Abstract
To enhance risk assessment for contaminated sites, incorporating bioavailability through bioaccessibility as a corrective factor to total concentration is essential to provide a more realistic estimate of exposure. While the main in vitro tests have been validated for As, Cd, and/or Pb, their potential for assessing the bioaccessibility of additional elements remains underexplored. In this study, the physicochemical parameters, pseudototal Cr and Ni concentrations, soil phase distribution, and oral bioaccessibility of twenty-seven soil samples were analysed using both the ISO 17924 standard and a simplified test based on hydrochloric acid. The results showed wide variability in terms of the concentrations (from 31 to 21,079 mg kg−1 for Cr, and from 26 to 11,663 mg kg−1 for Ni) and generally low bioaccessibility for Cr and Ni, with levels below 20% and 30%, respectively. Bioaccessibility variability was greater for anthropogenic soils, while geogenic enriched soils exhibited low bioaccessibility. The soil parameters had an influence on bioaccessibility, but the effects depended on the soils of interest. Sequential extractions provided the most comprehensive explanation for bioaccessibility. Cr and Ni were mostly associated with the residual fraction, indicating limited bioaccessibility. Ni was distributed in all phases, whereas Cr was absent from the most mobile phase, which may explain the lower bioaccessibility of Cr compared to that of Ni. The study showed promising results for the use of the simplified test to predict Cr and Ni bioaccessibility, and its importance for more accurate human exposure evaluation and effective soil management practices.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Anthropogenic sources are releasing an increasing amount of potentially toxic elements (PTEs) into the environment as a result of increasing industrialization and urbanization (Lwin et al., 2022). Because of these multiple sources in soils, such as industrial activities, mining, and the use of pesticides and fertilizers, PTEs have become a growing public health concern worldwide (Ayangbenro & Babalola, 2017; Kumar et al., 2022). However, there are also many areas with a high geochemical background which people live without known adverse effects, probably due to the low mobility and bioavailability of PTEs (Barsby et al., 2012; Fernández-Caliani et al., 2020; Juhasz et al., 2007). Chromium (Cr) and nickel (Ni) are two PTEs that are widely distributed in soils. These PTEs are needed in small amounts for biological functions but can have adverse effects on human health, such as damage to the respiratory, digestive, and nervous systems if the exposure dose is too high (ATSDR, 2005, 2012). The ingestion of soil particles is one of the main contamination pathways, especially for children who are more likely to engage in hand⁃to⁃mouth behaviour (Dudka & Miller, 1999). There is now a consensus that using total (or pseudototal) concentrations can lead to an overestimation of exposure (Babaahmadifooladi et al., 2020; Mehta et al., 2019; Wang et al., 2023) and can lead to high management costs and overly stringent restrictions for local residents (Zhong & Jiang, 2017). Indeed, only the bioavailable fraction can lead to adverse effects. It corresponds to the fraction of the contaminant that reaches systemic circulation after dissolution from the soil matrix in gastric juice and absorption by the intestinal epithelium (Semple et al., 2004). However, the measurement of oral bioavailability requires in vivo experiments (e.g., piglets, mice, and monkeys), which are lengthy, expensive, and ethically questionable. Bioaccessibility (BAc) corresponds to the fraction of contaminants in soil that are dissolved by digestive fluids and represents the maximum amount that can reach systemic circulation and produce toxic effects (Paustenbach, 2000). Numerous in vitro tests have been developed to simulate this dissolution step (e.g., PBET, Ruby et al., 1996; SBRC, Kelley et al., 2002; Method 1340, US EPA, 2017; SBET, Medlin, 1997; IVG, Rodriguez et al., 1999; DIN, Hack et al., 2002; UBM; Denys et al., 2012; ISO 17924, 2019; RIVM, Oomen et al., 2003).
Many studies have shown the importance of taking bioavailability into account by measuring BAc rather than pseudototal concentrations to avoid risk overestimation. As and Pb, and to a lesser extent Cd, are the most widely studied PTEs in the literature and most in vitro tests have been validated relative to in vivo models for them (Li et al., 2019). However, other PTEs, such as Cr and Ni, are also of interest since studies in the literature have shown that the BAc of Cr and Ni was mostly less than 20% for Cr and less than 30% for Ni (Billmann et al., 2023; Bourliva et al., 2021; Ding & Hu, 2014; Gu et al., 2016). Moreover, the presence of Cr and Ni in soils is often associated with health risks due to their occurrence in numerous legacy sites.
Among in vitro tests showing the maximal in vitro in vivo criteria, the unified bioaccessibility method (UBM) has been validated for As, Cd, and Pb (Denys et al., 2012; Li et al., 2019) and is also standardized (ISO 17924, 2019). The UBM test is therefore considered a reference method for assessing the oral BAc in many countries, especially in Europe. Previous work (Pelfrêne et al., 2020) was conducted to develop a simplified test based on extracting PTEs with an HCl solution for predicting the BAc of As, Cd and Pb. This test is simpler, faster and can be used in screening to obtain a first approximation of BAc.
This paper presents an investigation of the BAc of Cr and Ni in 27 soil samples from different geographical origins (metropolitan France and French overseas departments, Belgium, Greece, and Northern Ireland), PTE sources (anthropogenic and, geogenic), and contexts (e.g., industrial, mining, and urban) over a wide range of concentrations and soil physicochemical parameters. The aim of this work was to highlight the importance of measuring the oral BAc of Cr and Ni to assess more realistic population exposure and health risks. More specifically, the BAc of Cd and Ni was estimated using both the UBM and simplified HCl tests. Specific attention was given to the contamination origin, physicochemical soil parameters, PTE phase distribution and geochemistry, which were assessed to understand how BAc is affected by these parameters.
Materials and methods
Soil sample collection and characterization of soil parameters
A total of 27 soil samples were collected in the same way at 13 different contaminated sites located in metropolitan France, its overseas departments and Europe. These sites have different uses, origins, and sources of contamination (Table 1). These samples also included a wide range of physicochemical parameters and concentrations of Cr and Ni. For each site, a composite topsoil sample was constituted and sent to the laboratory where all soil samples were prepared using soft techniques (oven drying at 40 °C and then sieving to 2 mm). A portion was then sieved to 250 µm using a sieve shaker (AS200 Control, Retsch).
The pseudototal concentrations of PTEs were obtained by acid digestion with aqua regia (6 mL HCl and 2 mL HNO3) added to 0.4 g of soil, dried and sieved to 250 µm in a closed microwave oven (NF 13657) and determined by inductively coupled plasma with optical emission spectrometry (ICP OES, Agilent). The limits of quantification averaged 5 µg/L for Cr and 10 µg/L for Ni. Unlike hydrofluoric acid mineralization (determination of total concentration), aqua regia does not dissolve silicates; therefore, only the pseudototal concentration can be determined. Triplicates were made for each soil sample. The soil pH was measured in a mixture (1:5, v/v) of 2 mm sieved matrix and water (NF ISO 10390). The total carbonate content (CaCO3) of the < 250 µm soil fraction was determined via the volumetric method (NF ISO 10693). The method consists of quantifying the release of CO2 produced by the reaction between carbonates present in the soil sample and hydrochloric acid. Assimilable phosphorus, expressed as P2O5, was cold extracted from the 2 mm sieved matrix according to NF X31161. Organic matter (OM) was determined in the < 250 µm soil fraction with the NF ISO 10694 standard (i.e., determination of organic carbon by dry combustion). The exchangeable bases (EBs) Na+, K+, Ca2+ and Mg2+ were determined in the < 250 µm soil fraction by saturation of the binding sites with a barium chloride solution of 0.1 mol L−1. The cation exchange capacity (CEC) was determined after the extraction of EBs by saturation with a known amount of magnesium sulfate (0.02 mol L−1) and by measuring the excess Mg. Single extraction with a mixture of solutions (0.111 mol L−1 sodium bicarbonate, 0.267 mol L−1 sodium tricitrate, and 200 g L−1 sodium dithionite) was performed on the 250 µm soil fraction to extract free Fe, Mn, and Al oxides (i.e. oxides not bonded to silicates) (Mehra & Jackson, 1960). The EBs, Mg and oxide concentrations were measured using an atomic absorption spectrometer (AAS, AA-6800 Shimadzu). Blanks and certified materials were run in each series to control the quality of the analysis. For the pseudototal concentrations, the SRM NIST2710a was used as a certified material and the recovery averaged 91% for Cr and 132% for Ni.
