Abstract
Estuarine ecosystems of the Bay of Bengal, India, are considered as the most productive environment, which have been persistently threatened by substantial anthropogenic activity. This study aims to investigate the metal contamination in the sediment of two estuaries and possible biomagnifications in the indigenous edible oyster Saccostrea cucullata and related health hazards due to its consumption. The accumulative ecological risks indicated that the sediment is moderate to strongly contaminated with cadmium and lead. The sediment pollution index and pollution load index suggested that the sediment possesses a little ecological stress on the exposed flora and fauna. The statistical interpretation highlights the most metals which have a similar source of origin and are bound to the finer fractions of the sediment, except nickel. Bioaccumulation of sediment-associated Cu and Zn in oyster reflects their potential biomagnifications through aquatic food chain. HPI range was below the critical limit of safe human consumption. The non-carcinogenic (THQ) and carcinogenic (CR) health hazards were estimated from the PTDI provided by USEPA. Except Cr, Hg and Zn, the THQ of all other metals was > 1 suggesting detrimental non-carcinogenic health effects on humans. The TCR of Cr and Cd above safety limit indicates the exposed population might be under severe carcinogenic threat due to those metals.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The rapid industrial growth coupled with the development of modern technologies has resulted in the introduction of diverse toxic substances, including toxic metals into the environment. Toxic metal ions are imperishable and have lethal effects on living beings when their quantity surpasses a certain threshold level (Zuzolo et al. 2017). While few metals are essential for body metabolism, the presence of the metals like Cr, Cd, Pb, Mn and Ni above the threshold limits exhibits carcinogenic and genotoxic effects on humans (Fuentes-Gandara et al. 2016). The industrial or harbour zone typically acts as a reservoir of various pollutants which are responsible for creating anthropogenic pressure on the coastal environment. The condition is more critical for estuaries of the developing countries like India due to industrial bloom, because of which the marine environment is rapidly deteriorating. Estuaries are regarded as the transitional zone of ocean, river and land, which control a wide range of organic matter present in water and sediment from riverine discharge with great efficiency. The heavy metal concentration in estuarine sediment is greatly dependant on particle size and organic substances present in the sediment. Geogenic sources like weathering of rocks, natural erosion and anthropogenic sources such as disposal of solid wastes, vehicular emission, waste discharges from industries and urban areas, mining action, sewage sludge, landfill leachates and port transport activities are the major sources of estuarine sediment contamination. However, the heavy metal content in sediment, based on the total metal content, has been considered in many countries as a criterion of sediment pollution (Barrio-Parra et al. 2017). The recently published reports are also more focussed on metal contamination level of estuarine sediment by using various contamination indices such as geoaccumulation index (Igeo), contamination factor (CF), enrichment factor (EF), pollution load index (PLI), sediment pollution index (SPI). These indices, though they provide precise information on the toxic effects imposed on marine biota, they do not predict the ability of the toxic metals to get transferred into the food chain (Ávila et al. 2017). The total metal content in the sediment does not necessarily correspond to the bioavailable metal form in natural environment. Bioavailability of any metal is the fraction of total metal content that can be assimilated into the biota (bioaccumulation). Bioavailability is significantly influenced by the chemical speciation of sediment, like non-residual metal complexes (water soluble, convertible metal complexes, metals that are bound to iron and manganese oxide, carbonate bound metals and metals bound to organic matter) (Kadhum et al. 2017). Residual metal complexes (metals within the structure of the sediment and minerals) are, however, considered as inert and inaccessible to biota. Sediment acts as the source of food and shelter to benthic organisms, which promotes the accumulation of metals in the benthic oysters through aquatic food web. Seasonal and spatial patterns also greatly influence the biological response of the organisms to these toxic metal ions.
According to the need of the body, the macro- or microtrace elements are necessary for the nutritional supplement in human but their superfluity is toxic to the body as well (Bilandžić et al. 2014). Therefore, a standard dietary intake rate has been prescribed for the normal functioning of the human body intakes, which includes the recommended dietary intake, the maximum intake rate, the tolerable range of upper intake level and oral reference dose (Institute of Medicine 2001; US EPA 2015). Therefore, it is essential to know the chemical composition and contents of the elements in a food in order to minimise probable adverse health effects in humans. Seafood like oysters is significant source of protein and contains all the vital elements for human growth like fat (4%), carbohydrate (6%), vitamins (A, B, B12, etc.), minerals (Na, K, P, Ca), essential trace elements (selenium, zinc, iron and magnesium) and polyunsaturated omega 3 fatty acids (Mason et al. 2014). Out of the 4.7 million tons of annual consumption of oysters all around the world, about 92% of world production of oyster comes from Asia. In India, the commercial production of oysters is blooming and more than 50,000 tons of oysters is cultured annually for consumption (Oyster world congress report 2012). The available species of edible oysters in India are Saccostrea cucullata, Crassostrea rivularis, Crassostrea gryphoides, Crassostrea madrasensis. Sedentary oysters are constantly exposed to water and sediment contaminants as they live near the sediment–water interface. They are known to accumulate higher metal concentration than marine fishes due to their filter feeding behaviour, long lifespan and sedentary life (Stanković et al. 2012). Oysters have been widely used to study the metal pollution as they can accumulate metals from contaminated marine environments. Nevertheless, our previous study reported that the consumption of marine fishes of Odisha coast is a major contributor of heavy metal intake in coastal population (Satapathy and Panda 2017). Therefore, the knowledge of the elemental contents in edible oysters in these study areas is essential to minimise potential adverse health effects. The risks arise from heavy metal can be categorised into both carcinogenic and non-carcinogenic risks. The non-carcinogenic risk assessment was adopted by many researcher using target hazard quotient (THQ) and toxicity equivalent factor (Praveena and Aris 2018). The THQ-based risk assessment method is a probabilistic method of experiencing adverse health effects on exposed population, which provide a realistic probability of risk level after a prolonged exposure. Carcinogenic effect is determined by dose–effect relationship and comparing between exposure concentration and threshold limit for adverse effect (Solomon et al. 2013). In this context, along with the estimation of risk on aquatic organisms, the human health risks associated with consumption of oyster Saccostrea cucullata of both the sites have also been explored.
The objectives of this study include (i) to establish a general perception of toxic metal bioavailability, their source and pattern of distribution in estuaries nearer to industrial establishments; (ii) to estimate the potential carcinogenic and non-carcinogenic threat associated with ingestion of edible oysters using the average daily intake (EDI) and the target hazard quotient (THQ); (iii) to suggest some guidelines for the safety measure of the estuarine ecosystem and create awareness.
