Abstract
Seafood has been generally considered to be the main diet exposure source of metal(loid)s. We evaluated health risk of mercury (Hg), arsenic (As), cadmium (Cd), lead (Pb), chromium (Cr), nickel (Ni), copper (Cu), and zinc (Zn) through consumption of cooked seafood based on bioaccessibility, which was obtained by physiologically based extraction test method. Results showed that cooking practices could decrease metal(loid)s concentration from seafood (by 6.0–45.7%). Metal(loid)s release from seafood in this study followed the descending order of Hg > Zn > Ni > Cd > Pb > As > Cu > Cr. On average, cooking lowered the bioaccessibility of As, Hg, Cd, Pb, Ni, Cr, Cu, and Zn by 15.2, 26.1, 30.9, 30.7, 25.7, 31.2, 17.6, and 22.4%, respectively. Health risk calculation results showed that Cr, Ni, and Zn in seafood species in this study were within the human health benefits range. Hg, Cd, Pb, and Cu exposure from cooked seafood was within the safe dose. However, we found that there is a potential of having cancer (especially bladder and lung cancer) for people exposure to iAs from seafood based on bioaccessible contents the first time.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Metal(loid)s, including heavy metals and metalloid, are commonly found in the environment, and some of them are highly toxic, persistent, and non-degradable and deemed serious pollutants (El-Kady and Abdel-Wahhab 2018). Metal(loid)s in the environment originate from natural processes as well as anthropogenic activities (Ip et al. 2005). Some metal(loid)s (e.g., zinc, nickel) are essential for human health but may be toxic when taken in excess (Chen et al. 2018). However, other metal(loid)s such as arsenic, lead, mercury, and cadmium are extremely detrimental even at low contents and some are even classified as class 1 non-threshold carcinogen (de Filipps et al. 1979; Fowler et al. 2015; Gao et al. 2018).
Except for occupational exposure, dietary intake of heavy metals via food has been believed to be the predominant way of human exposure to environmental metal(loid)s (Chen et al. 2018). It has been reported that metal(loid)s contents are high in certain types of fish although trace levels can be found in nearly all types of seafood (FAO 2014; Gu et al. 2015; Hu et al. 2018; Keshavarzia et al. 2018). Thus, seafood has been generally considered to be the main diet exposure source of metal(loid)s. The health risk assessment of metal(loid)s from food has been widely studied in recent years (Burger et al. 2002; Nfon et al. 2009; Sobhanardakani 2017; Alamdar et al. 2017; Hu et al. 2018; Keshavarzia et al. 2018; Zhong et al. 2018). Researchers usually investigated the health risk of target heavy metals based on their initial contents in food matrices (Islam et al. 2015). However, food is consumed cooked in most cases. It has been revealed that food processing practices affect (lower or increase) the concentration of the elements (Atta et al. 1997; Schmidt et al. 2015; Praveena and Omar 2017; Houlbrèque et al. 2011; Hajeb et al. 2014). For instance, it has been shown that washing could release part of the arsenic from food (Naito et al. 2015). Perelló et al. (2008) reported that the metal(loid)s contents in meat and fish reduced after cooking processes, especially for mercury. Schmidt et al. (2015) found that frying could decrease about 33% of the total mercury from fish.
Bioaccessibility is a maximum bioavailability of the target element and can be conducted in vitro (Moreda-Piñeiro et al. 2011). The bioaccessibility of many metal(loid)s was lower than 100% in general, which has been demonstrated by previous studies (EFSA 2012; Alves et al. 2018). It would be less realistic to use the initial contents of metal(loid)s for health risk assessment. Moreover, in our previous study, we found that methylated As in shellfish could be demethylated and brought about the increase in iAs (Liao et al. 2018). Hence, it is highly necessary to evaluate the risk of health exposure to metal(loid)s from seafood based on bioaccessibility. Besides, it has been also reported that cooking could result in a change of metal bioaccessibility from food. For instance, Amiard et al. (2008) showed that cooking reduced metal(loid)s bioaccessibility in shellfish. Nonetheless, to our knowledge, there was still a lack of health risk assessment of mixed metals from seafood after processing and in vitro gastrointestinal digestion.
Consequently, the present study aimed to solve problems as follows: (1) to investigate the impact of various cooking processes on the contents and bioaccessibility of metal(loid)s in seafood; (2) to assess multiple metal(loid)s bioaccessibility from raw and cooked seafood; (3) to evaluate the health risk of metal(loid)s from seafood in Guangzhou City after cooking and in vitro gastrointestinal digestion on the basis of target hazard quotient as well as target carcinogenic risk.
Materials and methods
Samples
Seafood samples, including raw fish (tilapia, 3 × 5 samples; snapper, 3 × 5 samples; salmon, 3 × 5 samples; pomfret, 3 × 5 samples; croaker, 5 × 5 samples; turbot, 3 × 5 samples; tiger grouper, 3 × 5 samples; and anchovy, 5 × 5 samples) and raw crustacea (mantis shrimp, 2.0 kg of samples; red shrimp, 2.0 kg of samples; prawn, 2.0 kg of samples; spotted crab, 3 × 5 samples; red crab, 3 × 5 samples; clam, 4.0 kg of samples; scallop, 4.0 kg of samples; and oyster, 4.0 kg of samples) were purchased randomly from both markets and grocery from Guangzhou City in China. Shells, skin, and bones of the collected samples were removed. Tools used for cutting and homogenizing tissues were all stainless. In consideration of the initial contents of the analyzed elements, part of seafood samples were chosen for processing, including boiling, steaming, baking, frying. The chosen food tissues for cooking were uniform in shape (radius = 4 cm; thickness = 1 cm). In the boiling process, the seafood tissues were soaked in boiling ultrapure water (100 °C) for 15 min in a Teflon saucepot. For steaming, the seafood tissues were processed for 10 min in a steamer under temperature of 100 °C. The mass ratio of water to seafood ratio was 1:1 for boiling and steaming. Baking was performed in a commercial baking oven without anything for 10 min under temperature of 130 °C. In the process of frying, the seafood tissues was spread in a Teflon pan with peanut oil for 10 min (the ratio of oil to seafood ratio was 0.5:1) and the oil temperature was up to 125 °C. All samples (raw and cooked) were divided into two subsamples: One part was stored in a freezer under temperature of − 80 °C immediately, and the other part was freeze-dried firstly (for 48 h at − 80 °C and 0.770 Pa) and afterward stored in the same freezer until further experiments.