Chemical extraction methods
Sequential chemical extraction
Cr and Ni fractionation was estimated using a three-step sequential extraction procedure recommended by the Standard Measurement and Testing Program (SM&T) of the European Community, formerly BCR (Rauret et al., 1999). The fractions F1, F2, F3, and F4 were defined as (a) exchangeable, water- and acid-soluble (0.11 mol L−1 acetic acid), (b) reducible (0.5 mol L−1 hydroxylammonium chloride), (c) oxidizable (8.8 mol L−1 H2O2, followed by 1.0 mol L−1 ammonium acetate at pH 2), and (d) residual (aqua regia), respectively. Each suspension was mixed in a mechanical horizontal shaker for 16 h. Sequential extraction methods were used to assess PTE association and distribution with the different solid phase components in soils and to provide knowledge on PTE affinity to the soil components and the strength with which they are bound. Soil samples were digested in successive extraction solutions to mobilize element fractions with decreasing mobility and availability. The first step was targeting the PTEs contained in the soil solution, carbonates and exchangeable PTEs. These PTEs are considered readily mobile and bioavailable (F1). The second and third steps involved targeting the PTEs occluded in Fe, Mn and Al oxides and hydroxides (F2), or complexed with OM and sulfides (F3). The PTEs bound to these compounds are generally less available, depending on the soil matrix and the strength of the interaction between the PTE and the compounds. The final step consisted of targeting the PTEs contained in the residual fraction (F4), which is the least readily available fraction because it corresponds to the remaining share of PTEs bound to nonsilicate compounds (Žemberyová et al., 2006). BCR CRM 701 was used as standard reference material. The sum of recoveries of the reference material extraction steps was between 76% and 103% of the pseudototal concentrations for Cr and Ni.
In vitro oral BAc measurements of Cr and Ni
The BAc of Cr and Ni in the soil samples (dried and sieved to < 250 mm particle size) was assessed first using the UBM, which is a validated and standardized in vitro extraction method (Unified Bioaccessibility Method; ISO 17924; Denys et al., 2012). The test consisted of two parallel sequential extractions and provided samples for analysis from both the gastric (G) and gastrointestinal (GI) phases. The fluids used in this protocol consisted of organic and inorganic reagents with a composition close to that of human digestive fluid (Pelfrêne et al., 2020), and were prepared one day before the extractions were performed. Triplicates were made for each sample in both the gastric and gastrointestinal phases. This measurement was performed on a mass of 0.6 g of soil to which 9 ml of saliva fluid was added, followed by 13.5 ml of gastric fluid. The pH of the suspension was then adjusted to 1.20 ± 0.05 by adding HCl (37%). After end-over-end shaking at 37 °C for 1 h, the G phase was obtained by centrifuging the suspension at 4500 × g for 5 min. The intestinal fraction was measured by adding a liquid simulating intestinal digestive fluid, i.e., 27 ml of duodenal solution and 9 ml of bile solution. The pH of the suspension was adjusted to 6.3 ± 0.5 by adding NaOH (10 M). The suspension was then homogenized at 37 °C for 4 h and centrifuged as before. The resulting supernatants were acidified and analysed by inductively coupled plasma mass spectrometry (ICP MS) to determine the bioaccessible PTE concentrations in both phases. Quality control was based on the use of blanks and the NRC-CNRC PACS-3 reference material. The BAc of the PTEs was expressed in mg kg−1 and as a percentage of the pseudototal concentration. The limits of quantification were 0.19 µg/L for Cr and 0.13 µg/L for Ni on average.
In the second approach, a simplified test based on simple extraction with diluted HCl (Pelfrêne et al., 2020), developed to predict the oral BAc of As, Cd, and Pb in both the G and GI phases, was carried out on 27 soil samples to measure the extractable concentrations of Cr and Ni. For HCl extraction, 0.03 g of soil was weighed in triplicate and placed in polypropylene tubes before adding 25 mL of the HCl solution (0.65% v/v). The tubes were then placed in an ultrasonic bath (Elmasonic S120, Grosseron, France) for 15 min at room temperature. To mimic digestion, the tubes were placed in a thermostatic chamber for 1 h at 37 °C before being filtered (0.45 µm of porosity). The extractable concentrations of Cr and Ni were measured by ICP MS. The data were log-transformed to improve the distribution and linearity of the linear regression between the concentrations extracted by the UBM test and the HCl test. Quality control was based on the use of blanks and the PACS-3 reference material. The extractable fractions of PTEs determined by the HCl test are expressed in mg kg−1 and in % of pseudototal concentrations. The limit of quantification was 0.4 µg/L for both elements on average.
Chemical speciation of Cr.
Chromium speciation was determined by Cr(VI) determination via alkaline digestion and ion chromatography with spectrophotometric detection (NF ISO 15192). Cr(VI) was measured spectrophotometrically at 540 nm after postcolumn derivatization with 1,5-diphenylcarbazide (DPC) in acidic solution. To verify the accuracy of the method, the following quality controls were performed: (i) analysis of a sample in duplicate to check the accuracy of the method; (ii) doping of the sample with Cr(III) to check the absence of possible oxidation processes; and (iii) doping of the sample with soluble Cr(VI) to check the absence of possible reduction processes. The Cr(III) content is the difference between the pseudototal and Cr(VI) concentrations.
Statistical analysis.
Statistical analysis of the data was performed using XLSTAT 2022.1.1 (Addinsoft). Spearman correlation was performed to study the influence of soil parameters on bioaccessibility and significant correlations at the 0.05 level are highlighted. Spearman’s nonparametric statistical test is used to test the effect of one quantitative variable on another and to detect a trend of any form. This test determines whether the two variables are related. The Wilcoxon–Mann–Whitney Rank Sum two-sided test (U test) was performed to study the difference between anthropogenic (n = 19) and naturally enriched (n = 8) soils according to Cr and Ni BAc (G and GI phases), and soil physicochemical parameters and their soil phase distributions and significant differences between the variable means at the 0.05 level are highlighted and presented in SM1.
Results and discussion
Physicochemical properties of the soil
The pseudototal concentrations of Cr and Ni and the physicochemical parameters of the 27 soil samples are presented in Table 1. The pseudototal concentrations varied from 26 to 11,663 mg kg−1 for Ni and from 31 to 21,079 for Cr. The speciation of Cr was assessed, and the Cr (VI) concentrations varied from < 0.1 (LQ) to 1012 mg kg−1, which corresponds to a range from 0.02 to 13.5% of the pseudototal concentrations; this indicates that Cr was mostly found in the + 3 oxidation state which corresponds to its cationic form. The physicochemical parameters showed relatively high variability, highlighting the wide heterogeneity of the soils. The soil pH varied from acidic (5.1) to basic (9.3). These soils also contained wide ranges of total carbonates, organic matter (OM) and assimilable phosphorus (P2O5) contents, from < 0.1 to 516 g kg−1, 1 to 27%, and < 6 to 2383 mg kg−1 respectively. The CEC was between 2 and 104 cmol + kg−1, which indicates the number of cation sites (e.g. CrIII and Ni2+) retained. The exchangeable bases (EBs) measured were Ca+, Mg+, Na+, and K+ and were between 2–101, 0.1–20, 0.01–0.12 and 0.1–1.3 cmol + kg−1, respectively. The concentrations of Fe, Al and Mn oxides were determined to be between 4776–87, 280, 170–15,863 and 18–6896 mg kg−1, respectively. The role of oxides is very important, as certain associations, such as Cr(III) with Mn-oxides, can induce oxidation to Cr(VI) (Garnier et al., 2013). The diverse soil characteristics make these sites ideal for studying the links between PTE BAc, soil characteristics and PTE properties.
