Abstract
On small and medium karstic coastal islands in the Adriatic Sea, brackish lakes are often the only source of freshwater. Therefore, it is important to adequately evaluate the biogeochemical processes occurring in these complex water systems, as well as to determine the origin of contaminants present. In this study, the distribution and origin of trace metals (Tl, Hg, Cd, Pb, Cu, Zn, Ni, Co) and organic matter in the water column, sediment, and surrounding soil of the brackish lakes on Mljet Island, South Adriatic Sea, Croatia, were evaluated. Thallium and mercury concentrations in the lake water were up to two orders of magnitude higher compared to ranges found in the adjacent coastal sea water. Elevated thallium concentrations were of anthropogenic origin resulting from previous use of rodenticide, while elevated mercury content was naturally enhanced. Levels for the other metals were characteristic of uncontaminated water systems. Speciation modelling showed that dissolved trace metals such as Cu, Pb, and Zn were mostly associated with organic matter, while Tl, Co, and Ni were present predominantly as free ions and inorganic complexes. The presence of organic matter (OM) clearly influenced the speciation and distribution of some trace metals. OM was characterised by the determination of the complexing capacity for Cu ions (CuCC), surface active substances, and catalytically active compounds. Reduced sulphur species (glutathione and other thiols) representing significant Cu-binding ligands were determined and discussed as well.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The people of the Mljet Island (Adriatic Sea, Croatia) have not yet been connected with a mainland potable water supply network. Local authorities desalinise brackish water from drill holes, which local community uses for personal and agricultural purposes. In several places, on the southern part of the island, surface water collects in lakes formed by sea-level rise during a postglacial period, approximately 10,000 years ago (Gušić et al. 1995; Wunsam et al. 1999). These lakes (“blatine”—muds in local term) are connected hydrologically with the coastal Adriatic Sea surface water but only through the porous karsticated carbonated rock. Therefore, lakes are brackish with salinity ranging between 1 and 25, depending on the vicinity and connection to sea water and the season. The distance from the brackish lakes to the sea ranges approximately 100–1500 m. The main sources of freshwater to the lakes are small submerged springs and rainwater run-off from the surrounding hills. Local communities use the lakes for agriculture irrigation. The biogeochemistry of natural waters cannot be explained without examining trace metals and their speciation. Although trace metals exist naturally in water systems, anthropogenic influences, such as mining, fossil fuel combustion, industrial processes, agriculture, and tourism, can change their concentrations as well as their speciation (Oursel et al. 2013). Moreover, these human pressures are considered to further enhance trace metal bioavailability.
So far, various karstic and geochemical features were investigated on the Mljet Island, including anchialine caves (Cuculić et al. 2009, 2011; Kwokal et al. 2014), subterranean estuaries (stratified water column) in a cavernous geological setting (Bishop et al. 2015). Among other elements, water from the anchialine caves of the Mljet Island was found to contain naturally elevated levels of mercury (Cuculić et al. 2009; Kwokal et al. 2014) linked to leaching of guano from bat communities inhabiting these caves. Once introduced into the aquatic environment, Hg is redistributed throughout the water column, deposited and accumulated in sediments, and biomagnified along the trophic chain (Fitzgerald et al. 1991; Selin 2009). Although mercury is one of the most toxic elements and biogeochemical processes in coastal regions related to this element have been extensively studied worldwide (Mason and Lawrence 1999; Wang et al. 2012; Fantozzi et al. 2013; Kwokal et al. 2014), its distribution and speciation in brackish environments of Croatia is still poorly explored.
Thallium(I) sulphate was extensively used on the Mljet Island as a rodenticide (Barun et al. 2011). However, Tl abundance and biogeochemistry in the area have not yet been studied. Thallium can be toxic (Lin and Nriagu 1998) and its toxicity depends on its redox condition. The TlIII is more toxic (up to 3 orders of magnitude) compared to TlI, which is thermodynamically more stable and less reactive (Ospina-Alvarez et al. 2015). It was shown that TlI was the major species in suspended particulate matter, while TlIII was detected in larger particulate fraction (>0.45 µm) (Ospina-Alvarez et al. 2015). Therefore, it could be speculated that Tl will be present in the shallow, brackish system in both oxidation states.
It has been established that peroxo-radicals (produced by photochemical reactions) and organics could influence the redox equilibrium of various elements in the water column, e.g. Fe and Cu (Moffett and Zafiriou 1993), although mainly stabilising in the lower oxidation state. Elevated TlI concentrations are of great environmental concern since the metal ion has a low affinity for organic matter and remains in its ionic (free) fraction. Its inability to form complexes is even applied in electrochemical measurements (the stability of its redox potential upon adding the complexing agents), and TlI is used as internal standard for normalisation of the current of other investigated metal ions (Pižeta et al. 1996).
The concentration of free ionic metals, i.e. their most toxic form, in aquatic systems is regulated by organic complexation, which affects their bioavailability (Buck et al. 2007). For example, up to 99% of copper ions occur complexed with organic ligands in shallow aquatic environments (Croot 2003). The extent of copper complexation with the organic ligands can be determined by titration with copper ions in a controlled electrochemical procedure (Plavšić et al. 1982). With the predominance of thiol compounds, different reduced sulphur species (RSS) could be present in the water when organisms are stressed with metal ions. Moreover, RSS can also be important copper complexing ligands (Laglera and van den Berg 2003). Generally, the bioavailability of metals in the water column is not determined only by their total concentration, but also by the content of organic matter and the presence of various complexing ligands.
The shortage of potable water sources on karstic islands remains a considerable challenge and requires proper measures. Hence, the assessment of the actual metal concentrations and their speciation in such environments is of utmost importance. Moreover, identifying the sources of metals in brackish water environments as well as their forms (free or complexed) is essential to understand biogeochemical cycles in these important aquatic systems.
Thus, the aims of this study were as follows: (1) to determine total and dissolved metal concentration in brackish lakes from the Mljet Island; (2) to determine the metal concentration in associate sediments and surrounding bedrocks and soils; (3) to estimate organic and inorganic species of measured elements in studied lake waters; and (4) to characterise organic matter by the determination of the complexing capacity for Cu ions (CuCC), surface active substances (SAS), and catalytically active compounds (CAC).
