Abstract
Geophagy, the intentional consumption of soil, has been observed in humans and numerous other animal species. Geophagy has been posited to be adaptive, i.e., consumed soil protects against gastrointestinal distress and/or supplements micronutrients. We conducted a field experiment in the Budongo Forest, Uganda, to investigate geophagic behaviors, including soil preference, the quantity of soil eaten, and competition for access to preferred soils. We placed pairs of artificial tree stumps at two existing geophagy sites. One stump contained soil from the surrounding area, Sonso, that could supplement bioavailable iron. The other stump contained soil from a neighboring community, Waibira, that was richer in clay minerals, which could provide protection from plant secondary compounds. We monitored activity and engagement with the stumps for 10 days using camera traps. After 5 days, we reversed the type of soil that was in the stumps at both sites (i.e., a crossover design). Only Colobus guereza (black-and-white colobus monkeys) interacted with the stumps. These monkeys used visual and olfactory cues to select between the two soils and exclusively ate the clay-rich soil, consuming 9.67 kg of soil over 4.33 h. Our findings lend the greatest plausibility to the protection hypothesis. Additionally, monkeys competed for access to the stumps, and 13% of the videos captured aggression, including pushing, excluding, and chasing other individuals from the experimental stumps. Nine episodes of vigilance and flight behavior were also observed. Given that intentionally ingested soil is a valuable resource that may confer health benefits, geophagy sites should be conserved and protected.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Understanding nonhuman primate culture, language, learning, and social interaction is inherently challenging. Unlike human subjects, whom researchers can interview, wildlife researchers need creative techniques to understand the motivation for and consequences of complex animal behaviors. One such technique is the field experiment (Gruber et al. 2009). Previous nonhuman primate field experiments have been transformative for understanding differences in cultural knowledge between populations of chimpanzees (Gruber et al. 2009), responses to potentially contaminated material in Japanese macaques (Sarabian and MacIntosh 2015), fecal avoidance in cercopithecoid primates (Sarabian et al. 2020), transmission of tool use in chimpanzees (Matsuzawa 1994; Biro et al. 2003; Sirianni et al. 2018; Lamon et al. 2018), variation in calls using bio-acoustic playback (Cheney and Seyfarth 1982; Hauser 1998; Zuberbühler 2000; Fischer et al. 2013; Caselli et al. 2018), efficiency of nut-cracking in capuchin monkeys (Fragaszy et al. 2010), social relationships and cognition (Cheney et al. 1986), and social learning (Botting et al. 2018; Bono et al. 2018). However, despite their importance, few field experiments have been conducted on nonhuman primates because they have the potential to alter an animal’s social and physical world.
One primate behavior that remains an enigma is geophagy, the purposeful consumption of earth (e.g., soil from the forest floor, termite and other insect mounds, earthen bricks). This behavior is common across the animal kingdom; it has been observed in humans on all inhabited continents and in over 300 species of mammals, birds, and reptiles (Young et al. 2011). Among nonhuman primates, 136 species are known to eat earthen materials (Pebsworth et al. 2019b). Within the genus Colobus, researchers have observed geophagy in four of five recognized species and several subspecies (Pebsworth et al. 2019b). Yet, despite its ubiquity, little is known about the motivations for and the consequences of geophagy.
Two adaptive hypotheses have been posited to explain the motivation for geophagy. The first, and perhaps most intuitive, is that soil supplements micronutrients that may be lacking in the diet (Kreulen 1985; Pebsworth et al. 2019b). Micronutrient deficiencies can adversely affect overall primate health, growth, reproduction, and disease resistance (Rode et al. 2003). Previous geophagy studies have therefore suggested that soil consumed by nonhuman primates may supplement a variety of micronutrients (Krishnamani and Mahaney 2000; Pebsworth et al. 2019b). To test the supplementation hypothesis, it is critical to demonstrate micronutrient bioavailability (i.e., the proportion of nutrients that are absorbed by the body and enter circulation) (Pebsworth et al. 2019b).
The second hypothesis is that soil protects against gastrointestinal (GI) distress (e.g., nausea, diarrhea, vomiting) and/or infection (Young 2010; Pebsworth et al. 2019b). Previous studies have demonstrated that clay minerals can bind directly to agents that cause GI distress (Gilardi et al. 1999; Dominy et al. 2004), strengthen the luminal epithelium of the GI tract (Said et al. 1980; González et al. 2004), and lyse bacterial cells (Papaioannou et al. 2005). Further, soils with a high proportion of clay minerals have the capacity to adsorb polar plant secondary compounds (PSCs), like alkaloids and phenolics (Johns 1986; Ta et al. 2018; Pebsworth et al. 2019a), that can negatively affect plant palatability and the function of proteolytic enzymes necessary for digestion (Hladik 1977; DeGabriel et al. 2009). Indeed, several species of nonhuman primates regularly consume clay-rich soils that can adsorb PSCs (Setz et al. 1999; Wakibara et al. 2001; Ta et al. 2018; Pebsworth et al. 2019b). To test the protection hypothesis, it is critical to analyze soil texture (percentage of sand, silt, clay) and the type of clay minerals present and their ability to adsorb PSCs.