Cr and Ni solid-phase distribution in the soils studied
The results for the solid-phase distribution of Cr and Ni in the soil samples are shown in Fig. 1, and SM1 presents the differences between the mean distributions in anthropogenic and geogenic soils. Figure 1a shows the general solid-phase distribution of Cr and Ni among the four fractions, while Fig. 1b shows the specific distribution in each soil, differentiating the soil origin. Values are expressed as the percentage of pseudototal concentrations for each PTE.
a and b Fractionation of Cr and Ni (expressed in % of pseudototal concentration) in comparison to the pseudototal concentrations (expressed in mg kg−1) in the 27 soils (F1, exchangeable, water- and acid-soluble fraction; F2, reducible fraction; F3, oxidizable fraction; F4, residual fraction; B, Belgium; MF, Metropolitan France; FD, France DROM; GR, Greece; and NI, Northern Ireland)
Cr was mainly present in the residual fraction (Fig. 1a, b), with 29–96% of the Cr bound to F4 (mean 69%). Cr tended to be more prevalent in F3 (3–55%; mean 23%) than in F2 (0.1–29%; mean 8%). Cr was virtually absent in F1 (< 1.5%). When comparing the Cr distribution in soils with anthropogenic and geogenic PTE origins (Fig. 1a), a similar distribution pattern was shown for both sources, where most Cr was found in F4 and virtually no Cr was found in F1. However, the proportion of Cr bound to F2 and F3 was significantly lower, and the Cr bound to the residual fraction was greater in Cr-enriched soils (F2: 2%; F3: 12%; and F4: 86%) than anthropogenically contaminated soils (F2: 11%; F3: 27%; and F4: 61%) (SM1). As the contamination of these soils was not very recent, it is possible that the absence of Cr in F1 may be the result of rapid leaching of Cr after its release into the environment, before it had time to pass into the less mobile phases.
Ni was also mainly present in the residual fraction F4 (Fig. 1), with values ranging from 26 to 96% (mean 64%). Ni was more likely to be found in F2 (2–51%; mean 23%) than in F1 (0.3–24%; mean 5%) or F3 (1–42%; mean 11%). When comparing the Ni distribution in soils with anthropogenic and geogenic PTE origins (Fig. 1a), a similar distribution pattern was shown for both origins, where most Ni was found in F4. However, the proportion of Ni bound to F1 and F2 was significantly lower, and the Ni bound to the residual fraction was greater in geogenic soils (F2: 6%; F4: 84%) than in anthropogenic soils (F2: 25%; F4: 56%) (SM1).
In contrast to Cr, Ni was found in all estimated fractions. Since fractions F1 and F2 were more easily solubilized, it can be assumed that Ni was more available than Cr. Mendoza et al. (2017) reported similar distributions in suburban soils, where Cr was mainly present in the residual fraction (F4) or in the oxidable fraction (F3) and was absent from the soluble (F1) and reducible (F2) fractions; additionally, Ni was mainly found in F4 followed by F3, and to a lesser extent in F2 (0–15%), and F1 (0–5%). These results provided a general idea of the distribution, but they were not precise, as the recovery was not always 100%. This is in accordance with a study of Zemberyova et al. (2006), in which it was shown that the recovery was lower for Cr and Ni than for other PTEs with the BCR method on Slovak reference material soils.
Cr and Ni in vitro oral bioaccessibility
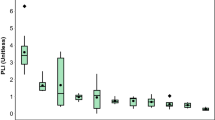
The distributions of Cr and Ni BAc (min, max and mean values expressed in % of pseudototal concentrations) in the soils studied in comparison to the data from the literature are presented in Table 2. Only BAc measured with the UBM was selected for a relevant comparison, as it has been shown that BAc ranges can vary depending on the in vitro test used (e.g., Kierulf et al., 2022; Li et al., 2019; Oomen et al., 2002). However, it is important to note that bioaccessibility ranges can also vary depending on the source of contamination, the context of use or the soil matrix. Figure 2 presents the BAc values for each soil sample studied while differentiating their origin.
Bioaccessible fractions of Cr and Ni (mean and standard deviation values expressed in % of pseudototal concentrations; n = 3) in the gastric (G) and gastrointestinal (GI) phases in comparison to the pseudototal concentrations (expressed in mg kg−1); B, Belgium; MF, metropolitan France; FD, France DROM; GR, Greece; and NI, Northern Ireland)
Chromium
The BAc of Cr ranged from 4 to 638 mg kg−1 in the G phase and from 1 to 188 mg kg−1 in the GI phase. Expressed in % of the pseudototal concentrations, the mean values of BAc of Cr during the G phase were 10% and decreased to 1.2% in the GI phase. The Cr in the study soils was mostly Cr(III), which corresponds to the cationic form (Table 1). With the exception of the MF17 soil, which had a BAc of 80% in the G phase, our results are consistent with the findings of the literature on the percentages of BAc (Table 2). The soils studied were also differentiated according to their geogenic or anthropogenic origin to determine whether this parameter affects BAc (Fig. 1), and differences in means were analysed and are shown in SM1. In geogenic soils, the BAc of Cr in the G phase was 1.7% on average, and that in the GI phase was 0.6%. In anthropogenic soils, the BAc of Cr in the G phase was 13% on average, and in the GI phase it was 1% on average. Globally, Cr BAc was significantly lower for geogenically enriched soils than for anthropogenic soils (SM1).
In our study, the BAc of Cr was always greater in the G phase than in the GI phase. The BAc in the GI phase was very low, which means that almost all the Cr solubilized in the G phase precipitated, complexed or readsorbed, which was the result of the increase in the pH of the solution, which changed from acidic to neutral (Fernández-Caliani et al., 2019). The same partition was also observed by other studies when the UBM test was used (Table 2). However, for other in vitro tests (e.g., SBRC and PBET), a higher Cr concentration was sometimes observed in the GI phase than in the G phase (Karadaş and Kara, 2011; Mendoza et al., 2017; Pereira et al., 2020). Indeed, various in vitro methods influence bioaccessibility (Billmann et al., 2023; Kierulf et al., 2022). For other tests, the pH in the GI phase was adjusted to 7, which is slightly greater than that for the UBM test (pH GI = 6.3) and could therefore influence the behaviour of a redox-sensitive PTE such as Cr. The higher the pH of the environment in which Cr is found, the more likely it is that redox equilibrium will lead to the formation of Cr(VI), which could explain the higher BAc in the GI phase of the tests in which the pH was adjusted to 7. These differences could also be linked to variations in the digestive fluid compositions of in vitro tests. It is possible that the more complex composition of the GI phase in the UBM test was responsible for this much greater Cr readsorption than in the other in vitro tests with fewer constituents in the GI phase.
The influence of the pseudototal concentration, Cr(VI) content, soil physicochemical parameters and distribution among soil phases on Cr and Ni BAc was analysed by Spearman correlation, and the rank correlation coefficients (r) are presented in Table 3. A second Spearman correlation was performed to separate anthropogenic from geogenic enriched soils, and the results are shown in the supplementary material (Table SM2). When correlations are carried out on smaller samples (e.g. when anthropogenic and geogenic soils are analysed separately), it is important to remain cautious and not overinterpret correlations. Nevertheless, these correlations can still provide information on trends, insofar as the Spearman test correlations are significant (p < 0.05).
