Abstract
Industrial horticulture can release pesticides and trace metals/metalloids to terrestrial and aquatic environments. To assess long-term and more recent land contamination from an expanding horticultural region, we sampled soils from chemical mixing, crop production, and drainage areas, as well as retention reservoirs (dam) sediments, from 3 blueberry farms with varying land-use history in subtropical Australia. Soils were analysed for 97 different pesticides and trace metal/metalloid contents. The most recent farm had fungicides propiconazole and cyprodinil contents that may compromise soil invertebrate survival and/or nutrient recycling (5–125 mg kg−1). A site previously used to cultivate bananas had 6 dam sediment subsamples with arsenic contents over sediment quality guidelines (SQG); however, the soil content values were just below Australian health investigation levels (100 mg kg−1). Arsenic is suspected to originate from pesticide application during previous banana cultivation in the region. Dam sediment cores at all sites had mercury contents over the SQG likely due to fungicides or fertiliser impurities. Mean contents of mercury from dam sediments (141 ± 15.5 µg kg−1) were greater than terrestrial soils (78 ± 6.5 µg kg−1), and sediment profiles suggest mercury retention in anoxic sediments. Soils in chemical mixing areas at two sites were contaminated with copper and zinc which were above the national soil ecological investigation levels. Based on toxicity data, distribution, persistence, and mobility, we identified the fungicide cyprodinil, mercury, and phosphorus as contaminants of the greatest concern in this intensive horticulture area of Australia. Additional sampling (spatial, chemical speciation, biotic) is required to support mitigation efforts of the emerging contamination in the rapidly expanding blueberry farms of this region of Australia.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pesticides, fertilizers, and other products containing contaminants are often applied directly to crops or soils in which they grow. Since many contaminants exhibit the affinity to bind to soil particles rapidly, the accumulation of contaminants occurs in the soil underlying the crops. Indeed, agricultural fields are known sites of increased nutrient, metal, and pesticide accumulation (He et al. 2005; Fang et al. 2007; Haynes and Naidu 1998). While agricultural soils may be the predominant (or first) sink for contaminants, these substances may become mobile under changing environmental conditions. For instance, rainfall leaches contaminants from the soils to surface or groundwaters and increases soil erosion from agricultural landscapes (Santos et al. 2011; Brodie and Mitchell 2005). Areas receiving runoff or sediment from agricultural lands, such as engineered dams or estuaries, can also become sinks of the drainage (Kreuger et al. 1999; Conrad et al. 2019). Sampling crop production areas and adjacent waterways can, therefore, provide an improved understanding of the extent of environmental impact from industrial agriculture.
Pesticide residues may not undergo rapid degradation and can become persistent environmental pollutants. Accumulation and overexposure of pesticides within the natural environment can cause wildlife and human health decline (Hernández et al. 2011; Schäfer et al. 2012). Examples of pesticides with long environmental retention times include aldrin, dieldrin, DDT, chlordane, endosulfan, heptachlor, and many others. In addition to pesticides, some trace metals/metalloids, such as lead (Pb), mercury (Hg), and arsenic (As), as well as excess nutrients from fertilizers such as nitrogen (N) and phosphorus (P) are associated with agricultural products. If present in sufficient amounts, pesticides, trace metals, and nutrients alter trophic interactions leading to decreased biomass and biodiversity in terrestrial and aquatic systems (Tilman 1999; He et al. 2005; Bennett et al. 2001).
Unsustainable agricultural practices after World War II, in combination with climate change and export-oriented production mentalities, have increased environmental and economic pressures on Australian farmers (Lawrence et al. 2013). Australian agricultural ideology has been described as “competitive productivism- unsubsidised, highly productive agriculture” (Dibden et al. 2009) driven by neoliberalist dogma (Lawrence et al. 2013). Productivism in Australia can be characterised by the intensification of production via increased use of external inputs, the ownership of the majority of farmland by a minority of farmers, and specialisation of regions with particular farm industries and expertise (Argent 2002).
Our study region is no exception to this rule. Coastal northern New South Wales (NSW) has a long history of intense banana cultivation. However, due to the opportunity for increased profit margins, the blueberry industry rapidly began to replace banana horticulture in this region around the year 2000 (exemplified in Fig. 1). Australian blueberry industry objectives focus on increasing domestic consumption, exports, and yields per hectare by 10% each year, with only a not-explicitly defined goal of increasing sustainability (ABGA 2018). Blueberry industry fertilisation guidelines are dated and based on data from overseas (Doughty et al. 1988), while other recommendations are relatively homogeneous strategies for growth and disease control across various landscapes, soil types, and hydrological regimes (Wilk and Ireland 2008; Simpson 2019). Additionally, many of the farmers in this region have generational knowledge of banana cultivation techniques, which may not be applicable to current blueberry horticulture.
Aerial photography time series of the Sandy Beach catchment (red line) and Hearnes Lake estuary in subtropical northern NSW demonstrating the land-use percentage change in banana to blueberry cultivation between 1956 and 2017. Sites 1 and 2 from this study are located in this catchment (pink). ArcGIS software used to quantify farm type. Banana farms: yellow polygons and percentages; blueberry farms: blue polygons and percentages. Images provided by New South Wales DPI
Here, we quantify contaminant levels in various soil and sediment types on blueberry farms with different land-use histories. We identify pesticide, trace metal/metalloids, and nutrient contaminants of concern in recently established, rapidly expanding horticultural landscapes in the subtropical East coast of Australia. Data derived from our sampling efforts will further the knowledge of the extent of land contamination from productivist-mentality horticulture in Australia.
Materials and methods
Study site
Samples were collected from the Coffs Harbour region in northern New South Wales, Australia. For > 100 years, the predominant horticultural industry in the region was banana cultivation, with the epicentre being the town of Coffs Harbour (30.2986° S, 153.1094° E). The climate in this region is characterised by mild summer and winter temperatures with seasonal rains in spring and autumn. Banana plantations were developed amongst subtropical rainforest on nearly every steep, north-facing slope of the coastal Great Dividing mountain range.
After nearly a century of intense banana cultivation in the region, public health concerns surrounding the industry arose. In the 1980s, higher than average congenital disabilities and cancer rates were attributed to pesticide exposure from banana cultivation (Short 1994). Bloodborne concentrations of organochlorine (OC) pesticides in Coffs Harbour children were higher than other more populous cities in Australia and other nations (Budd 1995). Despite these observations, data on the extent of environmental contamination from nearly a century of banana cultivation are lacking. Conrad et al. (2017) reported sediment enrichments with copper (Cu) coinciding with the beginning of aerial spraying of fungicides on banana farms in 1956 (Hedditch 2014).
More recently, a large-scale land-use change from banana to blueberry farms occurred in Coffs Harbour (demonstrated in Fig. 1). Blueberry cultivation area doubled between 2010 and 2012 (Brazelton 2013), with a > 400% increase in blueberry farms in the Coffs Harbour region (White et al. 2018b). Due to a lack of strict regulations and reporting mechanisms of farm practices, many uncertainties remain regarding the environmental impact of blueberry cultivation. Recent studies identified high levels of dissolved N and heavy metals in waterways draining blueberry farms (White et al. 2018a, b; Conrad et al. 2020). The land-use change from banana to blueberry farming also increased sedimentation rates and trace metal and P sediment pollution in a downstream estuary (Conrad et al. 2019). Here, we sampled soil and dam sediment cores from blueberry farms in the Coffs Harbour region to better understand the extent of agricultural contamination nearer to their source of potential introduction. To our knowledge, these represent the first pesticide observations in soil samples since the expansion of the blueberry industry in this region.
Sample collection
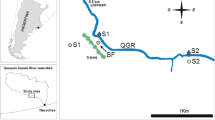
We sampled three farms (hereby referred to as Sites 1, 2, and 3). Sites 1 and 2 are located in the coastal Sandy Beach catchment (Fig. 2) and have a history of banana cultivation since the 1940s. Both sites have been growing blueberries since ~ 2002. Site 3 is located further inland in the Bucca Bucca Creek catchment (Fig. 2) with cattle grazing as the previous land use. A blueberry farm was constructed at Site 3 in 2013.
We sampled various locations within each site. NSW state regulations require that all agricultural chemicals are contained in a lockable indoor area (Browne 2016). Local farms typically have a shed that contains agricultural chemicals and a nearby area specially designated for the mixing of chemicals. Due to the increased chemical exposure at these ‘mixing sheds’, greater accumulation of contaminants is expected here. We took soil cores near chemical mix sheds at each site (hereby termed ‘mix shed’ cores). To assess soil quality in growing areas, we took soil cores from crop production areas (hereby termed ‘field’ cores). Lastly, at Site 2, we took a soil core from a steeply sloping ditch, which drained a mixing shed that was used previously during banana cultivation. This mixing shed has been out of use since 1980. All terrestrial soil cores were 30 cm in length and taken with a 10 cm diameter metal or PVC tube. We collected a total of 7 terrestrial soil cores between 4 and 6 March 2019 (Fig. 2). The terrestrial soil cores were sectioned into intervals from 0 to 7.5 cm, 7.5 to 15 cm, and 15 to 30 cm depths following the Environmental Protection Authority (EPA) protocol (EPA 1997). Each subsection was frozen directly after collection and then dried at 105 °C before analysis.