Metal(loid) concentrations are shown as μg g−1 wet weight (ww) or μg g−1 dry weight (dw). Based on the fresh and dry weights, the moisture contents in seafood could be obtained.
Reagents
High-purity water (resistivity was above 18.2 mΩ cm), which was produced from a water purification system named Milli-Q Element from USA, was used to prepare all solutions. Nitric acid was of chromatographically pure grade. Other chemicals were of guaranteed reagent grade (GR).
In vitro digestion
A physiologically based extraction test (PBET) model was used to study the bioaccessibility of metal(loid)s from raw and cooked homogenized seafood samples. The detailed information of the PBET model was described in our previous study (Liao et al. 2018). Gastric digestion phase: Raw or cooked dried seafood samples (5.0 g) were transferred into a brown bottle, which contained 500 mL of gastric digestion solution (500 mL of ultrapure water with 0.625 g of porcine pepsin, 210 μL of lactic acid, 0.25 mL of glacial acetic acid, and 0.25 g of malic acid, pH value was 2). Then, argon gas was ventilated into the solution in a shaking incubator at 150 rpm with stable temperature (37 °C) for 1 h, and the digestion liquid was sampled. Intestinal phase: The pH value was regulated to 5.3 using saturated sodium bicarbonate, and then, porcine bile (mass ratio of seafood samples to bile mass was 1:0.175) and pancreatin (mass ratio of seafood samples mass to pancreatin mass was 1:0.05) were added. Afterward, the pH value was regulated to 7 using 1.0 mol L−1 of sodium hydroxide and the mixture was digested for 2 h (150 rpm and 37 °C). After gastric and gastrointestinal digestion, the corresponding bioaccessible fractions were centrifuged (5000×g) for 20 min and then filtered through a filter (0.22 μm) for testing.
Determination of metal(loid)s
Extraction of total contents of metal(loid)s in raw and cooked seafood samples followed previously described method (Liao et al. 2018; Liao et al. 2019a, b): 0.2 g of samples were added into Teflon reactors, followed by 5 mL of 14 mol L−1 HNO3 and stand for 6 h. Next, 1 mL H2O2 (30% v/v) was added into the mixture, followed by being incubated in a microwave system for 30 min (1600 W, 180 °C).
The metal(loid)s concentrations in seafood samples and the bioaccessible fraction were determined using a quadrupole inductively coupled plasma mass spectrometry unit (ICP-MS; Agilent 7700x, Agilent, USA) (Dolan et al. 2003). An environmental calibration standards solution of multi-elements (Agilent, USA) was used for the determination of metal(loid)s in seafood simultaneously by ICP-MS. Concentration of Hg, As, Cd, Pb, Cr, Ni, Cu, and Zn in blank solution (5% HNO3) was determined for 11 times, and the corresponding mean value was 0.027, 0.0049, 0.015, 0.0096 0.0035, 0.0073, 0.014, and 0.016 μg L−1, respectively. And the detection limit of the metal(loid)s was the value of three times of the standard deviation of the concentration in 5% HNO3 solution (Klaue et al. 1999). The detection limit of Hg, As, Cd, Pb, Cr, Ni, Cu, and Zn was 0.0135, 0.0022, 0.0013, 0.0014, 0.0119, 0.0023, 0.0058, and 0.0082 μg L−1, respectively. Internal standard elements were 45Sc, 72Ge, 115In, and 209Bi. Extraction and determination procedure of metal(loid)s in the certified reference materials [GBW 10068, GBW 10024, and GBW 10050, China; DORM-4, fish protein, Canada] were the same with samples. Recovery rates of these metal(loid)s ranged from 86 to 112%.
HPLC-ICP-MS method was use to detect the contents of inorganic As (arsenious acid, i.e., iAsIII and arsenic acid, i.e., iAsV) and methylmercury (MeHg) (Liao et al. 2018). For determination of inorganic As (iAs), A Prin-cen Specia Fast Column (4.6 mm × 100 mm, Prin-cen Scientific Ltd., China) and mobile phase of ammonium nitrate solution (NH4NO3) with dual concentrations alternately (solution concentration A: 8 mmol L−1; concentration B: 20 mmol L−1; injection volume: 30 μL; flow rate: 1.2 mL min−1) were used. For identification of MeHg, a CNW Athena C18 Column (4.6 × 150 mm, 5 μm, 120 Å, ANPEL Laboratory Technologies (Shanghai) Inc., China) and single mobile phase (solution: 0.6 mol L−1 ammonium acetate with 0.1% L-cystine and 5% methanol; injection volume = 80 μL; flow rate = 0.9 mL min−1) were adopted.
Preparation of calibration standards for the iAsIII and iAsV used stock solution of arsenious acid (GBW 08666) and arsenic acid (GBW 08667), respectively. Detection limits for the iAsIII and iAsV on the ICP-MS instrument were 0.0083 and 0.0052 μg L−1, respectively. The extraction of iAs was as follows: Raw and cooked seafood samples (0.5 g) were transferred into centrifuged tubes containing 20 mL of 0.01 mol L−1 HNO3 solution, followed by ultrasonic-assisted extraction for an hour. Then, the mixture was centrifuged for 20 min (5000×g) and the supernatant was filtered through a cellulose acetate disk filter (0.22 μm) for testing. Extraction procedure of iAs from the standard reference material (SRM 1568b, rice flour, USA) and blank solutions was the same with samples. The recovery rate for iAs in SRM 1568b was 80–115%.