Considering all 27 soils, a negative correlation was found between pseudototal Cr and bioaccessible Cr in both phases (G: r = − 0.49; GI: r = − 0.47, p < 0.05; Table 3). This negative correlation can be explained by the mostly low Cr BAc, particularly for highly contaminated soils such as geogenic soils or anthropogenic soils such as MF3, MF8 and MF13. Indeed, the negative correlation was much greater when considering only geogenic enriched soils (G: r = − 0.81; GI: r = − 0.74, p < 0.05) (Table SM2), which is consistent with studies showing that the bioaccessibility of geogenic PTEs tends to be low (Barsby et al., 2012; Fernández-Caliani et al., 2020; Juhasz et al., 2007). Moreover, soils MF14, MF17 and FM18 (anthropogenic sources) had high G BAc and among the lowest pseudototal concentrations which could be responsible for the negative correlation with anthropogenic soils (G: r = − 0.56, p < 0.05) (Table SM2).
The Spearman analysis only revealed the influence of the soil parameters on the BAc of Cr in the G phase (Table 3). In the GI phase, Cr BAc was always < 4%, which might explain why the influence of the soil parameters was limited, and no correlation was detected. The BAc of Cr in the G phase was positively correlated with soil pH, P2O5 and CaCO3 (Cr G: r = 0.48; r = 0.57; r = 0.60, p < 0.05). The influence of soil pH and CaCO3 might be linked because a high soil pH often also indicates a higher CaCO3 content. As the pH of the extraction solution is adjusted to 1.2 with HCl in the G phase, a high soil pH and CaCO3 content indicates that more HCl needs to be added to adjust the pH of the solution. As carbonates are easily dissolved in the acidic environment of the G phase, the Cr contained inside is released, and the Cr becomes more bioaccessible. Considering all soils, the results showed that a high P2O5 concentration induced a greater BAc; this is in accordance with a study by Li et al. (2017), in which they observed an increase in As BAc when phosphate was added, demonstrating that phosphate can influence BAc, but more research is needed to confirm this positive correlation. Among the analysed oxides, only the Mn-oxide content had a negative influence on Cr BAc in the G phase (G: r = − 0.46, p < 0.05) (Table 3), whereas oxides are normally known to represent retention sites that limit BAc. The influence might be diluted by other mechanisms, such as stronger affinities for other soil components. The study of the mineralogy though X-ray diffraction (XRD) analysis might help to better understand the interactions between Cr and the different types of oxides as seen in a study by Cox et al. (2017).
Considering all the soils, no significant correlation between the Cr(VI) content and bioaccessible Cr (%) was detected (Table 3). Because many of them contained less than 1 mg of Cr(VI) kg−1, a second Spearman correlation was carried out considering only the 11 soils containing a Cr(VI) concentration greater than 1 mg kg−1 (data not shown). It is important to recognize the weakness of this study resulting from the insufficient sample size, which makes it impossible to draw definite conclusions. It does, however, raise the possibility of future studies. The results showed that the Cr(VI) content seemed to have an influence on bioaccessible Cr, especially in the GI phase where the positive correlation was significant (r = 0.87, p < 0.05). The acidic environment of the G phase may favour the reduction of Cr(VI) to Cr(III) (Broadway et al., 2010). The freshly reduced Cr(III) in the G phase could become more bioaccessible in the GI phase than the original Cr(III), which may form more stable complexes or absorb on soil matrix compounds. Even if the solution pH increases to 6.3 in the GI phase, the Eh-pH diagram shows that at this pH, only a small amount of Cr(VI) should be formed again as the redox potential must be at least 0.6 to favour the oxidation of Cr(III) to Cr(VI) (Hlihor et al., 2009).
Among the parameters that can explain Cr BAc, the distribution by sequential extraction can help to better clarify the soil physicochemical parameters. As seen before, the general distribution of Cr in the four fractions of the sequential extraction order was ranked as follows: F4 > > F3 > F2 > > F1. For geogenically enriched soils, the low BAc could be explained by the high Cr content found in the residual fraction F4; this is consistent with the negative correlation found between the F4 fraction and bioaccessible Cr in both fractions (Cr G: r = − 0.93; GI: r = − 0.86, p < 0.05) (Table SM2). However, for anthropogenically contaminated soils, the distribution in F4 was not always consistent with that of Cr BAc in the G phase (Table SM2). In MF1, MF2, MF4, MF8, MF9, and MF13, Cr had a low BAc (< 10%), even if Cr was not mostly located in the residual fraction. In those soils, Cr was mostly found in F3 bound to oxidizable compounds, as most Cr was in the Cr(III) oxidation form, which can form strongly stable complexes with the carboxyl group in OM (Stewart et al., 2003); this could explain the low BAc. Considering all soils, when comparing the patterns of distribution (Fig. 1b) and BAc (Fig. 2), bioaccessible Cr seemed to be related to its distribution in F2 and, to a lesser extent, in F3. This finding is consistent with the positive correlations found between bioaccessible Cr and F2 (G: r = 0.59; GI: r = 0.74, p < 0.05) and F3 (G: r = 0.53; GI: r = 0.53, p < 0.05) (Table 3). Bioaccessible Cr was probably associated with reducible soil compounds, i.e., Fe/Mn oxyhydroxides and oxidable soil compounds, i.e., organic matter.
The highest BAc was observed in MF17, with a percentage of 80% in the G phase, whereas 81% of Cr was contained in the F4 fractionn which generally corresponds to the least mobile fraction. In the GI phase, BAc decreased to 0.2%. This soil sample was characterized by the highest content of P2O5 (Table 1). Phosphate compounds might be dissolved in the acidic G phase, and at higher pH in the GI phase, the dissolved phosphate could precipitate with dissolved PTEs, explaining the wide variation in bioaccessibility between these two phases (Grøn & Andersen, 2003). The inconsistency between the high Cr BAc in the G phase and the main distribution in the residual fraction can be explained by the difference in acid used in the sequential extraction protocol: acetic acid (0.11 M) and the hydrochloric acid used to adjust the pH (1.2) in the bioaccessibility tests. As hydrochloric acid is stronger than acetic acid, it is possible that the extractants used in the sequential extraction are not strong enough to solubilize the Cr in the soil and that only mineralization (F4) was able to extract the Cr from the soil matrix. In fact, this inconsistency may reflect the limitations of sequential extraction methods including a lack of efficiency and selectivity of extractants, readsorption or precipitation of target species after mobilization, and changes in the valence of redox-sensitive target species during extraction. Indeed, sequential extractions provide operationally defined speciation data that generally reflect trends and major differences in PTE speciation among different soils but do not necessarily provide accurate estimates of concentrations of the various target species. Inconsistencies have already been observed in comparison to Cr speciation studies using synchrotron X-ray techniques (Elzinga & Cirmo, 2010).
Nickel
The bioaccessible concentrations of Ni (Fig. 2) ranged from 1 to 431 mg kg−1 in the G phase and from 1 to 412 mg kg−1 in the GI phase. Expressed in % of the pseudototal concentrations, the mean values of the BAc of Ni during the G phase were 17% and decreased to 13% in the GI phase (Fig. 2; Table 2). In the geogenic soils, the average BAc Ni content in the G phase was 4% and that in the GI phase was 3%. In anthropogenic soils, the average BAc of Ni in the G phase was 23% and that in the GI phase was 17%.