Materials and methods
Description of the study area
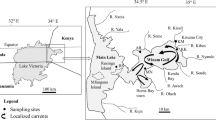
Mljet Island (Fig. 1) is situated in the South Adriatic Sea, Croatia. It has an area of 100 km2 and is 37 km long and 3 km wide. The island’s north–west part was proclaimed a national park (NP) in 1960. The geology of Mljet is characterised by limestones and dolomites, which are porous and semi-porous rocks, respectively, and occurrences of flysch, an impervious, thin-layered sedimentary rock, serving as a hydraulic barrier (Gušić et al. 1995).
Blatska blatina (Blato Lake) (Fig. 1) is the biggest brackish lake and is situated near the village of Blato. Its surface area varies from 0.08 km2 during dry seasons to 0.2 km2 in the wet periods. Near the northern edge of the Blato Lake there is a round well with a depth of 15 m and diameter of 20 m. Even in summer, during times of droughts, this well remains filled with water (Fig. 1). Moreover, in this brackish environment, local people fish for eels that are at times present in high abundance, confirming a hydrological connection to the sea. The other investigated brackish water bodies, the Požur Lake, the Lokva Lake (only brackish lake inside NP), and the Kozarica Lake (Fig. 1), are smaller with water quantity strongly dependent on weather conditions. They are also connected with the coastal sea water through the karstic material.
Sampling and physico-chemical parameters
Sampling campaigns were carried out in May, June, July, and September of 2012. Water samples were collected in brackish lakes Blato, Prožur, Lokva NP, and Kozarica and in nearby coastal surface sea water in the Kozarica port (Fig. 1). Depth profile water samples were taken in the Blato Lake at 0.2, 1.5, 2, 3, 6, and 6.5 m. Water samples for trace metal determination were collected using a clean sampling technique (Horowitz 1997), in pre-washed 1-L fluorinated ethylene propylene (FEP) bottles (Thermo Fisher Scientific, Waltham, USA). It has been shown that trace metals exhibit low adsorption ability for FEP (Cuculić and Branica 1996). Water samples for Hg analysis were collected in 1-L (DURAN, Wertheim, Germany) pre-washed borosilicate glass bottles. A Uwatec Aladin Teac 2 dive computer (depth accuracy ± 5 cm) (Henggart, Switzerland) was used for determining sampling depths. Coordinates of sampling sites (accuracy ± 5 m) were identified by GPS Map 76 CSx (Garmin Ltd, Kansas City, USA). Hach HQ40D (Hach Company, Loveland, USA) was used for an in situ analysis of physico-chemical parameters, salinity (S), pH, dissolved oxygen (DO), and water temperature (t). Sensor calibration was performed prior to each sampling, and salinity was expressed according the Practical Salinity Scale and quoted without unit.
Bedrock samples were collected from the area of the Mljet NP. The topsoil and sediment samples were collected in and around brackish lakes Blato, Prožur, and Kozarica (Fig. 1) and stored in plastic bags. Sediments were sampled by a diver with hand-driven acrylic corers. At each location, approximately a half kilogram of sample was taken. All solid samples were homogenised and analysed as total fraction. Subsamples of sediments, soils, and rocks of approximately 0.2 g were digested using a mixture of concentrated Suprapur® perchloric (1 mL) and nitric acid (10 mL) (all Merck), in closed Teflon crucibles (V = 35 cm3) on a hotplate, at a temperature of ~180 °C (Martinčić et al. 1989).
Analysis of metal ions
Total metal concentrations in the water were determined in unfiltered samples, while dissolved metals were measured in filtered samples (0.45-µm cellulose nitrate membrane filters) (Sartorius, Göttingen, Germany). Prior to voltammetric measurements, all water samples for trace metals were acidified (pH < 2) (Suprapur® HNO3, Merck Millipore, Billerica, USA) and UV-irradiated with 150-W mercury lamp for 24 h in order to destroy stable organic complexes. Trace metal determinations in water and solid samples were obtained by μAUTOLAB potentiostat (Metrohm Autolab, Utrecht, The Netherlands) connected with a Metrohm 663 VA Stand three-electrode system, while a hanging mercury drop was used as a working electrode. A 25 mL water sample was measured in the electroanalytical cell. Differential pulse anodic stripping voltammetry (DPASV) was used for Cu, Cd, Pb, and Zn analyses (Cuculić et al. 2009, 2011), with standard addition method for measurement in water and solid samples. Thallium was determined separately, due to Cd interferences, also using DPASV. Voltammetric parameters were as follows: modulation time 0.04 s, interval time 0.1 s, modulation amplitude 20 mV, and step potential 2 mV. The deposition time was 600 s at −0.85 V for Cu, Cd, Tl, and Pb, while for Zn deposition time was 60 s at −1.2 V.
The limits of quantification (LOQ) in water were 1, 5, 2, 15, and 10 ng L−1 for Cd, Cu, Pb, Tl, and Zn, respectively (10 σ rule with 10-min deposition time; standard addition method, pH < 2), assessed in Milli-Q® water (Merck Millipore). Adsorptive cathodic stripping voltammetry (ACSV) and standard addition method were used to measure concentrations of Ni and Co. Voltammetric parameters were as follows: modulation time 0.04 s, interval time 0.1 s, modulation amplitude 20 mV, step potential 2 mV, and deposition time 240 s at −0.75 V. The limits of quantification (LOQ) of Ni and Co were 10 and 1 ng L−1, respectively. The precision of the applied method for Co, Cd, Cu, Ni, Pb, and Zn was checked measuring the standard reference material (SRM) for trace metals in ocean sea water (NASS-6, National Research Council of Canada). Thallium precision was obtained using SRM trace elements in water (1643e, National Institute of Standards and Technology (NIST), USA). The limit of detection for metals in solid matrices were 0.01 mg kg−1, while for Tl was 0.1 mg kg−1. Quality control for metals in sediment was obtained by using SRM 2702 (NIST). All concentrations were measured in triplicate and were found to be within 10% of certified values.
Concentrations of total (not filtered) mercury (HgTOT) in all samples were analysed by cold vapour atomic absorption spectrometry (CVAAS) with Elemental Mercury Detector 3200 (Thermo Separation Products, USA). The method used was described earlier in detail by Kwokal et al. (2014).