Although the supplementation and protection hypotheses are often considered separately, several authors have concluded that geophagy is multifunctional and that protection and supplementation co-occur (Davies and Baillie 1988; Pebsworth et al. 2019a). Yet previous nonhuman primate studies have not been able to rigorously test these hypotheses concurrently due to imprecise estimation of the amount and type of soil consumed.
Accurate measurement of soil consumption is critical for quantifying exposure and determining potential physiological impacts. In human geophagy studies, soil ingestion has been estimated using (1) the tracer element method, which measures the amount of common soil elements (i.e., Al, Ce, La, Si) in an individual’s feces and urine; (2) the biokinetic model comparison method, which measures the concentration of an element (e.g., Pb) in blood; and (3) the survey response method, in which questions about soil ingestion are combined with tests for a tracer element (Doyle et al. 2012). None of these techniques are practical for free-ranging animals since the accurate measurement of the quantity of soil consumed requires the collection of all voided material containing digested soil. Measuring the amount of soil consumed in feces is further complicated because soil can remain in the GI tract for several days, and animals may consume additional soil before soil previously consumed has been completely excreted. Previous nonhuman primate geophagy studies have therefore used qualitative descriptions, like a “handful,” “mouthful,” or “a bite,” to estimate the amount of soil consumed (Krishnamani 1994; Klein et al. 2008). These descriptions, however, are imprecise. Field experiments can overcome these limitations through direct observation and weighing the amount of soil consumed.
Black-and-white colobus monkeys (Colobus guereza) have particular behaviors and diets that make them an ideal species for the examination of multiple hypotheses about geophagy. For instance, black-and-white colobus monkeys eat highly digestible foods that are broken down via anaerobic fermentation in the foregut, creating volatile fatty acids. These compounds can lead to a decrease in the forestomach pH and may cause fatal acidosis (Goltenboth 1976). Further, the diet of black-and-white colobus monkeys also contains PSCs that can cause GI distress. Although there is evidence that clay minerals may offer some protection against acidosis (Davies and Baillie 1988) and PSCs (Hladik and Gueguen 1974; Oates 1978), no studies have investigated this in colobus monkeys. Additionally, while previous studies have analyzed the chemical and mineralogical content of soils consumed by colobus monkeys (Oates 1978; Fashing et al. 2007), none have assessed bioavailability.
We, therefore, sought to fill these knowledge gaps by conducting a field experiment in which we concurrently offered known amounts of iron- and clay-rich soils from existing geophagy sites to black-and-white colobus monkeys living in the Budongo Forest Reserve, Uganda. Our primary objective was to characterize geophagic behaviors among these monkeys, including soil preference, quantity of soil consumed, and competition for access to preferred soils. We focused on iron as a key micronutrient because it was reported as low in the black-and-white colobus monkeys’ diet (Rode et al. 2003), has been hypothesized as a geophagy stimulus (Mahaney et al. 1990), and its bioavailability has been previously assessed (Pebsworth et al. 2019a). Because the soils were a finite resource that had the potential to confer beneficial micronutrients and/or protection from GI distress, we predicted that competition among black-and-white colobus monkeys for access (i.e., aggression) to the soils would increase over time.
Materials and methods
Study site and subjects
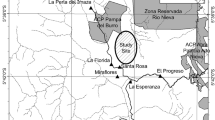
We conducted a geophagy experiment for 10 days (14–23 October 2016) in the Budongo Forest Reserve (1.617–2.0°N, 31.367–31.766°E) (Fig. 1). This reserve is a moist, semi-deciduous tropical forest located in the Masindi District of western Uganda. The reserve is home to over 95 vertebrate species (Plumptre, unpublished data), some of whom eat soil from permanent geophagy sites and termite mounds (e.g., chimpanzees, bushbuck, duiker, black-and-white colobus). Previously we had identified geophagy sites in the Sonso and Waibira communities by direct observation and camera trap monitoring in 2015–2016 (Reynolds et al. 2015, 2019; Pebsworth et al. 2019a) (Fig. 1).
Two experimental stumps were placed at two fixed geophagy study sites (G6, 4/6) in the Budongo Forest Reserve. One stump contained soil higher in clay minerals and the other contained soil higher in bioavailable iron. Black-and-white colobus monkeys were observed routinely eating both soils in prior studies
Black-and-white colobus monkeys live in small troops containing multiple females and one or more adult males. In multi-male troops, one male is dominant, and interaction between males is agonistic (Bocian 1997). The average troop size in the Budongo Forest ranges from eight to ten individuals (Marler 1969; Schel et al. 2010). Intergroup aggression among colobus monkeys functions to defend mates and food resources directly (Fashing 2001). Between 2008 and 2009, researchers identified approximately 60 different colobus groups within the Budongo Forest Reserve. These groups were found 150–200 m apart (Schel et al. 2010), indicating that there was considerable home range overlap among them (i.e., there was potential for the troops to interact and compete for resources).
Colobus monkeys are folivore/frugivores and possess a chambered stomach (Chivers and Hladik 1980). Leaves and leaf buds typically dominate their diet (Oates 1978). There is no published dietary information for black-and-white colobus monkeys from Budongo. Data from eight groups within Kibale National Park (located in Uganda), however, showed that 78.5–94.0% of foraging effort is spent on leaves (Harris and Chapman 2007). At Budongo, black-and-white colobus monkeys are the preferred prey of chimpanzees and crown eagles (Newton-Fisher et al. 2002; Schel et al. 2010; Hobaiter et al. 2017).