In addition to sampling terrestrial soils, we assessed sediment quality in retention reservoirs (hereby referred to as dams) that capture water for reuse on the farms. Construction of dams at Sites 1 and 2 was completed in 1976, while Site 3 dam was constructed in 2013. Dam bottom sediments were collected from each site (Fig. 2) on 4 March 2019 using a 6.8 cm inner diameter Aquatic Research Instruments Universal weighted gravity corer. Dam cores were collected from depths between 4.0 and 4.2 m. Maximum water depths of the dams were ~ 6 m. Dam sediments were extruded in 1 cm intervals using the provided extruding device from Aquatic Research Instruments. Sediments were frozen, freeze-dried, and weighed to obtain dry bulk density (DBD) before further analyses.
Contaminant analysis
Soil organochlorine (OC) pesticide content was determined by placing 10 g of sample into a tumbler bottle. Anhydrous sodium sulphate (Na2SO4) was added until the mixture was free-flowing. To assess soil matrix effects on contaminant recovery, each sample was spiked with dibromo-DDE universal pesticide surrogate. Next, 30 mL of 1:1 dichloromethane (DCM): acetone solution was added. Bottles were tumbled for 1 h. The resulting extract was concentrated under N2 gas to 1 mL and placed into a 1.5 mL vial. Samples were run on an Agilent 5975 Gas Chromatograph/Mass Selective Detector (GC/MSD). Limits of reporting (LOR) ranged from 0.05 to 0.2 mg kg−1. Blank (free of contaminant) samples of a similar soil matrix were performed before analysis. Reference material was spiked with 0.5 mg kg−1 of each target OC pesticide. Spike recoveries ranged from 77 to 108% recoveries. During analysis, a sample with a representative array of target analytes was split to assess potential matrix interference and homogeneity of samples. The sample was spiked with OC pesticides at contents between 0.5 and 2 mg kg−1. Spike recoveries ranged from 78.5 to 124% recovery.
Synthetic pyrethroids were extracted in the same manner as the OC pesticides. Samples were run on an Agilent 59,755 GC/MSD in selected ion monitoring (SIM) mode. LOR was 0.05 mg kg−1. Blanks and reference spikes were performed in the same manner as the OC pesticides. Spike recoveries ranged from 80.5 to 115% recoveries. Representative samples were split to test for homogeneity and matrix interference and spiked at contents of 0.5 mg kg−1. One analyte (bioresmethrin) had a recovery of 22.8% and was therefore discarded from the analysis. All other spike recoveries ranged from 81.4 to 115%.
To determine the organophosphate (OP) and carbamate pesticide, triazine, urea, chloroacetanilide herbicide, aminopyrimidine, benzimidazole, and conazole fungicide soil contents, 5 g of sample was placed into a 50 mL polypropylene centrifuge tube. Four millilitres of acetonitrile (ACN) was added, followed by 6 mL of methanol (MeOH). Centrifuge tubes were vigorously hand shaken and vortexed to mix contents. Tubes were then either sonicated for 15 min or tumbled for 1 h. Tubes were centrifuged at 3000 rpm for 5 min. Twenty-five microlitres of the supernatant was pipetted into a 10 mL glass tube containing 2 mL of Milli-Q water and 25 µL MeOH. Samples were briefly vortexed and filtered into 1.5 mL microcentrifuge tubes. Samples were run on an Applied Biosystems/MDS Sciex API 5000 Liquid Chromatography/mass spectrometry/mass spectrometry (LC/MSMS) with Qjet ion guide. LORs ranged from 0.001 to 1 mg kg−1. Blank samples of a similar soil matrix were run before analysis. Reference materials were spiked with standards to contents between 0.01 and 4 mg kg−1. Reference material spike recoveries ranged from 63.2 to 103%. Representative samples were split and spiked with contents of contaminants ranging from 0.01 mg kg−1 to 4 mg kg−1. Recoveries ranged from 72.8 to 102%.
All samples (terrestrial soil and dam sediment cores) were analysed for trace metals, metalloids, phosphorous, and other major ions using the methodology outlined in Conrad et al. (2019) and references therein. We used the Australian and New Zealand Environment Conservation Council (ANZECC) sediment quality guidelines (SQG) (Simpson et al. 2013) as threshold values to assess the extent of trace metal/metalloid contamination in dam sediments. The ANZECC SQG have two levels of trigger values: a default guideline value (DGV) and guideline value-high (GV-high). The DGV represents a concentration below which there is low probability of toxic effects. The GV-high represents a concentration above which there is a high probably of deleterious effects. Below GV-high values, toxic effects are still possible. Where no ANZECC SQG existed, we used values from the literature. SQG for selenium (Se) and P were obtained from Van Derveer and Canton (1997) and Persaud et al. (1993), respectively. To compare geologic and anthropogenic fractions of trace metals, metalloids, and phosphorus in dam sediments, enrichment factors were calculated using aluminium (Al) as the reference material as outlined in Conrad et al. (2017).
Due to lack of stringent agricultural soil quality guidelines, we adopted the Australian National Environment Protection Measures (NEPM) policy framework to develop ecological investigation levels (EIL) of contaminants in terrestrial soil (NEPC 2011e). The NEPM framework uses species specific distribution toxicity data to protect a predetermined percentage of terrestrial invertebrate, plant, and microbial species (percentage of species protected depends on land use) (NEPC 2011c). The NEPM classifications lack specific EILs for agricultural lands, but suggest adopting 80% species protection to ensure the preservation of essential soil processes (nutrient cycling, etc.) (NEPC 2011d). The urban and residential public space EIL values also protect 80% of species, and therefore, we adopted the EIL values for this land use as a surrogate for agricultural land use (NEPC 2011d). The EIL values correspond to the lowest observed effect concentrations (LOEC) and to 30% effective concentration (EC30) toxicity data (NEPC 2011b). Where the current 2011 version of the NEPM framework did not update the toxicity or bioavailability data to yield soil specific EIL values, we used the 1999 EIL values (NEPM 1999). Since the NEPM framework lacked toxicity guidelines for Co, we adopted the Co soil quality guidelines of Moen et al. (1986).
The EIL for Zn, Pb, and Cu developed using the 2011 NEPM framework are soil specific (NEPC 2011e) based upon soil pH and/or cationic exchange capacity (CEC). We used NSW eSPADE online soil database to survey nearby average soil pH values. Soil pH from samples on and nearby sites 1 and 2 was between 5.0 and 6.0 in the surface (0–22 cm), respectively, and 5.5–6.0 in the subsoil (eSPADE 2020). In samples near site 3, soil pH in topsoils (0–20 cm) and subsoils (20–30 cm) ranged from 5.0 to 6.0 (eSPADE 2020). To be conservative and adopt the highest degree of environmental protection, we derived an EIL using a pH of 5.0. We calculated base CEC by dividing the soil contents of Mg, K, Na, and Ca by their milliequivalents per 100 g (mEq 100 g−1) of soil. To calculate mEq 100 g−1, we calculated the gram equivalent weight of each ion by dividing the atomic mass by its valence charge. We then converted equivalents to mEq 100 g−1 of soil by multiplying by a factor of 10. Since we did not obtain buffer pH or NH3+ soil contents, our estimates of CEC do not include acidic cations. Therefore, our estimates of CEC are underestimated, resulting in a generation of a conservative EIL value. Mean base CEC in all terrestrial soil samples was 17.3 (sample specific CEC values in supplemental materials), but we rounded the CEC value to 20 to comply with the NEPM guidelines (NEPC 2011d).
The NEPM framework also provides health investigation levels (HIL) to investigate potential human health risks that may arise from chronic exposure to certain contaminants. HIL are intentionally conservative, and exceedances of HIL signify further appropriate investigation to fully evaluate human health risks from long-term soil exposure (NEPC 2011b). We opted to use the HIL-A values from NEPC (2011a) and NEPM (1999) when more recent HIL were not available. HIL-A values are guidelines for standard residential garden/accessible soils and home grown produce that comprises less than 10% of vegetable and fruit intake. We assumed the consumers fruit intake from produce of these farms would account for less than 10% of their diet. The HIL-A provide the lowest concentrations at which to investigate human health risks further.
Results
Terrestrial soil cores
Pesticides
We detected ten out of 97 analysed pesticides in the terrestrial soil cores (Table 1). By weight, 98% of the pesticide mass we detected occurred in the Site 3 mix shed core, with only small amounts of pesticides in the other cores. Ethoprophos and dimethoate were the OP pesticides detected. Ethoprophos was detected in low contents at the Sites 1 and 2 mix sheds and Site 2 drainage ditch (Table 1). No ethoprophos was detected at Site 3. Dimethoate was found exclusively at Site 3 mix shed in low contents. Methomyl content was the greatest along the deeper sediments of the Site 3 mix shed core (0.074, 0.011, and 0.089 mg kg−1 with increasing depth). Bifenthrin was present in relatively greater contents than the other pesticides. Bifenthrin in the Site 3 mix shed core followed a trend of decreasing content with depth (Table 1). Prometryn was detected in the surface interval of the Site 3 mix shed just above the detection limit (0.004 mg kg−1).
Fungicides were the most abundant pesticides detected. Propiconazole was found in mix shed and field cores at Sites 2 and 3. Propiconazole in the Site 3 mix shed had the highest content of any pesticide we detected (125 mg kg−1). Cyprodinil had the highest spatial distribution of any contaminant found in our testing, being detected in the surface intervals all mix shed and field cores (Table 1). Pyrimethanil contents were ≤ 0.10 mg kg−1 in Site 1 field core. Carbendazim contents were low in all mix shed cores and Site 3 field cores.