The detection limits for HgTOT in water and solid samples were 0.01 ng L−1 and 0.001 mg kg−1, respectively. These measurements were based on three standard deviations of a blank measurement (Milli-Q® water). Precision of the method in water samples was verified by analysing coastal sea water reference material CRM 579 (EU Standards, Measurements and Testing Programme). For total Hg in sediment samples, SRM 2702 was measured (NIST) and tested mass fractions were determined to be within 5% of the CRM value. All quality assurance data are given in Online Resource 1.
Analysis of organic matter
All organic species were determined from 25 mL of sample. Sample acidification, where needed, was obtained by the addition of Suprapur® HCl. Copper complexing capacities (CuCC) were determined by the DPASV method of Plavšić et al. (1982). The electrochemical instrument and the electrode system for analysis of organic matter were the same as for metal ion analysis, with the electrode used in static mercury drop mode (SMDE). Calculations were made according to the linear transformation of the measured data (Ružić 1982; van den Berg 1982). Experimental conditions were as follows: modulation time 0.04 s, interval time 0.31 s, modulation amplitude 25 mV, and step potential 5 mV. The deposition time was 60 s at −0.6 V. A solution of acidified (pH = 2), UV-irradiated sample was titrated with copper ions, to determine the sensitivity, S, of the method for copper. A sample at natural pH was titrated in the same way with the increasing amount of copper ions, equilibrated for 20 min and measured three consecutive times. Oxido-reduction reaction of Cu ions proceeds in two steps: Cu(0), from deposited amalgam, during the stripping step, is oxidised to Cu(I) at the potential of ~−0.2 V. Subsequently, the Cu(I) is oxidised to Cu(II) at a potential of ~+0.05 V (Krznarić et al. 1992). Ligands measured were mostly different thiols (Laglera and van den Berg 2003; Strmečki et al. 2010b) and sulphide ions (S2−) (Plavšić et al. 2011) which complexes do not dissociate during the applied stirring rate in the deposition step.
The surface active substances (SAS) were determined by phase selective alternating current voltammetry (PSACV) (Ćosović 1985; Plavšić and Ćosović 1994). Surfactant activities were expressed in the equivalents of the model SAS, Triton-X-100 (MW–600), and the detection limit was 0.03 mg L−1.
The reduced sulphur species (RSS) were determined by square wave cathodic stripping voltammetry (SWCSV) (Ciglenečki and Ćosović 1996). The model calibrating compound was glutathione with detection limit of 0.006 mg L−1.
The catalytically active compounds (CAC) were determined by electrochemical method of constant current chronopotentiometric stripping analysis (CSA) (Strmečki et al. 2010a). They were measured at natural pH. Results were given in the equivalents of a model protein, human serum albumin (HSA, Sigma, USA, MW = 67 kDa) with detection limit of 0.0026 mg L−1.
Statistical analysis
The data were treated statistically using STATISTICA 7.0 (StatSoft, Inc.) and SigmaPlot 11.0 (Systat Software, Inc.) software for Windows. The multivariate principal component analysis (PCA) was performed on data matrix consisting of the element concentrations in the lake waters. The correlations between the concentrations of elements in the water and the salinity were expressed as Pearson correlation coefficients (c.c.). Differences in the water composition concerning the location (lake), depth, and analysed phase (total and dissolved) were tested by analysis of variance (ANOVA) on ranks. The level of significance was set at p < 0.05.
Results and discussion
Salinity, dissolved oxygen, and temperature
Vertical profiles of the physico-chemical parameters (salinity, dissolved oxygen, and temperature) from the Blato Lake (May 2012) and nearby sea water (closest to the Kozarica Lake) are presented in Fig. 2. Sea water temperature decreased by one degree, from 20 °C at 0.2 m to 19 °C at 3 m, with a slight increase in DO (10.2–10.5 mg L−1). Dissolved oxygen values indicate an oxic marine system. Sea water surface salinity (S = 35.0) suggested intrusions of freshwater from the Kozarica Lake, while below 0.4 m salinity (S = 38) was comparable to southern Adriatic sea water (Cuculić et al. 2009, 2011; Kwokal et al. 2014). In the Blato Lake, surface water temperatures and DO (25.4 °C, 15.8 mg L−1) were found to be similar to those in the Kozarica Lake (25.8 °C, 16.1 mg L−1), and indicative of the well aerated and oxic surface of these brackish water bodies. Measured salinities in the Blato Lake were the lowest compared to other brackish water systems, probably due to a larger karstic freshwater input. DO decreased significantly, from 15.8 mg L−1 at the surface, to 1.7 mg L−1 at 9 m, with the biggest change between 8 and 9 m where hypoxic conditions prevailed (Fig. 2).
Metals in topsoil, sediment, and bedrock
The trace metal results for the bedrock, sediment and topsoil samples, and a literature comparison are given in Table 1 and in Online Resource 2. The amounts of all metals in the bedrock samples, classified as limestones and dolomites (Gušić et al. 1995), were within the range or below values characteristic of unaltered carbonate rocks (Connor and Shacklette 1975). Furthermore, no significantly elevated metal content was recorded in brackish sediments, compared to the results reported for unpolluted Adriatic Sea sediments (Martinčić et al. 1989; Cuculić et al. 2009), with the exception of Tl in the Blato Lake. Namely, thallium amounts in the Blato Lake sediment varied between 0.7 and 1.5 mg kg−1, which is up to two times higher compared to an average value reported for the Adriatic Sea sediments (0.69 mg kg−1) and far above values found in the Rijeka harbour sediments (0.17–0.69 mg kg−1, Cukrov et al. 2011).
In topsoil samples, levels of Cd, Co, Pb, Ni, and Zn elements were found in accordance with the literature data for Croatian soils (Miko et al. 1999) and Geochemical Atlas of Europe (FOREGS, Salminen 2005). Only for Cu and Tl, levels in topsoil samples around the Blato Lake were significantly above the aforementioned literature data. Agricultural activity was responsible for the elevated Cu mass fractions found in the topsoil samples since they were collected from gardens and vineyards.