Experimental design
The two soils from the Sonso study sites (G6 and 4/6) were physically, chemically, and mineralogically similar to each other, but different from the Waibira soil. In terms of color, as determined using the Munsell soil color chart, the Waibira soil had a neutral hue (7/N), while the G6 soil had a green-yellow hue (7/5GY) (Munsell Color 2010) (Fig. 2b). Soil color is indicative of minerals from the parent material, organic matter, iron, and moisture. Soil from Waibira thus contained a higher percentage of 2:1 clay minerals (e.g., montmorillonite), which we measured with X-ray diffraction (Table 1). In the laboratory, soil from Waibira had a stronger capacity to adsorb phenolic and alkaloid compounds than soil from sites G6 and 4/6. We measured the adsorption of PSCs [expressed as average gallic acid equivalent adsorbed (μg/mg soil)] using the Folin-Ciocalteu method (Table 1) (Pebsworth et al. 2019a). We removed approximately 95% of micronutrients found in the soil with a HNO3/HClO4 extraction and measured the concentration of iron using inductively coupled plasma (Table 1). We also analyzed the soils for bioavailable iron using Caco-2 cell experiments.
We created experimental stumps of known weight. a A hole was carved into the flat surface of the stump to house a known quantity of soil from permanent geophagy sites. A smaller hole was drilled into the side of the stump to allow moisture to drain. b One stump was filled with approximately 5 kg of iron-rich soil from that particular area (left), and the other was filled with approximately 5 kg of clay-rich soil from a neighboring area, Waibira (right)
Researchers have conducted several field experiments at Budongo (Gruber et al. 2009; Lamon et al. 2018), but this was the first geophagy field experiment to be carried out there. We first created four similar-looking experimental stumps from dead wood found in the Budongo Forest Reserve, and carved a 15 × 15 × 10-cm hole into the flat surface of each; the stumps were cleaned with an antiseptic liquid to prevent the transmission of human pathogens (Fig. 2a). We then selected two sites where geophagy was regularly occurring and placed two stumps at each (Fig. 1). We partially buried each stump into the surrounding soil and placed leaves and branches around its base to make it look natural (Fig. 2b).
At each study site, one stump was filled with approximately 5 kg of iron-rich soil from that particular geophagy site and the other was filled with approximately 5 kg of clay-rich soil from the geophagy site in the neighboring community of Waibira (Fig. 2b). On the sixth day of the experiment, we reversed the type of soil that was in the stumps at both sites (i.e., a crossover design), keeping the stump position the same to ensure that any observed differences in behavior were related to soil type, not stump design or stump preference. We checked the stumps daily and replenished the soil in the evening or the day after animals consumed it. The first 5-day period of the experiment was 14–18 October, and the second was 19–23 October 2016.
Monitoring behavior
The field experiment was actively monitored for all 10 days with Bushnell Trophy camera traps (one at each site) that were activated by infrared motion and heat detection. We positioned the cameras approximately 20 cm above the ground near the experimental stumps and programmed them to take 59-s videos, with a 1-s interval between videos, for a total of 480 h. Cameras were synchronized by date and time.
For each video, the first author documented the stump from which the animal(s) consumed soil, how many animals were present, age-class and sex (when possible), and signs of aggression. We defined “aggression” as an individual pushing, hitting, excluding, and chasing away others from the stumps. Aggression that was not associated with competition for access to the stump was not coded.
Statistical analysis
We used χ2-tests to evaluate our prediction that if soil was a valued resource, intra- and inter-troop aggression would increase over time. Using χ2-tests, we determined whether the number of aggressive events at the experimental stumps were statistically different between the first 5 days and the last 5 days (p < 0.05). We ran basic statistics in Stata 15 (Stata Statistical Software: Release 15, StataCorp. 2017; StataCorp., College Station, TX).
Ethical note
We obtained research clearance for the study from the Uganda National Council of Science and Technology and the Uganda Wildlife Authority (permit NS 548). We also obtained ethical permission from the University of Texas at San Antonio’s Institutional Animal Care and Use Committee. We followed all applicable international, national, and institutional guidelines, and complied with all recommendations from the University of Texas at San Antonio. All study activities conformed to the Association for the Study of Animal Behaviour/Animal Behavior Society guidelines for the Treatment of Animals in Behavioral Research and Teaching. For instance, we used camera traps to reduce human contact and limited the number of experimental days to minimize any potential effects of the study.
Results
Soil consumption and quantity eaten
Although other species of animals have been observed eating soil in the Budongo Forest Reserve (e.g., chimpanzee, bushbuck, red duiker, blue duiker) (Pebsworth, unpublished data), the camera traps only recorded black-and-white colobus monkeys eating soil at the experimental stumps. Additionally, while monkeys were present at both sites, interaction with the stumps was only recorded at site G6 (Fig. 1).
After a brief exploration of both stumps (looking, smelling), black-and-white colobus monkeys exclusively ate the clay-rich soil from Waibira, even after reversing the soils in the stumps (Table 2). The monkeys ate 4.52 kg of soil on 14–15 October 2016, and 5.15 kg of soil on 22 October 2016. On average, the monkeys ate approximately 2.23 kg of soil/h. Although we were unable to determine when stumps were depleted, there was evidence that the monkeys continued to interact with the stump even when empty.