The aryl urea herbicide diuron was detected in small amounts in all Site 2 cores. Site 2 field core had diuron contents of 0.010 and 0.014 mg kg−1 at depths 7.5–15 and 15–30, respectively. Site 2 drainage ditch cores had diuron contents of 0.02 and 0.012 mg kg−1 in 0–7.5 and 7.5–15 cm, respectively.
Trace metals/metalloids and phosphorous
None of the soil As, Pb, Cd, Cr, Ni, Hg, or Co soil contents exceeded the EIL (Table 2). No soil contents from any sample exceeded the HIL. Eleven out of 12 samples from the Sites 1 and 2 mix shed and field cores exceeded the EIL for Mn. Mean Mn contents in the Sites 1 and 2 mix shed and Site 1 exceeded the EIL by two- to threefold. All site 3 soil samples were below the EIL for Mn. A sample at Site 2 was close to exceeding EIL and HIL for As (Table 2). Two samples from the Site 2 drainage ditch core would have exceeded the EIL for As if we used the 95% species protection (EIL value: 40 mg kg−1). Cu contents in the surface interval (100.3 mg kg−1) of Site 3 mix shed exceeded the EIL value of 85 mg kg−1. All samples of Zn from the Site 2 mix shed core exceeded the EIL (mean Zn content: 406 ± 117 mg kg−1). Site 1 mix shed had the highest mean P contents (1273 ± 507 mg kg−1). The surface contents here exceeded the EIL.
Dam sediment cores
The EFs of Pb, Cr, Cu, and Ni reflect natural variation (Zhang and Liu 2002), and their contents did not exceed the ANZECC DGV. In the Site 2 core, surface sediment (0–5.5 cm depth) As contents exceeded the ANZECC DGV of 20 mg kg−1 (Table 3). Moderate enrichments (2 < EF < 4) of As were observed in all cores (Fig. 4, Zhang and Liu 2002). At Site 2, Cd contents and EF were elevated from 2.5 to 5.5 cm depth (EF: 6–12 at these depths). No Cd contents exceeded the ANZECC DGV (Table 3). Due to the low Se contents in the bottom interval at Site 3, there was high EF throughout this core (Fig. 4). Despite the severe enrichments at Site 3, no Se contents exceeded the Se sediment quality guideline from Van Derveer and Canton (1997). There was no Zn enrichment, and no Zn contents in the dam cores exceeded the ANZECC DGV (Table 3). Mn contents were an order of magnitude greater at Site 2 (Table 3). Mn was enriched in surface sediments of Site 3 by a factor of ~ 9 (Fig. 4). Three samples from Site 1, six from Site 2, and four from Site 3 exceeded the DGV for Hg. The highest Hg contents were at the bottom intervals of the Site 2 core (Fig. 3) and exceeded ANZECC DGV (Table 3).
Mean phosphorus (P) contents exceeded our proxy sediment quality guidelines (from Persaud et al. 1993) at Site 2 (Table 3) and were moderately enriched (EF ~ 4, Fig. 4). At Site 1, P contents increased ~ fivefold from 2.5 cm to the surface (~ 50–250 mg kg−1) and were moderate to severely enriched (EF ~ 4–10). P contents in the surface interval at Site 3 (2161 mg kg−1) exceeded the SQG high value (2000 mg kg−1). Due to the relatively high Al contents in this layer (1.54%), there was only moderate enrichment of P (EF ~ 4), despite the high P contents.
Discussion
Pesticide residues in terrestrial cores
Reports of blueberry fungal outbreak were greater in subtropical than in temperate regions of Australia (Waters et al. 2008), which may explain why the highest contents of all pesticides were fungicides in the Site 3 mix shed core (propiconazole and cyprodinil). However, complications with fungus may differ at microclimate scales. Different fungal problems persist in blueberries at inland and coastal farms in this region (personal communication with landowners). Proximity to the coast positively influences blueberry water uptake and daily growing period (Hunt et al. 2009), which may allow improved fungal resilience at coastal farms. Site 3 does not receive a sea breeze, as do Sites 1 and 2. Seasonal droughts that persist inland and constant reception of salt spray at coastal locations may be responsible differences in fungal communities between coastal and inland locations (Lowry et al. 2008). Therefore, these small-scale environmental regimes may drive environmental contaminant distribution.
Chemical mixing sheds seem to be hot spots for pesticide accumulation and dispersal. 64% of all detected residues were found in mixing sheds, and four pesticides (bifenthrin, dimethoate, methomyl, and prometryn) were found exclusively in the mixing sheds. Further sampling of mixing sheds may reveal contamination on other farms in this region. Because pesticides do not occur naturally, their detection signifies that horticultural activities have altered soil composition. However, differences in degradation rates, toxicity, and mobility within the environment (discussed below) will create variable concerns over the environmental impacts of each pesticide. More widespread, repeated sampling of growth and other use areas will lead to an improved understanding of the spatial extent, the impact of degradation products, and half-lives of pesticides from horticulture in this region.
Fungicides
Propiconazole found in the soils of the Site 3 mix shed represented the highest pesticide content detected (mean contents 58.2 ± 38.9 mg kg−1). At contents of 100 mg kg−1, propiconazole reduces nitrogen mineralisation (Vašíčková et al. 2019; Authority et al. 2017) and agricultural soil phosphatase activity by 46%, decreasing phosphate availability to plants (Kalam et al. 2004). Propiconazole content in the surface interval of the Site 3 mix shed (125 mg kg−1) exceeded this threshold. If propiconazole contents in the horticultural areas reach levels similar to in the Site 3 mix shed, the uptake of nutrients by the crops may become reduced. Propiconazole contents that we observed were greater than the no observed effect concentrations for annelid and arthropod species (Vašíčková et al. 2019), meaning soil invertebrate health at Site 3 may be negatively affected. Propiconazole contents in Site 3 mix shed were higher than observations from corn and soybean fields from the Midwestern United States, while our field cores had orders of magnitude lower or similar contents depending on the timing of propiconazole application (Edwards et al. 2016).
The fungicide cyprodinil was the most widespread of all pesticides, found in trace amounts in mix shed and field cores at sites 1 and 2, and significant levels at Site 3. The highest contents of cyprodinil (in the Site 3 mix shed) were above the 50% effective concentration (EC50, 7.75 mg kg−1) which reduced non-target macrophyte growth by 50% (Azimonti et al. 2015). Cyprodinil contents were reduced in the Site 3 field core, signifying deleterious effects in crop production areas are not likely; however, long-term contamination of mix shed land is possible as > 75% of cyprodinil applications can be irreversibly bound to soil organic material (Arias et al. 2005). As expected, we would not find cyprodinil in the Site 2 drainage ditch because the area upstream has been discontinued as a chemical storage/mixing area since 1980 (personal communication with landowner) and cyprodinil was introduced to the market in 1998 (USEPA 1998). Cyprodinil was detected at higher contents in field cores than mix shed cores at Sites 1 and 2 (Table 1), perhaps indicating an additional mixing area or increased accumulation in growing areas due to repeated applications. Bermúdez-Couso et al. (2007) reported surface sediment contents of cyprodinil at 0.462 mg kg−1 in a Spanish vineyard, orders of magnitude lower than the mean contents found in the Site 3 mix shed core (8.5 mg kg−1), but elevated compared other soil contents (see Table 1). Cyprodinil contents may decrease via chemical degradation over 3–4 months (Authority 2006); however, the toxicity of the breakdown products can be greater than the parent products (Nicol et al. 2016). Isolated contents which may inhibit plant growth, the widespread spatial distribution across our study sites, and the toxic breakdown products make cyprodinil a pesticide to monitor in the future.
Two other fungicides, pyrimethanil and carbendazim, were found in low contents (0.006–0.044 mg kg−1) at all three sites. These fungicides degrade into their by-products within 4 weeks (Agüera et al. 2000; Baude et al. 1974). Carbendazim detection may have resulted from direct application to blueberries or from the degradation of benomyl (Baude et al. 1974), a fungicide previously used in banana cultivation, but discontinued since 2006 (APVMA 2020). Pyrimethanil degradation products have low toxicity (Sirtori et al. 2012) but may leach into subsurface water under typical field conditions (Komárek et al. 2010).
Propiconazole, cyprodinil, and carbendazim bind to organic and clay particles and are generally immobile in soil (Thorstensen et al. 2001; Komárek et al. 2010; Ahmad 2018). Erosion could deliver soil-bound fungicides into nearby waterways. Erosion of soils from the Site 3 mix shed is of particular concern due to the high contents of fungicides and the lack of connectivity into the dam at Site 3 (Fig. 2). Soils eroded from the Site 3 mix shed may therefore be transported into a nearby natural waterway (Bucca Bucca Creek).
Herbicides
Diuron is a water-soluble aryl urea herbicide commonly used in Australia, with regulations regarding timing and concentrations of application (APVMA 2012). To mitigate aquatic ecosystem harm from diuron runoff, the Australian Pesticides and Veterinary Medicines Authority (APVMA) regulated diuron use to a general use concentration of 450 g of active constituent ha−1 (Holmes 2014; APVMA 2012). However, according to the APVMA, there is no approval for the use of diuron on blueberries. Diuron at Site 2 may be remnant from previous land use as a banana farm. Alternatively, farmers in this region may still be applying diuron on blueberry farms following long-established habits practised during decades of banana cultivation.