For all soils, the BAc of Ni was not dependent on the pseudototal concentration (Table 3) but was positively correlated with soil pH, P2O5 and CaCO3 (Ni G: r = 0.60; r = 0.49; r = 0.57; Ni GI: r = 0.65; r = 0.46; r = 0.59, p < 0.05) and negatively correlated with OM (Ni G: r = − 0.40; Ni GI: r = − 0.50, p < 0.05). The same influence of OM was observed in a study by Palmer et al. (2013). This can be explained by the retention of the cationic element on negatively charged OM sites (Cempel & Nikel, 2006). No correlation was found for Cr indicating that Ni might have a greater affinity for OM than Cr in its cationic form. For Cr, the influence of soil pH and CaCO3 might be linked and a high P2O5 could be responsible for a higher BAc, however, further research needs to be conducted on the influence of phosphorus on BAc. All analysed oxides were negatively correlated with Ni BAc in both phases (Table 3). Indeed, oxides are known to represent good retention sites for PTEs. When considering anthropogenic and geogenic enriched soils, virtually no correlation was found between Ni BAc and soil parameters except for OM in geogenic soils (G: r = − 0.76; GI: r = − 0.95, p < 0.05) (Table SM2); this emphasizes that the influence of soil parameters is highly complex and can vary from one study to another and from one source of contamination to another.
Usually, divalent metallic cations, such as Cd and Pb, are extracted more in the G phase and have a tendency to reprecipitate, reform complexes or readsorb in the GI phase (Ellickson et al., 2001). However, in some soils, the BAc of Ni in the G phase was equal to that in the GI phase (Fig. 2), e.g., B1, MF1, MF2, MF3, MF5, MF14 and MF15. MF12 and geogenic enriched soils also seem to have equal Ni extracted in G and GI but as bioaccessibility is very low in these soils, it is difficult to determine whether the difference is significant. To more thoroughly clarify the mechanisms behind this partition, relationships with the soil physicochemical parameters were studied by considering the ratio between the BAc of Ni in the G phase and that in the GI phase (Table 4).
The BAc G/BAc GI for Ni was negatively correlated with soil pH (r = − 0.62, p < 0.05) and positively correlated with OM, K+ and Fe- and Al-oxide contents (r = 0.63, r = 0.39, r = 0.44 and r = 0.51, respectively, p < 0.05). The BAc of Ni in the G and GI phases tended to be equal in soils with higher soil pH and lower OM and Al-oxide contents. It can be assumed that a higher soil pH can induce the addition of more HCl to adjust the pH to 1.2 in the G phase, which could promote the formation of soluble NiCl2 complexes in the GI phase rather than insoluble complexes with soil matrix compounds. Another hypothesis is that there might be competition with other cations or compounds (salts) in the GI fluids making the readsorption, precipitation or complexation more complicated. Indeed, the Ni adsorption affinity on oxides was weaker than that for Cr and Pb (Young, 2013), and both PTEs had high concentrations in studied soils (Table 1).
Among the parameters that can clarify Ni BAc, the distribution can help provide a better understanding in addition to soil physicochemical parameters.The order of the distribution of Ni in the four fractions was ranked as follows: F4 > > F2 ≥ F3 > F1. Similar to Cr, the globally low BAc could therefore be explained by the high Ni content found in the residual fraction; this is consistent with the significant negative correlation found between F4 and bioaccessible Ni in both phases (Ni G: r = − 0.85; GI: r = − 0.85, p < 0.05) (Table 3). Considering geogenically enriched soils, bioaccessible Ni was best explained by F1 + F2 (G: r = 0.76; GI: r = 0.95, p < 0.05) (Table SM2), indicating that bioaccessible Ni was associated with exchangeable and reducible compounds. However, in anthropogenically contaminated soils, bioaccessible Ni seemed to be less elucidated by its association with F2 reducible compounds than by its association with F1 and F3 (F1 G: r = 0.69; GI: r = 0.65; F2 G: r = 0.70; GI: r = 0.64, p < 0.05) (Table SM2). Depending on the origin of the contamination, the associations seemed to differ slightly.
Comparison of Cr and Ni
When a distinction is made between soils of geogenic and anthropogenic origin (Fig. 1), it appears that Cr and Ni BAc were generally lower for soils with naturally occurring PTEs (SM1). This is explained by the high proportion of Cr and Ni found in the residual fraction compared to the other fractions and the many immobilization mechanisms over time, as shown by several studies on BAc (Barsby et al., 2012; Chu et al., 2022; Ding et al., 2022; Fendorf et al., 2004; Ljung et al., 2007; Rinklebe & Shaheen, 2017; Stewart et al., 2003; Young, 2013). For anthropogenically contaminated soils, the association of Cr and Ni with the more mobile fractions (F1, F2 and F3) was more pronounced, explaining the tendency towards higher BAc (Fig. 1). However, considering the different sources among anthropogenic soils (i.e., urban wastelands, industrial sites, and mining areas), the limited number of available samples did not allow us to identify the influence of the source on bioaccessibility. As most soils studied were industrial, the comparison with the other contexts was therefore not particularly relevant because it was not necessarily representative. However, a meta-analysis by Kierulft et al., (2022) revealed that Cr had greater G phase BAc in industrial and urban soils than in mining, agricultural or rural soils.
In the present study, the BAc of Cr and Ni was generally low, and both in the literature and in this study, Cr tended to be less bioaccessible than Ni (Fig. 2; SM1). Indeed, the sequential extraction showed that Ni tended to be present in more easily available fractions (e.g., F1, F2) than Cr (Fig. 1a). This is consistent with a study by Cox et al. (2017), in which an analysis of the mineralogy showed that Ni was more widely dispersed within the soils, with a proportion of pseudototal Ni found in more easily soluble compounds (carbonates and weathering products: secondary iron oxides and precursor clay minerals) when Cr concentrations were principally related to noneasily solubilized compounds (e.g. recalcitrant chrome spinel and primary iron oxides).
In our study, only a few correlations between soil matrix compounds and BAc were established and varied according to the contamination origin. Similar results were observed in a study by Różański et al. (2021). A previous literature review showed that BAc seems to be site dependent. The influence of a single soil parameter or statistical model mostly differs from one study to another (Billmann et al., 2023). Soil matrices are complex, and many reactions can occur within them. Soil compounds can influence the bioaccessibility of Cr, Ni, and each other. In addition, the PTEs present can modify the bioaccessibility of other PTEs by competing with each other for adsorption sites.
Evaluation of the ability of the single-extraction method with HCl to predict the BAc of Cr and Ni
Because a previous study showed the efficiency of using HCl (0.65%) in a first-tier screening to predict the BAc of As, Cd, and Pb in both the G and GI phases (Pelfrêne et al., 2020), this single extraction method was assessed for Cr and Ni. The study of the correlation between the HCl concentration and the UBM for Cr and Ni provided additional information on a validated and standardized method (UBM for As, Cd, and Pb) or on methods in progress (HCl) to begin extending their fields of use to these two PTEs. Figure 3 presents the results obtained for the extractable concentrations of Cr and Ni by HCl in comparison with the pseudototal concentrations. The extractable Cr and Ni concentrations ranged from 6 to 1531 mg kg−1 and 2 to 829 mg kg−1, respectively, and the extractable percentages of the pseudototal Cr and Ni concentrations ranged from 0.1 to 87% and 2 to 79% respectively. The extractable concentrations of Cr and Ni by HCl were slightly greater than those for the UBM G phase, but globally, both patterns were similar. The relationships between the extractable concentrations of Cr and Ni by using the UBM in the G and GI phases and those by HCl were investigated and are presented in Fig. 4. The best relationships were for Ni in both phases (G: R2 = 0.96 and GI: R2 = 0.94). In the G phase, the relationship for Cr was also good (R2 = 0.93), but in the GI phase, the relationship was weaker (R2 = 0.45). Ni has cationic behaviour, similar to that of Cd and Pb, in which the HCl test also had a good relationship with the UBM test (Pelfrêne et al., 2020). Cr BAc in the GI phase was low compared to that in the G phase due to several processes of complexation, precipitation, and readsorption in the higher pH environment of the intestinal compartment. Indeed, the acidic environment with this single extractant could explain the poor relationship between the extractable concentrations of HCl and the UBM GI phase. This poor correlation was mainly due to two soil samples (MF7 and MF17). Indeed, when these two points were removed, the relationship was much better with an R2 value of 0.80, because the amount of extracted Cr in the GI phase was practically ≤ 1 when the amount of extracted Cr in the G phase was 211 and 303 mg kg−1 and the amount of extracted Cr with HCl was 357 and 329 mg kg−1 for FM7 and FM17, respectively. FM7 was characterized by a high OM content (23%) and high iron-oxide content (56 g kg−1). FM17 was characterized by a high P2O5 (2.4 g kg−1) content, high CEC and exchangeable Ca+ (80 and 76 cmol + kg−1, respectively). No distinguishable differences were found between the physicochemical parameters of the analysed soils. Qian et al., (2024) showed that in the G phase, the transformation of Cr(VI) to Cr(III) could be the main process, while in the GI phase, the precipitation of Cr(III) could dominate the transformation. The low Cr BAc in the GI phase could be explained by strong reprecipitation due to increased solution pH.
