Abstract
Geophagy, the intentional consumption of earth materials, has been recorded in humans and other animals. It has been hypothesized that geophagy is an adaptive behavior, and that clay minerals commonly found in eaten soil can provide protection from toxins and/or supplement micronutrients. To test these hypotheses, we monitored chimpanzee geophagy using camera traps in four permanent sites at the Budongo Forest Reserve, Uganda, from October 2015–October 2016. We also collected plants, and soil chimpanzees were observed eating. We analyzed 10 plant and 45 soil samples to characterize geophagic behavior and geophagic soil and determine (1) whether micronutrients are available from the soil under physiological conditions and if iron is bioavailable, (2) the concentration of phenolic compounds in plants, and (3) if consumed soils are able to adsorb these phenolics. Chimpanzees ate soil and drank clay-infused water containing 1:1 and 2:1 clay minerals and > 30% sand. Under physiological conditions, the soils released calcium, iron, and magnesium. In vitro Caco-2 experiments found that five times more iron was bioavailable from three of four soil samples found at the base of trees. Plant samples contained approximately 60 μg/mg gallic acid equivalent. Soil from one site contained 10 times more 2:1 clay minerals, which were better at removing phenolics present in their diet. We suggest that geophagy may provide bioavailable iron and protection from phenolics, which have increased in plants over the last 20 years. In summary, geophagy within the Sonso community is multifunctional and may be an important self-medicative behavior.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Humans and other animals eat a variety of nutritive and non-nutritive materials to sate cravings and physiological needs. However, little is known about geophagy, the deliberate consumption of weathered earth materials, (e.g., soil from the forest floor, termite and other insect mounds, earthen bricks). Geophagy has been observed in over 300 species of mammals, birds, and reptiles (Young et al. 2011) and more than 130 species of non-human primates (NHP) (Pebsworth et al. 2019). Within the genus Pan, researchers have documented geophagy in Pan paniscus (bonobo) (Kano and Mulavwa 1984), Pan troglodytes schweinfurthii (East African chimpanzee) (Mahaney et al. 2005; Reynolds et al. 2015), Pan troglodytes troglodytes (Central African chimpanzee) (Basabose 2002), and Pan troglodytes versus (Western chimpanzee) (Gašperšič and Pruetz 2011).
Despite the high prevalence of geophagy across many species, the causes and consequences of this behavior among chimpanzees and other animals are not well understood. Two adaptive hypotheses have been posited: that soil provides supplementation of micronutrients that may be lacking in the diet and that it confers protection against plant secondary compounds (Gilardi et al. 1999; Johns 1986; Ta et al. 2018), parasites (Bicca-Marques and Calegaro-Marques 1994; Knezevich 1998), and pathogens (Ketch et al. 2001).
Under the supplementation hypothesis, soil provides micronutrients that are deficient in the diet (Kreulen 1985). Micronutrient deficiencies can adversely affect health and increase the risk of disease (Rode et al. 2003). A corollary is that if geophagy supplements micronutrients, it would occur more frequently when animals require more nutrients and might be prone to deficiencies, e.g., while pregnant or lactating. Previous chimpanzee geophagy studies have documented that mineral acquisition is plausible, but did not analyze eaten soil under physiological conditions (Mahaney et al. 1996, 1997, 2005; Reynolds et al. 2015). One primate study has used in vitro Caco-2 experiments (Pebsworth et al. 2013). It showed that even though iron was present in soil eaten by chacma baboons, the iron was not bioavailable. While it has not been suggested for non-human animal geophagy, it should be noted that soil eating by humans has been associated with micronutrient deficiencies rather than corrections (Abrahams 1997; Miller et al. 2016; Reid 1992; Severance et al. 1988; Young 2010).
Under the protection hypothesis, geophagy is considered a behavioral strategy to mitigate GI distress. Most consumed soils contain clay minerals, which can provide protection by directly adsorbing agents that cause GI distress (Dominy et al. 2004; Gilardi et al. 1999; Ta et al. 2018), reinforcing the luminal epithelium of the GI tract (Gilardi et al. 1999; González et al. 2004; Said et al. 1980), and lysing bacterial cells (Papaioannou et al. 2005). Plant secondary compounds naturally occur in many dietary items consumed by NHPs and can result in both chronic and temporary GI distress, including nausea, diarrhea, and vomiting. In addition to causing GI distress, plant secondary compounds can reduce the palatability of a plant and inhibit proteolytic enzymes that are important for breaking down proteins into amino acids (DeGabriel et al. 2009; Gurian et al. 1992; Hladik 1977; Oates 1978). Non-human primates use a variety of physiological, morphological, and behavioral adaptations to contend with plant secondary compounds. Studies have shown that soil eaten by NHPs adsorbed polar plant secondary compounds like alkaloids and phenolics (Johns 1986; Ta et al. 2018); however, nonpolar compounds like terpenes were poorly adsorbed (Ta et al. 2018). These studies used pure commercially available compounds that resembled those found in the diet but did not use plant extracts eaten by humans or NHPs.
A corollary is that if geophagy were protective, it would occur more frequently among individuals with diets high in plant secondary compounds. Previous chimpanzee geophagy studies have explored the protection aspect by assessing adsorption of toxins thought to be found in the diet (Aufreiter et al. 2001). This study tested soil protection by measuring whether alkaloids were adsorbed by soil eaten by chimpanzees. They tested four pure alkaloid compounds that resembled those found in the diet but did not create extracts from plants eaten by chimpanzees.
Previous chimpanzee geophagy studies have tested the supplementation hypothesis by digesting soil samples with an acid (ammonium oxalate) that resulted in dissolution of the soil elements by the GI tract but did not realistically simulate GI conditions (Aufreiter et al. 2001). Another study used “aqua regia” to digest soil samples, which also does not simulate GI conditions (Reynolds et al. 2015). Consequently, these results may have overestimated the amount of micronutrients that were available to chimpanzees. To date, no studies have used an in vitro technique to determine whether micronutrients present in soil eaten by chimpanzees were bioavailable. Such tests demonstrate bioavailability under in vitro conditions. Further, in vivo testing is required to show that micronutrients are, in fact, bioavailable.
Therefore, the objective of this study was to improve our understanding of geophagy and its potential for micronutrient supplementation and protection by examining geophagy among East African chimpanzees living in the Budongo Forest. We used the following innovative techniques: camera traps to monitor permanent geophagy sites, a soil digestion that simulated physiological conditions, extracts created from plants eaten by the chimpanzees, and in vitro Caco-2 cell assay to assess iron bioavailability. We posited that:
- 1.