In all topsoil samples from around the Blato Lake, Tl concentrations were similar, ranging from 1.67 to 1.76 mg kg−1, which is above the environmental screening value (1 mg kg−1) defined by USEPA and above the maximum permissible concentration (1.3 mg kg−1) agreed by Dutch standards. Although literature data for Tl in uncontaminated soils range from 0.01 to 3 mg kg−1 (Wenqi et al. 1992), most soils contain Tl at concentrations of less than 1 mg kg−1 (Fergusson 1990). However, low thallium levels (0.02–0.09 mg kg−1) in carbonate rocks (dolomites and limestones) of the study area indicate that the bedrock could not be the significant source of elevated Tl amounts in studied soil samples.
The latter could be attributed to use of rodenticide, in the form of thallium(I) sulphate, during the 1900s. Namely, in 1910, the mongoose (Herpestes auropunctatus), one of the worst invasive small mammal species (Barun et al. 2011), was introduced to the Mljet Island to control the poisonous snakes inhabiting the island. The mongoose introduction has had detrimental effects on some native species and changed the behaviour of ship rats (Rattus rattus), which were introduced much earlier to the island. As a result of the mongoose introduction, rats modified their daily activities to avoid mongoose predation and became even more widespread requiring the use of rodent control. Nonetheless, the applications of thallium salts were prohibited in 1975 in the USA due to the non-selective nature of their toxicity.
Trace metals in water
The trace metal concentration ranges for studied brackish lakes and coastal sea water are presented in Table 2, and compared to the annual average concentrations (AAC) and maximum allowable concentrations (MAC) of trace metals in surface waters, defined by national and European legislation (Official Gazette 2013, 2015; European Union Directive 2013). Additionally, the raw data for all metal concentrations are reported in Online Resource 2.
Studied lakes displayed differences in terms of levels of measured elements. For Zn, Cd, Pb, and Co, measured concentrations showed statistically significant differences (p < 0.05) between studied lakes. On the other hand, the same was not observed for Cu, Ni, and Hg. Thallium could not be included in the comparison, due to a limited set of data.
For all measured elements, distribution along the water column displayed no significant difference, regardless of the lake system, with exception of Cu. Only for copper, statistically significant difference in measured concentrations between different sampling depths was observed. Subsurface layer (at 20 cm depth) displayed overall highest range of measured values, while at other sampling depths Cu concentrations displayed lower variability. Overall highest copper concentrations, both dissolved and total, in the subsurface layer (at 20 cm depth) were measured in the Kozarica Lake (949 and 951 ng L−1, respectively) and the Lokva Lake (1196 and 1214 ng L−1, respectively). Moreover, no statistically significant differences (p > 0.05) between total and dissolved concentrations, for all measured elements, were observed.
Despite the observed differences in the spatial distribution, concentrations were within the range for non-contaminated waters as specified by national and European legislation for all studied elements, with exception of Tl. Moreover, the average concentrations of Cd, Pb, Hg, and Ni from the brackish lakes were tenfold and 100-fold lower when compared to MAC and AAC values, respectively. However, when compared to other brackish lakes and coastal sea water, concentrations of mercury and, particularly, thallium were elevated in Blato Lake water.
By some standards, Tl in the range 5–10 ng L−1 is characteristic for uncontaminated freshwater systems and concentrations of 20–50 ng L−1 are considered polluted ones (Lin and Nriagu 1998). For unpolluted sea water, Tl ranges from 10 to 20 ng L−1 (Lin and Nriagu 1999). The average (74 ng L−1) and minimum (33 ng L−1) Tl concentrations (Table 2) in studied brackish waters were significantly above those values reported for unpolluted freshwater. Unfortunately, Croatia, as well as the European Union, has no legislation or limits for admissible Tl concentrations in natural waters. However, USEPA defined the maximum contamination level for drinking water to be 2 µg L−1 and maximum contaminant level goal of 0.5 µg L−1 (USEPA 2005), significantly above the measured values.
The observed elevated thallium concentrations, both dissolved and total, in the lake water samples could be attributed to higher levels of this element in studied soils, i.e. the earlier use of rodenticide on the Mljet Island.
In order to identify the major sources of elements in the studied lakes, the PCA was performed on the element data set. The eigenvalues of the first three principal components (PCs) were found larger than 1, indicating their significance. The first three PCs explained 76.8% of the total variability among the 8 variables; the first component (PC1) contributed 33.0%, while the second (PC2) and the third (PC3) components contributed 22.8 and 21.0% of the total variance, respectively. Results of the PCA were presented on the PC1 versus PC2 and PC1 versus PC3 plots (Fig. 3). The greatest negative PC1 loadings (<−0.70) displayed Co, Cu, and Ni (Fig. 3a). Their common grouping combined with their rather low levels characteristic for unpolluted systems point to their common natural origin.
The greatest positive effect on PC2 is exerted by Hg and Tl (Fig. 3a). The latter suggests that PC2 differentiates elements which levels are influenced by additional factors. As stated previously, measured levels of Tl in the lake waters are a combined effect of the natural background and application of rodenticide for purposes of rodent control. On the other hand, the elevated HgTOT concentration (14 ng L−1) in the water column of Blato Lake likely originated from the airborne input (wet/dry precipitation), as this was the reason for high mercury concentrations measured during periods of high rainfall and strong eastern winds, in Veliko jezero and Malo jezero (lakes) in the Mljet NP (Cuculić et al. 2009). Additionally, elevated HgTOT in the brackish lakes could be a result of bat guano leaching. Namely, bats readily inhabit anchialine caves on the Mljet Island. Previous studies (Cuculić et al. 2011) demonstrated that bat guano leaching is responsible for elevated concentrations of trace metal in waters of the anchialine caves on the Mljet Island, whereby at some locations extremely high HgTOT concentrations in the waters (300–1000 ng L−1) were recorded (Kwokal et al. 2014). The elevated Hg is most likely the result of bioaccumulation from the bat prey, given that bats are insectivorous mammals that consume the equivalent of their own body mass daily (Pavlinić et al. 2010).
The greatest negative PC3 loadings displayed Cd and Pb (Fig. 3b). Moreover, Cd and Pb were the only elements whose concentrations were found to be positively correlated to the salinity (c.c. 0.63 and 0.83, respectively, p < 0.05). The latter suggests that in addition to natural background levels, their distribution along the water column, particularly Cd, is determined by the salinity as well.