Our observed soil diuron contents of 0.01–0.02 mg kg−1 were an order of magnitude below soil invertebrate toxicity values (0.7 mg kg−1 EC10, Campiche et al. 2006) and lower than what was reported in other soils. Soil diuron contents in grass seed production areas in the Northwestern United States were 0.38 mg kg−1, estimated to be 46% of the applied diuron (Field et al. 2003). A study of Queensland sugarcane soils found that 267 days after diuron application, the surface soil (0–15 cm depth) content was 0.009 mg kg−1 (Stork et al. 2008). These diuron contents were slightly lower than our 0–15 cm soil contents (0.010–0.020 mg kg−1) from Site 2 mix shed and drainage ditch cores, possibly indicating the application of diuron at Site 2 over the last year. Stork et al. (2008) estimated their observed diuron contents to be equivalent to 22 g of diuron applied ha−1 year−1 based upon assumptions of time since application, soil density, and depth. This application rate estimate falls well below the 2012 AVPMA diuron general use guideline of 450 g ha−1, possibly signifying diuron application rates at Site 2 could be similar. Our low, but detectable, soil diuron content and the pesticide’s water solubility may indicate that it has leached to waterways, depleting the soil contents (Yang et al. 2006; Stork et al. 2008; Liu et al. 2010). Long soil retention times (Moncada 2004) and its potential to be transported long distances in surface waters (Sakugawa et al. 2010) lead us to suggest monitoring of diuron concentrations in the aquatic environment, especially after rain events (Conrad et al. 2020).
Another herbicide, prometryn, was detected in the surface interval of the Site 3 mix shed core (0.004 mg kg−1). Prometryn photodegrades rapidly in the aqueous phase, especially with UV light (Jiang et al. 2017). Prometryn is typically applied wet; therefore, prometryn may quickly degrade in sunlight before accumulation in soils occurs. We detected one residue of prometryn just above the limit of detection. Prometryn use is not likely widespread in blueberry farms in this region.
Organophosphates
Ethoprophos in the Site 2 drainage ditch core may have originated from previous land use. Ethoprophos was a common pesticide used in banana cultivation (Collins et al. 1991). Repeated applications of ethoprophos promote an increased rate of biodegradation by soil microbes, albeit with a reduction of the desired pest control effect (Smelt et al. 1987). Populations of some target pest species in this region are resistant to ethoprophos treatments (Collins et al. 1991). The combination of increased biodegradation rates after repeated exposure and lowering of pest control effect may lead to more frequent, less efficient applications of ethoprophos.
Our observed ethoprophos contents are low when compared to the literature, possibly due to rapid biodegradation occurring after repeated treatments. The ethoprophos contents we observed are below NOEC concentrations for sensitive invertebrate species (Leitão et al. 2014). Smelt et al. (1987) found mean contents of 1.22 mg kg−1 in the top 25 cm of Dutch potato fields (compared to mean contents of 0.0085 mg kg−1 from our cores). Ethoprophos residues disappeared completely below 25 cm depth after 474 days (Boesten and van der Pas 2000; Boesten and Gottesbüren 2000). Data on application and degradation rates of ethoprophos are not available but would allow for more efficient ethoprophos use on blueberry farms in northern NSW.
Dimethoate was present in the Site 3 mix shed core at all depths. Dimethoate has low soil persistence and is water-soluble, with risk for runoff into surface and groundwaters (Van Scoy et al. 2016). Degradation rates of dimethoate in soil vary, from between 4 days to over 2 years, depending on soil organic material content (El Beit et al. 1981; Martikainen 1996; Bohn 1964). Runoff into groundwater or rapid degradation may explain the low soil contents observed. The soil contents we observed were not great enough to indicate obvious toxicity. The literature reports contents between 7 and 9 mg kg−1 cause mortality in soil invertebrates, while 3–4.5 mg kg−1 was sufficient to reduce invertebrate biomass (Martikainen 1996; Owojori et al. 2019). Daily dimethoate intake between 2 and 18 mg kg−1 is safe for human consumption (Sanderson and Edson 1964). Soil dimethoate was only found in the mixing shed of Site 3, meaning it may not persist for long periods in the growing areas, thus not permitting significant dimethoate assimilation into the food chain.
Other pesticides
Site 3 mix shed was the only area where we detected bifenthrin. Bifenthrin has low water solubility and binds strongly to organic matter; therefore, it is immobile in the soil column, has low groundwater leaching potential, and can persist in the soil for years (Kamble and Saran 2005). While bifenthrin contents were greatest in the surface sediments of the Site 3 mix shed, we detected residues, albeit decreasing, in the lower sediment intervals of the Site 3 mix shed core. It is unclear if soil bifenthrin contents are from a single application or exist as residues from repeated applications. Bifenthrin application rates between 10 and 15 mg kg−1 (11.4 mg kg−1 in Site 3 surface soils) did not reduce mycorrhizal colonisation in corn plants (Corkidi et al. 2009). However, the effect of bifenthrin on fungal recruitment in blueberry plants may differ due to the ericoid mode of mycorrhizal symbiosis in blueberries. While detrimental effects to fungal root communities are not expected, further research can confidently ascertain bifenthrin’s effects on blueberry plant symbiotic relationships. Our detected soil contents of bifenthrin are lower than other studies. Soils from potato fields of western Canada had mean soil bifenthrin contents of 872.25 + 62.98 mg kg−1 329 days after application, equivalent to 35% of the field application rate of 349 g active ingredient ha−1 (van Herk et al. 2013). While the application rate of bifenthrin is unknown, our soil bifenthrin contents were an order of magnitude lower than reported by van Herk et al. (2013).
Contents of methomyl were the greatest in the bottom (15–30 cm) layer of the Site 3 mix shed core. This result may be demonstrative of mobility in the soil column due to methomyl’s high water solubility and low soil retention affinity (Van Scoy et al. 2013). Degradation of methomyl from field soils is typically < 1 month and occurs via microbial breakdown (Van Scoy et al. 2013; Harvey Jr and Pease 1973). Reported methomyl soil contents from same-day applications were 1.272 ± 0.1 in soils of tomato plants (Malhat et al. 2015) and 0.025–0.035 mg kg−1 in sandy loam soils (Bisht et al. 2015). Methomyl disappeared within 15 days after application in both of these studies. Our soil contents of methomyl are within this range (mean contents 0.058 mg kg−1). Our results suggest recent environmental exposure to methomyl at the Site 3 mix shed. More sampling is needed to investigate the degradation rates and leaching potential of methomyl in our blueberry horticultural setting.
Trace metals/metalloids and phosphorus
High contents of As in the surface sediments of the site 2 dam may be from recently deposited eroded soils containing As, or result from the re-precipitation of As released via reductive dissolution with Fe species from deeper in the sediment column (Arsic et al. 2018; Sun et al. 2016). Content of As in the terrestrial soils did not exceed the EIL for 80% species protection, or the HIL (both 100 mg kg−1). However, As in the Site 2 drainage ditch core was just under this concentration (99.2 mg kg−1 in 7.5–15 cm). The NEPM guidelines suggest adopting 95% species protection to protect crop species (NEPC 2011d). We did not use this level of protection as there was no obvious phytotoxicity occurring on the blueberry plants at the time of sampling. Instead, we adopted 80% species protection which is designed to preserve soil nutrient recycling processes essential to optimal growth (NEPC 2011d). If health of the blueberry plants deteriorates, the 95% species protection EIL may need to be adopted. The As contents at the surface of the Site 2 drainage ditch would exceed this 95% protection EIL (> 40 mg kg−1).
We presume that the relatively high As contents observed in soils and dam sediments at Site 2 are remnant from As pesticides used in livestock and banana cultivation as previously noted in this region (Smith et al. 1998, 2003). The highest As contents were observed in the steeply sloping drainage ditch, probably due to its location downstream of the chemical mixing shed used during times of banana cultivation. Arsenic contamination is apparent in the soils from previous land use, even over 15 years after the change in the horticultural regime at Site 2. If plant health or productivity becomes compromised at Site 2, As should be considered a potential toxicant due to the elevated contents and evidence of mobility through the terrestrial and aquatic environments.
High levels of P at the chemical mixing sheds likely evidence prolonged fertilizer exposure at these locations. High levels of P in terrestrial soils (such as what we observed in the site 1 mix shed) could be expected in a long-term horticultural setting (Mishra et al. 2017). Once the clay-rich kandosol soils of this region saturate with phosphate ions, P availability in the soil and leeching to ground or surface waters will increase (Muukkonen et al. 2007), although P in the terrestrial environment at Site 1 does not appear to have contaminated dam sediments as is evident at Sites 2 and 3 (DGV exceeded in dam cores at these locations and GV-high exceeded at Site 3 surface sediments). Increased P accumulation in these sediments is likely due to the deposition of nearby eroded fertilised soils (Conrad et al. 2019). Content of P in the surface sediment of the Site 3 dam core exceeded the SQG high range value and was ninefold higher than the sediment below. The high P contents in the top sediment layer likely indicate rapid P accumulation after blueberry cultivation began in 2013. Elevated P in the sediments can cause eutrophication even after extraneous inputs are ceased, due to persistent diffusion of sediment P to the water column (Carpenter 2005). P inputs after surface runoff and dam overflow events were sufficient to invert limiting nutrients and cause algal blooms in a natural waterway downstream of Sites 1 and 2 (White et al. 2018a).