Under physiological conditions, consumed soils would not provide micronutrients.
- 2.
Consumed soils would adsorb the plant secondary compounds that were found in these chimpanzees’ diet.
- 3.
Because micronutrient demands are not equivalent, we posit that female chimpanzees would spend more time at geophagy sites.
Methods
Study site and subjects
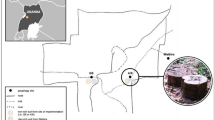
The Budongo Forest Reserve (1.617–2.0°N, 31.367–31.766°E) is a moist semi-deciduous tropical forest located in the Masindi District of western Uganda (Eggeling 1947; Plumptre and Reynolds 1996). There are four main forest types: Cynometra-dominated, mixed, colonizing, and swamp (Eggeling 1947). Rainfall averages 1600 mm per year and exhibits a bimodal pattern, with a main annual dry season occurring during December–February (Reynolds 2005). A trail system has been cut across the local floodplain tracts that cross the main study area of the forest. Trails are 0.5 m wide and are cut 100 m apart forming 100 m × 100 m blocks. The trails form a grid that is labeled with numbers and letters. Humans, chimpanzees, and other animals use this trail system (Fig. 1).
From September 2015 to October 2016, we observed the Sonso community of chimpanzees (Pan troglodytes schweinfurthii) who live within and around the Budongo Forest Reserve. During the study, there were 59 members of the community: 22 adult females (8 lactating), 11 adult males, 18 juveniles, and 8 infants.
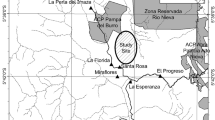
The chimpanzees had a varied diet that included fruits, leaves, bark, meat, and soil from permanent geophagy sites, fine silt along riverbanks (Reynolds et al. 1998), and termite mounds (Reynolds et al. 1998, 2015; Tweheyo et al. 2006). The permanent geophagy sites are located along the River Sonso, which contained clean, clear (not clay-infused) water (Fig. 2). After it rains, clay particles become suspended in standing water. Because clay is a component of soil, we include the consumption of clay-infused water as geophagy.
We monitored four permanent geophagy sites used by the Sonso community of chimpanzees (Fig. 2). Three were located in the Sonso community’s home range (N6, G6, and 4/6) and are located at the base of trees. N6 is at the western edge of the chimpanzee’s home range; G6 and 4/6 are in the core area of their home range. The fourth site was located in Waibira, which is not in the community’s core range but rather the periphery of their home range. This site was not located at the base of trees.
All applicable international, national, and institutional guidelines for the care and use of the chimpanzees were followed. All research undertaken and reported complied with The University of Texas at San Antonio’s Institutional Animal Care and Use Committees (IACUC) and approved in protocol #PA005-10/18.
Camera traps
We monitored behavior at four permanent geophagy sites using Bushnell Trophy camera traps. Cameras were activated by infrared motion and heat detection. We positioned the cameras approximately 20 cm above the ground near fixed locations where chimpanzees and other animals were observed eating soil. Cameras operated continuously, except when the memory card became full, batteries failed, or a camera malfunctioned. We programmed camera traps to take 59-s videos with a 1-s interval between videos, and all cameras were synchronized by date and time. Cameras were checked several times per month to ensure they were functional and to exchange batteries and SD cards.
For each video, we documented date, time, site, visibility, whether the chimpanzees stopped at the geophagy sites or were in transit. When the chimpanzees stopped at the geophagy sites, we documented age-class and the sex of adult individuals, whether there was standing water, and use of plant material (leaves or moss) to extract soil and clay-infused water.
Plant collection and plant secondary compound extraction
We collected and dried ten dietary items known to contain plant secondary compounds that the chimpanzees ate during the study. Dried plant samples were exported with Uganda National Council of Science and Technology (UNCST) permit NS 548, and also received phytosanitary certification from the Ugandan Ministry of Agriculture on Export license #1804. At the University of Ottawa, they were ground with a Wiley mill to pass through a 30 mesh screen and extracted with 80% ethanol (1:20 w:v), filtered, then dried using a rotary evaporator and a lyophilizer, as described elsewhere (Spoor et al. 2006).
Clay-infused water collection and screening
Two samples of standing clay-infused water were collected at all four geophagy sites when water began to accumulate during the rainy season (n = 8). We screened these samples in the field for available iron using a ferrozine assay test (see Bioavailability of Iron).
Soil collection and preparation
Forty-five soil samples were collected at four permanent sites exactly where chimpanzees had been observed and camera traps captured them eating soil (Fig. 2). No control samples were collected because there were no obvious areas in the camera traps view that were avoided by the chimpanzees. Using a clean trowel, approximately 150 g of soil was collected and placed in a sealable polyethylene bag. Samples were labeled and photographed in accordance with best practices (Young et al. 2008). Each sample was subsequently dried and homogenized.
Physical characterization of soil
Forty-five soil samples were analyzed and archived at Makerere University, Kampala, Uganda. Makerere University’s Soils Department processed and analyzed the samples for pH and particle size. The samples were air-dried, pounded and then passed through a 2-mm sieve to remove any debris. Soil pH was measured in a 1:2.5 soil/water ratio. Particle size distribution was measured using the hydrometer method (Bouyoucos 1962).
Soil samples received phytosanitary certification from the Ugandan Ministry of Agriculture on Export license #1803 and were shipped to the James Hutton Institute, the University of Ottawa, and Cornell University, where mineralogical, chemical, and bioavailable iron analyses were conducted.
Soil mineralogical and chemical characterization
From the 45 samples collected, we selected eight samples that were representative of the samples collected at the geophagy sites. We characterized the mineralogical composition of the bulk and < 2 μm clay size fraction using these eight soil samples (one from N6, two from G6, two from 4/6, and three from Waibira) using quantitative X-ray diffraction (XRD) procedures at the James Hutton Institute. The number of samples analyzed is proportional to the frequency of geophagy at the site, the size of the site, and how many areas within the site were used by the chimpanzees. Preparation and analysis followed techniques described elsewhere (Hillier 1999; Omotoso et al. 2006).
The same eight bulk soil samples were also analyzed for total major element geochemistry by X-ray fluorescence spectrometry (XRF) on fused glass beads (Phillips PW2404), total organic carbon content by combustion (Thermo Finnegan Elemental Analyzer FlashEA 1112 Series), and oxalate extractable iron, aluminum, silicon, manganese, and phosphorus (Farmer et al. 1983).