Materials and method
Study area
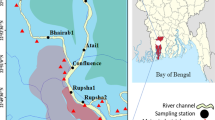
Both study sites are considered as the most dynamic and productive estuarine environment of Bay of Bengal due to their location and ecological habitat (Fig. 1). The climatic condition of both the estuaries is dominated by NE and SW monsoon. The Mahanadi estuary (19°47′N and 20°30′N–80°33′E and 86°49′E) is the largest estuary of Odisha formed by river Mahanadi. This shallow brackish water estuary spread over an area of about 104 sq. Km. of the coastline along the Bay of Bengal. The shoreline of sampling site “Paradip mouth region” is sandy while upstream sediment consists of a mixture of sand and clay. This area is rich in mangrove vegetation together with mudflats, and thus rich in fishery resources, and plays a vital role in the economy of the local residents with annual fishery production is about 550 tonnes (Odisha fishery policy 2015). Its location is in the close proximity of the port city Paradip, where along with port activity, a number of major industrial establishments of eastern India like PPL, IFFCO, IOCL, PPT, HPCL, Essar steel are located. The industrial activity near the upstream of Mahanadi estuary, large agricultural runoff and mining activity from the mineral belts influence the geochemical condition of this site. In Sambalpur, Cuttack and Paradip region, there is a proliferated industrial zone and the sewage discharges to Mahanadi river are prominent. The drainage basin area is approximately 1.42 × 105 km2, which results a total runoff of 50 × 109 m3. Classified as a partially mixed coastal plain, the pollution level of this study site is significantly affected by riverine discharge due to rainfall variation. The second study area, the Hooghly estuary, is the first deltaic offshoot of Ganges, India, which lies between 21°31′N 87°45–88°45′E in the West Bengal district and is associated with Sundarbans, the world’s largest mangrove biosphere. The mixing zone of this funnel-shaped estuary extends up to 80 km. Geomorphologically, it is comprised of coastal dunes, sand flats, mud flats, etc. It is very shallow with average depth of only 6 m and tidal mixing is intense. The soil is composed of silty clay loams, sandy clays, loams, and soil with organic and peat deposits (Banerjee et al. 2012). Alluvial soils impregnated with salts are found along the coast. The soil pH averages 8.0. The major pollution sources of this estuary are due to Farakka Barrage, port activities of Haldia and Kolkata, dredging and discharge of sewage from Haldia town and Kolkata metropolis. Untreated domestic wastes of around 397 tons/day are being discharged to Hooghly from Kolkata metropolis. Forty major industries at a stretch of 92 km (Jute, Pulp and papers mills, brick kiln, textile industries, rubber, distilleries, tannery and thermal power plants) discharge their wastewater into the estuary either directly or through municipal drainage. Despite a number of contemporary studies focusing on pollution and other ecological aspects, there has been a lack of complete study on the risk assessment of both the study sites.
Sample collection and processing
Appropriate sampling methods according to the Ministry of Earth Sciences (MoES), India, have been followed to accommodate various samples. Sampling plan included forty numbers of sediment samples collected from the selected hotspots of both the sites using a handheld GPS. The sediment samples were collected using Peterson grab from the same places where bivalve beds were located. In the laboratory, the texture and the organic carbon of the sediment were analysed following the method suggested by Ingram (1970). The proposed sequential extraction methods by the European Community Bureau of Reference have been slightly modified for metal speciation study in the sediments. This method can efficiently separate non-residual and residual metal complexes. Sequential extraction method was performed in triplicate for each sample to ensure accuracy of the experiment.
Oysters were manually collected from the sampling sites with the help of local fisherman twice a year during summer and winter. Their habitat was uniformly dispersed throughout the bottom and was well exposed to wave current. In the laboratory, these samples were identified, depurated and washed in milli-Q water to remove any external debris, attached to their shells. Twenty living individuals of each site were isolated for metal analysis and health risk assessment. Each bivalve was cut by the abductor’s muscle and was put on a filter paper to extract the internal water. The obtained tissues (after discarding the shells) were ground to a fine paste, freeze-dried and homogenised. The tissue sample was treated with 10 cm3 of mixtures of HNO3, H2O2 and HCl (7:3:1 ratio) in a microwave digester. The residues were redissolved using 2% HNO3. The digested samples were filtered; volume was made up to 50 ml and stored at 4 °C until analysis. Both the sediment and the oyster tissue samples were run in AAS (SHIMADZU AA-7000) against known standards.
Prior to analysis, all the used glass-wares and Teflon vessels were precleaned in 10% HNO3. The instrument calibration standards were made from mono-element certified reference solution for ICP grade (Merck, Germany). The samples were analysed in triplicate for precision. Each analysis included one blank reagent, a reference tissue or sediment sample and triplicate no of samples. Standard reference material of National Institute of Standard and Technology for both sediment (SRM 320) and mussel (ERM-CE278 K) were used. The spiked recovery of all metals was within the range of 97.2–102.12%.
The observed values were expressed as mean ± S.D and statistically analysed (STATISTICA Version 6.1.478.0) using two ways ANOVA. Duncan multiple range test, box plot, PCA, Pearson correlation and factor analysis was performed using SPSS 11.5 (SPSS Inc., Chicago IL, USA). Significant differences were determined at the P < 0.05 level.
Sampling was carried out twice a year during two different seasons: summer (April) and winter (December) to observe if there is any seasonal variation in the pollution level due to human interference. During winter, the concentration of nutrients in water was quite low. The additive values of NO2, NO3, NH3 in both the sites during winter were < 0.3 µmol/L, total phosphate (IP + OP) < 1 µmol/L and SSC (suspended solid concentration) ranged between (7.2–9.04mg/L). During summer, the level of nutrients (NO2 + NO3 + NH3) increased to 18 µmol/L. SSC and total phosphate level increased to 23.28mg/L, 5 µmol/L-respectively. Average salinity was 28 PSU and pH of water was 8. However, the seasonal variation of metal content in sediment was insignificant because of minimal external input; therefore, instead of choosing both seasons for various risk assessment, the average metal concentrations of both the seasons were taken into account. The tissue metal content in oysters, from both the sites, showed seasonal discrepancy for the reasons described in the discussion section afterwards. Therefore, their metal content was represented seasonally.
Results and discussion
Metal concentration of major elements (Cr, Cu, Cd, Pb, Zn, Fe, Mn and Ni) in the sediments followed by texture (sand, silt and clay) and organic carbon of both the study sites was assessed and was presented in (Table S1). The Hg content in the sediment was below detection limit. The ternary plot (Fig. 2) indicates that the sediment textural distribution in Mahanadi estuary was almost sandy. The reason includes the segregation of finer particles due to tidal and wave action in the near-shore and thus the sand particles are allowed to get deposited. Further, the mixture of both fresh and saline water reduces the velocity of the transporting agent, which led to the sand deposition in the mouth of the estuary (Magesh et al. 2011). The textural pattern of Hooghly estuary shows a unique distribution pattern where the upper end has high sand content but in the middle part the silt percentage increases. The middle part of the estuary is considered as low-energy environment that favours the deposition of silt.