Speciation modelling
Modelled organic and inorganic species of the dissolved trace metals in the Blato brackish lake and nearby surface sea water are presented in Table 3. Trace metal speciation was calculated with Visual MINTEQ 3.1 software according to the major and trace element composition (Gustafsson 2001). Trace metal interactions with an organic matter were estimated with Stockholm humic model (SHM) implemented in the software. Fulvic acid (FA) and humic acid (HA) parameters given by the software were used together with the measured dissolved organic carbon (DOC) concentrations 3 and 6.5 mg L−1 in surface sea water and Blato brackish lake, respectively (Cuculić et al. 2011). It was presumed that 80% of active dissolved organic matter (DOM) is FA, according to Bronk and Sipler (2014). Concentrations of dissolved trace metals measured in May from the brackish lake were used for speciation modelling. For the sea water model, the mean values of dissolved trace metal concentrations from Table 2 were used. As specified above, Tl concentrations were not available for sea water samples, so a dissolved Tl concentration of 15 ng L−1 was used for the speciation calculation. Moreover, due to a higher stability of Tl(I) and published results (Moffett and Zafiriou 1993; Ospina-Alvarez et al. 2015), Tl(I) speciation was obtained. Sea water salinity of 38.0 was used to calculate Cl− and Na+ concentrations, 19.4 and 10.8 g L−1, respectively, as well as K+ (0.40 g L−1), Ca2+ (0.41 g L−1), Mg2+ (1.29 g L−1), and SO4 2− (2.7 g L−1). Salinity of 2.0 was used for calculation of major ions distribution in Blato brackish water (Cl− 0.97 g L−1, Na+ 0.54 g L−1, K+ 0.02 g L−1, Ca2+ 0.02 g L−1, Mg2+ 0.06 g L−1, and SO4 2− 0.12 g L−1). This model estimates speciation of metals associated with organic matter concerning humic substances; hence, the speciation of Co, Cd, and Ni inorganic fraction could be slightly overestimated.
Modelling suggests differences in the organic and inorganic distribution of dissolved trace metals, as well as between brackish and sea water systems. The fraction of organically bound metals reflects their affinity for association with organic ligands in order Cu > Pb > Zn > Co > Cd > Ni > Tl and Cu > Pb > Zn > Cd > Co > Ni > Tl, for sea water and brackish lake, respectively. Copper and lead were found almost completely bound to organic matter, both in the lake and in the sea water, supporting earlier reports (Millero 2001; Hirose 2006; Stockdale et al. 2011). In general, organic portions of all dissolved metals, except thallium, were more pronounced in the brackish lake in comparison with the sea water and governed by lower Cl− amount and higher DOC content.
The concentration of dissolved trace metals bonded with organic ligands was determined in this study as the complexing capacity for copper ions—CuCC (chapter 3.5). Among other trace metals, copper forms the most stable organic complexes and has been the metal of choice in most environmental studies (Laglera and van den Berg 2003; Plavšić et al. 2009a; Strmečki et al. 2014). Moreover, the concentration of trace metals in the organic metal complexes regulates the bioavailability of trace metal ions. As reported earlier, free metal ions can more easily cross through biological membrane compared to organic metal complexes (Sunda and Guillard 1976; Sunda and Huntsman 1998).
Inorganic speciation in sea water revealed that fractions of free (hydrated) ions, representing a bioavailable form of metals, decline in order Co > Ni > Tl > Zn > Cu > Cd > Pb, while in the brackish lake, the Ni-free ionic fraction was the largest (94.2% of diss. Ni). In both water systems, modelling indicated that more than half of total dissolved concentrations of cobalt, nickel, and thallium were present in the free (ionic) state. However, their bioavailable fractions could be considered as non-toxic, since dissolved Cu, Cd, Ni, Co, Zn, and Pb concentrations were mainly in the range of uncontaminated eastern coastal Adriatic Sea (Cuculić et al. 2009; Mlakar et al. 2015). Moreover, a major inorganic portion of dissolved Pb was bound to carbonates and to chlorides in lesser extent, while dissolved Cd was characterised by stable chloro complexes (more than 19 and 89% in the brackish lake and the sea water, respectively), confirming earlier studies (Millero 2001; Waeles et al. 2009).
Speciation of Tl+ did not show any significant organic complexation, neither in sea water nor in brackish lake, confirming previous findings (Lin and Nriagu 1998; Moffett and Zafiriou 1993). Free ionic Tl+ was found to be the dominant Tl(I) species in both water bodies, 50.5 and 94.3% in sea water and the brackish lake, respectively, and potentially highly toxic. Thallium(I) chloride was also present in high proportion in sea water (44.7%), due to high chloride concentration, while in brackish lake this species was not significantly presented (5.2%), similar to [TlSO4]− ionic form with only 0.5 and 4.9% in brackish lake and sea water, respectively.
Depleted DO concentration in the bottom layer of brackish water column and the presence of different reduced sulphur species (RSS), as discussed in details below, in the hypoxic/anoxic water layer near the bottom of the brackish lake were likely responsible for the thallium partitioning at the sediment/water interface and stabilising Tl+ in the water column. Moreover, due to the constant salinity, concentrations of major inorganic ligands were uniform throughout the water column; hence, they could not be the controlling factor of Tl sediment/water partitioning. Additionally, organic complexation of Tl+ ions is already known to be insignificant (Pižeta et al. 1996).
Calculation further confirmed that oxido-reduction conditions, rather than organic ligands distribution, regulate thallium sediment/water partitioning, as hypoxic, or even anoxic conditions prevail in bottom water layer in Blato brackish lake (Fig. 2), which is in favour of more stable Tl+ oxidation state.
SAS, CuCC, CAC, and RSS
Surface active substance concentrations, in the range from 0.232 to 0.402 mg L−1 in equiv. of Triton-X-100 (Table 4), were elevated in comparison with the other karstic area in the Adriatic (Plavšić et al. 2009a). These concentrations are also higher than the concentrations of SAS in North Adriatic (Plavšić et al. 2009b). The SAS influence the fate of metal ions and other micro- and macro-constituents on different phase boundaries like sediment (particles)/water; biological membranes/water and especially the biggest one air/sea water interface (Ćosović 2005). Surfaces are modified with the adsorbed SAS, and the interactions with/on the surfaces are changed. A part of SAS could in addition complex metal ions (Plavšić and Ćosović 1994).
