Abstract
The high-Andean mountain of northern Chile host numerous water systems that is in risk due to increased mining activities. Total and dissolved Cd, Cr, Cu, Hg, Ni, Pb, Se, and Zn in water, and Cd, Cu, Fe, Ni, Pb, Zn, As, Mo, Al, and V in sediments of 21 aquatic systems (rivers, saline lakes, salt flats), were studied. The presence of Pb, Cd, and As in waters and sediments could be explained, in part, by mining activities. Waters are not suitable for human consumption or irrigation due to high content of Cu and As and high pH that exceed Chilean water quality guideline values. The use of different background reference values influences noticeably the conclusion related to environmental quality of sediments, measured with different environmental indexes. The local geological background suggest that Cd, Mo, Pb, and As generate some degree of contamination, while the use of unpolluted systems as background suggest that all metals measured in sediments represent a low contamination risk. The use of background values of local unpolluted systems seems to be more realistic than geological formation or Upper Continental Crust reference values to assess the environmental condition. The ecological risk assessment suggests that Cd and As are threat for communities living in these aquatic environments. However, these systems support abundant wildlife, developing unique extreme ecosystems with great potential for non-consumptive use such as special interest tourism and conservation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
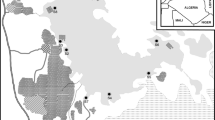
The Andes Mountains is an imposing obstacle to tropospheric circulation, establishing a climatic barrier between east and west of South America. In the semi-arid region of northern Chile, the magnitude of the mountain range generates particular meteorological conditions — such as the altiplano or high-Andean climate — that strongly affect the water availability and the atmospheric circulation at continental-scale (Garreaud et al. 2003). The high Andes (19°S–27°S) is extremely dry, and the precipitation (100–400 mm year−1) is concentrated mainly in the austral summer, when the increased sunlight alone destabilizes the local troposphere, strengthening the easterly winds and enhancing the moisture transportation from the core of the continent (Grosjean et al. 1995; Vuille et al. 2000; Falvey and Garreaud 2005). The high-Andean mountain located between 25°S and 29°S has 24 endorheic basins at altitudes > 3000 MASL, (Fig. 1). These high-Andean water systems have their origins in the tectonic activity, successive glaciations, and volcanism, also impacted by intense solar radiation, low atmospheric humidity, and a strong supply of wind-borne allochthonous material (Vila and Mühlhauser 1987). Nevertheless, these endorheic basins in the high Andes are fed by liquid and solid precipitation forming rivers, saline lakes, and evaporitic basins commonly called salt flats (locally, salares). The salares are in an advanced stage of lacustrine evolution, with > 50% of the surface covered by a salt crust, or a solid layer of various mineral (Chong 1988). These aquatic systems sustain complex ecosystems, often with highly diverse flora and fauna, as well as local productive activities like tourism, but mainly industrial mining, and currently lithium exploitation.

(Source: www.geovirtual.cl); B altitudinal location of the systems studied. For rivers, the altitude corresponds to the sampling point.
 Indicate mining activities
Indicate mining activities
Location of water systems studied in the high-Andean Atacama, northern Chile. A Altitudinal profile from the coast to the Andes Mountains
The chemical composition of high-Andean water systems depends on the local climate and geology. Whereas climate involves water supply, sunlight, and temperature; geology plays a significant role in basins closure, through structural system and volcanic activity (Houston et al. 2011), weathering processes and the leaching of rocks and soils in the basin, specific contributions of possible exposed mineral manifestations, and eventual anthropogenic factors (Chong 1988). Consequently, bottom sediments accumulated in the water systems play an essential role in their chemical dynamics, acting as either sources or sinks of nutrients and other substances that determine the area’s trophic status and biodiversity (Trolle et al. 2010). On the other hand, different studies indicate that lagoons of remote “pristine” mountain regions that are far away from direct human activities are very critical for the deposition of atmospheric pollutants (Fernández et al. 2000; Usenko et al. 2007; Van Drooge et al. 2011; Vilanova et al. 2001; Bandowe et al. 2018). Moreover, large-scale human activities (e.g., industrial centers, mining, extensive agriculture) generate regional-level pollutants that may affect water quality for human consumption at local scale (Choque-Quispe et al. 2021, 2022), as well as water bodies in protected remote areas (Rizzo et al. 2010). These and others studies use different environmental and ecological risk indices (geoaccumulation index, contamination factor, potential ecological risk, and others) to assess the health status of aquatic systems (Cheng et al. 2015; Cheng et al. 2015; Tapia et al. 2018; Guédron et al. 2021; Custodio et al. 2022, and many others).
