Abstract
Fate of thiodicarb and its major metabolite in sandy loam soil were studied by applying thiodicarb (Larvin 75 WP) at 500 and 1000 g a. i. ha−1 under laboratory conditions. Samples drawn periodically were analysed on GC-FTD equipped with capillary column. The average initial deposits of total thiodicarb (thiodicarb and methomyl) were 0.025 and 0.035 mg kg−1 at single and double dosages, respectively. Residues of thiodicarb reached below the determination level (BDL) of 0.005 mg kg−1 after 15 days. Half-life periods for total thiodicarb were calculated to be 5.90 and 8.29 days at two doses, respectively, following first-order kinetics.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pesticides are intensively used in agriculture and much effort is extended to manage and reduce possible deleterious effects on the environment. The soil compartment has a major influence on the fate and behaviour of pesticides applied to crops. Understanding the fate of pesticides in soil is fundamental to the accurate assessment of their environmental behaviour and vital in ensuring the safe use of new and existing products. Alongside sorption, degradation is the second important process used to predict the fate of pesticides in soils (Boesten and van der Linden 1991). Variability in degradation rate is expected, and numerous studies have provided evidence for field-to-field variation in the degradation rates of pesticides (Price et al. 2001; Walker et al. 2001; Dyson et al. 2002). Systematic methods that allow a relative assessment of off-site impacts of pesticides are of great value to many people, including pesticide users, natural resource managers and regulators, as an aid in choosing the pesticides and practices with the least detrimental impact on the environment (Levitan et al. 1995; Van der Werf 1996). Risk indicators are regarded as useful tools in minimizing off-site impacts of pesticides and can assist in decision making and policy formulation (Reus et al. 2002). Generic methods for assessing pesticide effects on the environment are currently imperfectly developed and consequently are a field of current interest (Levitan 1997; Reus et al. 2002). Several approaches and tools have been developed to carry out the relative assessment of pesticides impact on the environment (Van der Werf 1996; Levitan 1997; Sanchez-Bayo et al. 2002; Reus et al. 2002; Brown et al. 2003).
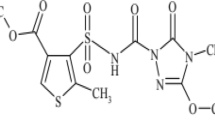
Thiodicarb, dimethyl N,N-[thiobis(methyliminocarbonyloxy)]bis-(ethanimidothiolate), is a sulfenylated biscarbamate insecticide. Thiodicarb has very low persistence in soil under aerobic laboratory conditions. Under the same conditions, methomyl also show low persistence in soils. Thiodicarb is low to high mobile and methomyl is very high mobile in soil (Summary of the EFSA Scientific Report 2005). Thiodicarb is a broad spectrum compound closely related to methomyl and less phytotoxicity. It is said to be effective against cutworms (Osbourne and Schwartz 1981). It acts by inhibiting acetyl cholinesterase activity and is used on many crops to control lepidopterous and other pests (Khosawinah and College 1978).
Perusal of literature revealed that not much information is available on fate of thiodicarb in soil. This experiment has therefore been undertaken to study the fate of thiodicarb and its metabolite, methomyl in sandy loam soil at different doses under laboratory conditions.
Material and methods
Chemicals and reagents
The technical grade analytical standards of thiodicarb (99.9) and methomyl (99.9) were procured from Sigma-Aldrich, India. Thiodicarb (Larvin75WP) formulation of Bayer company used for field application was purchased from local market. Analysis of acetone extract of the formulation showed the presence of thiodicarb, and none of its metabolic products and no interfering peak were observed in the vicinity of the retention time of the compounds being studied. Moreover, the concentration of thiodicarb was found to be accurate with respect to its purity as claimed by the manufacturers, Bayer Crop Science, Germany. Sodium chloride (ASC reagent grade ≥99.9 %) was obtained from Merck, Darmstadt, Germany. Sodium sulphate anhydrous (AR grade) was from S.D. fine Chemicals, Mumbai. All common solvents were redistilled in all-glass apparatus before use. The suitability of the solvents and other chemicals was ensured by running reagent blanks before actual analysis.