Preparation of calibration standards for MeHg used MeHg chloride stock solution (o2si smart solutions®; 1000 mg L−1). Concerning the extraction of MeHg, seafood samples (0.5 g) were transferred into centrifuged tubes containing 20 mL of 2 mol L−1 HCl solution, followed by ultrasonic-assisted extraction for an hour. Then, the mixture was centrifuged for 20 min (5000×g). Sampled 2 mL of the supernatant afterward was added to Hg species separation mobile phase carrier solution (2 mL), and the pH was adjusted to 7.0 with 0.310 mL ammonium hydroxide (25%). Next, a cellulose acetate disk filter (0.22 μm) was used to filter the solution for next testing. Extraction procedure of MeHg from the certified reference material (CRM DORM-4, fish protein, Canada) and blank solutions was the same with samples. The recoveries of MeHg in DORM-4 were 90–113%.
The concentration of all the elements in used oil was also determined by ICP-MS, which were below the detected line.
Health benefit/risk calculation
To calculate the resident daily intake of all metal(loid)s through consumption of seafood in Guangzhou City, the established daily intake (EDI) was used in this study and calculated by the following formula:
where EDI is the established daily intake of metal(loid)s from seafood (μg d−1 kg−1), Ci is the concentration of metal(loid)s in food (μg g−1), and DC is the daily consumption of food (g d−1). Fish and crustacea consumption in Guangzhou was 41.3 g d−1 and 31.2 g d−1 for adults, respectively (Ma 2004). BW is average body weight (60.0 kg for adults in Guangzhou City) (Wang 2005; MEP, 2013).
To calculate the hazardous exposure to metal(loid)s via consumption of seafood by the consumers, the target hazard quotient (THQ) was obtained used the following formulas (Zhong et al. 2018):
where THQ is the target hazard quotient (unitless), and EF is the exposure frequency (365 days/year). ED is exposure duration (70 years) (Wang 2005), i.e., the average lifetime. RfD is the tolerable daily intake of metal(loid), and the value for As, iAs, Hg, MeHg, Pb, Cd, Cu, Ni, Cr, and Zn is 30, 2.1, 0.5, 0.2, 4, 1, 40, 20 1500, and 300 μg d−1 kg−1, respectively (Keshavarzia et al. 2018; Sobhanardakani 2017; Hu et al. 2018). AT is the average exposure time (365 days/year × ED).
Besides, we calculated the total THQ (tTHQ, unitless) of metal(loid) in seafood through the following equation (Sobhanardakani 2017):
Among the studied elements, arsenic and inorganic arsenic compounds are identified as carcinogenic to humans (being classified as Group 1 in the IARC Monographs) (IARC 2018). Target cancer risk (TR, unitless) for iAs could be calculated by the following formulas (Zhong et al. 2018):
where TR is the target cancer risk, and CFS is the cancer slope factor. USEPA proposed a CFS value of 1.5 (mg kg−1 d−1)−1 (skin cancer) and 25.7 (mg kg−1 d−1)−1 (bladder and lung cancer) for iAs (Hu et al. 2018). The acceptable TR ranged from 10−4 to 10−6 (Gao et al. 2018).
The parameters used for health/risk calculation are shown in Table 1.
Statistical analyses
SPSS 21.0 (SPSS, Chicago, IL, USA) and OriginLab 9.0 (OriginLab, USA) were used for statistical analysis. A one-way analysis of variance (ANOVA), followed by the least significant difference (LSD) test were served to describe differences in values among treated groups. All data in this study are shown as the means or the mean ± standard deviation (SD). If p < 0.05, the means were considered significantly different.
Results and discussion
Contents of metal(loid)s in raw and cooked seafood
The concentrations of metal(loid)s (μg g−1 ww) were determined in the raw seafood samples collected in Guangzhou market and are presented in Table 2.
We found a wide range of concentrations of arsenic (As) in the seafood samples, with the highest mean concentration of As in crabs (35.30 μg g−1 ww) and the lowest in clam (0.15 μg g−1 ww). It was found that iAs could be detected in almost all seafood samples in this study, and iAs was mainly in the form of iAsV in raw samples. Nonetheless, iAs contributed far less to the tAs in seafood samples: The contents of iAs in the edible muscle of fish, shrimp, crab, and shellfish samples were < DL–0.018, 0.00078–0.0085, < DL–0.0066, and 0.011–0.022 μg·g−1 ww, respectively. All values of iAs in seafood samples were under the limit recommended by the Chinese National Standard Agency (GB 2762-2017): The limit contents of iAs content in fish and other seafood were 0.1 and 0.5 μg g−1 ww, respectively. The contents of iAs in most kinds of seafood were comparable with the results reported by previous research which concluded that iAs is at very low contents (< 0.01 μg g−1) in seafood (El-Kady and Abdel-Wahhab 2018).
As described in Table 2, mean concentration of mercury (Hg) (μg g−1 ww) in seafood followed the descending order of: crab (0.068 ± 0.0028) > fish (0.042 ± 0.038) > shrimp (0.021 ± 0.0040) > shellfish (0.0082 ± 0.0043). The highest mean concentration of Hg was observed in snapper (0.12 ± 0.038), and the lowest was found in anchovy (0.0046 ± 0.00055). The concentration ratios of MeHg to the total Hg in seafood samples were in the range of 40.2–100%. Correspondingly, the contents of MeHg in seafood samples were from 0.0021 to 0.084 μg g−1 ww, which were under the limit recommended by the GB (2762-2017) in China (carnivorous fish: 1.0 μg g−1 ww; other seafood: 0.5 μg g−1 ww) and similar to the previous research (EFSA 2012).