A small proportion of the High-Andean water bodies of the Atacama region are under the control of the National System of Protected Wild Areas (SNASPE). Only the Nevado Tres Cruces National Park is legally protected (Escudero et al. 2016). The low proportion of protected areas may be due, in part, to a lack of scientific information about high-Andean water systems, making it difficult to demonstrate the fragility and unique condition of these systems and impeding a better-founded justification of their need for protection. Moreover, the subsistence of this territory is uncertain due to the increased importance of lithium mining. Recently, seven high-Andean aquatic systems of the Atacama Region have been authorized for lithium exploitation. These systems are Isla, Parinas, Grande, Agua Amarga, Aguilar, Maricunga, and Piedra Parada (see Fig. 1). Economic development normally does not consider the ecological richness of natural environments, mostly due to the lack of scientific information about the structure and function of these fragile, unique, and extreme systems.
Another concern related to aquatic systems management is the fact that in Chile, the Water Quality Guidelines for marine and continental systems are focused only on the protection of human health (primary guidelines), while the ecosystems protection guidelines (secondary guidelines) have not been fully developed. On the other hand, Chile still does not have Sediment Quality Guidelines for aquatic systems. This situation is a major obstacle to progress in the implementation of sustainable development strategies. In this regard, environmental agencies of countries with a robust legislation advise that environmental standards must be implemented on the basis of scientific information generated locally in each system that is intended to be protected (ANZECC 2000).
In order to generate sustainable territorial development and contribute with a deeper understanding of the High-Andean zone, the present work aims to generate and analyze chemical data on the water and sediments of these aquatic systems, describing differences and similarities between them. Besides, we evaluate their environmental condition using different indexes and background reference values in order to provide a solid baseline to analyze eventual natural or anthropogenic alterations of these extreme ecosystems.
Materials and methods
Climate and geology of the study zone
According to the Köppen-Geiger climatic classification (Sarricolea et al. 2017), the north of Chile between 25° and 28°S is climatically composed from west to east by a cold semi-arid zone with winter rain and oceanic influence (BSk’(s)); a cold desert with winter rain zone (BWk(s)) that to the north becomes cold desert (BWk), alternated with a hot desert with winter rain climate (BWh(s)) in coastal zones and valleys; a cold semi-arid zone with winter rain (BSk(s)) with specific tundra zones with winter rain (ET(s)); a cold semi-arid zone (BSk); a tundra zone (ET); and an ice cap climate (EF) to ice cap climate with summer rain (EF(w)) in areas that exceed 6000 MASL. (Fig. 2). In particular, the study basin contains the climates BSk, ET, ET(w), EF and EF(w), and is delimited by the isohyets of 100 and 200 mm·year–1 (DGA 1987) (Fig. 2). There is no public meteorological station in the basin, and the closest station, “Pastos Grandes” (27°06′51″S, 69°33′51″W, 2260 MASL; Fig. 2), is located to the west of the basin in the Intermediate Valley (Fig. 1). Since 1967, this station has registered an average precipitation of 31 mm year–1, with maximums of 150 and 145 mm year–1 in 1987 and 1997, respectively, while in 1968, 1969, 1985, 1988, and 2003 no rainfall was recorded (data from https://explorador.cr2.cl/).