Geophagic behavior
A camera trap captured 260 videos of black-and-white colobus monkeys interacting with the experimental stumps on 3 days. Of these, one or more monkeys were eating soil in 242 (93%) videos. In the remaining 18 videos, monkeys were smelling the soil (n = 6), licking the soil and stump (n = 3), or chewing bark of the experimental stump (n = 9). For example, one monkey was recorded smelling both stumps and then eating only the clay-rich soil from Waibira. Two of the three licking events occurred at the end of the experiment, apparently for the removal of remaining soil from the outside of the stump that housed clay-rich soil. When the clay-rich soil was fully exhausted, two monkeys were recorded smelling the iron-rich soil and then departing, while another smelled the iron-rich soil, placed its head into the stump that had contained clay-rich soil, and proceeded to lick the outside of the stump.
The number of individuals varied considerably at the stumps, from one to 11 (Fig. 3a; Table 2). In total, the black-and-white colobus monkeys spent 4.33 h interacting exclusively with the experimental stumps and consumed only the clay-rich soil from Waibira. Sex identification was difficult, as distinguishing features (e.g., nipples, penis, ischial callosities) were often concealed. Nonetheless, adult females were obvious in 51% of videos, adult females with an infant in 47%, juveniles in 18%, and adult males in 1% (Fig. 3b). During this experiment, only one chimpanzee, a female with an infant, interacted with an experimental stump; however, by the time she arrived, all the clay-rich soil had been eaten.
Two experimental stumps were visited by two troops of black-and-white colobus monkeys. a The number of individuals at the stumps varied from one to 11 during the video clip, b adult females with an infant were present in 47% of videos, c aggression occurred between adults and juveniles, and d monkeys exhibited vigilant behavior
More videos recorded geophagy during the second 5 days than the first 5 days of the experiment (Table 2). Even though the monkeys spent more time at the stump during the second period and ate more soil then, the rate of soil consumption during the first and second period remained the same (0.04 kg/min). Observed aggression also increased across the two periods (p = 0.012) (Table 2). In the videos showing aggression, some individuals had less access, appeared younger, and were pushed away, excluded, or chased from the experimental stumps (Fig. 3c; Table 2).
Discussion
In this first geophagy field experiment at Budongo Forest Reserve, we used camera trap videos and well-characterized soils to examine soil preference in Colobus guereza. We allowed monkeys to choose between clay-rich and iron-rich soils, and they exclusively consumed the clay-rich soil. This finding lends the greatest plausibility to the hypothesis that soil was eaten for protection against GI distress. To distinguish between soils, monkeys looked at and smelled both experimental stumps, but few licked the soil before eating it. This suggests that the black-and-white colobus monkeys primarily selected soils using visual and olfactory cues.
Physiological studies have demonstrated that the olfaction of some nonhuman primates is equivalent to that of dogs and rodents (Laska and Freyer 1997; Laska 2000) and plays an essential role in the location of food and assessment of its quality (Nevo and Heymann 2015; Melin et al. 2019). The distinctive smell of wet earth is most commonly cited by human geophagists as the main attractant for soil consumption (Young et al. 2010; Huebl et al. 2016). Our experiment demonstrated that black-and-white colobus selected soil with a higher clay content. We do not know why these monkeys chose the clay-rich soil, but our findings suggest that the smell of clay could have been a stimulus for geophagy. Given that some monkeys were observed licking the soils, soil texture (i.e., sand, silt, clay content) may also be important.
In addition to clay, it has been hypothesized that salts, lime, and organic matter contained in soil may provide olfactory stimulation for geophagy (Stambolic-Robb 1997; Krishnamani and Mahaney 2000). The soil that we provided in this experiment lacked organic matter, although the monkeys may have been responding to the smell of available salt in the soil. Interestingly, when chacma baboons could select between soils of varying degrees of saltiness, they chose soil with the least amount of sodium and the highest percentage of clay minerals (Pebsworth et al. 2012). This behavior, however, has not been tested in black-and-white colobus monkeys (Oates 1978; Rode et al. 2003).
Mahaney et al. (1990) also suggested that the smell of organically bound iron may lead gorillas to iron-rich soil. In our experiment, the monkeys did not consume the iron-rich soil; therefore, we conclude that the smell of iron was not a stimulus for geophagy here. We primarily focused on iron as a key micronutrient because its content is generally low in natural foods and it may be deficient in the black-and-white colobus monkeys’ diet (Rode et al. 2003). It was clear that, despite large amounts of iron in the Waibira soil, very little was bioavailable, which suggests that the clay fraction may have bound trace iron (Table 1). The Caco-2 cell experiments revealed that the amount of bioavailable iron present in the Waibira soils was below the level of a quality control cell that lacked soil (Pebsworth et al. 2019a). Naturally occurring plant compounds can decrease micronutrient bioavailability: oxalate and phytate inhibit calcium absorption (Gibson et al. 2010); phytate inhibits zinc absorption; and polyphenols, phytate, calcium, legume proteins, and casein inhibit iron absorption (Hambidge 2010). Future research should determine the bioavailability of micronutrients other than iron contained in soil eaten by nonhuman primates.