In Fig. 4, the CuCC results for Blato Lake in June are presented. The small peak at the potential of ~−0.4 V in Fig. 4a is a consequence of the presence of Pb ions in the sample and is visible only in the acidified sample (as at neutral or alkaline pH, Pb ions are also bound either to particles or to the organics). In Fig. 4b, the additions of copper ions to the sample of alkaline pH resulted in two voltammetric waves. These two waves are the consequence of the occurrence of two oxidation states of Cu ions (CuI and CuII) resulting from the low salinity of the sample and the proceeding of the oxidation step in two sequences. The voltammetric (oxidation) Cu peak, which was stabilised under experimental conditions, increased with further additions of Cu ions, and total ligand concentration (L T) and log K (apparent stability constant) were calculated by the linearisation method of Ružić–van den Berg.
CuCC determination for Blato, sample 4, June 2012, pH = 9.0, is presented. a voltammograms for additions of Cu at pH 2; b voltammograms for additions of Cu at pH 9.0; c Titration lines obtained as results of (a) and (b); d data from (c) presented in the form of Ružić–van den Berg plot from which the L T and log K are calculated
The CAC fluctuate between zero concentration and 0.021 mg L−1 equiv. (Table 4), similar to values measured in the sea water samples from North Adriatic (Strmečki et al. 2010a, Strmecki et al. 2014), and are usually associated with increased biological activity. The “H” peaks (catalytic waves) for the Blato Lake in June 2012 are presented in Online Resource 3. The “H” peaks with the prolonged accumulation are moved to more positive potentials which reflect their “easier” appearance, i.e. the catalytic reaction is favoured. A characteristic of a catalytic “H” peak is its occurrence at a more positive potential with prolonged accumulation time. Organic compounds determined as CAC are efficient ligands for metal complexation (Strmečki et al. 2010b).
The concentration of sulphur species (RSS) in studied brackish lakes was high and contributed to the elevated CuCC values (0.43–2.13 μM Cu2+ in June), and inhibited the determination of CuCC in July (Table 4). Similar observation was made earlier for the karstic marine Rogoznica Lake (Croatian middle Adriatic coast), a stratified lake with permanent anoxia below 8 m (Plavšić et al. 2011), which prevented the determination of CuCC in the anoxic water layer. The high RSS concentration interfered with the electrochemical determination of copper ions.
As the metal complexing capacity increases, the bioavailable fraction decreases, relating directly to the metal toxicity. The exact chemical species of the organic ligands remain unidentified; however, the ligands incorporating sulphur ions (e.g. of the glutathione type) could represent a significant portion of ligand pool, responsible for the copper complexing capacity. For other measured trace metals (excluding Tl and Hg), the organic ligands with other functional groups (without sulphur), like amino groups and/or carboxylic and phenolic type, could be significant for regulating metal ion distribution at the sediment/water interface.
Conclusions
Anthropogenic influence and natural processes of soil leaching and dry/wet precipitation were responsible for elevated trace metals (Tl and Hg, in particular), SAS, CuCC, CAC, and RSS concentrations in the brackish water environments on the Mljet Island. Elevated Tl concentrations were anthropogenically influenced by earlier rodenticide usage on the nearby fields. Speciation modelling points to prevalence of Tl(I) in free ionic form which could be toxic for organisms. Such bioavailable fraction of Tl could enter the food chain through the use of water from the lakes as potable water and for the agriculture irrigation. Naturally elevated mercury concentrations in the brackish lakes originate from the atmospheric input and/or from the bat guano leaching, previously reported to impact marine lakes in the Mljet NP. Other trace metal concentrations were in the ranges characteristic for uncontaminated water systems, while sporadically elevated levels of Cu in topsoils around brackish systems were attributed to agricultural activities. Speciation modelling confirmed that the major portions of dissolved Cu, Pb, and Zn were bound to organic matter, while high percentages of free (not complexed) ionic Co, Ni, and Tl occur in both water systems, brackish and sea water. Organic ligands, sulphur species, and surface active substances were found to regulate metal speciation. Reduced sulphur species (e.g. glutathione and other thiols) represent the significant portion of Cu-binding ligands.
Our results elucidate the importance of investigating the water resources available on an inhabited coastal island. For some islands deprived of municipal water sources, brackish lakes are potentially a vital source of freshwater. A specific approach is critical as each of the islands and the water available exhibits their own features. Investigations of trace metals and organic matter distribution in these brackish systems are essential to provide accurate data about their geochemistry and speciation. The interpretation of geochemical data and modelling are important prerequisites for the distinction between natural and anthropogenic sources of contamination and their possible abatement.
References
Barun, A., Simberloff, D., Tvrtković, N., & Pascal, M. (2011). Impact of the introduced small Indian mongoose (Herpestres auropunctatus) on abundance and activity time of the introduced ship rat (Rattus rattus) and the small mammal community on Adriatic islands, Croatia. NeoBiota, 11, 51–61.
Bishop, R. E., Humphreys, W. F., Cukrov, N., Žic, V., Boxshall, G. A., Cukrov, M., et al. (2015). ‘Anchialine’ redefined as a subterranean estuary in a crevicular or cavernous geological setting. Journal of Crustacean Biology, 35, 511–514.
Bronk, D. A., & Sipler, R. E. (2014). Dynamics of Dissolved Organic Nitrogen. In D. A. Hansell & C. A. Carlson (Eds.), Biogeochemistry of marine dissolved organic matter (pp. 127–232). London: Academic Press.
Buck, K. N., Ross, J. R. M., Flegal, A. R., & Bruland, K. W. (2007). A review of total dissolved copper and its chemical speciation in San Francisco Bay, California. Environmental Research, 105, 5–19.
Ciglenečki, I., & Ćosović, B. (1996). Electrochemical study of sulphur species in seawater and marine phytoplankton cultures. Marine Chemistry, 52, 87–97.