Up: map with the climatic zones of northern Chile (Sarricolea et al. 2017) and isohyets (DGA 1987) between 25° and 28°S. Climates: BSk: cold semi-arid; BSk(s): cold semi-arid with winter rain; BSk’(s): cold semi-arid with winter rain and oceanic influence; BWh(s): hot desert with winter rain; BWk: cold desert; BWk(s): cold desert with winter rain; ET: tundra; ET(s): tundra with winter rain; ET(w): summer rain tundra climate; EF: ice cap; EF(w): ice cap with summer rain. Meteorological station: “Pastos Grandes” (27°06″51″S, 69°33′51″W, 2260 m.a.s.l (https://explorador.cr2.cl/). Down, geological map at 1:1,000,000 (SERNAGEOMIN 2003) and location of Pleistocene and Holocene volcanoes (Global Volcanism Program 2013) in northern Chile, between 25° and 28°S. Volc.-Sed.: Volcano sedimentary. In the legend, only the outcrops that represent an area ≥ 0.5% of the basin are indicated, together with lagoons (Lag) and meadows (Vega). The detail of the legend is shown in Table 2 (Supplementary material)
The Atacama Desert between 25.5°S and 27.5°S, in northern Chile, comprises five major continental morphostructural units (Fig. 1), from west to east: Coastal Mountain Range (or Coastal Cordillera); Intermediate Valley (or Central Depression); Foothills (or Precordillera); Salt Flats Valley (or Pre-Andean Depression) —including the endorheic basin studied—and Western Cordillera (Arriagada et al. 2006; Kay and Coira 2009). The Coastal Cordillera is largely composed of Jurassic to Middle Cretaceous igneous and sedimentary rocks; the Central Depression consists of a basin filled by Oligocene to Pliocene sediments; the Precordillera is composed of Paleozoic basement and Mesozoic to Eocene volcanic and sedimentary rocks; the Pre-Andean Depression is filled by Miocene to Holocene sediments; and Western Cordillera consists of an active magmatic arc, formed by a series of Pliocene to Holocene stratovolcanoes where peaks reach over 6000 m, which marks the western limit of the high Andes (Fig. 2) (Stern 2004; Clarke 2006; Arriagada et al. 2006; Kay and Coira 2009; Tapia et al. 2018).
In accordance with Andean volcanism segmentation, the study area is in the Central Andes Volcanic Zone (CVZ), which corresponds to a region between 15°S and 28°S where the Nazca plate is subducted beneath the South American plate with a moderate slab angle (~ 25–30°) (Barazangi and Isacks 1979) at a current rate of ~ 6–8 cm year–1 (DeMets et al. 1990; Kraemer et al. 1999; Kendrick et al. 2003; Stern 2004). In this zone, the continental crust is characterized by being extremely thick, reaching values > 70 km along the main magmatic arc (Beck et al. 1996; Trumbull et al. 1999; Yuan et al. 2002). According to Davidson et al. (1991), the volcanic activity in the CVZ is an expression of interaction between subduction-related partial melts derived from the subcontinental mantle and those generated within the continental crust. Particularly, the Cenozoic volcanism in the CVZ comprises three main volcanic associations, all of which are present in the basin studied and represent more than half of the basin area: (i) Early Miocene to Pleistocene andesite-dacite main arc stratovolcanoes; (ii) Early Miocene to Pliocene silicic ignimbrite deposits and lava domes present in both main arc and back-arc regions; and (iii) small Pliocene to Pleistocene basaltic centers in the back arc (Fig. 2, Supplementary material) (Schnurr et al. 2007).
Sampling and laboratory procedures
A total of 65 water samples and 70 sediment samples from 21 aquatic systems of the high-Andean Atacama Region, northern Chile were collected (Fig. 1), during the austral summer of 2015. In cases of saline lakes and salt flats, each system was divided into 3 sections covering its entire surface, and into each section at least one samples was taken. In the case of the rivers, the samples were taken in three different sectors, where the current was slow. Surface water samples was collected with an acid rinsed plastic jar, while surface sediment samples were taken with an acid rinsed spoon. These samples were stored and transported in inert containers treated with nitric acid and abundant deionized water to avoid contamination. The salinity and pH were measured in situ, using a YSI multiparameter probe, model 6600 (YSI 2012).