Wild black-and-white colobus monkeys competed for access to soil from Waibira containing a higher percentage of 2:1 clay minerals than soil from within their home range (G6), which contained more bioavailable iron. Camera trap videos documented that more monkeys were present, more soil was eaten, and more time was spent at the experimental stumps during the second half of the experiment (Table 2). The videos also documented intra-troop aggression at the experimental stumps. Some individuals had fewer opportunities to consume the clay-rich soil in the stump, likely due to factors such as rank and age. The experimental stumps represented a poorly distributed, novel food source that could be monopolized, which caused feeding competition (Harris 2006). These observations supported our prediction that competition among black-and-white colobus monkeys for soil would increase over time. These results do not support either the protection hypothesis or the supplementation hypothesis, but do reinforce that soil is a valuable resource for these monkeys.
In addition to aggression, black-and-white colobus displayed vigilance, perhaps in response to competition and risk of predation (Fig. 3d). Vigilance is defined as “any visual search directed beyond arm’s reach” (Treves 1999). Typical vigilant behaviors include a heightened state of awareness, watching, listening intently, and looking up (Cords 1990). Based on the presence/absence of recognizable individuals and behavior, it appeared that two troops of black-and-white colobus monkeys interacted with the geophagy experiment. Camera traps did not record inter-troop interactions at the stumps, but these may have taken place beyond the camera’s view.
That soil is a valued resource was further supported by the risk of predation to the colobus monkeys when they accessed it. Descending to the ground might also be perceived as a risky behavior. Colobus monkeys are highly arboreal, and their locomotion is awkward while on the ground. Furthermore, black-and-white colobus monkeys are a preferred prey item for chimpanzees at Budongo, so their vigilance may have been in response to a risk of predation (Schel et al. 2010; Hobaiter et al. 2017). Escape is much easier for a colobus monkey when in the canopy than on the ground. Observations similar to those of the present study were recorded for brown spider monkeys, who descended to saladeros (licks) to eat soil even though there was a risk of jaguar, puma, and ocelot predation (Link et al. 2011).
In sum, the black-and-white colobus monkeys in this experiment preferentially selected and consumed clay-rich over iron-rich soil, in support of the protection hypothesis. The consumed clay-rich soil had the potential to bind polar PSCs, which commonly occur in the black-and-white colobus’s diet (Ta et al. 2018; Pebsworth et al. 2019a), and adjust gut pH (Oates 1978; Davies and Baillie 1988). Curiously, the monkeys did not eat the iron-rich soil in the experimental stump, even when the clay-rich soil was exhausted. What remains unclear is whether the monkeys ignored the iron-rich soil in the stump because they knew where else to find it, they preferred the clay-rich soil, or they preferred to eat a novel food item.
We do not know why chimpanzees did not interact with the experimental stumps. Over the last decade, both habituated and non-habituated chimpanzees have engaged with these types of field experiments (Gruber et al. 2016). One possibility is that the chimpanzees perceived the stumps as unnatural and avoided them. Another possibility is that chimpanzees in the Budongo Forest Reserve do not eat the iron-rich soil there but instead drink standing water at G6 and 4/6 that is infused with clay and iron (Pebsworth et al. 2019a).
This experiment had several strengths, including that the soils eaten by the monkeys were well described, i.e., a laboratory ran a battery of soil analyses, and there was a marked difference between the two soils with regards to their potential for detoxification, protection, and iron supplementation (Pebsworth et al. 2019a). We also used camera traps to document geophagy continually (e.g., which species, duration) without introducing observer bias. To our knowledge, this is the first field experiment to establish not only soil preference, but also the quantity of soil consumed by nonhuman primates.
This experiment also had several weaknesses, including that these monkeys were not habituated to human presence, nor were they routinely followed. Thus we did not know their diet nor the social hierarchy or composition of the troop members. Additionally, Waibira was outside the monkeys’ home range, thus the soil obtained there was novel to them. As such, it is possible that participation in this experiment would have been more appealing to less neophobic monkeys. The hole carved into the stump was also small, which may have prevented some monkeys from participating.
Despite these shortcomings, this experiment yielded insights about geophagy and its potential role as a means of self-medicating in black-and-white colobus. We cannot definitively draw any conclusions as to why one soil was selected and the other not. The behavior observed at the stumps, however, suggests that the consumed soil was chosen based on its color, smell, and in limited cases, taste, and texture. Once eaten, the soil had the potential to serve multiple functions, e.g., protection against GI distress, and supplementation of micronutrients.
We recommend that this geophagy field experiment be repeated at Budongo and other research sites to ensure that the findings reported here are not a consequence of stochastic variation between the time periods in the experiment. The number of experimental trials should be limited to avoid altering patterns of movement and increasing intra- and inter-troop aggression.
One consequence of global climate change is a decline in the nutritional composition of leaves and an increase in PSCs (Marsh et al. 2013). This experiment demonstrates that soil is an important dietary resource, and we advocate for the conservation and preservation of geophagy sites where animals congregate to eat soil. In a time of unprecedented habitat destruction and intra- and interspecies competition for finite resources, geophagy sites may become increasingly vital to animal health and survival.