Isolated contamination of Zn and Cu (Sites 2 and 3 mix shed, respectively) is probably introduced to soils from agricultural treatment products. P fertilisers can contain Zn impurities that can accumulate to undesirable levels in the environment after persistent applications (Nziguheba and Smolders 2008). Elevated Cu in surface sediments may be from Cu containing fungicides used on blueberry farms (Simpson 2019). Surface sediments at Site 3 also had high contents of other fungicides (propiconazole and cyprodinil, Table 1). Background values of Zn and Cu from regional soils need to be established to confidently determine the toxicity of the added concentrations of these contaminants (NEPC 2011e). While we lack exact knowledge of background concentrations of these metals, it is likely observed Zn contents were within the added concentration range that caused Zn toxicity in wheat crops of Australia (Warne et al. 2008). Despite Cu concentrations exceeding the EIL, our total Cu concentrations were less than added Cu concentrations causing wheat toxicity in Australian soils (Warne et al. 2008). Higher spatial resolution sampling of farm soils and crop biomass (similar to Brennan 2001 and/or Warne et al. 2008) is necessary to reveal if Zn and Cu have accumulated to levels detrimental to plant health or soil processes in the growing areas. Zn and Cu are doubtful to be a risk to human health at this time as contents were orders of magnitude below HIL.
While Cd did not exceed any of our adopted guidelines, there is reason to further investigate Cd transport in this horticultural industry. Cd is typically introduced to the soil environment as a fertiliser impurity (Mortvedt and Osborn 1982). Moderate-to-severe enrichments in dam sediments at Sites 1 and 2 demonstrate mobility of Cd from farm soils to the aquatic environment. Health risk of Cd should be further assessed due to its unique bioaccumulative nature. Cd can exceed health guidelines in food at soil contents < 1 mg kg−1 (Jinadasa et al. 1997; McLaughlin et al. 1994). Unlike other metals/metalloids (As, Cu, Cr, Hg, Ni, Pb, and Zn), Cd may reach levels of concern in food products before phytotoxicity is observed. Tests of Cd contents in fertilisers and blueberry plants need to be undertaken to exclude potential Cd toxicity.
Contents of Mn similar to what we observed, especially in the Site 1 and 2 field cores, caused lethal and sublethal effects in regenerative (wheat and grass) species used to remediate contaminated horticultural land (Paschke et al. 2005). Mn in the slightly acidic (pH 5.0–6.0) and anaerobic regions of the thick clay soils at these sites may have increased bioavailability (Paschke et al. 2005), which could, depending on redox state of Mn, lead to increased uptake by the roots and inhibiting the uptake of Ca and Mg, potentially reducing blueberry growth (Langheinrich et al. 1992). If horticulture is abandoned at these sites, Mn contamination may cause mortality or inhibit growth in potential restorative grass species (Paschke et al. 2005). Mn accumulation in the crop production areas of Site 1 and 2 is probably from organic fertiliser soil amendments applied during decades of horticulture. If Mn toxicity to blueberries becomes a concern, farmers should consider growing the Mn-resistant Legacy or Brigitta highbush blueberry (Vaccinium corymbosum) cultivars (Millaleo et al. 2019). Due to relatively high soil contents and uncertainties in the spatial extent and biotic effects of Mn contamination, we suggest further research on Mn uptake into blueberry and native vegetation and Mn speciation in legacy horticultural soils.
Dam cores contained 13 sediment subsamples that exceeded the DGV for Hg. The elevated Hg in dam bottom sediments, especially at Site 2 (Fig. 3), could be from atmospheric Hg deposition during dry periods or episodic rain events (Shanley and Bishop 2012; Conrad et al. 2020). Mercury stored in the horticultural soils (from fungicides or fertiliser impurities, AVPMA 2020) may also leach or erode with soils and flocculate to dam sediments with organic material or sulphides (Belzile et al. 2008). Due to the high clay content soils of this region, soil saturation causes overland runoff and flushing of chemicals from the shallow root zone of soils when rainfall exceeds ~ 30 mm in 24 h (White et al. 2020). Dams at sites 1 and 3 are intersected by first order streams and may receive eroded soils and surface runoff from the growing areas. Dams at Sites 1 and 2 are supplied with treated sewage wastewater which is pumped throughout the farms for irrigation. The recycled wastewater has been shown to be a significant source of Hg and other trace metals in this area (Conrad et al. 2021).
The Hg profile in dam sediments suggests decreasing Hg deposition in the more recent sediments (Outridge and Wang 2015). Hg is mostly immobile in sediments, and profiles of Hg in sediments typically follow patterns of deposition (Lockhart et al. 2000; Rydberg et al. 2008). Bioactive methyl-Hg production in the anoxic Hg-rich layer may be low due to loss via diagenetic processes (Rydberg et al. 2008). Terrestrial soils had Hg contents below or within natural ranges reported in the literature (50–350 µg kg−1, Grigal 2003; Rundgren et al. 1992).
Contaminants of concern
To identify an overall concern for each specific contaminant, we used multiple lines of evidence based on pesticide and metal/metalloid toxicity, the magnitude of soil/sediment contents (compared to toxicity data or environmental guidelines), spatial distribution among our study sites, as well as persistence and mobility within the soil and sediment environments. We generated a rating of overall concern (Table 4). The conclusions presented in Table 4 represent contaminants of environmental concern. Only As was present in levels that can indicate possible human health risks. Pesticide toxicity was qualified based on comparing our observed soil contents to toxicity data from the literature. However, due to their inherent aspects of toxicity and xenobiotic nature, we believe that the detection of any pesticide represents a potential risk. Data limitations, as well as uncertainties and conflicts in the literature, have prevented us from confidently qualifying the overall concern for some pesticides. For instance, the literature reports variable degradation rates and solubility/desorption behaviours for cyprodinil, dimethoate, and propiconazole (for example, cyprodinil: Ahmad 2018 and Rhodes and Long 1974). This highlights our limited knowledge about the degradation and transport of pesticides and the need for further research in order to refine our risk assessment framework.
Overall, the contaminants of the greatest concern were cyprodinil, Hg, and P (Table 4). Undetected pesticides were classified as uncertain, as their absence from our samples does not signify their absence from other horticultural landscapes in the region. We classified propiconazole and diuron concern as moderate (due to contents likely to interrupt soil nutrient recycling processes and mobility into the aquatic phase). Contaminations of As, Mn, Cd, and Zn were localised and were not clearly present in levels hazardous to human health. For this reason, we classified As, Mn, Cd, and Zn concern as moderate. These elements were enriched in sediments downstream of Sites 1 and 2, suggesting their mobility across terrestrial and aquatic environments (Conrad et al. 2019). More extensive sampling will reveal the full extent of trace metal, metalloid, and pesticide contamination.
Conclusions
This work identified pesticides associated with blueberry and banana horticultural practices in subtropical northern NSW, Australia. Results highlighted that:
-
(1)
On-farm dams may provide a valuable contaminant retention service. Our data indicated dams are an efficient sink for As, P, and Hg. Dam cores from all three sites contained high contents of these elements relative to terrestrial soils and/or SQG and displayed various degrees of enrichment with As and P. Anoxic dam sediments seemed to be the predominant Hg sink.
-
(2)
Land previously used for banana cultivation had severe As contamination, likely from the historical use of As pesticides on banana farms in this region. Land historically used for cultivating bananas should be surveyed to assess if exposure to soil contents is hazardous to farm workers or accumulating in food products.
-
(3)
Chemical mixing sheds were sites of increased contaminant accumulation and need special attention during their construction and when designing contaminant monitoring programs.
-
(4)
The fungicide cyprodinil, Hg, and P were contaminants of greatest concern based upon their relatively high soil/sediment contents, extensive distributions amongst our study sites, potential for negative effects on biota, long environmental retention times, and mobility between the terrestrial/aquatic environment.
Although we collected 79 soil and sediment samples that were analysed for over 110 trace metals and pesticides, our sampling regime was restricted spatially. To build confidence in broadly identifying horticultural contaminants of regional concern, we recommend more spatially and temporally extensive sampling of farm soils and adjacent drainage basins.
Availability of data and material
Data are available in the manuscript or as supplementary material.
References
ABGA. (2018). Australian Blueberry Industry Strategic Investment Plan 2018–2022. In A. B. G. Association (Ed.), (pp. 1–16). Lindendale, NSW, Australia: Australian Blueberry Growers’ Association.
Agüera, A., Almansa, E., Tejedor, A., Fernández-Alba, A. R., Malato, S., & Maldonado, M. I. (2000). Photocatalytic pilot scale degradation study of pyrimethanil and of its main degradation products in waters by means of solid-phase extraction followed by gas and liquid chromatography with mass spectrometry detection. Environmental Science and Technology, 34(8), 1563–1571. https://doi.org/10.1021/es990112u.
Ahmad, K. S. (2018). Sorption-desorption characteristics of benzimidazole based fungicide benomyl on physicochemical properties of selected Pakistani soils and their minerals. Biological Sciences-PJSIR, 61(2), 59–67.
APVMA. (2012). Diuron final review report. In A. P. a. V. M. Authority (Ed.), (pp. 1–112). Canberra, ACT, Australia.
APVMA. (2020). AVPMA Gazette No. 12. In A. P. a. V. M. Authority (Ed.), (Vol. 25, p. 25). Sydney, NSW: Commonwealth of Australia.
Argent, N. (2002). From pillar to post? In search of the post-productivist countryside in Australia. Australian Geographer, 33(1), 97–114.
Arias, M., Torrente, A., López, E., Soto, B., & Simal-Gándara, J. (2005). Adsorption–desorption dynamics of cyprodinil and fludioxonil in vineyard soils. Journal of Agricultural and Food Chemistry, 53(14), 5675–5681.