Supplementation and detoxification analyses
Simulated digestion procedures
We digested the 10 plant and 8 soil samples using a modified, physiologically based extraction method (Gilardi et al. 1999; Klein et al. 2008; Ruby et al. 1996). Soil collected at geophagy sites (1 g) was suspended in 50 mL of simulated gastric fluid (SGF) with pepsin (pH = 1.2). 2.5-mL aliquots were drawn and treated with varying concentrations of each plant extract. Samples were incubated under agitation (250 rpm) at 37 °C for 1 h with simulated gastric fluid (SGF). Next, the pH was adjusted to 5.5–6.0 using 1.0 M sodium bicarbonate (NaHCO3) to mimic intestinal conditions and 2.5 mL of simulated intestinal fluid (SIF) with pancreatin was added to each sample. The samples were then incubated for 2 h at 37 °C under agitation. After incubation, the samples were centrifuged at 3900 rpm. For each sample, a 2-mL aliquot of the supernatant was transferred to a Falcon tube where 2 mL of methanol was added to precipitate the enzymes. These samples were then centrifuged at 3900 rpm to yield the final supernatant for chemical analyses. Simulated digestive fluids were prepared according to USP specifications (US Pharmacopeia 2017).
Element release from soil under biological conditions
We used two methods to examine elements released from the digested soils. The first method was the simulated physiological digestion, as described above but without pepsin and pancreatin. The second method was modified from Gilardi et al. (1999), where 1 g of soil was digested under agitation for 1 h in 0.01 M hydrochloric acid (HCl). The rationale for using the second method was to measure the concentration of Na, K, and P released from the soils as the simulated digestive fluids contain NaCl, NaOH, and KH2PO4. After digestion, the soil samples were analyzed by inductively coupled plasma emission spectroscopy (ICP-ES) and mass spectrometry (ICP-MS) at the University of Ottawa (ICP-ES: Agilent (Varian) VistaPro ICP spectrometer; ICP-MS: Agilent 8800 triple quadrupole ICP-MS). The limit of detection (LOD) was the following ppm: Al 0.1, Ba 0.0, Ca 0.2, Fe 0.0, K 0.6, Mg 0.0, Mn 0.0, Na 0.3, P 0.5, S 0.8, Sr 0.0.
Bioavailability of iron
We assessed bioavailability of iron using two different techniques: ferrozine assay and the Caco-2 cell culture model. The colorimetric ferrozine assay measures Fe+2 and Fe+3 in water (Riemer et al. 2004), and we tested two clay-infused water samples at each geophagy site. Ferrous ions form a complex with the chromogen ferrozine, and the intensity of the color is proportional to the iron concentration in the sample. We measured bioavailable iron in four soil samples, one per geophagy site, using the Caco-2 cell culture model in which ferritin levels of colon cells grown in vitro approximate bioavailability (Glahn et al. 1998). Fewer samples were analyzed due to expense and limited soil sample. Preparation and analysis techniques are as previously described (Pebsworth et al. 2013; Seim et al. 2013).
Phenolics and detoxification trials
We measured total phenolics of ten Ugandan plant extracts using the Folin–Ciocalteu reagent with gallic acid as the standard and 50% methanol as the blank (control) as previously described (Spoor et al. 2006). Subsequently, we determined the average gallic acid equivalents (GAE) adsorbed from plant extracts by soils from three of the four geophagy sites (G6, 4/6, WB) and two reference clay minerals: kaolinite (1:1 clay mineral) and montmorillonite (2:1 clay mineral). N6 was excluded as little geophagy occurred there. We compared mean adsorption of phenolics for the 10 relevant plant extracts by site and two reference clay minerals (sample 15 from WB, 9 from G6, and 5 from 4/6, kaolinite, and montmorillonite).
Statistical analyses
We ran basic statistics in Stata 15 (StataCorp. 2017. Stata Statistical Software: Release 15. College Station, TX: StataCorp LLC). We used X2 to test whether any age-class or sex frequented geophagy sites more frequently than would be anticipated if each group equally visited sites, proportional to their representation in the community. We set alpha to 0.01.
We used the Kruskal–Wallis test with a post hoc Dunn’s test to determine whether there was a difference in phenolic adsorption between geophagy sites and two reference clay minerals: montmorillonite and kaolinite. We set alpha to 0.05.
Results
Camera traps
Four camera traps operated from October 1, 2015 to October 26, 2016 (n = 1568 camera trap days), of which some cameras were non-operational for 203 camera trap days (13.0%). During this time, cameras captured 1185 videos of chimpanzees at four permanent geophagy sites (Fig. 2). Of these videos, 108 (9.1%) were of chimpanzees moving through the sites and were excluded from the analyses (n = 1077). The majority of videos were taken at sites 4/6 (n = 516) and G6 (n = 361), which are located within the core area of the chimpanzee’s home range (Newton-Fisher 2003) (Fig. 2). The fewest videos were captured at N6 (n = 28), which is located at the western edge of the Sonso community’s home range. The fourth site, Waibira, is located at the eastern edge of the community’s home range and captured 172 videos (Fig. 2).
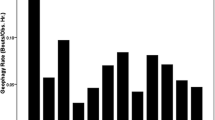
We assessed chimpanzee presence at geophagy sites by age-class and sex: adult females were present in 43.0% of videos, adult males 11.9%, juveniles 31.4%, and infants 13.7% (Fig. 3). Infants were not observed eating soil but represent the number of lactating females present. These frequencies are significantly different than expected under the null hypothesis (X2 = 37.09, df = 3, p < 0.0001). Adult females (lactating and non-lactating) spent more time than expected at geophagy sites eating soil or drinking clay-infused water and adult males and juveniles spent less time than expected.
Clay-infused water consumption
Chimpanzees primarily consumed clay-infused water at sites 4/6, G6, and N6. Visibility was poor in approximately 3.1% of the videos, but the remaining videos showed chimpanzees using several techniques to collect clay-infused water: (1) they picked and masticated leaves or moss and used them as a “sponge” and then placed the sponge into their mouths, (2) they put their heads into holes located at the base of tree roots and suctioned the clay-infused water out, and (3) they placed their hands into the holes and drank out of their hands or off the tips of their fingers.
Soil consumption
The chimpanzees primarily consumed soil at one site, Waibira (WB) 90.2% versus 9.8% at other sites. Depending on the season, they used four different techniques to eat soil or drink clay-infused water (Fig. 4A–D).
Chimpanzee geophagy feeding behavior at Waibira. A Chimpanzees dipped their hands or their fingers into the soft soil and licked the soil off, B while suspended, chimpanzees suctioned up clay-infused water with their mouths, C they picked and masticated leaves and then used them as a “sponge” or napkin and then placed the sponge into their mouths, D they broke off clumps of dry soil and ate them directly (Photo credit, PA Pebsworth)
Soil physical characterization
Particle size distribution analysis demonstrated that soils at the four geophagy sites contained large quantities of sand. The eight soil samples were classified as sandy loam, sandy clay, loamy sand, and silt loam (Table 1).