References
Biro D, Inoue-Nakamura N, Tonooka R et al (2003) Cultural innovation and transmission of tool use in wild chimpanzees: evidence from field experiments. Anim Cogn 6:213–223. https://doi.org/10.1007/s10071-003-0183-x
Bocian CM (1997) Niche separation of black-and-white colobus monkeys (Colobus angolensis and C. guereza) in the Ituri Forest. Doctoral dissertation, the City University of New York
Bono AEJ, Whiten A, van Schaik C et al (2018) Payoff- and sex-biased social learning interact in a wild primate population. Curr Biol 28:2800–2805.e4. https://doi.org/10.1016/j.cub.2018.06.015
Botting J, Whiten A, Grampp M, van de Waal E (2018) Field experiments with wild primates reveal no consistent dominance-based bias in social learning. Anim Behav 136:1–12. https://doi.org/10.1016/j.anbehav.2017.11.025
Caselli CB, Ayres PHB, Castro SCN et al (2018) The role of extragroup encounters in a Neotropical, cooperative breeding primate, the common marmoset: a field playback experiment. Anim Behav 136:137–146. https://doi.org/10.1016/j.anbehav.2017.12.009
Cheney DL, Seyfarth RM (1982) How vervet monkeys perceive their grunts: field playback experiments. Anim Behav 30:739–751. https://doi.org/10.1016/S0003-3472(82)80146-2
Cheney DL, Seyfarth R, Smuts B (1986) Social relationships and social cognition in nonhuman primates. Science 234:1361–1366. https://doi.org/10.1126/science.3538419
Chivers DJ, Hladik CM (1980) Morphology of the gastrointestinal tract in primates: comparisons with other mammals in relation to diet. J Morphol 166:337–386. https://doi.org/10.1002/jmor.1051660306
Color M (2010) Munsell soil color charts: with genuine Munsell color chips. Grand Rapids, MI
Cords M (1990) Vigilance and mixed-species association of some East African forest monkeys. Behav Ecol Sociobiol. https://doi.org/10.1007/BF00178323
Davies AG, Baillie IC (1988) Soil-eating by red leaf monkeys (Presbytis rubicunda) in Sabah, northern Borneo. Biotropica 20:252. https://doi.org/10.2307/2388241
DeGabriel JL, Moore BD, Foley WJ, Johnson CN (2009) The effects of plant defensive chemistry on nutrient availability predict reproductive success in a mammal. Ecology 90:711–719
Dominy NJ, Davoust E, Minekus M (2004) Adaptive function of soil consumption: an in vitro study modeling the human stomach and small intestine. J Exp Biol 207:319–324
Doyle JR, Blais JM, Holmes RD, White PA (2012) A soil ingestion pilot study of a population following a traditional lifestyle typical of rural or wilderness areas. Sci Total Environ 424:110–120. https://doi.org/10.1016/j.scitotenv.2012.02.043
Fashing PJ (2001) Male and female strategies during intergroup encounters in guerezas (Colobus guereza): evidence for resource defense mediated through males and a comparison with other primates. Behav Ecol Sociobiol 50:219–230. https://doi.org/10.1007/s002650100358
Fashing PJ, Dierenfeld ES, Mowry CB (2007) Influence of plant and soil chemistry on food selection, ranging patterns, and biomass of Colobus guereza in Kakamega Forest, Kenya. Int J Primatol 28:673–703. https://doi.org/10.1007/s10764-006-9096-2
Fischer J, Noser R, Hammerschmidt K (2013) Bioacoustic field research: a primer to acoustic analyses and playback experiments with primates: bioacoustic field methods. Am J Primatol 75:643–663. https://doi.org/10.1002/ajp.22153
Fragaszy D, Pickering T, Liu Q et al (2010) Bearded capuchin monkeys’ and a human’s efficiency at cracking palm nuts with stone tools: field experiments. Anim Behav 79:321–332. https://doi.org/10.1016/j.anbehav.2009.11.004
Gibson RS, Bailey KB, Gibbs M, Ferguson EL (2010) A review of phytate, iron, zinc, and calcium concentrations in plant-based complementary foods used in low-income countries and implications for bioavailability. Food Nutr Bull 31:S134–S146. https://doi.org/10.1177/15648265100312S206
Gilardi J, Duffey S, Munn C, Tell L (1999) Biochemical functions of geophagy in parrots: detoxification of dietary toxins and cytoprotective effects. J Chem Ecol 25:897–922. https://doi.org/10.1023/A:1020857120217
Goltenboth R (1976) Non human primates (apes, monkeys and prosimians). In: Kloes HG, Lang EM (eds) The handbook of zoo medicine. Van Norstrand Reihold, New York, pp 46–85
González R, de Medina FS, Martínez-Augustin O et al (2004) Anti-inflammatory effect of diosmectite in hapten-induced colitis in the rat. Br J Pharmacol 141:951–960. https://doi.org/10.1038/sj.bjp.0705710
Gruber T, Muller MN, Strimling P et al (2009) Wild chimpanzees rely on cultural knowledge to solve an experimental honey acquisition task. Curr Biol 19:1806–1810. https://doi.org/10.1016/j.cub.2009.08.060
Gruber T, Zuberbühler K, Neumann C (2016) Travel fosters tool use in wild chimpanzees. eLife. https://doi.org/10.7554/eLife.16371
