.4 (Fig. 5b), which represents a risk reduction of at least 22%. Consequently, RBA adjustment had a dramatic effect on the overall human health risk estimates, suggesting that the default exposure assumption may, in fact, overestimate the exposure to geogenic trace elements.
Occupational cancer risk for As exposure (a) and hazard index (b), considering both default values (CR and HI) and RBA-adjusted values (CR´and HI´) derived from the in vivo–in vitro correlation models of Caboche (Cab) and Wragg et al. (Wra). The dashed lines indicate the regulatory limits to risk management
Conclusions
This study has shown that high total concentrations of naturally occurring trace elements in the chestnut soils are indicative of extensive metal accumulation by pedogenetic processes, but do not properly address the risk to the health of people involved in harvesting activities. The use of total trace element contents in the exposure assessment resulted in an overestimation of the occupational risk. In reality, the risk posed by incidental soil ingestion exposure is related to RBA and may depend on the geochemical partitioning of trace elements among the soil constituents. Only a limited fraction of As (3.1%), Pb (21.5%) and Cd (35.6%) was mobilized from the soil matrix by digestive fluids under simulated gastric conditions, becoming available for absorption into the bloodstream. The predicted RBA values of As (3.1–2.1%), Pb (17.8–17.5%) and Cd (34.4–23.3%), derived from validated regression models relating RBA and IVBA, are indicative of the bioavailable metal fraction that actually enters into the body across the intestinal membrane. The incorporation of RBA data in the risk calculations allowed for a more accurate assessment of the risk resulting from oral exposure on a site-specific basis. The RBA-adjusted exposure to carcinogen effects of As and the hazard index for non-carcinogens were within acceptable levels, so that human health risks are not expected to occur at any sampling location.
Availability of data and materials
The authors confirm that the data supporting the findings of this study are available within the manuscript.
References
Adriano, D. C., Wenzel, W. W., Vangronsveld, J., & Boland, N. S. (2004). Role of assisted natural remediation in environmental cleanup. Geoderma, 122, 121–142.
ATSDR (2019). Substance Priority List. Agency for Toxic Substances and Disease Registry. https://www.atsdr.cdc.gov/spl/. Accessed 15 november 2020
Bradham, K. D., Scheckel, K. G., Nelson, C. M., Seales, P. E., Lee, G. E., Hughes, M. F., Miller, B. W., Yeow, A., Gilmore, T., Serda, S. M., Harper, S., & Thomas, D. J. (2011). Relative bioavailability and bioaccessibility and speciation of arsenic in contaminated soils. Environmental Health Perspectives, 119, 1629–1634.
Caboche, J. (2009). Validation d’un test de mesure de bioaccesibilité. Application à quatre éléments traces métalliques dans le sols: As, Cd, Pb, et Sb. (p. 348). Thèse de doctorat.
Connor, J. A., Bowers, R. L., McHugh, T. H., & Spexet, A. H. (2007). RBCA Tool Kit for Chemical Releases. Software Guidance Manual. GSI Environ Inc.
Denys, S., Caboche, J., Tack, K., Rychen, G., Wragg, J., Cave, M., Jondreville, C., & Feidt, C. (2012). In vivo validation of the unified BARGE method to assess the bioaccessibility of arsenic, antimony, cadmium and lead in soils. Environmental Science & Technology, 46, 6252–6260.
Diamond, G. L., Bradham, K. D., Brattin, W. J., Burgess, M., Griffin, S., Hawkins, C. A., & Juhasz, A. L. (2016). Predicting oral relative bioavailability of arsenic in soil from in vitro bioaccessibility. Journal of Toxicology and Environmental Health, Part A, 79, 165–173.
Ellickson, K. M., Meeker, R. J., Gallo, M. A., Buckley, B. T., & Lioy, P. J. (2001). Oral bioavailability of lead and arsenic from a NIST standard reference soil material. Archives of Environmental Contamination and Toxicology, 40, 128–135.
Fernández-Caliani, J. C., Giráldez, M. I., & Rivera, M. B. (2019a). Source and geochemical partitioning of silver in a naturally-enriched soil. Applied Geochemistry, 103, 85–96.