Soil chemical characterization
The soil samples ranged in pH from 6.1 to 7.3. The oxalate extractable iron data showed that all eight samples ranged from 569 to 6403 ppm corresponding to between 6 and 28% of total iron in a poorly/non-crystalline form. Organic carbon contents were indicative of organic matter contents of 1% or less in most soils with two samples, one from G6 and one from 4/6 geophagy sites, having higher organic carbon contents (Table 2).
Soil mineralogical characterization
The XRD results for the soil samples showed large proportions of quartz, which presumably account for the large amounts of sand and silt recorded by particle size analysis.
The XRD analysis of the clay fraction (< 2 μm) indicated that kaolinite, a 1:1 clay mineral, an expandable (i.e., swelling) 2:1 clay mineral, and an appreciable amount of X-ray amorphous material were present in the samples (Table 2). The expandable clays from the Waibira site show features indicative of mixed-layering of smectite with kaolinite, whereas at the other locations the expandable clays appear more uniformly smectitic in character. Other minerals present included minor/trace amounts of feldspars, mainly potassium forms, and minor/trace rutile, anatase, and ilmenite. No crystalline iron oxides were detected.
The bulk soil geochemistry showed that total iron in the soils ranges from 9372 to 22,522 ppm (0.94–2.25%). Although a minor amount of total iron may occur in ilmenite, the combination of mineralogical and geochemical analyses indicated that the main location of iron must be in clay minerals, principally the expandable swelling clays, since no other minerals that can accommodate iron were present in the samples.
Element release from soil under biological conditions
Element release analyses using techniques that simulated biological conditions showed that some samples yielded calcium (Ca), iron (Fe), and magnesium (Mg) in quantities greater than 5 ppm (Table 3). These analyses also demonstrated that there was agreement between the two digestion methods with the exception of the elements (K, Na, P) that were present in the extraction fluid (Table 3). With regards to iron, HCl digestion values were consistently higher and ranged from 5.3 to 36.9 while the PBET method values ranged from 0.3 to 2.1.
The extractions also showed the presence of minor amounts of heavy metals in the soil samples. The range of values was as follows: arsenic (As) 0.26–1.79 ppm, cadmium (Cd) 0.33–2.24 ppm, chromium (Cr) 10.44–19.94 ppm, and lead (Pb) 7.13–11.20 ppm.
Bioavailability of iron
The ferrozine assay test suggested that clay-infused water from all four sites contained iron. The Waibira site showed the fastest and darkest change in color suggesting that more iron was present at this site than the other sites in Budongo.
The Caco-2 cell culture model showed, however, that iron found in geophagic soil at Waibira was not bioavailable, but was bioavailable at N6, G6, and 4/6 (Fig. 5).
Iron bioavailability in four geophagic soil samples measured in triplicate. Total iron (in parentheses) and ferritin formation values are mean ± SEM, n = 4. Total iron was calculated from ICP data and iron bioavailability, based on ferritin data from Caco-2 experiments. Baseline refers to cell ferritin levels in wells without soil, which is a quality control measure used to confirm that the media was free of contaminant iron
Phenolics and detoxification trials
Total phenolics of eight plants commonly eaten and two barks infrequently eaten by the chimpanzees revealed that GAE was high (Table 4).
The five earth samples (G6, 4/6, Waibira, and two reference clays) evaluated suggest that plant phenols found in leaves, seeds, bark, and flowers are better adsorbed than fruit (Table 5). The negative values are likely due to the lack of biological replicates (N = 1) and the limit of detection. It is unlikely that the negative values were caused by phenolics in the soil being released after digestion, as this would have been accounted for by the 50% methanol blank (control). More research is needed to determine what factors (e.g., presence of sugars, fiber) affect soil adsorption.
The soil from the Waibira site that contained more 2:1 clay minerals removed more phenolics and was comparable with montmorillonite. Soils from G6 and 4/6 removed fewer phenolics and were comparable to kaolinite (Fig. 6). Using the Kruskal–Wallis test with a post hoc Dunn’s test, WB 5 and montmorillonite were significantly different (p < 0.05) from kaolinite but not G6 9 or 4/6 5.
Discussion
In this study, we characterized both geophagic behavior and geophagic soil using cutting-edge techniques including 13 months of camera trap data, a soil digestion that simulated intestinal biochemistry, and Caco-2 in vitro experiments to assess bioavailability of iron. We found evidence in support of both the supplementation and protection hypotheses.
East African chimpanzees have been studied the longest, and more information on geophagy has been collected than for other subspecies of chimpanzees and bonobos (Goodall 1963; Mahaney et al. 1997; Nishida and Uehara 1983; Tweheyo et al. 2006). Bonobos have been observed eating termite mound soil, but little else is known (Kano and Mulavwa 1984). A dietary ecology study conducted on Central African chimpanzees documented geophagy from fecal samples and only 0.1% of samples contained soil (Basabose 2002). Geophagy has also been observed in Western chimpanzees, but only traces of soil were found in their diet (Gašperšič and Pruetz 2011). It is not possible to compare geophagy among chimpanzees, but all subspecies eat soil but seemingly to varying degrees and with different earth materials.
Chimpanzees are a fission–fusion society and do not move cohesively. In the past, researchers documented soil consumption using focal animal observation. This technique works well when chimpanzees do not return to the same source (e.g., termite mounds). However, camera traps are ideal when the same sites are used repeatedly. The Sonso community of chimpanzees residing in the Budongo Forest Reserve regularly frequented permanent geophagy sites to eat soil and drink clay-infused water. At three permanent geophagy sites, the chimpanzees primarily drank clay-infused water. It should be noted that if the chimpanzees were seeking only water, it was readily available nearby at the Sonso River (Fig. 2). The ferrozine-based assay indicated that there was iron present in clay-infused water at all four permanent sites. The fourth geophagy site was located in Waibira. At this site, chimpanzees ate soil which adsorbed phenolic compounds present in their diet.
Geophagy seems to be increasing in Budongo. A 10-month study conducted in 1998 observed geophagy four times in 8 months (Tweheyo et al. 2006). A subsequent 16-month study in 2000 observed geophagy three times. A third 16-month study conducted in 1997–1998 identified soil in 21.1% (n = 34) of fecal samples analyzed for diet (Fawcett 2000). Since 2000, the chimpanzees have become more habituated to humans and the frequency of geophagy also appears to have markedly increased (Reynolds et al. 2015). The 1077 episodes of geophagy captured on camera traps in this study underestimate soil eating, as cameras did not capture geophagy at termite mounds and other locations where the chimpanzees are known to eat soil (Reynolds et al. 2015; Tweheyo et al. 2006).