Hambidge KM (1432S) Micronutrient bioavailability: dietary reference intakes and a future perspective. Am J Clin Nutr 91:1430S–1432S. https://doi.org/10.3945/ajcn.2010.28674B
Harris TR (2006) Between-group contest competition for food in a highly folivorous population of black and white colobus monkeys (Colobus guereza). Behav Ecol Sociobiol 61:317–329. https://doi.org/10.1007/s00265-006-0261-6
Harris TR, Chapman CA (2007) Variation in diet and ranging of black and white colobus monkeys in Kibale National Park, Uganda. Primates 48:208–221. https://doi.org/10.1007/s10329-006-0036-8
Hauser MD (1998) Functional referents and acoustic similarity: field playback experiments with rhesus monkeys. Anim Behav 55:1647–1658. https://doi.org/10.1006/anbe.1997.0712
Hladik CM (1977) A comparative study of the feeding strategies of two sympatric species of leaf monkeys: Presbytis senex and Presbytis entellus. In: Clutton-Brock TH (ed) Primate ecology: studies of feeding and ranging behaviour in lemurs, monkeys and apes. Academic Press, London, pp 324–353
Hladik CM, Gueguen L (1974) Géophagie et nutrition minérale chez les primates sauvages. CR Acad Sci Paris Ser D 279:1393–1396
Hobaiter C, Samuni L, Mullins C et al (2017) Variation in hunting behaviour in neighbouring chimpanzee communities in the Budongo forest, Uganda. PLOS ONE 12:e0178065. https://doi.org/10.1371/journal.pone.0178065
Huebl L, Akello G, Kutalek R et al (2016) Geophagy in Northern Uganda: perspectives from consumers and clinicians. Am J Trop Med Hyg 95:1440–1449. https://doi.org/10.4269/ajtmh.15-0579
Johns T (1986) Detoxification function of geophagy and domestication of the potato. J Chem Ecol 12:635–646. https://doi.org/10.1007/BF01012098
Klein N, Fröhlich F, Krief S (2008) Geophagy: soil consumption enhances the bioactivities of plants eaten by chimpanzees. Naturwissenschaften 95:325–331. https://doi.org/10.1007/s00114-007-0333-0
Kreulen DA (1985) Lick use by large herbivores: a review of benefits and banes of soil consumption. Mammal Rev 15:107–123
Krishnamani R (1994) Diet composition of the bonnet macaque (Macaca radiata) in a tropical dry evergreen forest of southern India. Trop Biodivers 2:285–302
Krishnamani R, Mahaney WC (2000) Geophagy among primates: adaptive significance and ecological consequences. Anim Behav 59:899–915. https://doi.org/10.1006/anbe.1999.1376
Lamon N, Neumann C, Gier J et al (2018) Wild chimpanzees select tool material based on efficiency and knowledge. Proc R Soc B Biol Sci 285:20181715. https://doi.org/10.1098/rspb.2018.1715
Laska M (2000) “Microsmatic” primates revisited: olfactory sensitivity in the squirrel monkey. Chem Senses 25:47–53. https://doi.org/10.1093/chemse/25.1.47
Laska M, Freyer D (1997) Olfactory discrimination ability for aliphatic esters in squirrel monkeys and humans. Chem Senses 22:457–465. https://doi.org/10.1093/chemse/22.4.457
Link A, Galvis N, Fleming E, Di Fiore A (2011) Patterns of mineral lick visitation by spider monkeys and howler monkeys in Amazonia: are licks perceived as risky areas? Am J Primatol 73:386–396. https://doi.org/10.1002/ajp.20910
Mahaney WC, Watts D, Hancock RGV (1990) Geophagia by mountain gorillas (Gorilla gorilla beringei) in the Virunga Mountains, Rwanda. Primates 31:113–120
Marler P (1969) Colobus guereza: territoriality and group composition. Science 163:93–95. https://doi.org/10.1126/science.163.3862.93
Marsh LK, Chapman CA, Arroyo-Rodríguez V et al (2013) Primates in fragments 10 years later: once and future goals. In: Marsh LK, Chapman CA (eds) Primates in fragments. Springer New York, New York, pp 505–525
Matsuzawa T (1994) Field experiments on use of stone tools by chimpanzees in the wild. In: Wrangham RW, McGrew WC, de Waal FBM, Heltne PG (eds) Chimpanzee cultures. Harvard University Press, pp 351–370
Nevo O, Heymann EW (2015) Led by the nose: olfaction in primate feeding ecology: olfaction in primate feeding ecology. Evol Anthropol Issues News Rev 24:137–148. https://doi.org/10.1002/evan.21458
Newton-Fisher NE, Notman H, Reynolds V (2002) Hunting of mammalian prey by Budongo Forest chimpanzees. Folia Primatol (Basel) 73:281–283. https://doi.org/10.1159/000067454
Oates JF (1978) Water-plant and soil consumption by guereza monkeys (Colobus guereza): a relationship with minerals and toxins in the diet? Biotropica 10:241. https://doi.org/10.2307/2387676
Papaioannou D, Katsoulos PD, Panousis N, Karatzias H (2005) The role of natural and synthetic zeolites as feed additives on the prevention and/or the treatment of certain farm animal diseases: a review. Microporous Mesoporous Mater 84:161–170. https://doi.org/10.1016/j.micromeso.2005.05.030
Pebsworth PA, Bardi M, Huffman MA (2012) Geophagy in chacma baboons: patterns of soil consumption by age class, sex, and reproductive state. Am J Primatol 74:48–57. https://doi.org/10.1002/ajp.21008