The content (μg g−1 ww) of lead (Pb) in seafood samples in this study ranged from 0.00040 ± 0.00016 to 0.18 ± 0.00056. And we found that the highest level of Pb was in scallop (0.18 ± 0.00056), followed by the oyster (0.13 ± 0.0012) and silvery pomfret (0.090 ± 0.00076). The overall average contents of cadmium (Cd) across all samples were 0.00020 ± 0.000066 (salmon) to 1.90 ± 0.0021 (scallop). And the detected Cd levels in all seafood samples in this study followed the descending order of shellfish > shrimp > crab > fish. The range of concentration for chromium (Cr) was ranged (0.032 ± 0.0041)–(2.04 ± 0.053) μg g−1 ww in all studied seafood species, which were substantially lower than the limit of 2.0 μg g−1 in aquatic foods set by China. The maximum mean level of nickel (Ni) detected was 0.20 μg g−1 ww in scallop, and the minimum was 0.014 μg g−1 ww in prawn. The concentration (μg g−1 ww) of copper (Cu) ranged from 0.12 to 17.43, with the overall average value of 3.88. Zinc (Zn) was the most abundant metal in all seafood samples analyzed, whose average content was highest in spotted crab (118.84 ± 0.16 μg g−1 ww) and lowest in clam (1.52 ± 0.068 μg g−1 ww). There are no limits of Ni, Cu, and Zn for seafood in Chinese standards. FAO (1983) set that the maximum limits for Cu and Zn were both 30 μg g−1 ww, and we found that only Zn levels in some species (spotted crab, red crab, scallop, and oyster) were higher than the limit. Overall, the results of metal(loid)s had no conflict with previous studies. For instance, Ip et al. (2005) reported the level of Cd in fish, shrimp, crab, and shellfish collected from the Pearl River Estuary was (0.01–2.1) μg g−1 ww, which was comparable with results in this study. Gu et al. (2015) reported the contents of metal(loid)s (μg g−1 ww) collected from the South China Sea: 0.00054–0.027 (Pb), 0.00051–0.115 (Cd), 0.02–1.26 (Cr), 0.00083–0.057 (Ni), 0.12–1.13 (Cu), and 2.34–6.88 (Zn), respectively. The results of Pb levels in this study were similar to those of previous studies of fish collected from South California (Burger et al. 2002) and Sweden (Nfon et al. 2009), though lower than the investigations of fish from river Chenab (Alamdar et al. 2017).
To exclude the effect of moisture, we study the influence of cooking based on the dry weight. As shown in Fig. 1, cooking practices were found to have some effect on the metal(loid)s concentration. The four types of cooking methods could decrease As contents from seafood, and boiling released As at the most extent (25.8%), followed by frying (24.1%), steaming (18.5%), and baking (16.6%). Correspondingly, the contents of iAs lowered after cooking (by an average of 24.5%). In regard to Hg (total Hg), the trends of average decreased percent of Hg in cooked seafood were in the order of steaming (14.8%) < boiling (16.6%) < baking (38.6%) < frying (45.7%). In respect of Ni, Cu, and Zn levels in cooked seafood, there existed the same decreasing trends with Hg after cooking. And, the decreased percent in contents of Ni, Cu, and Zn was in the range of 14.8–39.0%, 3.1–36.8%, and 9.1–43.9%, respectively. Cooking practices also brought about a decrease in Pb concentration in seafood samples in this study (a mean reduction of 21.7%). In relation to Cd concentration in seafood muscles, cooking could decrease it on average of 22.0% (frying: 36.5%; boiling: 24.3%; baking: 17.1%; steaming: 10.1%). Moreover, results demonstrated that cooking could also decrease Cr contents from seafood (on average of 6.0%) and the difference between cooking methods was not significant (p > 0.05). There have been some studies on the cooking effect on metal(loid)s in food. Our results were comparable with the previous studies, which believed that cooking resulted in a decrease in total As, Hg, Cd, Ni, Pb, Zn, Cu, and Cr in food matrices (Atta et al. 1997; Schmidt et al. 2015; Praveena and Omar 2017). However, results in some previous researches also gave various results. For instance, a study of Houlbrèque et al. (2011) said that cooking led to a rise in total Cd content in Chilean mussels. In our previous studies, we have verified that soluble As dissolved into the cooking water caused the reduction of As in shellfish after cooking (Liao et al. 2018). Besides, we determined the contents of all mentioned metals in raw food, cooked food, and discarded liquid and found that As, Pb, Cd, Ni, Cr, Cu, and Zn could be detected in the fried oil, boiled water, and steamed water after cooking. According to the boiling point (for instance, the boiling point of AsCl3 and As2O3 is 130 °C and 456 °C, respectively; the boiling point of Cd is 765 °C), those metal(loid)s were hardly volatile during the thermal treatments performed in this study (< 130 °C) (Chem YQ). Heating procedure could promote protein degradation, resulting in the dissociation of As in water as free salts, soluble amino acids, and bound to proteins. Other metal(loid)s also have a high affinity for metallothionein, a protein with low molecular weight and high cysteine residue content (de Filipps 1979). Thus, cooking resulted in a decrease in As, Pb, Cd, Ni, Cr, Cu, and Zn contents in seafood muscles maybe due to the solubilization of them into the cooking liquid.
The metal(loid)s contents in various seafood samples (n ≥ 3) before and after cooking (mean ± standard deviation). Lowercase letters indicate significant differences at p < 0.05 among each type of seafood. Capital letters indicate significant differences at p < 0.05 among these types of raw and cooked seafood. (a: turbot A; b: turbot B; c: tiger grouper A; d: tiger grouper B; e: anchovy; f: snapper; g: yellow croaker; h: mantis shrimp; i: prawn; j: red shrimp; k: spotted crab; l: red crab; m: clam; n: scallop)
However, Hg release into cooking liquid from the seafood, especially as MeHg, is negligible. Further, we found that cooking affects the release of metal(loid)s from seafood in this study which followed the descending order of Hg > Zn > Ni > Cd > Pb > As > Cu > Cr. It is known that the affinity ability for metals is Hg > Cu > Pb > Cd > Ni > Zn (de Filipps 1979). Results in our study showed that after cooking, the trend of decrease percent of the target metal(loid) reversed that of its affinity ability with metallothionein, which was except for Hg. Some studies have reported that cooking could decrease contents of Hg in fish muscle, which was attributed to evaporation (Hajeb et al. 2014; Mieiro et al. 2016). Consequently, volatilization of Hg in seafood muscles during heating may be dominant.