Table 5 summarizes the regression models for Cr and Ni predicting bioaccessible concentrations in the G and GI phases from extractable concentrations by HCl. At first glance, this simplified test seems promising for predicting the BAc of Cr and Ni in both phases (to a lesser extent Cr in GI) and needs to be reinforced with a larger number of soil samples.
Conclusion
This paper presents an investigation of the oral BAc of Cr and Ni in 27 soil samples collected from various sites, including those of anthropogenic and geogenic origins, as well as from different environmental contexts, such as industrial, mining, and urban areas, and revealed a wide range of concentrations and soil physicochemical parameters. The results indicated that the BAc values of Cr and Ni were generally low, with values below 20% and 30%, respectively. BAc tended to be more variable for anthropogenic soils, whereas for geogenically enriched soils, BAc was always very low. Among the various soil parameters analysed in our study, factors such as soil pH, assimilable phosphorus, carbonates, organic matter, and oxides influenced the BAc of Cr and/or Ni. It is worth noting that these influencing parameters may vary from one study to another, as indicated in the literature. The influence of soil parameters is obvious, but distinguishing the influence of each parameter is very complicated because they may themselves be correlated. The distribution of Cr and Ni within the various soil-bearing phases revealed that these elements were predominantly associated with the residual fraction, which largely explains their limited BAc. Aside from the residual fraction, Cr was primarily found in the oxidizable fraction and was absent in the acid soluble fraction. In contrast, Ni was more prevalent in the reducible fraction and was distributed across all the fractions in the anthropogenically contaminated soils. Compared with physicochemical parameters, sequential extractions offered a more comprehensive explanation for BAc. However, for certain specific soils, mineralogical studies are recommended as a complement to overcome the limitations of sequential extraction. This study is of interest for assessing the potential use of the simplified HCl test as a first approach to predict the BAc of Cr and Ni in both phases. The results obtained for these 27 soil samples are very promising, although further research is required to refine the applicability of these results to a larger soil population and, more specifically, for certain Cr-contaminated soils. Globally, the results emphasize the importance of assessing the oral BAc of Cr and Ni to better assess human exposure, refine risk and adopt more effective soil management practices.
References
ATSDR. (2005). Toxicological profile for nickel. Agency for Toxic Substances and Disease Registry.
ATSDR. (2012). Toxicological profile for chromium. Agency for Toxic Substances and Disease Registry.
Ayangbenro, A. S., & Babalola, O. O. (2017). A new strategy for heavy metal polluted environments: A review of microbial biosorbents. International Journal of Environmental Research and Public Health, 14(1), 94. https://doi.org/10.3390/ijerph14010094
Babaahmadifooladi, M., Jacxsens, L., Van de Wiele, T., & Laing, G. D. (2020). Gap analysis of nickel bioaccessibility and bioavailability in different food matrices and its impact on the nickel exposure assessment. Food Research International, 129, 108866. https://doi.org/10.1016/j.foodres.2019.108866
Barsby, A., McKinley, J. M., Ofterdinger, U., Young, M., Cave, M. R., & Wragg, J. (2012). Bioaccessibility of trace elements in soils in Northern Ireland. Science of the Total Environment, 433, 398–417. https://doi.org/10.1016/j.scitotenv.2012.05.099
Billmann, M., Hulot, C., Pauget, B., Badreddine, R., Papin, A., & Pelfrêne, A. (2023). Oral bioaccessibility of PTEs in soils: A review of data, influencing factors and application in human health risk assessment. Science of the Total Environment, 896, 165263. https://doi.org/10.1016/j.scitotenv.2023.165263
Bourliva, A., Aidona, E., Papadopoulou, L., Ferreira da Silva, E., & Patinha, C. (2021). Levels, oral bioaccessibility and health risk of sand-bound potentially harmful elements (PHEs) in public playgrounds : Exploring magnetic properties as a pollution proxy. Environmental Pollution, 290, 118122. https://doi.org/10.1016/j.envpol.2021.118122
Broadway, A., Cave, M. R., Wragg, J., Fordyce, F. M., Bewley, R. J., Graham, M. C., Ngwenya, B. T., & Farmer, J. G. (2010). Determination of the bioaccessibility of chromium in Glasgow soil and the implications for human health risk assessment. Science of The Total Environment, 409(2), 267–277. https://doi.org/10.1016/j.scitotenv.2010.09.007
Cempel, M., & Nikel, G. (2006). Nickel: A review of its sources and environmental toxicology. Polish Journal of Environmental Studies, 15(3), 375–382.
Chu, Z., Lin, C., Yang, K., Cheng, H., Gu, X., Wang, B., & WuMa, L. J. (2022). Lability, bioaccessibility, and ecological and health risks of anthropogenic toxic heavy metals in the arid calcareous soil around a nonferrous metal smelting area. Chemosphere, 307, 136200. https://doi.org/10.1016/j.chemosphere.2022.136200
Cox, S. F., Rollinson, G., & McKinley, J. M. (2017). Mineralogical characterisation to improve understanding of oral bioaccessibility of Cr and Ni in basaltic soils in Northern Ireland. Journal of Geochemical Exploration, 183, 166–177. https://doi.org/10.1016/j.gexplo.2017.02.006
da Pereira, W. V. S., Teixeira, R. A., de Souza, E. S., de Moraes, A. L. F., Campos, W. E. O., do Amarante, C. B., do Martins, G. C., & Fernandes, A. R. (2020). Chemical fractionation and bioaccessibility of potentially toxic elements in area of artisanal gold mining in the Amazon. Journal of Environmental Management, 267, 110644.
Denys, S., Caboche, J., Tack, K., Rychen, G., Wragg, J., Cave, M., Jondreville, C., & Feidt, C. (2012). In vivo validation of the unified BARGE method to assess the bioaccessibility of arsenic, antimony, cadmium, and lead in soils. Environmental Science and Technology, 46, 6252–6260. https://doi.org/10.1021/es3006942
Ding, S., Guan, D.-X., Dai, Z.-H., Su, J., Teng, H. H., Ji, J., Liu, Y., Yang, Z., & Ma, L. Q. (2022). Nickel bioaccessibility in soils with high geochemical background and anthropogenic contamination. Environmental Pollution, 310, 119914. https://doi.org/10.1016/j.envpol.2022.119914
Ding, Z., & Hu, X. (2014). Ecological and human health risks from metal(loid)s in peri-urban soil in Nanjing, China. Environmental Geochemistry and Health, 36, 399–408. https://doi.org/10.1007/s10653-013-9568-1
Dudka, S., & Miller, W. P. (1999). Permissible concentrations of arsenic and lead in soils based on risk assessment. Water, Air, and Soil Pollution, 113, 127–132.