Arsic, M., Teasdale, P. R., Welsh, D. T., Johnston, S. G., Burton, E. D., Hockmann, K., et al. (2018). Diffusive gradients in thin films reveals differences in antimony and arsenic mobility in a contaminated wetland sediment during an oxic-anoxic transition. Environmental Science and Technology, 52(3), 1118–1127.
Authority, E. F. S., Arena, M., Auteri, D., Barmaz, S., Bellisai, G., Brancato, A., et al. (2017). Peer review of the pesticide risk assessment of the active substance propiconazole. Efsa Journal, 15(7), e04887.
Authority, E. F. S. (2006). Conclusion regarding the peer review of the pesticide risk assessment of the active substance cyprodinil. Efsa Journal, 4(1), 51r.
Azimonti, et al. (2015). Comparison of NOEC values to EC10/EC20 values, including confidence intervals, in aquatic and terrestrial ecotoxicological risk assessment. EFSA Supporting Publications, 12(12), 906E.
Baude, F. J., Pease, H. L., & Holt, R. F. (1974). Fate of benomyl on field soil and turf. Journal of Agricultural and Food Chemistry, 22(3), 413–418. https://doi.org/10.1021/jf60193a051.
Belzile, N., Lang, C.-Y., Chen, Y.-W., & Wang, M. (2008). The competitive role of organic carbon and dissolved sulfide in controlling the distribution of mercury in freshwater lake sediments. Science of the Total Environment, 405(1), 226–238. https://doi.org/10.1016/j.scitotenv.2008.06.034.
Bennett, E. M., Carpenter, S. R., & Caraco, N. F. (2001). Human Impact on Erodable Phosphorus and Eutrophication: A Global Perspective: Increasing accumulation of phosphorus in soil threatens rivers, lakes, and coastal oceans with eutrophication. AIBS Bulletin, 51(3), 227–234.
Bermúdez-Couso, A., Arias-Estévez, M., Nóvoa-Muñoz, J., López-Periago, E., Soto-González, B., & Simal-Gándara, J. (2007). Seasonal distributions of fungicides in soils and sediments of a small river basin partially devoted to vineyards. Water Research, 41(19), 4515–4525.
Bisht, S., Chauhan, R., Kumari, B., & Singh, R. (2015). Fate of thiodicarb and its metabolite methomyl in sandy loam soil under laboratory conditions. Environmental Monitoring and Assessment, 187(7), 429. https://doi.org/10.1007/s10661-015-4640-1.
Boesten, J. J. T. I., & Gottesbüren, B. (2000). Testing PESTLA using two modellers for bentazone and ethoprophos in a sandy soil. Agricultural Water Management, 44(1), 283–305. https://doi.org/10.1016/S0378-3774(99)00096-7.
Boesten, J. J. T. I., & van der Pas, L. J. T. (2000). Movement of water, bromide and the pesticides ethoprophos and bentazone in a sandy soil: The Vredepeel data set. Agricultural Water Management, 44(1), 21–42. https://doi.org/10.1016/S0378-3774(99)00082-7.
Bohn, W. (1964). The disappearance of dimethoate from soil. Journal of Economic Entomology, 57(6), 798–799.
Brazelton, C. (2013). World blueberry acreage & production. North American Blueberry Council. Available online: http://www.chilealimentos.com/2013/phocadownload/Aprocesados_congelados/nabc_2012-world-blueberry-acreage-production.pdf. Accessed 4 May 2015.
Brennan, R. (2001). Residual value of zinc fertiliser for production of wheat. Australian Journal of Experimental Agriculture, 41(4), 541–547.
Brodie, J. E., & Mitchell, A. W. (2005). Nutrients in Australian tropical rivers: Changes with agricultural development and implications for receiving environments. Marine & Freshwater Research, 56(3), 279–302. https://doi.org/10.1071/MF04081.
Browne, B. (2016). Storing pesticides safely on farm. In D. o. P. Industries (Ed.), (pp. 1–4). NSW, Australia: NSW DPI.
Budd, L. (1995). Toxic playground: Reducing the toxic chemica load on schools and childcare centres. In Children’s Health and Chemicals Total Environment Center Sydney, NSW, Australia, 1995 (Conference Proceedings ed., pp. 4–7).
Campiche, S., Becker-van Slooten, K., Ridreau, C., & Tarradellas, J. (2006). Effects of insect growth regulators on the nontarget soil arthropod Folsomia candida (Collembola). Ecotoxicology and Environmental Safety, 63(2), 216–225. https://doi.org/10.1016/j.ecoenv.2005.07.004.
Carpenter, S. R. (2005). Eutrophication of aquatic ecosystems: Bistability and soil phosphorus. Proceedings of the National Academy of Sciences, 102(29), 10002–10005.
Collins, P. J., Treverrow, N. L., & Lambkin, T. M. (1991). Organophosphorus insecticide resistance and its management in the banana weevil borer, Cosmopolites sordidus (Germar) (Coleoptera: Curculionidae), Australia. Crop Protection, 10(3), 215–221. https://doi.org/10.1016/0261-2194(91)90046-T.
Conrad, S. R., Santos, I. R., Brown, D. R., Sanders, L. M., van Santen, M. L., & Sanders, C. J. (2017). Mangrove sediments reveal records of development during the previous century (Coffs Creek estuary, Australia). Marine Pollution Bulletin, 122(1–2), 441–445.
Conrad, S. R., Santos, I. R., White, S. A., Hessey, S., & Sanders, C. J. (2020). Elevated dissolved heavy metal discharge following rainfall downstream of intensive horticulture. Applied Geochemistry, 113, 104490. https://doi.org/10.1016/j.apgeochem.2019.104490.
Conrad, S. R., Santos, I. R., White, S., & Sanders, C. J. (2019a). Nutrient and Trace Metal Fluxes into Estuarine Sediments Linked to Historical and Expanding Agricultural Activity (Hearnes Lake, Australia). Estuaries and Coasts, 42(4), 944–957. https://doi.org/10.1007/s12237-019-00541-1.
Conrad, S. R., Santos, I. R., White, S., & Sanders, C. J. (2019). Nutrient and trace metal fluxes into estuarine sediments linked to historical and expanding agricultural activity (Hearnes Lake, Australia). Estuaries and Coasts, 1–14.
Conrad, S. R., Santos, I. R., S.A., W., Woodrow, R., & Sanders, C. J. (2021). Cryptic night-time trace metal and metalloid contamination in an intensively cultivated coastal catchment. [Full Research Article]. Environmental Pollution, In Press (In Press).
Corkidi, L., Bohn, J., & Evans, M. (2009). Effects of bifenthrin on mycorrhizal colonization and growth of corn. HortTechnology, 19(4), 809–812.
Dibden, J., Potter, C., & Cocklin, C. (2009). Contesting the neoliberal project for agriculture: Productivist and multifunctional trajectories in the European Union and Australia. Journal of Rural Studies, 25(3), 299–308. https://doi.org/10.1016/j.jrurstud.2008.12.003.
Doughty, C. C., Adams, E. B., & Martin, L. W. (1988). Highbush blueberry production in Washington and Oregon. PNW-Pacific Northwest Extension Publication, Washington, Oregon, and Idaho State Universities, Cooperative Extension Service (USA).
Edwards, P. G., Murphy, T. M., & Lydy, M. J. (2016). Fate and transport of agriculturally applied fungicidal compounds, azoxystrobin and propiconazole. Chemosphere, 146, 450–457. https://doi.org/10.1016/j.chemosphere.2015.11.116.
El Beit, I. O. D., Verner Wheelock, J., & Cotton, D. E. (1981). Factors affecting soil residues of dieldrin, endosulfan, γ-HCH, dimethoate, and pyrolan. Ecotoxicology and Environmental Safety, 5(2), 135–160. https://doi.org/10.1016/0147-6513(81)90030-0.
EPA, N. (1997). Guidelines for assessing banana plantation sites. In E. P. Authoritiy (Ed.), (Vol. 1–27). Sydney, NSW: NSW Environmental Protection Authority.
eSpade version 2.1. (2020). New South Wales Department of Planning, Industry and Environment. https://www.environment.nsw.gov.au/eSpade2WebApp#. Accessed September 2020.
Fang, W., Jiang, X., Bian, Y.-R., Yao, F.-X., Gao, H.-J., Yu, G.-F., et al. (2007). Organochlorine pesticides in soils under different land usage in the Taihu Lake region, China. Journal of Environmental Sciences, 19(5), 584–590.
Field, J. A., Reed, R. L., Sawyer, T. E., Griffith, S. M., & Wigington, P. (2003). Diuron occurrence and distribution in soil and surface and ground water associated with grass seed production. Journal of Environmental Quality, 32(1), 171–179.
Grigal, D. (2003). Mercury sequestration in forests and peatlands. Journal of Environmental Quality, 32(2), 393–405.
Harvey, J., Jr., & Pease, H. L. (1973). Decomposition of methomyl in soil. Journal of Agricultural and Food Chemistry, 21(5), 784–786.
Haynes, R. J., & Naidu, R. (1998). Influence of lime, fertilizer and manure applications on soil organic matter content and soil physical conditions: A review. Nutrient Cycling in Agroecosystems, 51(2), 123–137. https://doi.org/10.1023/a:1009738307837.
He, Z. L., Yang, X. E., & Stoffella, P. J. (2005). Trace elements in agroecosystems and impacts on the environment. Journal of Trace Elements in Medicine and Biology, 19(2), 125–140. https://doi.org/10.1016/j.jtemb.2005.02.010.
Hedditch, R. (2014). Coffs Harbour 2014 Heritage Study.