Bioaccessibility of metal(loid)s in seafood
We selected some seafood samples (turbot, snapper, yellow croaker, mantis shrimp, and clam) for performing in vitro gastrointestinal digestion tests to estimate the metal(loid) bioaccessibility from raw and cooked seafood. The results are shown in Fig. 2.
In general, the bioaccessible concentration of all metal(loid)s was lower than the initial concentration for all selected seafood samples. For raw fish, the average bioaccessible content of As, Hg, Pb, Cd, Cr, Ni, Cu, and Zn was 1.02, 0.042, 0.031, 0.0010, 1.05, 0.13, 0.21, and 5.04 μg g−1 ww, respectively. And for raw shellfish, corresponding mean content of each element was 11.20, 0.028, 0.046, 0.46, 0.12, 0.096, 7.55, and 44.89 μg g−1 ww, respectively. Based on bioaccessible concentration, bioaccessibility of metal(loid)s was given here.
Results showed that As was highly bioaccessible in raw seafood, whose bioaccessibility ranged from 87.4 ± 2.4% (yellow croaker) to 98.4 ± 3.3% (snapper), with the average value of 92.8%. In regard to Hg, the highest bioaccessible percent in raw seafood was found in turbot (96.8 ± 3.3%) and the lowest was in clam (64.7 ± 3.5%). It was observed that the mean bioaccessibility of MeHg had a wide range (45.2–100.0%). As a whole, the highest bioaccessibility of Cd, Pb, and Ni was all observed in raw turbot, while the lowest was found in raw mantis shrimp. Cd, Pb, and Ni bioaccessibility was 60.0–99.4%, 78.9–93.8%, and 75.9–94.3%, respectively. In relation to Cr, it was revealed that bioaccessible fraction in raw seafood samples was highly variable between species in this study, which was from 20.2 ± 1.1% (snapper) to 87.6 ± 3.3% (clam). As for Cu, there was an average of 71.5% (70.4–89.1%) of the initial concentrations in raw seafood muscles that were bioaccessible. Moreover, above 90% of Zn in seafood were bioaccessible, which ranged from 93.2 to 100.0% in this study.
In general, cooking processes (boiling, steaming, baking, and frying in this study) could affect the bioaccessibility of all metal(loid)s in selected seafood muscles in this study (Fig. 3). For all analyzed seafood species, all cooking practices decreased the bioaccessibility of Hg and MeHg significantly (p < 0.01). On average, Hg bioaccessibility in seafood after cooking reduced by 14%, 14%, 22%, and 39% for boiling, steaming, baking, and frying, respectively. The average reduced percentage for MeHg was 24%, and the maximum value was 44% (after frying). The bioaccessibility of As from seafood decreased by 2.7–18.6%, 3.5–14.0%, 6.5–26.8%, and 12.9–35.2% after boiling, steaming, baking, and frying, respectively. On average, cooking lowered the bioaccessibility of Cd, Pb, Ni, Cu, and Zn by 30.9%, 30.7%, 25.7%, 17.6%, and 22.4%, respectively. And we found that bioaccessibility reduction of As, Cd, Pb, Ni, Cu, and Zn after cooking was all in this order: steaming < boiling < baking < frying. To mention specially, the reduction of bioaccessibility of Cu in seafood after steaming (− 0.4%) was not as obvious as that after boiling (12.5%), baking (20.0%), and frying (38.3%). Alves et al. (2018) also found that bioaccessible Cu in seafood seemed not to be affected by steam. In relation to Cr in seafood, baking (by 37.7%) and frying (by 57.6%) decreased its bioaccessibility greatly, while the effect of boiling (by 11.5%) and steaming (by 18.0%) was much smaller. Our results were similar to Amiard et al. (2008), who reported that cooking led to a decrease in Cd, Cu, Pb, and Zn bioaccessibility in shellfish.
Thermal treatment could change the nutritional structure of seafood samples and influence the dissolvability of these metal(loid)s as a consequence (Wu et al. 2018). Metals, as well as As, have a high affinity for sulfhydryl groups in peptides and proteins, and heat deactivates proteins and enhances the breaking of bonds between them and seafood proteins, which makes their solubilization easier (Cheyns et al. 2017; Matos et al. 2015; de Filipps 1979). In a word, we believed two mechanisms influence the bioaccessibility of these metal(loid)s in seafood after cooking: The one is protein denaturation and the other one is the liberation of soluble chemicals into the cooking solution, or of volatile forms into the air (Liao et al. 2018; Liao et al. 2019a, b). Hence, the determined process affecting metal(loid)s bioaccessibility in seafood during cooking is the freeing of soluble portions, which decreases bioaccessibility as a result. Consequently, more severe heating conditions (e.g., baking and frying) are more conducive to decreasing bioaccessible fractions of metal(loid)s in seafood.
Assessment of health risk
Till now, health risk assessment of mixed metal(loid)s from seafood based on bioaccessibility was still very less. Thus, daily intake of metal(loid)s was calculated based on the bioaccessible concentration of raw and cooked seafood in this study. For an adult in Guangzhou City (average body weight is 60 kg), the estimation of Cr and Ni intake through consumption of seafood species in this study was in the range of 0.24–51.3 and 0.18–15.3 μg d−1, respectively. The recommended daily intake of Cr and Ni was 50–200 and 100–300 μg, respectively (Gu et al. 2015). The estimated intake of Zn from crab collected in this study was up to 60.13 μg kg−1 d−1, followed by scallop (39.20 μg kg−1 d−1), which were much lower than the tolerable daily intake (300 μg kg−1 d−1). Thus, these metals (Cr, Ni, and Zn) in seafood species in this study were within the appropriate range, which is a benefit for human health.