The Fe and Mn composition in the sediment in both estuaries was higher compared to other elements, as the sources of both the elements are mostly from riverine influx and natural weathering effect of the rocks. The sources of pollution and environmental risk assessment of both the sites were assessed using various pollution indices, and the detail classification is described in Table 1. Geoaccumulation index, enrichment factor and contamination factor evaluate the contamination level of the trace elements, while PLI and SPI reflect the environmental risks associated with the sediment metal content.
The (Igeo) value quantifies the metal contamination level of sediment (Muller 1979).
Cn = metal concentration of the sample, the Bn = background value of the observed metal. The geochemical background value from (Wedepohl 1995) has been considered here as it represents the continental crustal average and this classification of sediment quality is acknowledged worldwide. The results (Fig. 3a) highlighted the Hooghly estuary was moderately contaminated with Cd and Pb and the possible sources of both these elements in the estuary are mainly from the nearby tannery and textile industries, various small- to medium-scale electronic manufacturing industries and electroplating industries. However, the contamination was negligible from the elements like Ni, Cu, Cr and Zn. The Igeo value of Mahanadi estuary shows the similar pattern of contamination, and this site was strongly contaminated with Cd. The fertiliser-based industrial effluents from IFFCO and PPL that are directly being discharged to the estuarine region enrich the Cd level in the sediment (Islam et al. 2018).
The probable source of trace elements in the sediment is determined by employing a geochemically standardised element as a reference like aluminium (Satapathy and Panda 2018). The effect of sedimentary input can be negated by normalising the metal of interest against a reference metal, which does not have an anthropogenic origin.
The order of enrichment of metals in the sediment of both the Hooghly and Mahanadi estuaries is Cr > Zn > Ni > Cu > Pb > Cd and Zn > Cu > Ni > Cr > Pb > Cd, respectively. The average EF of both the sites indicates moderate to strong enrichment of Pb and Cd (Fig. 3b), which are mostly derived from port activities and discharge of paint and other industries nearby. Minor enrichment was noticed for copper in Hooghly, and no enrichment was observed for Cr, Zn and Ni in both the sites, which indicates the deposition of the above-said metals was primarily from earth’s crust.
The CF value effectively determines the level of contamination (Hakanson 1980)
The observed CF (Fig. 3c) indicated that Pb and Cd contamination was considerable in Hooghly estuary, whereas in Mahanadi estuary only Cd reflected considerable contamination level. In Hooghly and Mahanadi estuary, moderate contamination was observed for Cu and Pb, respectively, which indicated both the above-said elements were associated with common anthropogenic sources. No contamination was observed for Cr, Zn and Ni.
PLI is obtained by calculating the nth root of the n number of CFs that was observed for each metal (Tomlinson et al. 1980).
Based on PLI (Fig. 4), most of the sampling stations of Hooghly estuary fall under polluted category, whereas Mahanadi estuary remains relatively uncontaminated at most of the stations. SPI is a multi-elemental approach for sediment quality assessment considering the trace metal concentrations along with metal toxicity (Singh et al. 2002).
EFm is the ratio between measured metal concentration and reconstructed background values. Wm is the toxicity weight which is constants for different elements (Cr, Zn = 1, Cu, Ni = 2, Pb = 5). The sediment classifications highlight that the sediment of both the estuaries (SPI range 2–5) falls under low polluted zone.
The interpretation of interrelationship between sediment quality and heavy metal concentration was done using bivariate Pearson correlation matrix (Table S2). In Hooghly estuary, most of the metals have a negative correlation with sand, whereas strong positive relationship was observed with silt except the metal Ni. The exception of strong binding of Ni with sand particles supports the hypothesis that coarsest grain fractions have a greater external area that provides the colloid coating. This low crystalline coating allows the metal with higher adsorption capacity like Ni to bind with sand fractions (Otero et al. 2013). The positive correlation was observed between OC vs Mn, Zn, Cd, Cu and Fe; Mn vs Zn, Cu, Cr; Cd vs Cr, Fe, Zn vs Cr, Fe. In both the estuaries, although all the metals positively correlated with organic carbon, the coefficient of determination was within (r2 = 0.28–0.482) which indicated that the heavy metal content does not always increase with the increase in organic matter. It is also dependent on the binding competition among trace metals and type of organic carbon (Rajkumar et al. 2018). A strong positive correlation was observed between silt and OC (P < 0.05). The correlation analysis of both the sites revealed that most of the metals exhibit a strong correlation among each other. Three principal components (PCs) were selected from the rotated varimax correlation matrix, considering the eigenvalue > 1 (Table 2). The percentage of total variance and the cumulative percentage was also taken into account. In Hooghly estuary, the total variance explained was 65.84%, whereas in Mahanadi estuary, the total variance was 77.20%. The range of communality for all the parameters was in between 0.56–0.92 with average communality of 0.87 for Hooghly. Similarly, the range of communality was within the range of 0.76–0.99 with average communality of 0.97 for Mahanadi. The PC1 is explained by strong positive loading (> 0.8) with silt and OC, as the fine-fractioned materials present in the silt facilitates the adsorption and deposition of the organic matters. A moderate positive loading (> 0.4) was also observed for variables like Fe, Cd, Zn, Mn, for which a common lithogenic origin of the above-said metals is confirmed. PC2 showed higher loading of Cu and Cr which indicates both the metals are influenced by human interference and the source composition controls their distribution. PC3 represents positive loading for Zn, Cr and Pb. This loading explains desorption mechanism of Zn, Cr and Pb from the sediment at a higher pH (Facchinelli et al. 2001). Three PCs were also extracted for the Mahanadi estuary. The PC1, again, represented a very strong loading (> 0.9) between silt, OC and Mn. Manganese in its oxide form shows strong affinity with silt-sized fractions. A significant positive association with clay and Zn correlates to the fact that the clay content of the sediment plays an effective role in zinc adsorption into the sediment. The dispersion of Zn is long, and there is a strong evidence of coprecipitation with Fe–Mn hydroxides. PC2 shows strong loading for Cd, Cr and Pb, which indicates a common origin of these toxic metals like the industrial and urban sewage discharges brought by the Mahanadi river to this estuary. PC3 represents a strong relationship between clay and nickel, which points to the fact that nickel binds well with clay fractions of the sediment in the form of nickel oxide.
Though there is a negligible seasonal difference in the sediment of both the estuaries, a visible seasonal discrepancy in metal content in the bivalve’s tissue was observed. Table 3a summarises the concentrations of some major trace metals in different species of oysters collected from different regions of the world at different times as well as the metal concentration in oysters in the current study. The seasonal metal concentration instability (Table 3b) over a wide range is explained by a number of general factors, in agreement to that perceived in this study.
-
1.