Fernández-Caliani, J. C., Giráldez, M. I., & Barba-Brioso, C. (2019b). Oral bioaccessibility and human health risk assessment of trace elements in agricultural soils impacted by acid mine drainage. Chemosphere, 237, 124441.
Galán, E., Fernández-Caliani, J. C., González, I., Aparicio, P., & Romero, A. (2008). Influence of geological setting on geochemical baselines of trace elements in soils. Application to soils of South-West Spain. Journal of Geochemical Exploration, 98, 89–106.
Giráldez, M. I., Fernández-Caliani, J. C., & Rivera, M. B. (2020). Geochemical behavior and fate of trace elements in naturally contaminated soils under projected land-use changes. Journal of Soils and Sediments, 20, 1413–1423.
Juhasz, A. L., Smith, E., Weber, J., Rees, M., Rofe, A., Kuchel, T., Sansom, L., & Naidu, R. (2007). In vitro assessment of arsenic bioaccessibility in contaminated (anthropogenic and geogenic) soils. Chemosphere, 69, 69–78.
Juhasz, A. L., Weber, J., & Smith, E. (2011). Predicting arsenic relative bioavailability in contaminated soils using meta analysis and relative bioavailability-bioaccessibility regression models. Environmental Science & Technology, 45, 10676–10683.
Juhasz, A. L., Smith, E., Nelson, C., Thomas, D. J., & Bradham, K. (2014). Variability associated with As in vivo-in vitro correlations when using different bioaccessibility methodologies. Environmental Science & Technology, 48, 11646–11653.
Li, J., Wei, Y., Zhao, L., Zhang, J., Shangguan, Y., Li, F., & Hou, H. (2014). Bioaccessibility of antimony and arsenic in highly polluted soils of the mine area and health risk assessment associated with oral ingestion exposure. Ecotoxicology and Environmental Safety, 110, 308–315.
Li, J., Li, K., Cui, X. Y., Basta, N. T., Li, L. P., Li, H. B., & Ma, L. Q. (2015). In vitro bioaccessibility and in vivo relative bioavailability in 12 contaminated soils: Method comparison and method development. Science of the Total Environment, 532, 812–820.
Mehta, N., Cipullo, S., Cocerva, T., Coulon, F., Dino, G. A., Ajmone-Marsan, F., Padoan, E., Cox, S. F., Cave, M. R., & De Luca, D. A. (2020). Incorporating oral bioaccessibility into human health risk assessment due to potentially toxic elements in extractive waste and contaminated soils from an abandoned mine site. Chemosphere, 255, 126927.
Nathanail, C. P., & Smith, R. (2007). Incorporating bioaccessibility in detailed quantitative human health risk assessments. Journal of Environmental Science and Health, Part A, 42, 1193–1202.
Ng, J. C., Juhasz, A., Smith, E., & Naidu, R. (2015). Assessing the bioavailability and bioaccessibility of metals and metalloids. Environmental Science and Pollution Research, 22, 8802–8825.
Oomen, A. G., Hack, A., Minekus, M., Zeijdner, E., Cornelis, C., Schoeters, G., et al. (2002). Comparison of five in vitro digestion models to study the bioaccessibility of soil contaminants. Environmental Science & Technology, 36, 3326–3334.
Pascaud, G., Leveque, T., Soubrand, M., Boussen, S., Joussein, E., & Dumat, C. (2014). Environmental and health risk assessment of Pb, Zn, As and Sb in soccer field soils and sediments from mine tailings: solid speciation and bioaccessibility. Environmental Science and Pollution Research, 21, 4254–4264.
Pelfrêne, A., Waterlot, C., Mazzuca, M., Nisse, C., Bidar, G., & Douay, F. (2011). Assessing Cd, Pb, Zn human bioaccessibility in smelter-contaminated agricultural topsoils (northern France). Environmental Geochemistry and Health, 33, 477–493.
Rivas-Martínez, S., Penas, A., Del Río, S., Díaz-González, T. E., & Rivas-Sáenz, S. (2017). Bioclimatology of the Iberian Peninsula and the Balearic Islands. In: J. Loidi (Ed.), The Vegetation of the Iberian Peninsula (vol. 1, pp. 29–80). Springer.