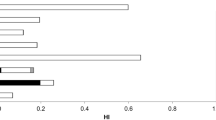
Daily intake of As, Hg, MeHg, Pb, Cd, and Cu from raw and cooked seafood was 0.027–13.24, 0.0003–0.10, 0.0002–0.065, 5.5 × 10−5–0.078, 0.0001–0.67, and 0.012–5.86 μg kg−1 d−1, respectively, based on bioaccessibility. These values were well below the maximum tolerable ones. As shown in Fig. 4, all values of tTHQ of the detected metal(loid)s were below 1.0 based on bioaccessible concentration while higher than 1.0 for spotted crab and scallop when calculated based on initial contents. Gu et al. (2015) also reported that the hazardous quotient values of Cd, Cr, Ni, Cu, and Zn were all less than 1, which revealed no significant adverse health effects with consumption of wild fishes captured from the South China Sea.
Total target hazard quotient (tTHQ) of hazardous metal(loid)s and through consumption of seafood in Guangzhou City based on bioaccessible contents (A) and initial contents (B), respectively. Least significant difference test demonstrated that there were significant differences at p < 0.05 among the THQ values for different cooking processes
Table 3 shows that TR values of skin cancer risk and TR values of bladder and lung cancer via exposure of iAs were in the range of 0–2.9 × 10−4 and 0–5.0 × 10−3, respectively. Range of acceptable risk levels for carcinogens is 10−4–10−6. Thus, results demonstrated that there is a potential of having bladder and lung cancer for adults via iAs exposure from some seafood species (e.g., clam, scallop, and oyster) in this study. In our previous study, we have verified that there existed As demethylation reaction during gastrointestinal digestion (Liao et al. 2018). Concerns should be focused particularly on those people who exposed to iAs from shellfish which can harm their bladder and lung.
Conclusion
This paper investigated the health risk of Hg, As, Cd, Pb, Cr, Ni, Cu, and Zn in seafood based on bioaccessibility in Guangzhou, China. The cooking effect was considered here. Results showed that cooking practices could decrease the metal(loid)s concentration from seafood. Decreased percent in contents of As, Hg, Cd, Pb, Ni, Cr, Cu, and Zn was in the range of 16.6–25.8, 14.8–45.7, 10.1–36.5, 1.6–49.4, 14.8–39.0, -5.4–21.6, 3.1–36.8, and 9.1–43.9%, respectively. On average, the release of metal(loid)s from seafood in this study followed the descending order of Hg > Zn > Ni > Cd > Pb > As > Cu > Cr. Cooking also brought about reduction of bioaccessibility of these metal(loid)s from seafood, and frying process decreased greatest. On average, cooking lowered the bioaccessibility of As, Hg, Cd, Pb, Ni, Cr, Cu, and Zn by 15.2, 26.1, 30.9, 30.7, 25.7, 31.2, 17.6, and 22.4%, respectively. Health risk calculation assessment results showed that Cr, Ni, and Zn in seafood species in this study were within the human health benefits range. And Hg, Cd, Pb, and Cu exposure from cooked seafood was within the safe dose. According to the total target hazardous quotient of mixed metal(loid)s, it seems the risk is tolerable. However, target cancer risk (TR) value of inorganic As from seafood showed that there is a potential of having cancer (especially bladder and lung cancer) for people exposure from iAs in seafood in this study.
Abbreviations
- C i :
-
Concentration of metal(loid)s in food (μg g−1)
- DC:
-
Daily consumption of food (g d−1)
- BW:
-
Average body weight (kg)
- THQ:
-
Target hazard quotient (unitless)
- EF:
-
Exposure frequency (days/year)
- ED:
-
Exposure duration (years)
- RfD:
-
The tolerable daily intake (μg d−1 kg−1)
- AT:
-
Average exposure time (days)
- TR:
-
Target cancer risk (unitless)
- CFS:
-
Cancer slope factor (mg kg−1 d−1)−1
- LSD:
-
Least significant difference
- SD:
-
Standard deviation
- DL:
-
Detected level
- SRM:
-
Standard reference material
- HPLC:
-
High-pressure liquid chromatography
- ICP:
-
Inductively coupled plasma
- MS:
-
Mass spectrometry
- dw:
-
Dry weight
- ww:
-
Wet weight
References
Alamdar, A., Eqani, S. A. M. A. S., Hanif, Nida, Ali, S. M., Fasola, M., Bokhari, H., et al. (2017). Human exposure to trace metals and arsenic via consumption of fish from river Chenab, Pakistan and associated health risks. Chemosphere, 168, 1004–1012. https://doi.org/10.1016/j.chemosphere.2016.10.110.
Alves, R. N., Maulvault, A. L., Barbosa, V. L., Fernandez-Tejedor, M., Tediosi, A., Kotterman, M., et al. (2018). Oral bioaccessibility of toxic and essential elements in raw and cooked commercial seafood species available in European markets. Food Chemistry, 267, 15–27. https://doi.org/10.1016/j.foodchem.2017.11.045.
Amiard, J. C., Amiard-Triquet, C., Charbonnier, L., Mesnil, A., Rainbow, P. S., & Wang, W. (2008). Bioaccessibility of essential and non-essential metals in commercial shellfish from Western Europe and Asia. Food and Chemical Toxicology, 46, 2010–2022. https://doi.org/10.1016/j.fct.2008.01.041.
ANVISA-Agência Nacional de Vigilância Sanitária (National Health Surveillance Agency). (2013). Alimentos. Portaria no 685, de 27 de agosto de 1998(*). Parcialmente revogada pela Resolução-RDC n. 42.