An increase in geophagy could be motivated by a need for protection from gastrointestinal distress caused by an increased concentration of plant secondary compounds over time. In 1998, Reynolds et al. reported the mean condensed tannin content for the top 12 foods consumed by the Budongo chimpanzees. At this time, no food item had greater than 30-ppm tannins and four had almost no tannin present. Although we can’t compare our total phenolics data with 1998 tannins data because of the difference in techniques, all plants tested contained appreciable levels of phenolic compounds. Additionally, results from Kibale National Park, also located in Uganda, have documented a decline in the nutritional quality of tropical leaves (Rothman et al. 2015). Indeed, it has been demonstrated that a consequence of climate change is a decline in the nutritional composition of leaves and greenhouse experiments have found that elevated CO2 levels resulted in a 19% increase in condensed tannins (Marsh et al. 2013). Studies have shown that tannins are potent inhibitors of iron bioavailability so an increase in dietary tannins could require additional iron to be consumed in order to meet iron requirements (Glahn et al. 2002).
Even though camera traps did not capture all geophagy episodes, they proved valuable at documenting chimpanzee soil-eating repertoire and patterns of geophagy unique to age-class and sex were revealed (Fig. 3). One such behavior was the chimpanzees’ use of leaves when eating soil (Fig. 4A, C). It is possible that soil or clay-infused water enhanced the pharmacological properties present in the leaves used (Klein et al. 2008). Alternatively, this community possesses considerable leaf technology (Gruber et al. 2009) and they may be using leaves to keep their hands clean, as well as absorbing clay-infused water.
Micronutrients were available from the soil but not in great concentrations (Table 3). Regardless, in addition to iron some soil samples had calcium and magnesium in quantities greater than 5 ppm, which suggests that some micronutrients were available from eaten soil.
Past human and non-human primate studies have found little bioavailable iron in geophagic soils (Pebsworth et al. 2013; Seim et al. 2013). For example, soil eaten by chacma baboons in South Africa contained approximately 6 ng ferritin/mg cell protein, which was just slightly greater than the baseline (Pebsworth et al. 2013). Conversely, three of four soil samples from permanent geophagy sites (4/6, G6, N6) contained approximately 20 ng ferritin/mg cell protein, i.e., appreciable amounts (Fig. 5). The fourth geophagy site, Waibira, had high total iron levels, but bioavailable iron levels were below the baseline. This indicates that components of the sample were able to complex iron and thus strongly inhibit iron bioavailability. The Caco-2 cell bioassay is extremely sensitive to bioavailable iron; thus, the trace amounts present in the baseline digest (containing only the digestive enzymes and culture media) typically yield cell ferritin levels in the 2–7 ng ferritin/mg cell protein range. From extensive experience, we know that values below baseline resulting from exposure to a sample indicate strong complexation of the iron in the sample and thus extending to the trace iron in the digest solutions (Ariza-Nieto et al. 2007).
Geophagy sites 4/6, G6, and N6 are located at the base of trees, and it is possible that trees exude phytochemicals that enhance iron bioavailability or that iron was more exchangeable at these sites (Kobayashi et al. 2018). It is also possible that iron was made bioavailable by soil bacterial activity. Cursory Illumina-based 16S sequencing revealed that microbial DNA from N6 contained abundant amounts of typical soil microbial populations (e.g., actinomycetes and acidobacteria) in and sparse amounts of iron oxidizers and iron reducers (Emerson, unpublished data). More microbiology work needs to be completed, but this result suggests that some microbiological iron mobilization is taking place at geophagy sites in Budongo. Previous studies have indicated that geophagy sites are typically on higher, well drained, and more mature segments of the landscape (Mahaney and Krishnamani 2003). The Budongo soils appear to be immature and are poorly drained during the rainy season, which could contribute to iron mobilization.
Ferrozine-assays indicated that clay-infused water found at all four permanent geophagy sites contained iron. It should be noted, however, that this form of iron may not be bioavailable. Iron in solution is often tightly bound to a compound and not exchangeable to the iron uptake transporters. There can also be large molar excess of a compound, relative to the iron, that outcompetes the iron transporter for the iron (Engle-Stone et al. 2005; Glahn et al. 1995; Hart et al. 2017). These findings are not consistent with our first hypothesis and instead suggest plausibility of chimpanzees eating soil to supplement micronutrients. More research is needed to determine the factor(s) that made iron bioavailable and whether the calcium and magnesium were bioavailable.
Phenolic compounds were present in all plant extracts that we tested (Table 4) and were bound by the 1:1 and 2:1 clay minerals found in the eaten soil (Table 2). Clay minerals were also consumed as a colloidal suspension in clay-infused water. They would have been concentrated because the sand would have settled and been excluded. Waibira soils were able to adsorb similar amounts of total phenolics as pure montmorillonite (Fig. 6). These soils contained more expandable 2:1 clay minerals (Table 2), which are better at adsorbing plant secondary compounds than kaolinite (Ta et al. 2018). During this study, the top four foods eaten by the chimpanzees were Cynometra alexandri, Ficus mucuso, Broussonetia papyrifera, and F. exasperata (Villioth 2019). All contained phenolic compounds which were adsorbed by soil from Waibira, with the exception of F. mucuso. These findings are consistent with our second hypothesis that chimpanzees consumed soil that adsorbed plant secondary compounds found in their diet.
Our data further indicate that adult females spent more time than expected at geophagy sites than adult males or juveniles (Fig. 3). Other non-human primate studies have also documented that females spend more time eating soil than males (Ampeng et al. 2016; Pebsworth et al. 2012; Wrangham et al. 2014; Zhao et al. 2013). This may be due to an increase in micronutrients required during pregnancy and while lactating. These findings are also consistent with our third hypothesis as female chimpanzees spent more time at geophagy sites than males and juveniles.
In conclusion, the soil eaten by chimpanzees contained elevated levels of iron that were bioavailable. The soils also contained 2:1 and 1:1 clay minerals, which could provide protection from GI distress. Sources of distress could include tannins and other plant secondary compounds. It is likely that these dietary toxins will increase with climate change. We urge future conservationists, park managers, and government policy makers to pay special attention to geophagy sites and other areas where animals congregate to eat soil. Such sites may become increasingly vital to animal health and survival and deserve special protection.