Total and dissolved As, Cd, Cr, Cu, Hg, Ni, Pb, Se, and Zn were measured in an accredited commercial laboratory according to ISO 17025, using the APHA 3030-B methodology for sample pre-treatment (Gottler 2017) and following the Chilean regulation NCH2313 (INN 1997).
Sediment samples were dried at 40° C and then separated for metal analyses. The fraction < 63 microns of the sediment was separated in a Retsch sieve shaker, model AS Basic 200. After that, about 0.2 and 0.5 g of the sieved fraction was digested in a Mars Xpress microwave digester, according to the EPA 3052 method. Finally, metal contents were measured in a Shimadzu 6300 Atomic Absorption Spectrophotometer, by flame technique, except for As, which was measured by Hydride Generation. A mixture of air-C2H2 gases was used to read Cd, Cu, Fe, Ni, Pb, Zn, As, and N2O–C2H2 (nitrous oxide-acetylene) for Mo, Al, and V. The technique was validated with the reading of metals in MESS-3 reference material, certified by the National Research Council of Canada (Table I, Supplementary data).
Environmental evaluation
Considering that the high-Andean aquatic ecosystems are not used for recreational purposes with direct contact, the metal content in water was evaluated using standards for irrigation and drinking waters according to Chilean legislation, established in NCh. 1333/78 and NCh. 409.01/84 (MOP, 1978, 2005).
Due to the lack of sediment quality guidelines in the Chilean legislation, metal content in this matrix was evaluated with different environmental indexes commonly used in scientific studies.
The geoaccumulation index (Igeo) was calculated using Eq. (1), according to Müller (1979).
where Cn is the current element concentration and Bn is the background concentration. The results of Igeo were classified according to the contamination scale proposed by Müller (1979); < 0 not polluted, 0–1 not polluted to moderately polluted, 1–2 moderately polluted, 2–3 moderately to heavily polluted, 3–4 heavily polluted, 4–5 heavily to extremely polluted, > 5 extremely polluted.
The degree of contamination (Eq. 2) and the ecological risk (Eq. 3) of some metals present in sediments of high-Andean water systems was evaluated according to Häkanson (1980).
where \({C}_{r}^{i}\) is the contamination index (CI) for element i, \({C }_{d}^{i}\) is the measured concentration of element i in lagoon surface sediments, and \({C}_{B}^{i}\) is the background concentration. The systems were classified according to the scale proposed by Häkanson (1980) and shown in Table 1.
where \({E}_{r}^{i}\) is the potential ecological risk (E) for a single element I, and \({T}_{r}^{i}\) is the toxicity response factor for the element i, where \({T}_{r}^{i}\) for As = 10, Cd = 30, Cu = 5, Ni = 2, Pb = 5, and Zn = 1 (Häkanson 1980). The results of \({E}_{r}^{i}\) were classified according to the contamination scale (Table 1) proposed by Häkanson (1980).
Finally, the potential ecological risk index (PERI) was calculated as the sum of \({E}_{r}^{i}\) and was categorized according the classification of Häkanson (1980) (Table 1).
Three background values were used in this study to evaluate the metal enrichment in sediments of Andean aquatic systems and their impact on natural ecosystems; reference values of local geological formation, concentrations measured in unpolluted systems of the high-Andean zone of Atacama Region, and values corresponding to Upper Continental Crust proposed by Rudnick and Gao (2014) (Table 2).
In the first case, and in order to obtain a representative local background of the basin, a composite is made from the geochemistry of igneous rocks published in different studies in the area (Brown 1991; Walker et al. 1991; Kay et al. 1994; Mpodozis et al. 1995, 1996; Trumbull et al. 1999; Richards et al. 2001; Schnurr et al. 2007; Naranjo et al. 2019) (Table II, Supplementary data).
To obtain a local background that is representative of the geochemistry of the basin, it is proposed to construct a composite from the weighting between the average chemistry of the geological outcrops in the basin and the relative surface area factor (RAF) of these (i.e., the ratio between the area of the outcrop and the total area of the outcrops considered). In this way, the concentration of the element E of the composite (Ec; Eq. 4) is defined by:
where EC is the concentration of element E in the composite; and Ei and RAFi are the mean concentration of element E and the relative surface area factor, in geological unit or outcrop i, respectively.