Hernández, A., Gil, F., Pla, A., Gómez, A., Lozano, D., Parrón, T., et al. (2011). Emerging human health concerns from chronic exposure to pesticide mixtures. Toxicology Letters (205), S4–S5.
Holmes, G. (2014). Australia’s pesticide environmental risk assessment failure: The case of diuron and sugarcane. Marine Pollution Bulletin, 88(1), 7–13. https://doi.org/10.1016/j.marpolbul.2014.08.007.
Hunt, J., Honeycutt, C., Starr, G., & Yarborough, D. (2009). Influence of Coastal Proximity on Evapotranspiration Rates and Crop Coefficients of Maine Lowbush Blueberry (Vaccinium angustifolium). International Journal of Fruit Science, 9(4), 323–343.
Jiang, C., Li, X. J., Wang, Y. R., Ma, L. Y., Wang, Y. K., Lu, Y. C., et al. (2017). Assessment of photodegradation of herbicide prometryn in soil. Water, Air, and Soil pollution, 228(4), 135.
Jinadasa, K., Milham, P., Hawkins, C., Cornish, P., Williams, P., Kaldor, C., et al. (1997). Survey of cadmium levels in vegetables and soils of Greater Sydney, Australia. Journal of Environmental Quality, 26(4), 924–933.
Kalam, A., Tah, J., & Mukherjee, A. K. (2004). Pesticide effects on microbial population and soil enzyme activities during vermicomposting of agricultural waste. Journal of Environmental Biology, 25(2), 201–208.
Kamble, S., & Saran, R. (2005). Effect of concentration on the adsorption of three termiticides in soil. Bulletin of Environmental Contamination and Toxicology, 75(6), 1077–1085.
Komárek, M., Čadková, E., Chrastný, V., Bordas, F., & Bollinger, J.-C. (2010). Contamination of vineyard soils with fungicides: A review of environmental and toxicological aspects. Environment International, 36(1), 138–151.
Kreuger, J., Peterson, M., & Lundgren, E. (1999). Agricultural inputs of pesticide residues to stream and pond sediments in a small catchment in southern Sweden. Bulletin of Environmental Contamination and Toxicology, 62(1), 55–62.
Langheinrich, U., Tischner, R., & Godbold, D. L. (1992). Influence of a high Mn supply on Norway spruce (Picea abies (L.) Karst.) seedlings in relation to the nitrogen source. Tree physiology, 10(3), 259–271.
Lawrence, G., Richards, C., & Lyons, K. (2013). Food security in Australia in an era of neoliberalism, productivism and climate change. Journal of Rural Studies, 29, 30–39. https://doi.org/10.1016/j.jrurstud.2011.12.005.
Leitão, S., Cerejeira, M. J., Van den Brink, P. J., & Sousa, J. P. (2014). Effects of azoxystrobin, chlorothalonil, and ethoprophos on the reproduction of three terrestrial invertebrates using a natural Mediterranean soil. Applied Soil Ecology, 76, 124–131. https://doi.org/10.1016/j.apsoil.2013.12.013.
Liu, Y., Xu, Z., Wu, X., Gui, W., & Zhu, G. (2010). Adsorption and desorption behavior of herbicide diuron on various Chinese cultivated soils. Journal of Hazardous Materials, 178(1–3), 462–468.
Lockhart, W., Macdonald, R., Outridge, P., Wilkinson, P., DeLaronde, J., & Rudd, J. (2000). Tests of the fidelity of lake sediment core records of mercury deposition to known histories of mercury contamination. Science of the Total Environment, 260(1–3), 171–180.
Lowry, D. B., Rockwood, R. C., & Willis, J. H. (2008). Ecological reproductive isolation of coast and inland races of Mimulus guttatus. Evolution: International Journal of Organic Evolution, 62(9), 2196–2214.
Malhat, F., Watanabe, H., & Youssef, A. (2015). Degradation profile and safety evaluation of methomyl residues in tomato and soil. Hellenic Plant Protection Journal, 8(2), 55–62.
Martikainen, E. (1996). Toxicity of dimethoate to some soil animal species in different soil types. Ecotoxicology and Environmental Safety, 33(2), 128–136. https://doi.org/10.1006/eesa.1996.0016.
McLaughlin, M. J., Palmer, L., Tiller, K., Beech, T. A., & Smart, M. (1994). Increased soil salinity causes elevated cadmium concentrations in field-grown potato tubers. Journal of Environmental Quality, 23(5), 1013–1018.
Millaleo, R., Alvear, M., Aguilera, P., González-Villagra, J., de la Luz Mora, M., Alberdi, M., et al. (2019). Mn toxicity differentially affects physiological and biochemical features in highbush blueberry (Vaccinium corymbosum L.) cultivars. Journal of Soil Science and Plant Nutrition, 1–11.
Mishra, G., Debnath, S., & Rawat, D. (2017). Managing phosphorus in terrestrial ecosystem: A review. European Journal of Biological Research, 7(3), 255–270.
Moen, J., Cornet, J., & Evers, C. (1986). Soil protection and remedial actions: Criteria for decision making and standardization of requirements. In Contaminated soil (pp. 441–448): Springer.
Moncada, A. (2004). Environmental fate of diuron. Sacramento, CA: California Department of Pesticide Regulation.
Mortvedt, J., & Osborn, G. (1982). Studies on the chemical form of cadmium contaminants in phosphate fertilizers. Soil Science, 134(3), 185–192.
Muukkonen, P., Hartikainen, H., Lahti, K., Särkelä, A., Puustinen, M., & Alakukku, L. (2007). Influence of no-tillage on the distribution and lability of phosphorus in Finnish clay soils. Agriculture, Ecosystems & Environment, 120(2–4), 299–306.
NEPC (2011a). National Environment Protection (Assessment of Site Contamination) Measure 2011 Schedule B7: Guideline on Health-Based Investigation Levels. In N. E. P. Council (Ed.), (pp. 1–60). Adelaide, Australia: Australian Commonwealth Government.
NEPC (2011b). National Environment Protection (Assessment of Soil Contamination) Measure 2011 Schedule B1: Guideline on Investigation Levels for Soil and Groundwater. In N. E. P. Council (Ed.), (pp. 1–61). Adelaide, Australia: Australian Commonwealth Government.
NEPC (2011c). National Environment Protection (Assessment of Soil Contamination) Measure 2011 Schedule B5a: Guideline on Ecological Risk Assessment. In N. E. P. Council (Ed.), (pp. 1–42). Adelaide, Australia: Australian Commonwealth Government.
NEPC (2011d). National Environment Protection (Assessment of Soil Contamination) Measure 2011 Schedule B5b: Methodology to Derive Ecological Investigation Levels in Contaminated Soils. In N. E. P. Council (Ed.), (Vol. Schedule B5b, pp. 1–87). Adelaide, Australia: Australian Commonwealth Government.
NEPC (2011e). National Environment Protection (Assessment of Soil Contamination) Measure 2011 Schedule B5c: Soil Quality guidelines for Arsenic, Chromium (III), Copper, DDT, Lead, Naphthalene, Nickel & Zinc. In N. E. P. Council (Ed.), (pp. 1–177). Adelaide, Australia: Australian Commonwealth Government.
NEPM. (1999). Investigation levels for soil and groundwater. Adelaide: National Environmental Protection Council Service Corporation.
Nicol, E., Chayata, H., Genty, C., Bouchonnet, S., & Bourcier, S. (2016). Photodegradation of cyprodinil under UV–visible irradiation–chemical and toxicological approaches. Rapid Communications in Mass Spectrometry, 30(19), 2201–2211.
Nziguheba, G., & Smolders, E. (2008). Inputs of trace elements in agricultural soils via phosphate fertilizers in European countries. Science of the Total Environment, 390(1), 53–57. https://doi.org/10.1016/j.scitotenv.2007.09.031.
Outridge, P. M., & Wang, F. (2015). The stability of metal profiles in freshwater and marine sediments. In Environmental contaminants (pp. 35–60): Springer.
Owojori, O. J., Ademosu, O. T., Jegede, O. O., Fajana, H. O., Kehinde, T. O., & Badejo, M. A. (2019). Tropical oribatid mites in soil toxicity testing: Optimization of test protocol and the effect of two model chemicals (cadmium and dimethoate) on Muliercula inexpectata. Chemosphere, 218, 948–954. https://doi.org/10.1016/j.chemosphere.2018.11.173.
Paschke, M. W., Valdecantos, A., & Redente, E. F. (2005). Manganese toxicity thresholds for restoration grass species. Environmental Pollution, 135(2), 313–322. https://doi.org/10.1016/j.envpol.2004.08.006.
Persaud, D., Jaagumagi, R., & Hayton, A. (1993). Guidelines for the protection and management of aquatic sediment quality in Ontario.
Rhodes, R. C., & Long, J. D. (1974). Run-off and mobility studies on benomyl in soils and turf. Bulletin of Environmental Contamination and Toxicology, 12(3), 385–393.
Rundgren, S., Rühling, Å., Schlüter, K., & Tyler, G. (1992). Mercury in soil: Distribution, speciation and biological effects. Nordic Council of Ministers.
Rydberg, J., Gälman, V., Renberg, I., Bindler, R., Lambertsson, L., & Martínez-Cortizas, A. (2008). Assessing the stability of mercury and methylmercury in a varied lake sediment deposit. Environmental Science and Technology, 42(12), 4391–4396.
Sakugawa, H., Tahara, K., Aoki, K., Arai, N., Nakatani, N., & Takeda, K. (2010). Studies on concentration, decomposition rate, half-life time and degradation products of herbicide diuron in river waters of Hiroshima Prefecture, Japan. Chikyukagaku (Geochemistry), 44(1), 1–15.