References
Abrahams, P. W. (1997). Geophagy (soil consumption) and iron supplementation in Uganda. Tropical Medicine & International Health: TM & IH,2(7), 617–623.
Ampeng, A., Shukor, M. N., Sahibin, A. R., Idris, W. M. R., Ahmad, S., Mohammad, H., et al. (2016). Patterns of mineral lick use by Northwest Bornean orangutans (Pongo pygmaeus pygmaeus) in the Lanjak Entimau Wildlife Sanctuary, Sarawak, Malaysia. European Journal of Wildlife Research,62(1), 147–150. https://doi.org/10.1007/s10344-015-0983-8.
Ariza-Nieto, M., Blair, M. W., Welch, R. M., & Glahn, R. P. (2007). Screening of iron bioavailability patterns in eight bean (Phaseolus vulgaris L.) genotypes using the Caco-2 cell in vitro model. Journal of Agricultural and Food Chemistry, 55(19), 7950–7956. https://doi.org/10.1021/jf070023y.
Aufreiter, S., Mahaney, W. C., Milner, M. W., Huffman, M. A., Hancock, R. G., Wink, M., et al. (2001). Mineralogical and chemical interactions of soils eaten by chimpanzees of the Mahale Mountains and Gombe Stream National Parks, Tanzania. Journal of Chemical Ecology,27(2), 285–311.
Basabose, A. K. (2002). Diet composition of chimpanzees inhabiting the Montane forest of Kahuzi, Democratic Republic of Congo. American Journal of Primatology,58(1), 1–21. https://doi.org/10.1002/ajp.10049.
Bicca-Marques, J. C., & Calegaro-Marques, C. (1994). A case of geophagy in the black howling monkey Alouatta caraya. Neotropical Primates,2, 7–9.
Bouyoucos, G. J. (1962). Hydrometer method improved for making particle size analysis of soils. Agronomy Journal,54, 464–465.
DeGabriel, J. L., Moore, B. D., Foley, W. J., & Johnson, C. N. (2009). The effects of plant defensive chemistry on nutrient availability predict reproductive success in a mammal. Ecology,90(3), 711–719.
Dominy, N. J., Davoust, E., & Minekus, M. (2004). Adaptive function of soil consumption: An in vitro study modeling the human stomach and small intestine. The Journal of Experimental Biology,207(Pt 2), 319–324.
Eggeling, W. J. (1947). Observations on the ecology of the Budongo Rain Forest, Uganda. The Journal of Ecology,34(1), 20. https://doi.org/10.2307/2256760.
Engle-Stone, R., Yeung, A., Welch, R., & Glahn, R. (2005). Meat and ascorbic acid can promote Fe availability from Fe-phytate but not from Fe-tannic acid complexes. Journal of Agricultural and Food Chemistry,53(26), 10276–10284. https://doi.org/10.1021/jf0518453.
Farmer, V. C., Russell, J. D., & Smith, B. F. L. (1983). Extraction of inorganic forms of translocated Al, Fe and Si from a podzol Bs horizon. Journal of Soil Science,34(3), 571–576. https://doi.org/10.1111/j.1365-2389.1983.tb01056.x.
Fawcett, K. A. (2000). Female relationships and food availability in a forest community of chimpanzees (Dissertation). Edinburgh: University of Edinburgh.
Gašperšič, M., & Pruetz, J. D. (2011). Chimpanzees in Bandafassi Arrondissement, southeastern Senegal: Field surveys as a basis for the sustainable community-based conservation. Pan-Africanism News,18, 23–25.
Gilardi, J., Duffey, S., Munn, C., & Tell, L. (1999). Biochemical functions of geophagy in parrots: Detoxification of dietary toxins and cytoprotective effects. Journal of Chemical Ecology,25(4), 897–922. https://doi.org/10.1023/A:1020857120217.
Glahn, R. P., Gangloff, M. B., van Campen, D. R., Miller, D. D., Wien, E. M., & Norvell, W. A. (1995). Bathophenanthrolene disulfonic acid and sodium dithionite effectively remove surface-bound iron from Caco-2 cell monolayers. The Journal of Nutrition,125(7), 1833–1840. https://doi.org/10.1093/jn/125.7.1833.
Glahn, R. P., Lee, O. A., Yeung, A., Goldman, M. I., & Miller, D. D. (1998). Caco-2 cell ferritin formation predicts nonradiolabeled food iron availability in an in vitro digestion/Caco-2 cell culture model. The Journal of Nutrition,128(9), 1555–1561.
Glahn, R. P., Wortley, G. M., South, P. K., & Miller, D. D. (2002). Inhibition of iron uptake by phytic acid, tannic acid, and ZnCl2: Studies using an in vitro digestion/Caco-2 cell model. Journal of Agricultural and Food Chemistry,50(2), 390–395. https://doi.org/10.1021/jf011046u.
González, R., de Medina, F. S., Martínez-Augustin, O., Nieto, A., Gálvez, J., Risco, S., et al. (2004). Anti-inflammatory effect of diosmectite in hapten-induced colitis in the rat. British Journal of Pharmacology,141(6), 951–960. https://doi.org/10.1038/sj.bjp.0705710.
Goodall, J. (1963). Feeding behaviour of wild chimpanzees: A preliminary report. In Symposium of the zoological society of London (Vol. 10, pp. 39–47).
Gruber, T., Muller, M. N., Strimling, P., Wrangham, R., & Zuberbühler, K. (2009). Wild chimpanzees rely on cultural knowledge to solve an experimental honey acquisition task. Current Biology,19(21), 1806–1810. https://doi.org/10.1016/j.cub.2009.08.060.
Gurian, E., O’Neil, P. L., & Price, C. S. (1992). Geophagy and its relation to tannin ingestion in rhesus macaques (Macaca mulatta). AAZPA Regional Proceedings,59, 152–159.
Hart, J. J., Tako, E., & Glahn, R. P. (2017). Characterization of polyphenol effects on inhibition and promotion of iron uptake by Caco-2 cells. Journal of Agricultural and Food Chemistry,65(16), 3285–3294. https://doi.org/10.1021/acs.jafc.6b05755.
Hillier, S. (1999). Use of an air brush to spray dry samples for X-ray powder diffraction. Clay Minerals,34(1), 127–135.
Hladik, C. M. (1977). A comparative study of the feeding strategies of two sympatric species of leaf monkeys: Presbytis senex and Presbytis entellus. In T. H. Clutton-Brock (Ed.), Primate ecology: Studies of feeding and ranging behaviour in lemurs, monkeys and apes (pp. 324–353). London: Academic Press.