Rivera, M. B., Fernández-Caliani, J. C., & Giráldez, M. I. (2015). Geoavailability of lithogenic trace elements of environmental concern and supergene enrichment in soils of the Sierra de Aracena Natural Park (SW Spain). Geoderma, 259–260, 164–173.
Rivera, M. B., Giráldez, M. I., & Fernández-Caliani, J. C. (2016). Assessing the environmental availability of heavy metals in geogenically contaminated soils of the Sierra de Aracena Natural Park (SW Spain). Is there a health risk? Science of the Total Environment, 560–561, 254–265.
Rodriguez, R. R., Basta, N. T., Casteel, S. W., & Pace, L. W. (1999). An in vitro gastrointestinal method to estimate bioavailable arsenic in contaminated soils and solid media. Environmental Science & Technology, 33, 642–649.
Ruby, M. V., Schoof, R., Brattin, W., Goldade, M., Post, G., Harnois, M., Mosby, D. E., Casteel, S. W., Berti, W., Carpenter, M., Edwards, D., Cragin, D., & Chappell, W. (1999). Advances in evaluating the oral bioavailability of inorganics in soil for use in human health risk assessment. Environmental Science & Technology, 33, 3697–3705.
USEPA (1989). Risk Assessment Guidance for Superfund. Vol. I, Human Health Evaluation Manual (Part A). U.S. Environmental Protection Agency, Washington, DC, EPA/540/1–89/002.
USEPA (2007). Guidance for Evaluating the Oral Bioavailability of Metals in Soils for Use in Human Health Risk Assessment. U.S. Environmental Protection Agency, Washington, DC, OSWER 9285.7–80.
USEPA (2011). Exposure Factors Handbook 2011 Edition. U.S. Environmental Protection Agency, Washington, DC, EPA/600/R-09/052F.
Tarazona, J. V., Fernández, M. D., & Vega, M. M. (2005). Regulation of contaminated soils in Spain. A new legal instrument. Journal of Soils and Sediments, 5, 121–124.
Wragg, J., Cave, M., Taylor, H., Basta, N., Brandon, E., Casteel, S., Gron, C., Oomen, A., & Van de Wiele, T. (2009). Inter-laboratory Trial of a Unified Bioaccessibility Procedure. British Geological Survey, Open Report OR/07/027.
Wragg, J., Cave, M., Basta, N., Brandon, E., Casteel, S., Denys, S., Gron, C., Oomen, A., Reimer, K., Tack, K., & Van de Wiele, T. (2011). An inter-laboratory trial of the unified BARGE bioaccessibility method for arsenic, cadmium and lead in soil. Science of the Total Environment, 409, 4016–4030.
Xia, Q., Peng, C., Lamb, D., Mallavarapu, M., Naidu, R., & Ng, J. C. (2016). Bioaccessibility of arsenic and cadmium assessed for in vitro bioaccessibility in spiked soils and their interaction during the Unified BARGE Method (UBM) extraction. Chemosphere, 147, 444–450.
Zhu, X., Li, M. Y., Chen, X. Q., Wang, J. Y., Li, L. Z., Tu, Ch., Luo, Y. M., Li, H. B., & Ma, L. Q. (2019). As, Cd, and Pb relative bioavailability in contaminated soils: Coupling mouse bioassay with UBM assay. Environment International, 130, 104875.
Funding
No funding was received for conducting this study.
Author information
Authors and Affiliations
Contributions
Sandra Fernández-Landero (SFL)—Inmaculada Giráldez (IG)—Juan Carlos Fernández-Caliani (JCFC). JCFC and IG designed the present study. SFL and IG performed analyses and processed the data. JCFC and SFL wrote the manuscript. All authors contributed to the interpretation of the data and discussed their implications.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Figure 1S
Schematic illustration of the gastric and gastrointestinal extraction procedures following the Unified BARGE Method. (PPTX 1480 kb)
Rights and permissions
About this article
Cite this article
Fernández-Landero, S., Giráldez, I. & Fernández-Caliani, J.C. Predicting the relative oral bioavailability of naturally occurring As, Cd and Pb from in vitro bioaccessibility measurement: implications for human soil ingestion exposure assessment. Environ Geochem Health 43, 4251–4264 (2021). https://doi.org/10.1007/s10653-021-00911-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-021-00911-4