In both the study sites, there was a considerable increase in metal concentration (Fe, Zn, Mn, Cu) in oysters during summer (prior to the reproduction period, considered to be a period of high storage of energy reserve), while the lowest accumulation was found during winter (during spawning, when the reserved energy has already been used in reproduction) (Taylor and Maher 2013). These above-said essential metals are involved in the extensive synthesis of biomolecules like protein, essential enzymes and respiratory pigments during the non-reproductive period (summer) and prepare their body for spawning. Kljaković-Gaspić et al. (2010) reported a similar trend of accumulation of heavy metals like Zn, Fe and Cu in mussel Mytilus galloprovincialis. The Cd bioaccumulation in bivalves also increases with temperature. The increased salinity, temperature, pH and higher organic carbon content during summer might have played a major role in the higher bioavailabilities of these metals (Genç et al. 2015).
-
2.
The amount of potential accumulation of toxic metal ions differed quantitatively for different sites. The inconsistency of metal ion accumulation can be ascribed to the unique environmental preexposure of the species to metals at different sites and individual characteristic difference (Luoma and Rainbow 2005).
-
3.
Metal bioaccumulation in the oyster’s tissue is mostly dependent on metal concentration present in surrounding environment and duration of exposure. Being the most abundant element in earth’s crust, Fe accumulation rate was highest irrespective of the seasons or sites (1241.5 ± 26.83 mg kg−1). Another reason of highest concentration of Fe was that it is stored in goethite form (α-FeO(OH)) in the radula (Cravo and Bebianno 2005). The metal accumulation of Cr, Cd, Pb, Ni was significantly lower than Zn accumulation due to its abundance in sediment compared to other metals. However, metal availability is not the only aspect of bioaccumulation as Cr and Zn both are abundant in nature, but uptake rates of Zn in oysters were greater than Cr due to the faster reaction rate of Zn with organic ligands, which results in higher uptake rate (Luoma and Rainbow 2005). Similarly, Cd bioaccumulation was also suppressed at the expense of substantial Zn inclusion. The oyster’s body limited the assimilation of metal ions like As and Pb because the involvement of those metals in metabolic process is negligible. Even though all metals are freely available in the surrounding environment, it was observed that bivalves selectively chose metals according to their biological needs.
The biota-sediment accumulation factor (BSAF) represents the amount of trace elements that was transferred to bivalves from the surrounding medium and was determined as follows:
Corg is the average metal concentration of organism and Csed is the average concentration of metal in associated sediment (Zhao et al. 2012). During summer, the accumulation of essential metals like Cu, Fe, Cr, Zn, Mn in oysters was higher compared to winter (P < 0.01). But Pb, Cd and Ni did not follow the seasonal variation pattern. Figure 5 represents the metal bioaccumulation efficiency of oysters in both the study sites. Cu is the metal with highest mean BAF followed by Zn. The lowest BAF was observed for Cr. In both the estuaries, the Zn and Cu bioaccumulation is directly related to their physiological need as i) both act as enzyme activator in tissue metabolism; ii) Cu is essential for haemocyanin (a protein for oxygen transport in the bodies of bivalves) formation; iii) higher content of Zn and Cu in their surrounding environment during summer in dissolved or suspended form could be another reason for higher uptake (Nwabueze and Oghenevwairhe 2012). The lesser Cu bioaccumulation during winter indicated that during reproduction the body is more into getting prepared for spawning than other metabolic activities which lead to the lesser requirement of Cu assimilation. The remarkably less Ni bioaccumulation signifies that the uptake rate and bioavailability of Ni are quite low. In oysters, Cd transport is accelerated by Zn ion (Chafik et al. 2001) and that’s why with higher Zn concentration, Cd accumulation was also higher. The BSAF of Cr, Pb, Mn exhibited insignificant variation which explains that the bioaccumulation of these metals from the surrounding environment is quite negligible as they have no role in body metabolism.
Human health risk assessment due to oyster meat consumption
The potential health hazard due to ingestion of contaminated seafood can be expressed as carcinogenic risks and non-carcinogenic risks. The non-carcinogenic risk is based on the target hazard quotient (THQ) which is derived from daily ingestion rate (EDI). The estimated daily intake of each metal (EDIm), average annual ingestion rate (EDIa), reference dose (Rfd) were calculated based on the EPA’s default data provided for safety limit of consumption for humans (Table 4). The EDI estimation is based on the metal concentration in tissue, consumption rate and average body weight. The risk assessment due to seafood consumption is based on the fact that the ingestion rate is equivalent to absorption rate (USEPA 1989) and cooking has no effect on the total metal content (Devi and Yadav 2018).
MS = adult meal size (approximately 120 g w.w), C = metal concentration (mg/Kg), BW = the average body weight is assumed as 60 kg, IR = annual average consumption rate, EF = exposure frequency (350 days/year), ED = exposure duration (30 years), RfDing = reference dose for ingestion mode of exposure (mg/kgday−1), AT = average time of exposure (30 years).
All the above-described constants were derived from the consumption limits and provisional daily intake limits, provided by USEPA 2008, integrated risk information system (IRIS) and USDOE 2011. The EDI range of trace elements due to seafood consumption of Mahanadi was 0.06(mg kg−1 BW), 0.15(mg kg−1 BW), 0.09(mg kg−1 BW), 0.1(mg kg−1 BW), 0.01(mg kg−1 BW), 0.006(mg kg−1 BW), 4.5(mg kg−1 BW), 0.13(mg kg−1 BW) and 0.0001(mg kg−1 BW) for Cu, Zn, Mn, Ni, Pb, Cd, Fe, Cr and Hg, respectively. Similarly, the EDI of oysters found from Hooghly was 0.07(mg kg−1 BW), 0.16(mg kg−1 BW), 0.19(mg kg−1 BW), 0.01(mg kg−1 BW), 0.01(mg kg−1 BW), 0.004(mg kg−1 BW), 1.6(mg kg−1 BW), 0.4(mg kg−1 BW), 0.00001(mg kg−1 BW) for Cu, Zn, Mn, Ni, Pb, Cd, Fe, Cr and Hg, respectively. The hexavalent form of Cr (Cr VI) is a carcinogen, whereas the trivalent Cr is considered to have no health effects. The Cr found in protein-rich seafood is mostly trivalent chromium, as it binds well with protein (B tassew et al. 2014). Seafood is consumed as the cheapest available protein source and the lower EDI of Cr in both the study sites reflects that the local population might be protein deficient. The EDIm results revealed that the EDI of all eight heavy metals except Fe was lower or near the borderline of RfD which suggests the oyster meat is a good supplement for iron-deficient population. The EDI of Cd from the oyster meat of both the sites reaches the borderline of RfD set by ATSDR, which might increase the risks of both breast and prostate gland cancer and endocrinal dysfunction among the sea food-consuming population (Akoto et al. 2014). The EDI of Ni, Mn, Cu, Cd in both the study areas was within the safety limit of reference dose set by USEPA 2011, demonstrating insignificant health issues related to those metals. The average daily intake of Pb in both the sites was above the RfD (ATSDR 2010) which may pose lead poisoning and may lead to conditions like IQ deficiency, neurological mutation and nephrological disorder mostly in infants (US-EPA 2000). However, further research is necessary before concluding any health risks to local population. The average THQ of Mn, Ni, Cd, Fe, Pb and Cu in oysters found in Mahanadi was > 1. Similarly, the THQ of Cu, Ni, Pb, Cd and Fe in oysters found in Hooghly was > 1, which suggests that oyster meat-consuming population of both the sites might experience adverse health threat due to these metals. In Mahanadi, the relative contribution of THQ of Mn and Ni to the total THQ represented that both were major risk contributors and accounted for 30.3% and 25.08% risks, respectively. Whereas in Hooghly, the major risk contributors to the total THQ were Cd (30.8%) and Pb (24.05%). In the oysters of Mahanadi, the risk contribution of Cu, Zn, Pb, Cr and Hg was < 10% of the total THQ which represents lesser health threat from these metals. The hazard index (HI) indicates health threats arising from a species due to the additive effects of various metals because of ionic bond interaction (Hallenbeck 1993). Mathematically, HI < 1 is considered to be safe and < 1 HI > 5 represents the concern health risk within limit. The HI > 5 represents a greater risk level. Considering the HI of both Mahanadi and Hooghly, it is strongly recommended to check the metal contamination level before consuming or exporting these oysters.