Connor, J.J., & Shacklette, H.T. (1975). Background geochemistry of some rocks, soils, plants, and vegetables in the conterminous United States. U.S. Geological Survey Professional Paper 574.
Ćosović, B. (1985). Aqueous surface chemistry. Adsorption characteristics of organic solutes. Electrochemical evaluation. In W. Stumm (Ed.), chemical processes in lakes (pp. 55–85). New York: Wiley.
Ćosović, B. (2005). Surface-active properties of the sea surface microlayer and consequences for pollution in the Mediterranean Sea. In A. Saliot (Ed.), The handbook of environmental chemistry (pp. 269–296). Berlin: Springer.
Croot, P. L. (2003). Seasonal cycle of copper speciation in Gullmar Fjord, Sweden. Limnology and Oceanography, 48, 764–776.
Cuculić, V., & Branica, M. (1996). Trace metals adsorption from seawater onto solid surfaces: analysis by anodic stripping voltammetry. Analyst, 121, 1127–1131.
Cuculić, V., Cukrov, N., Kwokal, Ž., & Mlakar, M. (2009). Natural and anthropogenic sources of Hg, Cd, Pb, Cu and Zn in seawater and sediment of Mljet National Park, Croatia. Estuarine, Coastal and Shelf Science, 81, 311–320.
Cuculić, V., Cukrov, N., Kwokal, Ž., & Mlakar, M. (2011). Distribution of trace metals in anchialine caves of Adriatic Sea, Croatia. Estuarine, Coastal and Shelf Science, 95, 253–263.
Cukrov, N., Frančišković-Bilinski, S., Hlača, B., & Barišić, D. (2011). A recent history of metal accumulation in the sediments of Rijeka harbor, Adriatic Sea, Croatia. Marine Pollution Bulletin, 62, 154–167.
European Union Directive, (2013). Directive 2013/39/EU of the European parliament and of the council amending Directives 2000/60/EC and 2008/105/EC as regard to priority substances in the field of water policy.
Fantozzi, L., Manca, G., Ammoscato, I., Pirrone, N., & Sprovieri, F. (2013). The cycling and sea-air exchange of mercury in the waters of the Eastern Mediterranean during the 2010 MED-OCEANOR cruise campaign. Science of the Total Environment, 448, 151–162.
Fergusson, J. E. (1990). The heavy elements: Chemistry, environmental impact and health effects. Oxford: Pergamon Press.
Fitzgerald, W. F., Mason, R. P., & Vandal, G. M. (1991). Atmospheric cycling and air-water exchange of mercury over mid-continental lacustrine regions. Water, Air, and Soil pollution, 56, 745–767.
Gušić, I., Velić, I., & Sokač, B. (1995). Geology of the Mljet Island. In P. Durbešić & A. Benović (Eds.), Proceedings of the symposium: Island of Mljet natural characteristics and social evaluation (pp. 35–53). Zagreb: Croatian Ecological Society. (in Croatian).
Gustafsson, J. P. (2001). Modeling the acid-base properties and metal complexation of humic substances with the stockholm humic model. Journal of Colloid and Interface Science, 244, 102–112.
Hirose, K. (2006). Chemical speciation of trace metals in seawater: A review. Analytical Sciences, 22, 1055–1063.
Horowitz, A. J. (1997). Some thoughts on problems associated with various sampling media used for environmental monitoring. Analyst, 122, 1193–1200.
Kabata-Pendias, A. (2011). Trace elements in soils and plants. Boca Raton: Taylor and Francis Group.
Krznarić, D., Plavšić, M., & Ćosović, B. (1992). Voltammetric investigations of copper processes in the presence of oxygen. Electroanalysis, 4, 143–150.
Kwokal, Ž., Cukrov, N., & Cuculić, V. (2014). Natural causes of changes in marine environment: mercury speciation and distribution in anchialine caves. Estuarine, Coastal and Shelf Science, 151, 10–20.
Laglera, L. M., & van den Berg, C. M. G. (2003). Copper complexation by thiol compounds in estuarine waters. Marine Chemistry, 82, 71–89.
Lin, T. S., & Nriagu, J. O. (1998). Speciation of thallium in natural waters. In J. O. Nriagu (Ed.), Thallium in the environment (pp. 31–43). New York: Wiley.
Lin, T. S., & Nriagu, J. O. (1999). Thallium speciation in the great lakes. Environmental Science and Technology, 33, 3394–3397.
Martinčić, D., Kwokal, Ž., Stoeppler, M., & Branica, M. (1989). Trace metals in sediments from the Adriatic Sea. Science of the Total Environment, 84, 135–147.
Mason, R. P., & Lawrence, A. L. (1999). Concentration, distribution, and bioavailability of mercury and methylmercury in sediments of Baltimore Harbor and Chesapeake Bay, Maryland, USA. Environmental Toxicology and Chemistry, 18, 2438–2447.
Miko, S., Durn, G., & Prohić, E. (1999). Evaluation of terra rossa geochemical baselines from Croatian karst regions. Journal of Geochemical Exploration, 1–2, 173–182.
Millero, F. (2001). Speciation of metals in natural waters. Geochemical Transactions, 2, 57–64.
Mlakar, M., Fiket, Ž., Geček, S., Cukrov, N., & Cuculić, V. (2015). Marine lake as in situ laboratory for studies of organic matter influence on speciation and distribution of trace metals. Continental Shelf Research, 103, 1–11.
Moffett, J. W., & Zafiriou, O. C. (1993). The photochemical decomposition of hydrogen-peroxide in surface waters of the eastern Caribbean and Orinoco River. Journal of Geophysical Research, 98, 2307–2313.
Official Gazette, (2013). Official journal of the Republic Croatia, No. 73.
Official Gazette, (2015). Official journal of the Republic Croatia, No. 78.
Ospina-Alvarez, N., Burakiewicz, P., Sadowska, M., & Krasnodebska-Ostrega, B. (2015). TlI and TlIII presence in suspended particulate matter: speciation analysis of thallium in wastewater. Environmental Chemistry, 12, 374–379.
Oursel, B., Garnier, C., Durrieu, G., Mounier, S., Omanović, D., & Lucas, Y. (2013). Dynamics and fates of trace metals chronically. Input in a Mediterranean coastal zone impacted by a large urban area. Marine Pollution Bulletin, 69, 137–149.