Ellickson, K. M., Meeker, R. J., Gallo, M. A., Buckley, B. T., & Lioy, P. J. (2001). Oral bioavailability of lead and arsenic from a NIST Standard reference soil material. Archives of Environmental Contamination and Toxicology, 40, 128–135. https://doi.org/10.1007/s002440010155
Elzinga, E. J., & Cirmo, A. (2010). Application of sequential extractions and X-ray absorption spectroscopy to determine the speciation of chromium in Northern New Jersey marsh soils developed in chromite ore processing residue (COPR). Journal of Hazardous Materials, 183, 145–154. https://doi.org/10.1016/j.jhazmat.2010.06.130
Fendorf, S., La Force, M. J., & Li, G. (2004). Temporal changes in soil partitioning and bioaccessibility of arsenic, chromium, and lead. Journal of Environmental Quality, 33, 2049–2055. https://doi.org/10.2134/jeq2004.2049
Fernández-Caliani, J. C., Fernández-Landero, S., & Inmaculada Giráldez, M. (2020). Human bioaccessibility of heavy metals and metalloids naturally occurring at high levels in chestnut grove soils of SW Spain. Proscience, 7(30), 34. https://doi.org/10.14644/ghc2020.005
Fernández-Caliani, J. C., Giraldez, M. I., & Barba-Brioso, C. (2019). Oral bioaccessibility and human health risk assessment of trace elements in agricultural soils impacted by acid mine drainage. Chemosphere, 237, 124441. https://doi.org/10.1016/j.chemosphere.2019.124441
Garnier, J., Quantin, C., Guimarães, E. M., Vantelon, D., Montargès-Pelletier, E., & Becquer, T. (2013). Cr(VI) genesis and dynamics in Ferralsols developed from ultramafic rocks: The case of Niquelândia, Brazil. Geoderma, 193–194, 256–264. https://doi.org/10.1016/j.geoderma.2012.08.031
Grøn, C., & Andersen, L. (2003). Human bioaccessibility of heavy metals and PAH from soil; Danish EPA. Technology Programme for Soil and Groundwater Contamination, 840, 113.
Gu, Y.-G., Gao, Y.-P., & Lin, Q. (2016). Contamination, bioaccessibility and human health risk of heavy metals in exposed-lawn soils from 28 urban parks in southern China’s largest city, Guangzhou. Applied Geochemistry, 67, 52–58. https://doi.org/10.1016/j.apgeochem.2016.02.004
Hack, A., Welge, P., Wittsiepe, J., & Wilhelm, M. (2002). Aufnahme und Bilanzierung (Bioverfügbarkeit) ausgewählter Bodenkontaminanten im Tiermodell (Minischwein). Forschungsbericht., 298(73), 227–324.
Hlihor, R., Apostol, L., Pavel, V., Smarande, C., Robu, B., Caliman, F., & Gavrilescu, M. (2009). Overview on chromium occurence in the environment and its remediation. Bulletin of the Polytechnic Institute of Iasi, Section Chemistry and Chemical Engineering, 3
ISO 17924 (2019). Soil Quality-Assessment of Human Exposure From Ingestion of Soil and Soil Material-Procedure for the Estimation of the Human Bioaccessibility/Bioavailability of Metals in Soil.
Juhasz, A. L., Smith, E., Weber, J., Rees, M., Rofe, A., Kuchel, T., Sansom, L., & Naidu, R. (2007). In vitro assessment of arsenic bioaccessibility in contaminated (anthropogenic and geogenic) soils. Chemosphere, 69, 69–78. https://doi.org/10.1016/j.chemosphere.2007.04.046
Karadaş, C., & Kara, D. (2011). In vitro gastro-intestinal method for the assessment of heavy metal bioavailability in contaminated soils. Environmental Science and Pollution Research, 18, 620–628. https://doi.org/10.1007/s11356-010-0404-1
Kelley, M. E., Brauning, S. E., Schoof, R. A., & Ruby, M. V. (2002). Assessing oral bioavailability of metals in soil. Battelle Press.
Kierulf, A., Ollson, C., Whitehead, C., Beauchemin, D., & Koch, I. (2022). Literature review and meta-analysis of gastric and intestinal bioaccessibility for nine inorganic elements in soils and soil-like media for use in human health risk assessment. International Journal of Hygiene and Environmental Health, 240, 113929. https://doi.org/10.1016/j.ijheh.2022.113929
Kumar, P., Gacem, A., Ahmad, M. T., Yadav, V. K., Singh, S., Yadav, K. K., Alam, M. M., Dawane, V., Piplode, S., Maurya, P., Ahn, Y., Jeon, B.-H., & Cabral-Pinto, M. M. S. (2022). Environmental and human health implications of metal(loid)s: Source identification, contamination, toxicity, and sustainable clean-up technologies. Frontiers in Environmental Science, 10, 949581. https://doi.org/10.3389/fenvs.2022.949581
Li, H.-B., Li, M.-Y., Zhao, D., Li, J., Li, S.-W., Xiang, P., Juhasz, A. L., & Ma, L. Q. (2019). Arsenic, lead, and cadmium bioaccessibility in contaminated soils: Measurements and validations. Critical Reviews in Environmental Science and Technology, 50(13), 1303–1338. https://doi.org/10.1080/10643389.2019.1656512
Li, S.-W., Liu, X., Sun, H.-J., Li, M.-Y., Zhao, D., Luo, J., Li, H.-B., & Ma, L. Q. (2017). Effect of phosphate amendment on relative bioavailability and bioaccessibility of lead and arsenic in contaminated soils. Journal of Hazardous Materials, 339, 256–263. https://doi.org/10.1016/j.jhazmat.2017.06.040
Ljung, K., Oomen, A., Duits, M., Selinus, O., & Berglund, M. (2007). Bioaccessibility of metals in urban playground soils. Journal of Environmental Science and Health, Part A, 42(9), 1241–1250. https://doi.org/10.1080/10934520701435684
Lwin, C. S., Kim, Y.-N., Lee, M., & Kim, K.-R. (2022). Coexistence of Cr and Ni in anthropogenic soils and their chemistry: Implication to proper management and remediation. Environmental Science and Pollution Research, 29, 62807–62821. https://doi.org/10.1007/s11356-022-21753-2
Medlin, E. A. (1997). An in vitro method for estimating the relative bioavailability of lead in humans (Doctoral dissertation, University of Colorado).
Mehra, O. P., & Jackson, M. L. (1960). Iron oxide removal from soils and clays by a dithionite-citrate system buffered with sodium bicarbonate. Clays and Clay Minerals, 7, 317–327.