Johns, T. (1986). Detoxification function of geophagy and domestication of the potato. Journal of Chemical Ecology,12(3), 635–646. https://doi.org/10.1007/BF01012098.
Kano, T., & Mulavwa, M. (1984). Feeding ecology of the pygmy chimpanzees (Pan paniscus) of Wamba. In R. L. Susman (Ed.), The Pygmy Chimpanzee: Evolutionary biology and behavior (pp. 233–274). Boston: Springer. https://doi.org/10.1007/978-1-4757-0082-4_10.
Ketch, L. A., Malloch, D., Mahaney, W. C., & Huffman, M. A. (2001). Comparative microbial analysis and clay mineralogy of soils eaten by chimpanzees (Pan troglodytes schweinfurthii) in Tanzania. Soil Biology and Biochemistry,33(2), 199–203.
Klein, N., Fröhlich, F., & Krief, S. (2008). Geophagy: soil consumption enhances the bioactivities of plants eaten by chimpanzees. Naturwissenschaften,95(4), 325–331. https://doi.org/10.1007/s00114-007-0333-0.
Knezevich, M. (1998). Geophagy as a therapeutic mediator of endoparasitism in a free-ranging group of rhesus macaques (Macaca mulatta). American Journal of Primatology,44(1), 71–82. https://doi.org/10.1002/(SICI)1098-2345(1998)44:1%3c71:AID-AJP6%3e3.0.CO;2-U.
Kobayashi, T., Nozoye, T., & Nishizawa, N. K. (2018). Iron transport and its regulation in plants. Free Radical Biology and Medicine,133, 11. https://doi.org/10.1016/j.freeradbiomed.2018.10.439.
Kreulen, D. A. (1985). Lick use by large herbivores: A review of benefits and banes of soil consumption. Mammal Review,15(3), 107–123.
Mahaney, W. C., Hancock, R. G. V., Aufreiter, S., & Huffman, M. A. (1996). Geochemistry and clay mineralogy of termite mound soil and the role of geophagy in chimpanzees of the Mahale Mountains, Tanzania. Primates,37(2), 121–134. https://doi.org/10.1007/BF02381400.
Mahaney, W. C., & Krishnamani, R. (2003). Understanding geophagy in animals: Standard procedures for sampling soils. Journal of Chemical Ecology,29(7), 1503–1523.
Mahaney, W. C., Milner, M. W., Aufreiter, S., Hancock, R. G. V., Wrangham, R., & Campbell, S. (2005). Soils consumed by chimpanzees of the Kanyawara community in the Kibale Forest, Uganda. International Journal of Primatology,26(6), 1375–1398. https://doi.org/10.1007/s10764-005-8857-7.
Mahaney, W. C., Milner, M. W., Sanmugadas, K., Hancock, R. G. V., Aufreiter, S., Wrangham, R., et al. (1997). Analysis of geophagy soils in Kibale Forest, Uganda. Primates,38(2), 159–176. https://doi.org/10.1007/BF02382006.
Marsh, L. K., Chapman, C. A., Arroyo-Rodríguez, V., Cobden, A. K., Dunn, J. C., Gabriel, D., et al. (2013). Primates in fragments 10 years later: Once and future goals. In L. K. Marsh & C. A. Chapman (Eds.), Primates in fragments (pp. 505–525). New York: Springer. https://doi.org/10.1007/978-1-4614-8839-2_34. Accessed 9 September 2015.
Miller, J. D., Collins, S., Krumdieck, N. R., Wekesa, P., Onono, M., & Young, S. L. (2016). Pica is associated with lower hemoglobin concentration in a cohort of pregnant Kenyan women of mixed HIV status. The FASEB Journal,30(1_supplement), 1149.
Newton-Fisher, N. E. (2003). The home range of the Sonso community of chimpanzees from the Budongo Forest, Uganda. African Journal of Ecology,41(2), 150–156. https://doi.org/10.1046/j.1365-2028.2003.00408.x.
Nishida, T., & Uehara, S. (1983). Natural diet of chimpanzees (Pan troglodytes schweinfurthii): Long-term record from the Mahale Mountains, Tanzania. African Study Monographs,3, 109–130.
Oates, J. F. (1978). Water-plant and soil consumption by guereza monkeys (Colobus guereza): A relationship with minerals and toxins in the diet? Biotropica,10(4), 241. https://doi.org/10.2307/2387676.
Omotoso, O., McCarty, D. K., Hillier, S., & Kleeberg, R. (2006). Some successful approaches to quantitative mineral analysis as revealed by the 3rd Reynolds Cup contest. Clays and Clay Minerals,54(6), 748–760. https://doi.org/10.1346/CCMN.2006.0540609.
Papaioannou, D., Katsoulos, P. D., Panousis, N., & Karatzias, H. (2005). The role of natural and synthetic zeolites as feed additives on the prevention and/or the treatment of certain farm animal diseases: A review. Microporous and Mesoporous Materials,84(1–3), 161–170. https://doi.org/10.1016/j.micromeso.2005.05.030.
Pebsworth, P. A., Bardi, M., & Huffman, M. A. (2012). Geophagy in chacma baboons: Patterns of soil consumption by age class, sex, and reproductive state. American Journal of Primatology,74(1), 48–57. https://doi.org/10.1002/ajp.21008.
Pebsworth, P. A., Huffman, M. A., Lambert, J. E., & Young, S. L. (2019). Geophagy among nonhuman primates: A systematic review of current knowledge and suggestions for future directions. American Journal of Physical Anthropology,1, 11. https://doi.org/10.1002/ajpa.23724.
Pebsworth, P. A., Seim, G. L., Huffman, M. A., Glahn, R. P., Tako, E., & Young, S. L. (2013). Soil consumed by chacma baboons is low in bioavailable iron and high in clay. Journal of Chemical Ecology,39(3), 447–449. https://doi.org/10.1007/s10886-013-0258-3.
Plumptre, A. J., & Reynolds, V. (1996). Censusing chimpanzees in the Budongo Forest, Uganda. International Journal of Primatology,17(1), 85–99. https://doi.org/10.1007/BF02696160.
Reid, R. M. (1992). Cultural and medical perspectives on geophagia. Medical Anthropology,13(4), 337–351. https://doi.org/10.1080/01459740.1992.9966056.
Reynolds, V. (2005). The chimpanzees of the Budongo forest: Ecology, behaviour, and conservation. Oxford: Oxford University Press.
Reynolds, V., Lloyd, A. W., English, C. J., Lyons, P., Dodd, H., Hobaiter, C., et al. (2015). Mineral acquisition from clay by Budongo Forest chimpanzees. PLoS ONE,10(7), e0134075. https://doi.org/10.1371/journal.pone.0134075.