For an approximate lifespan of 70 years, the carcinogenic risks are obtained from EDI and the cancer slope factor of ingestion mode of exposure (CSFing), provided by integrated risk information system (IRIS), USEPA. The cancer slope factor is the lifetime upper bound cancer risk due to the ingestion mode of exposure from a potential carcinogen (USEPA 2011). According to the USEPA, the CR below (1 × 10−6) in seafood represents negligible risk of developing cancer in human and CR above (1 × 10−3) requires minor remediation. TCR below (1 × 10−3) represents developing potential cancer risks on humans over a period of lifetime exposure. Lifetime target cancer risk can be calculated as follows (ATSDR 2010):
where EDI = estimated daily intake and CSFing is the cancer slope factor for ingestion mode of exposure.
The carcinogenic risk of toxic elements like Cr, Cd, Ni and Pb is presented in Table 5. The severity of carcinogenic risk is shown in Fig. 6 for both the sites, and it indicates that carcinogenic risk (CR) was highest for Cr followed by Cd in both the study sites. The oyster consumption of Mahanadi estuary might result in the increase in lifetime cancer risk due to Cr exposure which might report extra three cases of cancer per 100 no’s of population (3 × 10−2). Likewise, the possibility of developing cancer due to consumption of oyster of Hooghly is highest due to Cr toxicity (1 × 10−2). The lifetime cancer risk of Cd in both the sites increases up to extra 10 persons in 10,000 populations. The CR of Ni and Pb indicates the possible risk of developing cancer in seafood consumers due to these metals is negligible.
MPI is used for screening the pollution load in the marine organisms of different study sites (Javed and Usmani 2013). The critical MPI range is 100, and the MPI range above this limit is considered to create hazardous effects on humans.
where Cfn is the metal concentration of N th metal (mg/kg/dw). During sampling period, the MPI range of the bivalves of both the sites in both the seasons ranged between (16.67–33.69). The highest index was observed in bivalves of Mahanadi, which indicates the oysters of this site are more contaminated for human consumption. The observed MPI value was far below the critical limit in both the study sites.
The metal distribution pattern in the tissues was in the order of Fe > Mn > Cr ≅ Pb > Zn > Ni > Cu > Cd. The range of trace metal concentration was widely distributed, and most of the elements were above the permissible limit for edible mussel tissue set by different organisations (Table 6). Mn is a necessary element for cellular growth, reproduction and bone growth (Aschner and aschner 2005). However, its deficiency or superfluity can cause skeletal and reproductive abnormalities. Oysters and sponges are known to accumulate Mn in their body (Wafi 2015). As observed in our study, the seasonal average concentration in the oysters was much higher (43.75 and 79.1 mg kg−1) compared to the recommended permissible range of manganese concentrations (approximately 20.0 mg kg−1) in seafood provided by Turkish food codes. Adequate Mn intake range for adult men and women are 2.3 and 1.8 mgday−1, respectively (National Academy of Sciences 2001). Zn acts as an enzyme activator and is needed for biological metabolism in all the marine organisms. The insufficiency of Zn in body can lead to acute organ failure and excessive Zn intake can cause adverse effects. The RDA for Zn is 11 mgday −1 and 8 mg day −1 for adult man and woman, respectively, and the TDI (tolerable daily intake) is 40 mgday−1 (IOM 2001, 2003). The average Zn content in the oysters of both Mahanadi and Hooghly estuaries was observed as 81.5, 79.5 mg kg−1dw, which is far above the recommended Zn level by (FAO 2002, Turkish food code and EU 2006 and MOFL 2014). Fe is an essential element for all the living organisms. Its deficiency causes anaemia, and adequacy is responsible for different types of cancer, which ranges from 29% to 46% of cases (Antunes et al. 2018). Excess iron can also be harmful causing gastrointestinal disturbances, nausea and abdominal irritation if the intake rate is more than 20 mg kg−1day−1(IOM 2001). In severe cases, overdose of iron (ingestions of 60 mg/kg at a time) can lead to multiple organ failure, convulsions and even death (Chang and Rangan 2011). The UL (upper limit) of Fe is in children (< 8 yrs), and adults (14–70 yrs) is approximately 40–45 mgday−1(IOM 2003). Excess iron is harmful to aquatic organisms also who respire through gills as it forms “flocs” in gills which lead to respiratory discomfort (Peuranen et al. 1994). The major source of iron in coastal population is seafood, and the results indicate that Fe concentration is tremendously higher than the permissible limit compared to other metals. The average Cd level in the oysters of both Hooghly and Mahanadi estuary were 1.4 and 2.3 mg/kg dw, respectively, which was above the permissible limit of Cd (0.05 mg/kg) set by CODEX Alimentarius Commission 2006 on food additives. The Turkish food code recommended 0.1 mg/kg and the FAO, FSSI and Australian food standard code has recommended the range of Cd in seafood as 2 mg/kg. The source of cadmium into the water is mostly from painting industries, smelting, electroplating industries and fertiliser plants. The effect of excess long-term exposure to Cd includes renal failure, osteoporosis and prostate gland cancer (Gray et al. 2005). The seasonal average of Cu concentration in the oysters of both Mahanadi and Hooghly estuaries (36.9 and 32.45 mg kg−1, respectively) was above the recommended guideline of FAO 2002. According to the food standard committee of UK, the recommended Cu range in food should not exceed 20 mg kg−1 ww. Cu, though considered as an essential element for human body, its higher intake would result in renal and hepatic dysfunction (ATSDR 2010). The UL of Cu for children (1–3 yrs) is 1 mg kg−1 and adults of both male and female (19–70 yrs) is 10 mg kg−1(IOM 2003). The recommended daily limit of Cu intake is 990 mg/day. Comparing our results with the international food standards indicates that consuming these contaminated oysters might result in increased Cu contamination in humans. Hg presence in seafood is mainly methyl mercury as oysters and fishes are known to biomagnify methyl mercury in the food chain (Sharma et al. 2014). Many researchers have found that extremely low concentration of Hg is harmful to humans, affecting the nervous system especially to the children and foetus of pregnant women. Chronic exposure to methyl mercury can also lead to health effects like respiratory, hepatic and cardiac abnormalities (ATSDR 2010). In the present study, the Hg concentration was below the detection limit indicating least appreciable Hg pollution. Pb is an universal toxic metal mostly used in battery, metal products and electronic equipment manufacturing industries. Even a trace amount of Pb is toxic to humans, causing neurotoxicity and kidney diseases (ATSDR 2010). The maximum tolerable Pb intake is 1 mg kg−1 for shellfish. EC recommended limit of Pb is 0.2 mg kg−1 ww (EC 2002). The annual average concentration of Pb in the oyster meat from Mahanadi was within the safety range (2.10 mg kg−1) but the concentration was far above the recommended limit in the oysters of Hooghly estuary (9.2 mg kg−1) and thus unsafe for human consumption. Thus, this study raised a question mark over the possible health benefits from the seafood as the existences of harmful metal ions were beyond the safety limits of human intake. The authors challenge the conviction of considering oyster meat as one of the healthiest non-veg food, having low calorific value, rich in protein, minerals, vitamins and polyunsaturated fatty acid, which have immense health benefits.