Mehta, N., Cipullo, S., Cocerva, T., Coulon, F., Dino, G. A., Ajmone-Marsan, F., Padoan, E., Cox, S. F., Cave, M. R., & De Luca, D. A. (2020). Incorporating oral bioaccessibility into human health risk assessment due to potentially toxic elements in extractive waste and contaminated soils from an abandoned mine site. Chemosphere, 255, 126927. https://doi.org/10.1016/j.chemosphere.2020.126927
Mehta, N., Cocerva, T., Cipullo, S., Padoan, E., Dino, G. A., Ajmone-Marsan, F., Cox, S. F., Coulon, F., & De Luca, D. A. (2019). Linking oral bioaccessibility and solid phase distribution of potentially toxic elements in extractive waste and soil from an abandoned mine site: Case study in Campello Monti, NW Italy. Science of the Total Environment, 651, 2799–2810. https://doi.org/10.1016/j.scitotenv.2018.10.115
Mendoza, C. J., Garrido, R. T., Quilodrán, R. C., Segovia, C. M., & Parada, A. J. (2017). Evaluation of the bioaccessible gastric and intestinal fractions of heavy metals in contaminated soils by means of a simple bioaccessibility extraction test. Chemosphere, 176, 81–88. https://doi.org/10.1016/j.chemosphere.2017.02.066
Oomen, A. G., Hack, A., Minekus, M., Zeijdner, E., Cornelis, C., Schoeters, G., Verstraete, W., Van de Wiele, T., Wragg, J., Rompelberg, C. J. M., Sips, A. J. A. M., & Van Wijnen, J. H. (2002). Comparison of Five in vitro digestion models to study the bioaccessibility of soil contaminants. Environmental Science and Technology, 36(15), 3326–3334. https://doi.org/10.1021/es010204v
Oomen, A. G., Rompelberg, C. J. M., Bruil, M. A., Dobbe, C. J. G., Pereboom, D. P. K. H., & Sips, A. J. A. M. (2003). Development of an in vitro digestion model for estimating the bioaccessibility of soil contaminants. Archives of Environmental Contamination and Toxicology, 44, 281–287. https://doi.org/10.1007/s00244-002-1278-0
Palmer, S., Cox, S. F., McKinley, J. M., & Ofterdinger, U. (2014). Soil-geochemical factors controlling the distribution and oral bioaccessibility of nickel, vanadium and chromium in soil. Applied Geochemistry, 51, 255–267. https://doi.org/10.1016/j.apgeochem.2014.10.010
Palmer, S., Ofterdinger, U., McKinley, J. M., Cox, S., & Barsby, A. (2013). Correlation analysis as a tool to investigate the bioaccessibility of nickel, vanadium and zinc in Northern Ireland soils. Environmental Geochemistry and Health, 35, 569–584. https://doi.org/10.1007/s10653-013-9540-0
Paustenbach, D. J. (2000). The practice of exposure assessment: A state-of-the-art review. Journal of Toxicology and Environmental Health, Part B: Critical Reviews, 3(3), 179–291. https://doi.org/10.1080/10937400050045264
Pelfrêne, A., Sahmer, K., Waterlot, C., Glorennec, P., Douay, F., & Le Bot, B. (2020). Evaluation of single-extraction methods to estimate the oral bioaccessibility of metal(loid)s in soils. Science of the Total Environment, 727, 138553. https://doi.org/10.1016/j.scitotenv.2020.138553
Qian, Q., Liang, J., Ren, Z., Sima, J., Xu, X., Rinklebe, J., & Cao, X. (2024). Digestive fluid components affect speciation and bioaccessibility and the subsequent exposure risk of soil chromium from stomach to intestinal phase in in-vitro gastrointestinal digestion. Journal of Hazardous Materials, 463, 132882. https://doi.org/10.1016/j.jhazmat.2023.132882
Qin, J., Nworie, O. E., & Lin, C. (2016). Particle size effects on bioaccessible amounts of ingestible soil-borne toxic elements. Chemosphere, 159, 442–448. https://doi.org/10.1016/j.chemosphere.2016.06.034
Rauret, G., López-Sánchez, J. F., Sahuquillo, A., Rubio, R., Davidson, C., Ure, A., & Quevauviller, Ph. (1999). Improvement of the BCR three step sequential extraction procedure prior to the certification of new sediment and soil reference materials. Journal of Environmental Monitoring, 1, 57–61. https://doi.org/10.1039/a807854h
Rinklebe, J., & Shaheen, S. M. (2017). Redox chemistry of nickel in soils and sediments: A review. Chemosphere, 179, 265–278. https://doi.org/10.1016/j.chemosphere.2017.02.153
Rodriguez, R. R., Basta, N. T., Casteel, S. W., & Pace, L. W. (1999). An in vitro gastrointestinal method to estimate bioavailable arsenic in contaminated soils and solid media. Environmental Science and Technology, 33, 642–649. https://doi.org/10.1021/es980631h
Różański, S. Ł, Castejón, J. M. P., & McGahan, D. G. (2021). Child risk assessment of selected metal(loid)s from urban soils using in vitro UBM procedure. Ecological Indicators, 127, 107726. https://doi.org/10.1016/j.ecolind.2021.107726
Ruby, M. V., Davis, A., Schoof, R., Eberle, S., & Sellstone, C. M. (1996). Estimation of lead and arsenic bioavailability using a physiologically based extraction test. Environmental Science and Technology, 30, 422–430. https://doi.org/10.1021/es950057z
Semple, K. T., Doick, K. J., Jones, K. C., Burauel, P., Craven, A., & Harms, H. (2004). Defining bioavailability and bioaccessibility of contaminated soil and sediment is complicated. Environmental Science and Technology, 38(12), 228A-231A. https://doi.org/10.1021/es040548w
Stewart, M. A., Jardine, P. M., Brandt, C. C., Barnett, M. O., Fendorf, S. E., McKay, L. D., Mehlhorn, T. L., & Paul, K. (2003). Effects of contaminant concentration, aging, and soil properties on the bioaccessibility of Cr(III) and Cr(VI) in soil. Soil and Sediment Contamination: An International Journal, 12(1), 1–21. https://doi.org/10.1080/713610958
US EPA. (2017). Method 1340 (SW-846 Update VI). United States Environmental Protection Agency.
Wang, M., Xu, X., Han, Q., Lin, X., Yuan, H., Wang, M., Jiang, F., & Wang, W. (2023). Assessment of source-oriented health risk associated with the oral ingestion of heavy metals in dust within an iron/steel smelting-affected area of the North China Plain. Environmental Research, 237, 117101. https://doi.org/10.1016/j.envres.2023.117101
Wragg, J., Broadway, A., Cave, M. R., Fordyce, F. M., Palumbo-Roe, B., Beriro, D. J., Farmer, J. G., Graham, M. C., Ngwenya, B. T., & Bewley, R. J. F. (2017). Linkage between solid-phase apportionment and bioaccessible arsenic, chromium and lead in soil from Glasgow, Scotland, UK. Earth and Environmental Science Transactions of the Royal Society of Edinburgh, 108(2–3), 217–230. https://doi.org/10.1017/S1755691018000762
Young, S. D. (2013). Chemistry of heavy metals and metalloids in soils. In B. J. Alloway (Ed.), Heavy metals in soils (Vol. 22, pp. 51–95). Springer. https://doi.org/10.1007/978-94-007-4470-7_3
Žemberyová, M., Barteková, J., & Hagarová, I. (2006). The utilization of modified BCR three-step sequential extraction procedure for the fractionation of Cd, Cr, Cu, Ni, Pb and Zn in soil reference materials of different origins. Talanta, 70, 973–978. https://doi.org/10.1016/j.talanta.2006.05.057
Zhong, M., & Jiang, L. (2017). Refining health risk assessment by incorporating site-specific background concentration and bioaccessibility data of Nickel in soil. Science of the Total Environment, 581–582, 866–873. https://doi.org/10.1016/j.scitotenv.2017.01.036
Acknowledgements
The authors wish to thank Dr Karin Sahmer from JUNIA for her contribution to the statistical analyses. We also thank our research colleagues in France and abroad for providing us with Cr- and Ni- rich soil samples.
Funding
This work was supported by the ADEME (Agency for Ecological Transition) (Contract n°2172D0198-B).
Author information
Authors and Affiliations
Contributions
Madeleine Billmann: Conceptualization, data curation, formal analysis, writing—original draft. Aurélie Pelfrêne: Conceptualization, supervision, writing—original draft Corinne Hulot: Conceptualization, writing review & editing. Arnaud Papin: Writing—review & editing. Benjamin Pauget: Conceptualization, writing—review & editing.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Billmann, M., Pelfrêne, A., Hulot, C. et al. Toward a more realistic estimate of exposure to chromium and nickel in soils of geogenic and/or anthropogenic origin: importance of oral bioaccessibility. Environ Geochem Health 46, 273 (2024). https://doi.org/10.1007/s10653-024-02041-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10653-024-02041-z