Sanderson, D., & Edson, E. (1964). Toxicological properties of the organophosphorus insecticide dimethoate. Occupational and Environmental Medicine, 21(1), 52–64.
Santos, I. R., De Weys, J., & Eyre, B. D. (2011). Groundwater or floodwater? Assessing the pathways of metal exports from a coastal acid sulfate soil catchment. Environmental Science and Technology, 45(22), 9641–9648.
Schäfer, R. B., Bundschuh, M., Rouch, D. A., Szöcs, E., von der Ohe, P. C., Pettigrove, V., et al. (2012). Effects of pesticide toxicity, salinity and other environmental variables on selected ecosystem functions in streams and the relevance for ecosystem services. Science of the Total Environment, 415, 69–78. https://doi.org/10.1016/j.scitotenv.2011.05.063.
Shanley, J. B., & Bishop, K. (2012). Mercury cycling in terrestrial watersheds (Mercury in the environment: Pattern and process).
Short, K. (1994). Quick poison, slow poison: Pesticide risk in the lucky country. K. Short.
Simpson, M. (2019). Berry plant protection guide. In N. D. o. P. Industries (Ed.), Wollongbar Primary Industries Institute (pp. 1–100). Wollongbar, NSW: Department of Primary Industries.
Simpson, S., Batley, G., & Chariton, A. (2013). Revision of the ANZECC/ARMCANZ sediment quality guidelines. CSIRO Land and Water Report, 8(07), 128.
Sirtori, C., Zapata, A., Malato, S., & Agüera, A. (2012). Formation of chlorinated by-products during photo-Fenton degradation of pyrimethanil under saline conditions. Influence on toxicity and biodegradability. Journal of Hazardous Materials, 217–218, 217–223. https://doi.org/10.1016/j.jhazmat.2012.03.017.
Smelt, J. H., Crum, S. J. H., Teunissen, W., & Leistra, M. (1987). Accelerated transformation of aldicarb, oxamyl and ethoprophos after repeated soil treatments. Crop Protection, 6(5), 295–303. https://doi.org/10.1016/0261-2194(87)90058-5.
Smith, E. R. G., Naidu, R., & Alston, A. (1998). Arsenic in the soil environment. Academic Press.
Smith, E., Smith, J., Smith, L., Biswas, T., Correll, R., & Naidu, R. (2003). Arsenic in Australian environment: An overview. Journal of Environmental Science and Health, Part A, 38(1), 223–239.
Stork, P. R., Bennett, F. R., & Bell, M. J. (2008). The environmental fate of diuron under a conventional production regime in a sugarcane farm during the plant cane phase. Pest Management Science: Formerly Pesticide Science, 64(9), 954–963.
Sun, Q., Ding, S., Wang, Y., Xu, L., Wang, D., Chen, J., et al. (2016). In-situ characterization and assessment of arsenic mobility in lake sediments. Environmental Pollution, 214, 314–323. https://doi.org/10.1016/j.envpol.2016.04.039.
Thorstensen, C. W., Lode, O., Eklo, O. M., & Christiansen, A. (2001). Sorption of bentazone, dichlorprop, MCPA, and propiconazole in reference soils from Norway. Journal of Environmental Quality, 30(6), 2046–2052.
Tilman, D. (1999). Global environmental impacts of agricultural expansion: The need for sustainable and efficient practices. Proceedings of the National Academy of Sciences, 96(11), 5995–6000.
USEPA. (1998). Pesticide fact sheet: Cyprodinil. In P. a. T. S. Office of Prevention (Ed.), Pesticide Fact Sheet (pp. https://nepis.epa.gov/Exe/ZyNET.exe/P100BIX107.TXT?ZyActionD=ZyDocument&Client=EPA&Index=1995+Thru+1999&Docs=&Query=&Time=&EndTime=&SearchMethod=1991&TocRestrict=n&Toc=&TocEntry=&QField=&QFieldYear=&QFieldMonth=&QFieldDay=&IntQFieldOp=1990&ExtQFieldOp=1990&XmlQuery=&File=D%1993A%1995Czyfiles%1995CIndex%1920Data%1995C1995thru1999%1995CTxt%1995C00000031%00000035CP00000100BIX00000037.txt&User=ANONYMOUS&Password=anonymous&SortMethod=h%00000037C-&MaximumDocuments=00000031&FuzzyDegree=00000030&ImageQuality=r00000075g00000038/r00000075g00000038/x00000150y00000150g00000016/i00000425&Display=hpfr&DefSeekPage=x&SearchBack=ZyActionL&Back=ZyActionS&BackDesc=Results%00000020page&MaximumPages=00000031&ZyEntry=00000031&SeekPage=x&ZyPURL). Greensboro, North Carolina, USA: US EPA.
Van Derveer, W. D., & Canton, S. P. (1997). Selenium sediment toxicity thresholds and derivation of water quality criteria for freshwater biota of western streams. Environmental Toxicology and Chemistry, 16(6), 1260–1268.
van Herk, W. G., Vernon, R. S., & McGinnis, S. (2013). Response of the dusky wireworm, Agriotes obscurus (Coleoptera: Elateridae), to residual levels of bifenthrin in field soil. Journal of Pest Science, 86(1), 125–136.
Van Scoy, A., Pennell, A., & Zhang, X. (2016). Environmental fate and toxicology of dimethoate. In Reviews of environmental contamination and toxicology volume 237 (pp. 53–70). Springer.
Van Scoy, A. R., Yue, M., Deng, X., & Tjeerdema, R. S. (2013). Environmental fate and toxicology of methomyl. In Reviews of environmental contamination and toxicology (pp. 93–109). Springer.
Vašíčková, J., Hvězdová, M., Kosubová, P., & Hofman, J. (2019). Ecological risk assessment of pesticide residues in arable soils of the Czech Republic. Chemosphere, 216, 479–487. https://doi.org/10.1016/j.chemosphere.2018.10.158.
Warne, M. S. J., Heemsbergen, D., Stevens, D., McLaughlin, M., Cozens, G., Whatmuff, M., et al. (2008). Modeling the toxicity of copper and zinc salts to wheat in 14 soils. Environmental Toxicology and Chemistry: An International Journal, 27(4), 786–792.
Waters, E. K., Scagel, C. F., & McLean, C. (2008). A survey of Vaccinium cultural practices in Australia emphasizing implications for mycorrhizal infection. International Journal of Fruit Science, 8(1–2), 109–124.
White, S., Conrad, S., Woodrow, R., Tucker, J., Wong, W., Cook, P., et al. (2020). Nutrient transport and sources in headwater streams surrounded by intensive horticulture. National Marine Science Centre: Southern Cross University, Coffs Harbour, NSW.
White, S., Santos, I., Conrad, S., & Sanders, C. (2018a). Investigating water quality in Coffs coastal estuaries and the relationship to adjacent land use. Part 2: Water Quality. National Marine Science Centre, Southern Cross University, Coffs Harbour. NSW.
White, S., Santos, I., & Hessey, S. (2018b). Effect of intensive horticulture practices on surrounding headwater stream nitrate concentrations. Environmental Pollution, 243, 1036–1046. https://doi.org/10.1016/j.envpol.2018.08.074.
Wilk, P., & Ireland, G. (2008). Natural resource management guidelines for NSW Blueberry Growers. In N. DPI (Ed.), (pp. 1–16). Lismore, NSW: Graphiti-Design.
Yang, Y., Sheng, G., & Huang, M. (2006). Bioavailability of diuron in soil containing wheat-straw-derived char. Science of the Total Environment, 354(2), 170–178. https://doi.org/10.1016/j.scitotenv.2005.01.026.
Zhang, J., & Liu, C. (2002). Riverine composition and estuarine geochemistry of particulate metals in China—weathering features, anthropogenic impact and chemical fluxes. Estuarine, Coastal and Shelf Science, 54(6), 1051–1070.
Acknowledgements
We acknowledge the Gumbaynggirr people, traditional owners of the land on which this work took place. This project was funded by the Coffs Harbour City Council’s Environmental Levy program. We would like to acknowledge the contributions of Coffs Harbour City Council’s Samantha Hessey, Project Officer for the Orara River Rehabilitation Project & Regional State of the Environment Reporting for inspiring and supporting this project. We wish to thank the landowners who were kind enough to let us conduct sampling on their property and the efforts of Dylan Brown, Carolina Bueno, and Ceylena Holloway in both the laboratory and the field. Simon Proust provided insight in selecting sampling locations. Funding was provided by Australian Research Council (Grant Nos. FT170100327 and DE160100443).
Funding
Funding was provided by Coffs Harbour City Council’s Environmental Levy Grant Program. IRS and CJS receive funding from the Australian Research Council (FT170100327 and DE160100443).
Author information
Authors and Affiliations
Contributions
SC, CS, and SW collected and prepared samples for analysis. SC wrote the introduction, methods, results, discussion, and conclusions sections of the manuscript. SW assisted in sampling design and wrote the study site section of the manuscript. CS and IS critically revised the intellectual content and edited multiple drafts of the manuscript before submission.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Conrad, S.R., White, S.A., Santos, I.R. et al. Assessing pesticide, trace metal, and arsenic contamination in soils and dam sediments in a rapidly expanding horticultural area in Australia. Environ Geochem Health 43, 3189–3211 (2021). https://doi.org/10.1007/s10653-020-00803-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-020-00803-z