Preparation of standard solution
A standard stock solution of the parent compound thiodicarb and its metabolite, methomyl (1 mg mL−1), were prepared in acetone. The standard solutions required for constructing a calibration curve (2.00, 1.00, 0.50, 0.10 and 0.05 μg mL−1) were prepared from stock solution by serial dilution with acetone. All standard solutions were stored at −4 °C before use.
Laboratory experiment
For laboratory experiment, sandy loam soil was procured from the untreated field of Research Farm of CCS Haryana Agricultural University Hisar, Haryana, India. Soil was dried and sieved through 2-mm sieve before use to remove the debris. The experimental soil properties were sand, 78.0 %; silt, 10.2 %, clay, 11.8 %; EC, 0.21 dSm−1; P2O5, 15 Kg ha−1; pH 7.85 and organic carbon, 0.21 %. The quantity of thiodicarb formulation (Larvin 75WP) required per pot (4 Kg of soil) was calculated based on the soil weight of plough depth. The treatments were untreated control, single (T1) dose of 500 g a. i. ha−1 and double (T2) dose of 1000 g a. i. ha−1. Soil was treated with above doses, filled in separate pots in triplicate for each dose and control for each treatment. These pots were kept at field capacity moisture level of 1/3 bar tension. The moisture content in pot was checked periodically (3–4 days interval), and desired moisture content was maintained throughout the experimental period. Samples from each treatment were drawn and analysed periodically after 0 (1 h after treatment), 1, 3, 7, 15, 30, 60 and 90 days after treatment. From each pot, a thoroughly mixed soil sample was taken with steel auger and quartered to a required size (20 g) and analysed for degradation studies.
Well-ground representative soil sample (20 g) was taken in 250-ml Erlenmeyer flask added with 100-ml solution of acetone: water 1:1 v/v and shaken on mechanical shaker for 1.5 h. The extract was filtered through Whatman’s paper no.1 and partitioned thrice (75, 50, 30 ml) with dichloromethane after dilution with 10 % NaCl solution. The combined organic phase was first concentrated on rotary vacuum evaporator followed by gas manifold evaporator to near dryness. Final volume to 2 ml was made with n-hexane for gas chromatograph (GC) analysis.
Estimation
Thiodicarb residues were quantified using a GC (Shimadzu Model 2010) equipped with a flame thermionic detector (FTD) and HP-1 capillary column (30 m × 0.32 mm × 0.25 μm film thickness) of 5 % diphenyl and 95 % dimethyl polysiloxane. The GC operating parameters were as follows: column temperature, 100 °C (1 min) at 10 °C min−1 to 200 °C (0 min) at 20 °C min−1 to 260 °C (3 min); injection port, 270 °C; detector, 275 °C, O2 flow, 135 ml min−1; H2 flow 3.5 ml min−1 and carrier gas (N2) flow, 18 ml min−1. The flow rate of gas was 2 ml min−1 through the column with split ratio 1:10. The retention times observed for thiodicarb and methomyl were 4.258 and 4.334 min, respectively. Chromatogram is shown in Fig. 1.
Recovery experiment
In the present investigation, recovery experiments were carried out at different fortification levels to establish reliability and validity of analytical method and to know the efficiency of extraction and clean-up procedures. The control samples of soil were spiked at 0.05, 0.25 and 0.50 mg kg−1 and processed by following the methodology as described above. The average percent recoveries of thiodicarb and methomyl were 94.22 and 96.15, respectively. However, the recoveries of thiodicarb and methomyl at 0.005 mg kg−1 were 67.88 and 68.40 % (Table 1). Hence, correction factor was used for calculating the residues. Limit of detection was 0.001, and limit of determination/quantification was 0.005 mg kg−1 for thiodicarb as well as for methomyl.