Pavlinić, I., Đaković, M., & Tvrtković, N. (2010). Atlas of Croatian bats (Chiroptera), part I. Natura Croatica, 19, 295–337.
Pižeta, I., Omanović, D., & Branica, M. (1996). Application of thallium (I) as an internal standard redox process in voltammetric measurements. Analytica Chimica Acta, 331, 125–130.
Plavšić, M., Ciglenečki-Jušić, I., Strmečki, S., & Bura-Nakić, E. (2011). Seasonal distribution of organic matter and copper under stratified conditions in the karstic, marine, sulfide rich environment (Rogoznica Lake, Croatia). Estuarine, Coastal and Shelf Science, 92, 277–285.
Plavšić, M., & Ćosović, B. (1994). Influence of surface-active substances on the redox processes of metal ions: a contribution to the speciation analysis of metals in aquatic systems. Analytica Chimica Acta, 284, 539–545.
Plavšić, M., Gašparović, B., Strmečki, S., Vojvodić, V., & Tepić, N. (2009a). Copper complexing ligands and organic matter characterization in the northern Adriatic Sea. Estuarine, Coastal and Shelf Science, 85, 299–306.
Plavšić, M., Krznarić, D., & Branica, M. (1982). Determination of the apparent copper complexing capacity of seawater by anodic stripping voltammetry. Marine Chemistry, 11, 17–31.
Plavšić, M., Kwokal, Ž., Strmečki, S., Peharec, Ž., Omanović, D., & Branica, M. (2009b). Determination of copper complexing ligands in the Krka river estuary. Fresenius Environmental Bulletin, 8, 327–334.
Ružić, I. (1982). Theoretical aspects of the direct titration of natural waters and its information yield for trace metal speciation. Analytica Chimica Acta, 140, 99–113.
Salminen, R. (Ed.) (2005). Geochemical Atlas of Europe. part 1: Background information, methodology and maps. Geological Survey of Finland. Electronic version http://www.gtk.fi/publ/foregsatlas.
Selin, N. E. (2009). Global biogeochemical cycling of mercury: A review. Annual Review of Environment and Resources, 34, 43–63.
Stockdale, A., Tipping, E., Hamilton-Taylor, J., & Lofts, S. (2011). Trace metals in the open oceans: Speciation modelling based on humic-type ligands. Environmental Chemistry, 8, 304–319.
Strmečki, S., Dautović, J., & Plavšić, M. (2014). Constant current chronopotentiometric stripping characterization of organic matter in seawater from the northern Adriatic, Croatia. Environmental Chemistry, 11, 158–166.
Strmečki, S., Plavšić, M., & Ćosović, B. (2010a). Chronopotentiometric stripping analysis of”N-catalyst” in sodium chloride solution and seawater. Electroanalysis, 22, 91–98.
Strmečki, S., Plavšić, M., Steigenberger, S., & Passow, U. (2010b). Characterization of phytoplankton exudates and carbohydrates in relation to their complexation of copper, cadmium and iron. Marine Ecology Progress Series, 408, 33–46.
Sunda, W. G., & Guillard, R. R. L. (1976). Relationship between cupric ion activity and toxicity of copper to phytoplankton. Journal of Marine Research, 34(4), 511–529.
Sunda, W. G., & Huntsman, S. A. (1998). Interactions among Cu2+, Zn2+, and Mn2+ in controlling cellular Mn, Zn, and growth rate in the coastal alga Chlamydomonas. Limnology and Oceanography, 43(6), 1055–1064.
USEPA. (2005). National recommended water quality criteria. Washington, DC: EPA Office of Water.
Van den Berg, C. M. G. (1982). Determination of copper complexation with natural organic-ligands in sea-water by equilibration with MnO2: I theory. Marine Chemistry, 11, 307–322.
Waeles, M., Riso, R. D., Cabon, J.-Y., Maguer, J.-F., & L’Helguen, S. (2009). Speciation of dissolved copper and cadmium in the Loire estuary and over the North Biscay continental shelf in spring. Estuarine, Coastal and Shelf Science, 84, 139–146.
Wang, F., Macdonald, R. W., Armstrong, D. A., & Stern, G. A. (2012). Total and methylated mercury in the Beaufort Sea: The role of local and recent organic remineralization. Environmental Science and Technology, 46, 11821–11828.
Wenqi, Q., Yalei, C., & Jieshan, C. (1992). Indium and thallium background contents in soils in China. International Journal of Environmental Studies, 40, 311–315.
Wunsam, S., Schmid, R., & Müller, J. (1999). Holocene lake development of two Dalmatian lagoons (Malo and Veliko Jezero, Isle of Mljet) in respect to change in Adriatic Sea level and climate. Paleogeography, Paleoclimatology, Paleoecology, 146, 251–281.
Acknowledgements
We acknowledge the financial and professional support of the Mljet National Park and the financial support of the Croatian Academy of Science and Arts for the project “The study of biogeochemical processes in brackish lakes on the island of Mljet as a potential source of drinking water and water for irrigation”. Our special thanks go to Dr. Željka Fiket (from our Division) for the statistical analysis and to Professor Renée E. Bishop-Pierce (Penn State University, Worthington Scranton, USA) for editing grammar and syntax.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Online Resource 1
Quality assurance data for voltammetric measurements of trace metals (PDF 99 kb)
Online Resource 2
Complete raw data of trace metal concentration measurements from all three brackish lakes and Kozarica sea water, with sample depths (m) and salinities (XLS 50 kb)
Online Resource 3
Chronopotentiometric determination of catalytically active compounds, CAC (catalytic peaks „H“) for Blato brackish lake, June 2012. Experimental conditions: accumulation potential E a = −0.6 V, accumulation time t a = 60 s, I stripp.= −1 µA; max. time of measurement 5 s (PDF 105 kb)
Rights and permissions
About this article
Cite this article
Cuculić, V., Cukrov, N., Kwokal, Ž. et al. Assessing trace metal contamination and organic matter in the brackish lakes as the major source of potable water. Environ Geochem Health 40, 489–503 (2018). https://doi.org/10.1007/s10653-017-9935-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-017-9935-4