Conclusion highlights
This comprehensive investigation of trace metal contamination suggested that both the estuaries were moderate to strongly contaminated with Cd and Pb owing to substantial industrial discharges and urban sewage pollution sources nearby. However, physicochemical reactions involved in the binding behaviour of heavy metals with sediment matrices are complicated. Hence, it is inappropriate to predict the pollution level of a certain environment only based on statistical interpretation and on the available risk assessment methods. So to interpret the binding behaviour more efforts will be made in future to explore the fate of metals. The oyster consumption could result in a major exposure to metals. The THQ suggests that the Mn, Ni, Cd, Fe, Pb and Cu of Mahanadi and Cu, Ni, Pb, Cd and Fe of Hooghly might create severe non-carcinogenic risks for the oyster-consuming population. The carcinogenic risk of Cr followed by Cd was above the safety limit that indicates the probability of developing cancer in the coastal population due to these metals which is a critical public health issue particularly for high-risk groups, including women with iron deficit, children and indigenous people who consume organ meats. All the risk assessment methods are theoretical which provide the probabilistic results. The human blood and human hair analysis would give much more concise information regarding the presence of heavy metal in the body. Nevertheless, it is also essential to study the biomagnifications mechanism in the entire food web. To lessen the metal contamination in these areas, source identification of metal contamination is essential. In addition, to control the metal pollution along the coastal waters, rigorous monitoring, public awareness and a stringent government policy are essential.
References
Akoto, O. F., Bismark, E. D., & Adei, E. (2014). Concentrations and health risk assessments of heavy metals in fish from the Fosu Lagoon. International Journal Environmental Resources, 8(2), 403–410.
Alfonso, J. A., Handt, H., Mora, A., Vasquez, Y., Azocar, J., & Marcano, E. (2013). Temporal distribution of heavy metal concentrations in oysters Crassostrea rhizophorae from the central Venezuelan coast. Marine Pollution Bulletin, 73, 394–398.
Antunes, I. M. H. R., Albuquerque, M. T. D., Roque, N. Environ, & Health, Geochem. (2018). Spatial environmental risk evaluation of potential toxic elements in stream sediments. Environmental Geochemistry and Health, 40(6), 2573–2585.
Aschner, J. L., & Aschner, M. (2005). Nutritional aspects of manganese homeostasis. Mol. Asp. Med., 26, 353–362.
ATSDR. (2010). Agency for toxic substance and disease registry, public health assessment and health consultation. CENEX supply and marketing. Grant County, Washington: Incorporated, Quicy.
Ávila, P. F., Ferreira da Silva, E., & Candeias, C. (2017). Health risk assessment through consumption of vegetables rich in heavy metals: the case study of the surrounding villages from Panasqueira mine. Central Portugal Environ Geochem Health, 39(3), 565–589.
Banerjee, K., Senthilkumar, B., Purvaja, R., & Ramesh, R. (2012). Sedimentation and tracemetal distribution in selected locations of Sundarbans mangroves and Hooghly estuary. Environmental Geochemistry and Health, 34(1), 27–42. https://doi.org/10.1007/s10653-011-9388-0.
Barrio-Parra, F., Elío, J., De Miguel, E., García-González, J. E., Izquierdo, M., & Álvarez, R. (2017). Environmental risk assessment of cobalt and manganese from industrial sources in an estuarine system. Environmental Geochemistry and Health, 40(2), 737–748.
Bilandžić, N., Sedak, M., Dokić, M., Varenina, I., Solomun Kolanović, B., Božić, T., et al. (2014). Determination of zinc concentrations in foods of animal origin, fish and shellfish from Croatia and assessment of their contribution to dietary intake. Journal of Food Composition and Analysis, 35, 61–66.
Bray, D. J., Green, I., Golicher, D., & Herbert, R. J. H. (2015). Spatial variation of trace metals within intertidal beds of native mussels (Mytilus edulis) and non-native Pacific oysters (Crassostrea gigas): Implications for the food web? Hydrobiologia, 757, 235–249.
Chafik, A., Cheggour, M., Cossa, D., Benbrahim, S., & Sifeddine, M. (2001). Quality of Moroccan Atlantic coastal water monitoring and mussel watching. Aquatic Living Resources, 14(4), 239–249.
Chang, T. P. Y., & Rangan, C. (2011). Iron poisoning: A literature-based review of epidemiology, diagnosis, and management. Pediatric Emergency Care, 27, 978–985.
Chen, Y. M., Li, H. C., Tsao, T. M., Wang, L. C., & Chang, Y. (2014). Some selected heavy metal concentrations in water, sediment, and oysters in the Er-Ren estuary, Taiwan: chemical fractions and the implications for biomonitoring. Environmental Monitoring and Assessment, 186, 7023–7033.
Codex Alimentarius Commission. (2006). Report: International Conference Centre, Geneva, Switzerland. Joint FAO/WHO Food Standards Programme. www.codexalimentarius.org/input/download/report/662/al29_41e.pdf.
Cravo, M., & Bebianno, M. J. (2005). Bioaccumulation of metals in the soft tissues of Patella aspara: Application of metal/shell weight indices. Estuarine, Coastal and Shelf Science, 65, 571–586. https://doi.org/10.1016/j.ecss.2005.06.026.