Atta, M. B., El-Sebaie, L. A., Noaman, M. A., & Kassab, H. E. (1997). The effect of cooking on the content of heavy metals in fish (Tilapia nilotica). Food Chemistry, 58, 1–4. https://doi.org/10.1016/0308-8146(95)00205-7.
Burger, J., Gaines, K. F., Boring, C. S., Stephens, W. L., Snodgrass, J., Dixon, C., et al. (2002). Metal levels in fish from the Savannah River: Potential hazards to fish and other receptors. Environmental Research, 89(1), 85–97. https://doi.org/10.1006/enrs.2002.4330.
Chem YQ. Retrieved from December 15, 2019 from http://www.chemyq.com/En/xz/xz13/126102edfdj.htm.
Chen, L., Zhou, S. L., Shi, Y. X., Wang, C. H., Li, B. J., Li, Y., et al. (2018). Heavy metals in food crops, soil, and water in the Lihe Ricer Watershed of the Taihu Region and their potential health risks when ingested. Science of the Total Environment, 615, 141–149. https://doi.org/10.1016/j.scitotenv.2017.09.230.
Cheyns, K., Waegeneers, N., Van de Wiele, T., et al., 2017. Arsenic Release from Foodstuffs upon Food Preparation. Journal of Agricultural and Food Chemistry, 65(11), 2443–2453.
de Filipps, L. F. (1979). The effect of heavy metal compounds on permeability of Chlorella cells. ZP flanzenphysiol, 92, 39–49. https://doi.org/10.1016/S0044-328X(79)80151-8.
Dolan, S. P., Nortrup, D. A., Bolger, P. M., Capar, S. G. (2013). Analysis of dietary supplements for arsenic, cadmium, mercury, and lead using inductively coupled plasma mass spectrometry. Journal of Agricultural and Food Chemistry, 51(5), 1307–1312.
EC. (2006). European Commission Regulation N. 1881/2006 of 19 December 2006. Setting maximum levels of certain contaminants in foodstuff. Official Journal of the European Union: Legislation Series, 65, 5–24. Retrieved from http://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX:32006R1881.
EFSA. (2012). Scientific opinion on the risk for public health related to the presence of mercury and methylmercury in food. EFSA Journal, 10(12), 2985.
El-Kady, A. A., & Abdel-Wahhab, M. A. (2018). Occurrence of trace elements in foodstuffs and their health impact. Trends in Food Science & Technology, 75, 36–45. https://doi.org/10.1016/j.tifs.2018.03.001.
FAO. (1983). Compilation of legal limits for hazardous substances in fish and fishery products. In: FAO fishery circular No. 464 (pp. 5–10). Rome: Food and Agriculture Organization of the United Nations. Retrieved June 22, 2019 from https://trove.nla.gov.au/version/22206109.
FAO. (2014). The state of world fisheries and aquaculture opportunities and challenges. Rome: Food and Agriculture Organization of the United Nations. Retrieved June 22, 2019 from http://www.fao.org/3/a-i3720e.pdf.
Fowler, B.A., Alexander, J., & Oskarsson, A. (2015). Chapter 6—Toxic metals in food. In Handbook on the toxicology of metals (4th ed.). Academic Press, 123–140. https://doi.org/10.1016/C2011-0-07884-5
Gao, Y., Baisch, P., Mirlean, N., da Silva, Rodrigues, Júnior, F. M., Van Larebeke, N., et al. (2018). Arsenic speciation in fish and shellfish from the North Sea (Southern bight) and Açu Port area (Brazil) and health risks related to seafood consumption. Chemosphere, 191, 89–96. https://doi.org/10.1016/j.chemosphere.2017.10.031.
GB. (2762-2017). National food safety standard maximum levels of pollutants in foods. Beijing, China. (in Chinese)
Gu, Y., Lin, Q., Wang, X., Du, F., Yu, Z., & Huang, H. (2015). Heavy metal concentrations in wild fishes captured from the South China Sea and associated health risks. Marine Pollution Bulletin, 96, 508–512. https://doi.org/10.1016/j.marpolbul.2015.04.022.
Hajeb, P., Sloth, J. J., Shakibazadeh, Sh, Mahyudin, N. A., & Afsah-Hejri, L. (2014). Toxic elements in food: Occurrence, binding, and reduction approaches. Comprehensive Reviews in Food Science and Food Safety, 13, 457–472. https://doi.org/10.1111/1541-4337.12068.
Houlbrèque, F., Hervé-Fernández, P., Teyssié, J., Oberhaënsli, F., Boisson, F., & Jeffree, R. (2011). Cooking makes cadmium contained in Chilean mussels less bioaccessible to humans. Food Chemistry, 126, 917–921. https://doi.org/10.1016/j.foodchem.2010.11.078.
Hu, Y., Zhang, W., Chen, G., Cheng, H., & Tao, S. (2018). Public health risk of trace metals in fresh chicken meat products on the food markets of a major production region in southern China. Environmental Pollution, 234, 667–676. https://doi.org/10.1016/j.envpol.2017.12.006.
IARC. (2018). Agents classified by the IARC monographs (Vols. 1–121). Retrieved from June 12, 2019 from http://monographs.iarc.fr/ENG/Classification/.
Ip, C. C. M., Li, X. D., Zhang, G., Wong, C. S. C., & Zhang, W. L. (2005). Heavy metal and Pb isotopic compositions of aquatic organisms in the Pearl River Estuary, South China. Environmental Pollution, 138, 494–504. https://doi.org/10.1016/j.envpol.2005.04.016.
Islam, Md S, Ahmed, Md K, Habibullah-Al-Mamun, Md, & Raknuzzaman, M. (2015). The concentration, source and potential human health risk of heavy metals in the commonly consumed foods in Bangladesh. Ecotoxicology and Environment Safety, 122, 462–469. https://doi.org/10.1016/j.ecoenv.2015.09.022.