Reynolds, V., Plumptre, A. J., Greenham, J., & Harborne, J. (1998). Condensed tannins and sugars in the diet of chimpanzees (Pan troglodytes schweinfurthii) in the Budongo Forest, Uganda. Oecologia,115(3), 331–336. https://doi.org/10.1007/s004420050524.
Riemer, J., Hoepken, H. H., Czerwinska, H., Robinson, S. R., & Dringen, R. (2004). Colorimetric ferrozine-based assay for the quantitation of iron in cultured cells. Analytical Biochemistry,331(2), 370–375. https://doi.org/10.1016/j.ab.2004.03.049.
Rode, K. D., Chapman, C. A., Chapman, L. J., & McDowell, L. R. (2003). Mineral resource availability and consumption by colobus in Kibale National Park, Uganda. International Journal of Primatology,24(3), 541–573. https://doi.org/10.1023/A:1023788330155.
Rothman, J. M., Chapman, C. A., Struhsaker, T. T., Raubenheimer, D., Twinomugisha, D., & Waterman, P. G. (2015). Long-term declines in nutritional quality of tropical leaves. Ecology,96(3), 873–878. https://doi.org/10.1890/14-0391.1.
Ruby, M. V., Davis, A., Schoof, R., Eberle, S., & Sellstone, C. M. (1996). Estimation of lead and arsenic bioavailability using a physiologically based extraction test. Environmental Science and Technology,30(2), 422–430.
Said, S. A., Shibl, A. M., & Abdullah, M. E. (1980). Influence of various agents on adsorption capacity of kaolin for Pseudomonas aeruginosa toxin. Journal of Pharmaceutical Sciences,69(10), 1238–1239.
Seim, G. L., Ahn, C. I., Bodis, M. S., Luwedde, F., Miller, D. D., Hillier, S., et al. (2013). Bioavailability of iron in geophagic earths and clay minerals, and their effect on dietary iron absorption using an in vitro digestion/Caco-2 cell model. Food and Function,4(8), 1263. https://doi.org/10.1039/c3fo30380b.
Severance, H. W., Holt, T., Patrone, N. A., & Chapman, L. (1988). Profound muscle weakness and hypokalemia due to clay ingestion. Southern Medical Journal,81(2), 272–274.
Spoor, D. C. A., Martineau, L. C., Leduc, C., Benhaddou-Andaloussi, A., Meddah, B., Harris, C., et al. (2006). Selected plant species from the Cree pharmacopoeia of northern Quebec possess anti-diabetic potential. Canadian Journal of Physiology and Pharmacology,84(8–9), 847–858. https://doi.org/10.1139/y06-018.
Ta, C. A. K., Pebsworth, P. A., Liu, R., Hillier, S., Gray, N., Arnason, J. T., et al. (2018). Soil eaten by chacma baboons adsorbs polar plant secondary metabolites representative of those found in their diet. Environmental Geochemistry and Health,40(2), 803–813. https://doi.org/10.1007/s10653-017-0025-4.
Tweheyo, M., Reynolds, V., Huffman, M. A., Pebsworth, P. A., Goto, S., Mahaney, W. C., et al. (2006). Geophagy in chimpanzees (Pan troglodytes schweinfurthii) of the Budongo Forest Reserve, Uganda. In N. E. Newton-Fisher (Ed.), Primates of western Uganda (pp. 135–152). New York: Springer.
US Pharmacopeia. (2017). http://www.pharmacopeia.cn/v29240/usp29nf24s0_ris1s126.html. Accessed 1 June 2017.
Villioth, J. (2019). Foraging ecology in chimpanzees—A comparison of two communities from Budongo Forest (Doctoral dissertation). University of Kent, Canterbury.
Wrangham, R. W., Machanda, Z. P., Weaver, C., & Muller, M. (2014). The toxin-reduction hypothesis for geophagy: Evidence from pregnant chimpanzees. Presented at the 83rd Annual American Association of physical anthropologists, Calgary (Vol. 153, pp. 277–277). Alberta: American Journal of Physical Anthropology.
Young, S. L. (2010). Pica in pregnancy: New ideas about an old condition. Annual Review of Nutrition, 30(1), 403–422. https://doi.org/10.1146/annurev.nutr.012809.104713.
Young, S. L., Sherman, P. W., Lucks, J. B., & Pelto, G. H. (2011). Why on earth?: Evaluating hypotheses about the physiological functions of human geophagy. The Quarterly Review of Biology,86(2), 97–120. https://doi.org/10.1086/659884.
Young, S. L., Wilson, M. J., Miller, D., & Hillier, S. (2008). Toward a comprehensive approach to the collection and analysis of pica substances, with emphasis on geophagic materials. PLoS ONE,3(9), e3147. https://doi.org/10.1371/journal.pone.0003147.
Zhao, D., Huffman, M. A., & Li, B. (2013). First evidence of geophagy by golden snub-nosed monkeys (Rhinopithecus roxellana). Acta Theriologica Sinica,33(3), 282–285.
Acknowledgements
PAP thanks the Budongo Conservation Field Station staff, field assistants, and in particular Vernon Reynolds, Fred Babweteera, Klaus Zuberbühler, Catherine Hobaiter, Jakob Villioth, Geoffrey Muhanguzi, Geresomu Muhumuza, Jacob Alio, and Michael Jurua for field and data assistance. We also thank the Royal Zoological Society of Scotland who provided core funding and the Uganda Wildlife Authority and the Ugandan Council of Science and Technology for the opportunity of conduct research in Uganda. We thank David Emerson, Rui Liu, Nia Gray, Mary Bodis, Pei–Pei Chang, Nimal De Silva, Jean Bjornson, Christopher Boddy, and Joseph Ndawula for assistance with laboratory analyses. We further thank Thad Bartlett, Josh Miller, Chris and Diane West for manuscript assistance and logistical support. SH and RW acknowledge support of the Scottish Government’s Rural and Environment Science and Analytical Services Division (RESAS). JTA acknowledges support from the Canadian Natural Science and Engineering Research Council. Finally, we thank Environmental Geochemistry and Health, Professor William Mahaney, and two anonymous reviewers for their valuable advice and helpful comments on a previous version of this article.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pebsworth, P.A., Hillier, S., Wendler, R. et al. Geophagy among East African Chimpanzees: consumed soils provide protection from plant secondary compounds and bioavailable iron. Environ Geochem Health 41, 2911–2927 (2019). https://doi.org/10.1007/s10653-019-00366-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-019-00366-8