Devi, N. L., & Yadav, I. C. (2018). Chemometric evaluation of heavy metal pollutions in Patna region of the Ganges alluvial plain, India: implication for source apportionment and health risk assessment. Environmental Geochemistry and Health, 40(6), 2343–2358.
Environmental Protection Agency, Washington US-EPA. (2000). Risk-based concentration table. Philadelphia: United States Environmental Protection Agency.
EU. (2006). Maximum levels for certain contaminants in foodstuffs, Official Journal of the European Union, L 364/5.
Facchinelli, A., Sacchi, E., & Mallen, L. (2001). Multivariate statistical and GIS-based approach to identify heavy metal sources in soils. Environmental Pollution, 114(3), 313–324.
FAO/WHO. (2002). FAO/World Health Organization. Codex alimentarius—general standards for contaminants and toxins in food. Schedule 1 maximum and guideline levels for contaminants and toxins in food. Reference CX/FAC 02/16. Joint FAO/WHO Food Standards Programme, Codex Committee, Rotterdam, The Netherlands.
FSSAI. (2012). All fish and fish products. FSSAI lab parameters for imported food & methods for standardization. Order: F. No.06/QAS/2012?Important issues/FSSAI. http://www.fssai.gov.in/Portals/0/Pdf/Final Lab Parameters (21-08-2012).pdf.
Fuentes-Gandara, F., Pinedo-Hernandez, J., Marrugo-Negrete, J., & Diez, S. (2016). Human health impacts of exposure to metals through extreme consumption of fish from Colombian Caribbean Sea. Environmental Geochemistry and Health, 40, 229–242. https://doi.org/10.1007/s10653-016-9896-z.
Gao, S., & Wang, W. X. (2014). Oral bioaccessibility of toxic metals in contaminated oysters and relationships with metal internal sequestration. Ecotoxicology and Environmental Safety, 110C, 261–268.
Genç, T. O., Yilmaz, F., İnanan, B. E., Yorulmaz, B., & Utuk, G. (2015). Application of multi-metal bioaccumulation index and bioavailability of heavy metals in unio sp. (unionidae) collected from Tersakan River, Muğla, South-West Turkey. Fresenius Environmental Bulletin, 24(1a), 208–215.
Gray, M. A., Harrins, A., & Centeno, J. A. (2005). The role of cadmium, zinc, and selenium in prostate disease. In T. A. Moore, A. Black, J. A. Centeno, J. S. Harding, & D. A. Trumm (Eds.), Metal contaminants in New Zealand: Sources, treatments, and effects on ecology and human health (pp. 393–414). Christchurch: Resolutionz Press.
Hakanson, L. (1980). An ecological risk index for aquatic pollution control. A sedimentological approach. Water Research, 14, 975–1001.
Hallenbeck, W. H. (1993). Quantitative risk assessment for environmental and ocuupational health. Chelsea, MI: Lewis.
Ingram, R. L. (1970). Sieve analysis procedure in sedimentary petrology. New York: Willey Interscience.
Institute of Medicine. (2001). Dietary reference intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium and zinc. Food and Nutrition Board. Washington, DC: The National Academies Press.
IOM. (2001). Dietary reference intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. Institute of Medicine, Panel on Micronutrients. Washington, DC: National Academies Press.
IOM. (2003). Dietary reference intakes: Applications in dietary planning. Subcommittee on interpretation and uses of dietary reference intakes and the standing committee on the scientific evaluation of dietary reference intakes. Institute of Medicine of the National Academies (p. 248). Washington, DC: The National Academies Press.
Islam, M. A., Romić, D., Akber, M. A., et al. (2018). Trace metals accumulation in soil irrigated with polluted water and assessment of human health risk from vegetable consumption in Bangladesh. Environmental Geochemistry and Health, 40(1), 59–85.
Javed, M., & Usmani, N. (2013). Assessment of heavy metal (Cu, Ni, Fe Co, Mn, Cr, Zn) pollution in effluent dominated rivulet water and their effect on glycogen metabolism and histology of Mastacembelus armatus. Springer Plus, 2(1), 390.
Kadhum, S. A., Ishak, M. Y., & Zulkifli, S. Z. (2017). Estimation and influence of physicochemical properties and chemical fractions of surface sediment on the bio accessibility of Cd and Hg contaminant in Langat River, Malaysia. Environmental Geochemistry and Health, 39, 1145–1158.
Kljaković-Gaspić, Z., Herceg-Romanić, S., Kozul, D., & Veza, J. (2010). Bio monitoring of organochlorine compounds and trace metals along the Eastern Adriatic coast (Croatia) using Mytilus galloprovincialis. Marine Pollution Bulletin, 60(10), 1879–1889. https://doi.org/10.1016/j.marpolbul.2010.07.019.
Kumar, K. S., Sajwan, K. S., Richardson, J. P., & Kannan, K. (2008). Contamination profiles of heavy metals, organochlorine pesticides, polycyclic aromatic hydrocarbons and alkylphenols in sediment and oyster collected from marsh/estuarine Savannah GA. USA. Mar. Pollut. Bull., 56, 136–149.
Liang, L. N., He, B., Jiang, G. B., Chen, D. Y., & Yao, Z. W. (2004). Evaluation of mollusks as biomonitors to investigate heavy metal contaminations along the Chinese Bohai Sea. Science of the Total Environment, 324, 105–113.
Luoma, S. N., & Rainbow, P. (2005). Why is metal bioaccumulation so variable? Biodynamics as a unifying concept. Environmental Science and Technology, 39(7), 1921–1931.
Magesh, N. S., Chandrasekhar, N., & Roy, V. D. (2011). Spatial analysis of trace elements contamination in sediments of Tamiraparani estuary, southeastcoast of India. Estuarine Coastal Shelf Science, 92, 618–628.
Mason, S. L., Shi, J., Bekhit, A. E. D., & Gooneratne, R. (2014). Nutritional and toxicological studies of New Zealand Cookia sulcata. Journal of Food Composition and Analysis, 36, 79–84.
MOFL. (2014). Bangladesh Gazette, Bangladesh ministry of fisheries and livestock, SRO no. 233/Ayen.
Muller, G. (1979). Index of geoaccumulation in the sediments of the Rhine River. Geo. J., 2, 108–118.
National Academy of Sciences. (2001). Dietary reference intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. A report of the panel on micronutrients, subcommittees on upper reference levels of nutrients and of interpretation and use of dietary reference intakes, and the standing committee on the scientific evaluation of dietary reference intakes. National Academy Press, Washington, DC. www.nap.edu/books/0309072794/html/.
Nwabueze, A., & Oghenevwairhe, E. (2012). Heavy metal concentrations in the West African clam, Egeriaradiata (Lammark, 1804) from McIver market, Warri, Nigeria. International Journal of Science and Nature, 3(2), 309–315.