Keshavarzia, B., Hassanaghaei, M., Moorem, F., Mehr, M. R., Soltanian, S., Lahijanzadeh, A. R., et al. (2018). Heavy metal contamination and health risk assessment in three commercial fish species in the Persian Gulf. Marine Pollution Bulletin, 129, 245–252. https://doi.org/10.1016/j.marpolbul.2018.02.032.
Klaue, B., Blum, J. D. (1999). Trace analyses of arsenic in drinking water by inductively coupled plasma mass spectrometry: High resolution versus hydride generation. Analytical Chemistry, 71, 1408–1414.
Liao, W., Wang, G., Li, K. M., & Zhao, W. B. (2018). Change of arsenic speciation in shellfish after cooking and gastrointestinal digestion. Journal of Agricultural and Food Chemistry, 66, 7805–7814. https://doi.org/10.1021/acs.jafc.8b02441.
Liao, W., Wang, G., Li, K. M., Zhao, W. B., & Wu, Y. (2019a). Effect of cooking on speciation and in vitro bioaccessibility of Hg and As from rice, using ordinary and pressure cookers. Biological Trace Element Research, 187(1), 329–339. https://doi.org/10.1007/s12011-018-1345-7.
Liao, W., Wang, G., Zhao, W. B., Zhang, M., Wu, Y., Liu, X. W., et al. (2019b). Change in mercury speciation in seafood after cooking and gastrointestinal digestion. Journal of Hazardous Materials, 375(5), 130–137. https://doi.org/10.1016/j.jhazmat.2019.03.093.
Ma, W. J. (2004). Study on dietary nutrition and health status of residents in Guangdong province in 2002. Guangdong: Guangdong People’s Press. (in Chinese).
Matos, J., Lourenço, H. M., Brito, P., Maulvault, A. L., Martins, L. L., & Afonso, C. (2015). Influence of bioaccessibility of total mercury, methyl-mercury and selenium on the risk/benefit associated to the consumption of raw and cooked blue shark (Prionace glauca). Environmental Research, 143, 123–129. https://doi.org/10.1016/j.envres.2015.09.015.
Mieiro, C. L., Coelho, J. P., Dolbeth, M., et al., 2016. Fish and mercury: Influence of fish fillet culinary practices on human risk. Food Control, 60, 575–581.
Ministry of Environmental Protection (MEP). (2013). Exposure factors handbook of Chinese population. Beijing: China Environmental Science Press.
Moreda-Piñeiro, J., Moreda-Piñeiro, A., Romarís-Hortas, V., Moscoso-Pérez, C., López-Mahía, P., Muniategui-Lorenzo, S., et al. (2011). In-vivo and in vitro testing to assess the bioaccessibility and the bioavailability of arsenic, selenium and mercury species in food samples. Trends in Analytical Chemistry, 30(2), 324–345. https://doi.org/10.1016/j.trac.2010.09.008.
Naito, S., Matsumoto, E., Shindoh, K., & Nishimura, T. (2015). Effects of polishing, cooking, and storing on total arsenic and arsenic species concentrations in rice cultivated in Japan. Food Chemistry, 168, 294–301. https://doi.org/10.1016/j.foodchem.2014.07.060.
Nfon, E., Cousins, I. T., Jarvinen, O., Mukherjee, A. B., Verta, M., & Broman, D. (2009). Trophodynamics of mercury and other trace elements in a pelagic food chain from the Baltic Sea. Science of the Total Environment, 407, 6267–6274. https://doi.org/10.1016/j.scitotenv.2009.08.032.
Perelló, G., Martí-Cid, R., Llobet, J. M., & Domingo, J. L. (2008). Effects of various cooking processes on the concentrations of arsenic, cadmium, mercury, and lead in foods. Journal of Agricultural and Food Chemistry, 56, 11262–11269. https://doi.org/10.1021/jf802411q.
Praveena, S. M., & Omar, N. A. (2017). Heavy metal exposure from cooked rice grain ingestion and its potential health risks to humans from total and bioavailable forms analysis. Food Chemistry, 235, 203–211. https://doi.org/10.1016/j.foodchem.2017.05.049.
Schmidt, L., Bizzi, C. A., Duarte, F. A., Muller, E. I., Krupp, E., Feldmann, J., et al. (2015). Evaluation of Hg species after culinary treatments of fish. Food Control, 47, 413–419. https://doi.org/10.1016/j.foodcont.2014.07.040.
Sobhanardakani, S. (2017). Potential health risk assessment of heavy metals via consumption of caviar of Persian sturgeon. Marine Pollution Bulletin, 123, 34–38. https://doi.org/10.1016/j.marpolbul.2017.09.033.
Wang, L. D. (2005). Investigation of nutrition and health China residents: 2002 synthesis report. Beijing: People’s Medical Publishing House.
Wu, Z., Feng, X. B., Li, P., et al. (2018). Comparison of in vitro digestion methods for determining bioaccessibility of Hg in rice of China. Journal of Environmental Science, 68, 185–193. https://doi.org/10.1016/j.jes.2017.10.008.
Zhong, W., Zhang, Y., Wu, Z., Yang, R., Chen, X., Yang, J., et al. (2018). Health risk assessment of heavy metals in freshwater fish in the central and eastern North China. Ecotoxicology and Environmental Safety, 157, 343–349. https://doi.org/10.1016/j.ecoenv.2018.03.048.
Acknowledgements
This research was financially supported by the National Natural Science Foundation of China (No. 21207046), the Major Science and Technology Program for Water Pollution Control and Treatment (2017ZX07202006-002) and the Fundamental Research Program for the State Level Public Welfare Research Institutes (No. PM-zx703-201701-054).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liao, W., Zhao, W., Wu, Y. et al. Multiple metal(loid)s bioaccessibility from cooked seafood and health risk assessment. Environ Geochem Health 42, 4037–4050 (2020). https://doi.org/10.1007/s10653-020-00661-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-020-00661-9