Results and discussion
Residue data and percent dissipation of total thiodicarb (thiodicarb + methomyl) in soil under laboratory conditions at two treatments are given in Table 2. Residues of methomyl, a metabolite of thiodicarb, were also detected in soil at both the doses from 0 day onward. The average initial deposits of total residues estimated in soil were found to be 0.025 and 0.035 mg kg−1 at single and double dose, respectively. The residues dissipated by 0.019, 0.015, 0.011 and 0.007 mg kg−1 on 1, 3, 7 and 15 days after treatment, respectively, thereby recording 24, 40, 56 and 72 % dissipation in this period in case of single dose (T1). At double dose (T2), residues dissipated to 0.30, 0.027, 0.022, 0.010 and 0.005 mg kg−1 in 1, 3, 7, 15 and 30 days after treatment with percent dissipation of 14.28, 22.85, 37.14, 71.42 and 85.71, respectively. The residues reached below determination level (BDL) of 0.005 mg kg−1 after 30 and 60 days of treatment in case of single and double dose, respectively. Half-life (t1/2) of total thiodicarb calculated as per Hoskins (1961) was observed to be 5.90 and 8.29 days whereas for methomyl 8.25 and 9.46 days, in single and double dose, respectively (Table 3). Statistically analysed data showed that with increase in duration, significant reduction in residue levels was observed (CD = 0.002; p = 0.05). Interaction between dose and duration was found to be non-significant (CD = 0.003; p = 0.05) which suggested significantly less residues at each duration of lower dose (500 g a. i. ha−1) as compared to higher dose (1000 g a. i ha−1). The dissipation followed first-order kinetics (Fig. 2). Literature reveals that an acceptable first order kinetics is defined as r 2 > 0.7 (Organisation for Economic Co-operation and Development 1999). In present studies also, the linear relationship obtained by plotting of log residues versus time shows that the dissipation of thiodicarb followed a first order kinetics in both the treatments showing r 2 > 0.7. In the present study, value of r 2 is 0.956 and 0.942 for thiodicarb at single and double dose, respectively. Field study on the movement and degradation of thiodicarb and its metabolite methomyl was carried out by Jones et al. (1989) and reported the half-life of thiodicarb only a few hours in surface soils, while the half-life of methomyl was about 2 days in surface soils and about 0.5 to 1.6 months in sub-soils. The present results do not conform the results reported by Jones et al. (1989). Difference in results may be due to high temperature in the studying area and soil texture.
Chandra et al. (2009) carried out a study to know the fate of benfuracarb insecticide in mollisols and brinjal crop applying foliar application at 0.25 and 0.50 μg g−1 levels. At 0.25 μg g−1, benfuracarb persisted up to 7 days, and at 0.50 μg g−1, residues persisted up to 10 days in soil with the half-life values of 3.54 at 0.25 μg g−1 and 3.75 at 0.50 μg g−1. Difference in persistence pattern and half-life period is because the present studies are carried out under laboratory conditions. Chopra et al. (2013) investigated persistence and dissipation kinetics of thiodicarb (Larvin 75WP) applied at the time of flower initiation stage of cotton crop (H-1117) at 500 (T1) and 1000 g a. i. ha−1 (T2) during kharif season. The dissipation was 100 % in soil at both the doses after 35 days of application following a first-order kinetics. The half-life value was observed to be 7.27 days in T1 and 7.81 days in T2, respectively.
Conclusions
In soil under laboratory conditions, thiodicarb was found to be the main constituent, followed by its metabolite methomyl. Residue data showed that thiodicarb persisted up to 15 days and methomyl up to 30 days. Half-life period for total residues following two application of Larvin 75WP at 500 and 1000 g a. i. ha−1 were calculated to be 5.90 and 8.29 days, respectively, whereas of methomyl half-life was found to be 8.25 and 9.46 days showing thereby slightly more persistence than thiodicarb.
References
Boesten, J. J. T. I., & Van der Linden, A. M. A. (1991). Modelling the influence of sorption and transformation on pesticide leaching and persistence. Journal of Environmental Quality, 20, 425–435.
Brown, C. D., Hart, A., Lewis, K. A., & Dubus, I. G. (2003). Simulating the environmental fate of pesticides for a farm-level risk assessment system. Agronomie, 23, 67–74.