Meanwhile, relative surface area factor (RAFi; Eq. 5) is obtained by:
where Ai is the surface area of the geological unit or outcrop i.
As rock outcrops within the basin do not have their own chemical data, these are obtained and compiled from the geochemistry reported in papers. Meanwhile, the area of the geological outcrops is calculated using the free software QGIS 3.10, by using the $area function of the field calculator, available in the attribute table of the shapefile layer that contains the geological outcrops.
In the absence of all the elements of interest for each geological outcrop or not considering all the outcrops within the basin — or the analyzed area—for the calculation of the composite, the chemistry of the composite should be considered as the minimum or basal content of each element.
For the data reported as below detection level of the elements of interest, an element content equal to half the detection limit is considered. In particular, this correction was made in some samples of the pyroclastic units of acid chemistry (dacitic to rhyolitic). This is justified because Ni usually replaces Fe or Mg in olivine crystals, mainly, and to a lesser extent in pyroxene and hornblende crystals, minerals that are commonly absent in dacites and rhyolites (Best 2003).
In the case of metal background, based on unpolluted systems, the aquatic systems, such as Azufreras, Pedernales, and Negro Francisco, that have records of historical and current anthropogenic intervention, were not considered of this calculation. All the others systems, except the rivers that were not included in this procedure, were separated in saline lakes and salt-flats because their chemical processes can influence the precipitation of metals into the water column. For each case, the quartile Q3 was calculated and assumed as a valid background for the high-Andean aquatic systems. Other statistical methodologies like iterative 2σ technique (Gałuszka et al. 2015; Matschullat et al. 2000; Valdés et al. 2015) or mean values (Xi et al. 2020), require analysis of a large number of samples (more than used in this study), or information on metal content in surrounding sources of aquatic systems analyzed, respectively.
The sediment quality guidelines (SQG) were used to predict the adverse effects produced by contaminated sediments on aquatic organisms (US EPA 1992; Long et al. 1995; MacDonald et al. 2000). The objective of the SQG is to protect the organisms present in aquatic sediments from the deleterious effects produced by chemical substances (Crane 2003). In general, the SQG ranges for aquatic systems from different regions around the world differ by several orders of magnitude, which makes comparison difficult (Cheng et al. 2015). However, Mac Donald et al. (2000) proposed global SQG values obtained from the integration of aquatic systems data from different world regions applicable to systems without reference values. This proposal defines two limits: threshold effect concentration (TEC), under which no adverse effects on organisms are observed, and probable effect concentration (PEC), at which noticeable adverse effects are observed. Adverse effects may or may not occur between these limits (Table 3).
Statistical evaluation
Statistical parameters (mean, standard deviation, minimum, and maximum) were calculated in order to characterize the results of water and sediment measurements.
Similarity dendrogram analysis based on complete linkage methods (Euclidian distance) was performed with water and sediment variables, in order to evaluate the degree of correlation between the aquatic systems. This study explored the physical–chemical properties of water and sediment using a conglomerate analysis to search for similarities and differences among the aquatic systems of the high-Andean zone. This analysis allowed us to evaluate common characteristics in these systems and identify those that have their particularities that differentiate them from the rest. Although three types of high-Andean systems were studied, this analysis was focused on salt flats and lagoons, disregarding the data on the two rivers. For these analyses, Minitab 14 software was used.
Results and discussion
Saline water bodies have an essential geochemical impact on ecology, water resources, and economic activities (Deocampo and Renafult 2016). Also, these systems play an important role in the carbon cycle, since they can emit large amounts of carbon into the atmosphere and sequester an abundant amount of carbonate (Tranvik et al. 2009). In northern Chile, the water bodies of the high-Andean Atacama support important ecosystems of flora and fauna adapted to living in extreme conditions. However, economic activities (mainly mining) threaten the sustainability of these ecosystems.