Otero, X. L., Huerta-Diaz, M. A., De-La-Pena, S., Ferreira, T. O. (2013). Sand as a relevant fraction in geochemical studies in intertidal environments. Environ Monit Assess. Oyster World Congress report. (2012). Arcachon Bay, France. https://doi.org/10.1007/s10661-013-3146-y.
Paez-Osuna, F., & Osuna-Martínez, C. C. (2015). Bioavailability of cadmium, copper, mercury, lead, and zinc in subtropical coastal lagoons from the Southeast Gulf of California using mangrove oysters (Crassostrea corteziensis and Crassostrea palmula). Archives of Environmental Contamination and Toxicology, 68, 1–12.
Peuranen, S., Vuorinen, P. J., Vuorinen, M., & Hollender, A. (1994). The effects of iron, humic acids and low pH on the gills and physiology of Brown Trout (Salmo trutta). Annales Zoologici Fennici, 31, 389–396.
Praveena, S. M., & Aris, A. Z. (2018). Status, source identification, and health risks of potentially toxic element concentrations in road dust in a medium-sized city in a developing country. Environ Geo chem Health., 40(2), 749–762.
Rajkumar, H., Naik, P. K., & Rishi, M. S. (2018). Evaluation of heavy metal contamination in soil using geochemical indexing approaches and chemometric techniques. International Journal of Environmental Science and Technology. https://doi.org/10.1007/s13762-018-2081-4.
Satapathy, S., & Panda, C. R. (2017). Toxic metal ion in seafood: Meta-analysis of Human carcinogenic and non-carcinogenic threat assessment: A geomedical study from Dhamra and Puri, Odisha. Human and Ecological Risk Assessment, 23, 864–878.
Satapathy, S., Panda, C. R. (2018). Environmental source identification, environmental risk assessment and human health risks associated with toxic elements present in a coastal industrial environment, India. Environmental Geochem Health. https://doi.org/10.1007/s10653-018-0095-y.
Sharma, B., Singh, S., & Siddiqi, N. J. (2014). Biomedical implications of heavy metals induced imbalances in redox systems. BioMed Research International, 2014, 26.
Singh, M., Muller, G., & Singh, L. B. (2002). Heavy metals in freshly deposited stream sediment of rivers associated with urbanisation of the Ganga plain, India. Water, Air Soil Pollution, 141, 25–54.
Solomon, K. R., Giesy, J. P., LaPoint, T. W., Giddings, J. M., & Richards, R. P. (2013). Ecological risk assessment of atrazine in North American surface waters. Environmental Toxicology and Chemistry, 32, 10–11.
Stanković, S., Jović, M., Stanković, A. R., & Katsikas, L. (2012). Heavy metals in seafood mussels. Risks for human health. In E. Lichtfouse, J. Schwarzbauer, & D. Robert (Eds.), Environmental chemistry for a sustainable world nanotechnology and Health Risk, Part II (pp. 311–373). Netherlands: Springer.
Tassew, B., Ahmed, H., & Vegi, M. R. (2014). Determination of concentrations of selected heavy metals in Cow’s Milk: Borena Zone. Ethiopia, Journal of Health Science, 4(5), 105–112.
Taylor, A. M., & Maher, W. A. (2013). Exposure-dose-response of Tellina deltoidalis to metal contaminated estuarine sediments 1. Cadmium spiked sediments. Comparative Biochemistry and Physiology, Part C, 158, 44–55. https://doi.org/10.1016/j.cbpc.2013.04.005.
TFC. (2002). Turkish Food Codes, Official Gazette, 23 September, No: 24885,
Tomlinson, D., Wilson, J. G., Harris, C. R., & Jefferey, D. W. (1980). Problems in the assessment of heavy metal levels in estuaries and the formation of a pollution index. Helgolander Meeresuntersuchungen, 33(1–4), 566–575.
US EPA. (2015). US Environmental Protection Agency. Regional Screening Levels (RSLs) for Chemical Contaminants. Retrieved from: http://epa-prgs.ornl.gov/chemicals/download/ressoil_sl_table_01run_NOV2015.pdf. (18.01.16).
USDOE. (2011). The risk assessment information system (RAIS). Orkridge: U.S. Department of Energy Orkridge Operations Office (ORO).
USEPA. (1989). Agency, U.S.E.P. Risk assessment guidance for superfund-Human Health Evaluation Manual Part A, Interim Final, vol. I. Washington (DC)
USEPA. (2008). Environmental Protection Agency. Integrated Risk Information System. Boca Raton: CRC Press.
USEPA. (2011). Regional Screening Level (RSL) Summary Table. http://www.epa.gov/regshwmd/risk/human/Index.htm, last update: 6 th December.
US-EPA. (2000). Guidance for assessing chemical contamination data for use in fish advisories. Vol. II. Risk assessment and fish consumption limits.EPA/823-B94-004. United States.
Wafi, H. N. (2015). Assessment of Heavy Metals Contamination in the Mediterranean Sea Along Gaza Coast—A Case Study of Gaza Fishing Harbor (Master Thesis). Institute of Water and Environment, Al -Azhar University, Gaza, Palestine.
Wang, Z. H., Wang, X. N. (2014). The heavy metal contents in shellfish from South China Sea coast and its dietary exposure risk. Chin. Fish. Qual. Stand. 4, 14–20 (in Chinese).
Wedepohl, K. H. (1995). The composition of the continental crust. Cosmochim Acta, 59, 1217–1232.
Weng, N. Y., & Wang, W. X. (2014). Variations of trace metals in two estuarine environments with contrasting pollution histories. Science of the Total Environment, 485, 604–614.
Zhao, L., Yang, F., Yan, X., Huo, Z., & Zhang, G. (2012). Heavy metal concentrations in surface sediments and manila clams (Ruditapes philippinarum) from the Dalian coast, China after the Dalian Port oil spill. Biological Trace Elements Research, 149, 241–247.
Zuzolo, D., Cicchella, D., Catani, V., et al. (2017). Assessment of potentially harmful elements pollution in the Calore River basin (Southern Italy). Environmental Geochemistry and Health, 39(3), 531–548.
Acknowledgements
This work was supported by the Ministry of Earth Science (MoES), Govt of India. Thanks to the COMAPS team members, CSIR-IMMT for their cooperation in data preparation and sampling. Further Gratitude is extended to two anonymous reviewers and editor for their valuable suggestions.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Satapathy, S., Panda, C.R. & Jena, B.S. Risk-based prediction of metal toxicity in sediment and impact on human health due to consumption of seafood (Saccostrea cucullata) found in two highly industrialised coastal estuarine regions of Eastern India: a food safety issue. Environ Geochem Health 41, 1967–1985 (2019). https://doi.org/10.1007/s10653-019-00251-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-019-00251-4