Pebsworth PA, Hillier S, Wendler R et al (2019a) Geophagy among East African chimpanzees: consumed soils provide protection from plant secondary compounds and bioavailable iron. Environ Geochem Health. https://doi.org/10.1007/s10653-019-00366-8
Pebsworth PA, Huffman MA, Lambert JE, Young SL (2019b) Geophagy among nonhuman primates: a systematic review of current knowledge and suggestions for future directions. Am J Phys Anthropol. https://doi.org/10.1002/ajpa.23724
Reynolds V, Lloyd AW, English CJ et al (2015) Mineral acquisition from clay by Budongo Forest chimpanzees. PLOS ONE 10:e0134075. https://doi.org/10.1371/journal.pone.0134075
Reynolds V, Pascual-Garrido A, Lloyd AW et al (2019) Possible mineral contributions to the diet and health of wild chimpanzees in three East African forests. Am J Primatol. https://doi.org/10.1002/ajp.22978
Rode KD, Chapman CA, Chapman LJ, McDowell LR (2003) Mineral resource availability and consumption by colobus in Kibale National Park, Uganda. Int J Primatol 24:541–573. https://doi.org/10.1023/A:1023788330155
Said SA, Shibl AM, Abdullah ME (1980) Influence of various agents on adsorption capacity of kaolin for Pseudomonas aeruginosa toxin. J Pharm Sci 69:1238–1239
Sarabian C, MacIntosh AJJ (2015) Hygienic tendencies correlate with low geohelminth infection in free-ranging macaques. Biol Lett 11:20150757. https://doi.org/10.1098/rsbl.2015.0757
Sarabian C, Ngoubangoye B, MacIntosh AJJ (2020) Divergent strategies in faeces avoidance between two cercopithecoid primates. R Soc Open Sci 7:191861. https://doi.org/10.1098/rsos.191861
Schel AM, Candiotti A, Zuberbühler K (2010) Predator-deterring alarm call sequences in Guereza colobus monkeys are meaningful to conspecifics. Anim Behav 80:799–808. https://doi.org/10.1016/j.anbehav.2010.07.012
Setz E, Enzweiler J, Solferini V et al (1999) Geophagy in the golden-faced saki monkey (Pithecia pithecia chrysocephala) in the Central Amazon. J Zool 247:91–103
Sirianni G, Wittig RM, Gratton P et al (2018) Do chimpanzees anticipate an object’s weight? A field experiment on the kinematics of hammer-lifting movements in the nut-cracking Taï chimpanzees. Anim Cogn 21:109–118. https://doi.org/10.1007/s10071-017-1144-0
Stambolic-Robb A (1997) Geophagy amongst free-ranging Sumatran orang-utans (Pongo pygmaeus abelii) of Gunung Leuser National Park, and rehabilitated Bornean orang-utans (Pongo pygmaeus pygmaeus) of Sungai Wain Forest, Indonesia. Master’s thesis, York University
Ta CAK, Pebsworth PA, Liu R et al (2018) Soil eaten by chacma baboons adsorbs polar plant secondary metabolites representative of those found in their diet. Environ Geochem Health 40:803–813. https://doi.org/10.1007/s10653-017-0025-4
Treves A (1999) Within-group vigilance in red colobus and redtail monkeys. Am J Primatol 48:113–126
Wakibara JV, Huffman MA, Wink M et al (2001) The adaptive significance of geophagy for Japanese macaques (Macaca fuscata) at Arashiyama, Japan. Int J Primatol 22:495–520. https://doi.org/10.1023/A:1010763930475
Young SL (2010) Pica in pregnancy: new ideas about an old condition. Annu Rev Nutr 30:403–422. https://doi.org/10.1146/annurev.nutr.012809.104713
Young SL, Wilson MJ, Hillier S et al (2010) Differences and commonalities in physical, chemical and mineralogical properties of Zanzibari geophagic soils. J Chem Ecol 36:129–140. https://doi.org/10.1007/s10886-009-9729-y
Young SL, Sherman PW, Lucks JB, Pelto GH (2011) Why on earth? Evaluating hypotheses about the physiological functions of human geophagy. Q Rev Biol 86:97–120. https://doi.org/10.1086/659884
Zuberbühler K (2000) Causal cognition in a non-human primate: field playback experiments with Diana monkeys. Cognition 76:195–207. https://doi.org/10.1016/S0010-0277(00)00079-2
Acknowledgments
We thank the Budongo Conservation Field Station staff, field assistants, and in particular, Caroline Asiimwe, Geoffrey Muhanguzi, Bosco Chandia, Jacob Alio, and William Agani for field assistance. We also thank the Royal Zoological Society of Scotland who provided core funding and the Uganda Wildlife Authority, the Ugandan Council of Science and Technology, and the National Forestry Authority for the opportunity to conduct research in Uganda. Additionally, we thank associate editor of Primates, Michael Huffman, and two anonymous reviewers.
Funding
T. G. was supported by the Swiss National Science Foundation (grants CR13I1_162720 and P300PA_164678).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No competing interests to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Pebsworth, P.A., Gruber, T., Miller, J.D. et al. Selecting between iron-rich and clay-rich soils: a geophagy field experiment with black-and-white colobus monkeys in the Budongo Forest Reserve, Uganda. Primates 62, 133–142 (2021). https://doi.org/10.1007/s10329-020-00845-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10329-020-00845-y