Chandra, R., Srivastava, A., & Srivastava, P. C. (2009). Fate of benfuracarb insecticide in mollisols and brinjal crop. Bulletin of Environmental Contamination and Toxicology, 83(3), 348–351.
Chopra, I., Chauhan, R., & Kumari, B. (2013). Dissipation and persistence of thiodicarb in cotton. International Journal of Agricultural Science and Technology (IJAST), 1(2), 28–31.
Summary of the EFSA Scientific Report (2005). Conclusion regarding the peer review of the pesticide risk assessment of the active substancethiodicarb 55, 1–76.
Dyson, J. S., Beulke, S., Brown, C. D., & Lane, M. C. G. (2002). Adsorption and degradation of the weak acid mesotrione in soil and environmental fate implications. Journal of Environmental Quality, 31, 613–618.
Hoskins, W. M. (1961). Mathematical treatment of loss of pesticide residues. FAO Plant Protection Bulletin, 9, 163–168.
Jones, R. L., Hunt, T. W., Norris, F. A., & Harden, C. F. (1989). Field research studies on the movement and degradation of thiodicarb and its metabolite methomyl. Journal of Contaminant Hydrology, 4, 359–371.
Khosawinah, A. M. & College, P. R. (1978). Fats of single oral dose of C14-acetyl UC51762 in a lactating cow metabolism into natural products. Unpublished report No UCC 814 C21, submitted to WHO by research triangle park, North Carolina, USA.
Levitan, L. (1997). An overviewof pesticide impact and risk assessment systems in OECD workshop on pesticide risk indicators, Copenhagen, 21–23 April.
Levitan, L., Merwin, I., & Kovach, J. (1995). Assessing the relative environmental impacts of agricultural pesticides: the quest for a holistic method. Agriculture, Ecosystems and Environment, 55, 153–168.
Organisation for Economic Co-operation and Development. (1999). OECD guide lines for the testing of chemicals. Aerobic and anaerobic transformation in soil. Paris: OECD.
Osbourne, E., & Schwartz, P. (1981). Insecticides and Pheromones Used in the Midwest. A Short Re1212121view. Industrial and Engineering Chemistry Product Research and Development 20, 246–253.
Price, O. R., Walker, A., Wood, M. & Olivier, M. A. (2001). Using geostatics to evaluate spatial variation in pesticide/soil interactions. BCPC Symposium Proc No. 78, Pesticide Behaviour in soils and water. British Crop Protection Council Symposium, Farnham, Surrey, 233–238.
Reus, J., Leendertse, P., Bockstaller, C., Fomsgaard, I., Gutsche, V., Lewis, K., Nilsson, C., Pussemier, L., Trevisan, M., Van der Werf, H., Alfarroba, F., Blumel, S., Isart, J., McGrath, D., & Sepala, T. (2002). Comparison and evaluation of eight pesticide environmental risk indicators developed in Europe and recommendations for future use. Agriculture, Ecosystems and Environment, 90, 177–187.
Sanchez-Bayo, F., Baskaran, S., & Kennedy, I. R. (2002). Ecological relative risk (EcoRR): another approach for risk assessment of pesticides in agriculture. Agriculture, Ecosystems and Environment, 91, 37–57.
Van derWerf, H. M. G. (1996). Assessing the impact of pesticides on the environment. Agriculture, Ecosystems and Environment, 60, 81–96.
Walker, A., Jurado-Exposito, M., Bending, G. D., & Smith, V. J. R. (2001). Spatial variability in the degradation rate of isoproturon in soil. Environmental Pollution, 111, 407–415.
Acknowledgments
The authors wish to express their gratitude to the head of the Department of Entomology for providing research facilities.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bisht, S., Chauhan, R., Kumari, B. et al. Fate of thiodicarb and its metabolite methomyl in sandy loam soil under laboratory conditions. Environ Monit Assess 187, 429 (2015). https://doi.org/10.1007/s10661-015-4640-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-015-4640-1