To date, only three published articles (Risacher et al. 2002, 2003 and Tapia et al. 2018) and two technical reports (Risacher et al. 1999; Dept. Hydraulic Engineering 2009) address the water and sediment chemistry of some lakes and salt-flats in the high-Andean Atacama Region. Other works developed in some salt-flats of the Atacama Region have been focused on microbial communities (Escudero et al. 2013; 2018). Those works, and recent studies conducted in similar environments were used to discuss the results reported herein.
Composite geochemistry (local background)
The average chemical composition of the geological outcrops and the representative composite of the basin (or local background) are presented in Table II Supplementary material. For the calculation of the composite, igneous outcrops (volcanic or plutonic) were used with an area percentage greater than 0.5% of the basin surface, which are the most abundant rocks in the basin (> 60% of the basin area; Table II, Supplementary material). The use of sedimentary outcrops is ruled out for two reasons: (1) these outcrops do not usually have reported chemistry; (2) and, mainly, because most of these outcrops correspond to deposits (alluvial and piedmont deposits and sequences; Table II, Supplementary material), which are the product of weathering processes that fractionate and mobilize the elements (McLennan 2001; Brantley et al. 2007); so their chemistry would reflect dilution and/or concentration processes and not the chemistry of a pristine parent rock.
The outcrops that present a greater weight in the chemical composition of the composite are the M3i and Ms3i units, with an RAF of 0.29 and 0.26, respectively (Table II, Supplementary material), which correspond to volcanic rocks of intermediate chemistry (andesitic to dacitic). In rocks of this chemical affinity, the highest values of Al (9.77%) are found, shown by the OM3 unit; and the maximum contents of Ni (28.33 mh kg−1), V (150.50 mg kg−1), and Zn (96.00 mg kg−1), corresponding to the Q3i unit. Meanwhile, the highest Cu content is held by the CP3 unit (203.33 mg kg−1), while the Q3t pyroclastic volcanic unit has the highest Pb content (38.33 mg kg−1). Unfortunately, not all the outcrops have published values of As, Cd and Mo, so the content of these elements in the composite should be considered as a basal or minimum concentration for the basin.
The final values of geological background are showed in Table 2.
Water chemistry
The values of most of the parameters measured in the waters were below the detection limit (Table 4). Even though some authors suggest that values below detection limits can be used normally to evaluate water and sediment quality (Gochfeld et al. 2005; Helsel 2005; Williams and Antoine 2020), we chose not to use them, to avoid bias in the interpretation of the environmental condition. As a result, only pH, salinity, Cu, and As, that recorded measurable values in all systems, were used to evaluate water quality.
In terms of chemistry, salinity is the primary differentiating criterion to classify the three types of aquatic systems in the high-Andean Atacama. In the rivers, average salinity was 2.2 ppt; in lagoons, it was 47 ppt; and in salt flats, it was 58 ppt. However, the salinity of the lagoons varied widely, from 3 ppt (Santa Rosa, El Bayo, Negro Francisco) to 130 ppt (Verde, Bravas). Four salt flats — La Laguna, Maricunga, Aguilar, and Agua Amarga — presented salinities above 70 ppt; salinity in the other systems were below 40 ppt (Table 4). The type of water in the salt flats of the high-Andean Atacama depends on the geological characteristics of their basins and the evolutionary pathways of their systems. According to Risacher et al. (1999), the Atacama Region encompasses 12 sulfated salt flats (including two acid flats that contain much more sulfate than calcium), three mixed salt flats, and five calcic salt flats. Waters from carbonated pathways drain through volcanic rocks with no sulfur or mineralization, so they do not salinize much. Waters from calcic pathways drain through sedimentary terrain or calcium brines, becoming highly salty. The quality of waters from the alkaline sulfated pathway is better than that of waters from the neutral sulfated waters (more sulfur, lower quality). From an evolutionary viewpoint, the brackish lakes are in a stage preceding the formation of salt flats, and they will eventually develop a salt crust (Risacher et al. 2002). On the other hand, the salt flats in the high-Andean Atacama are in a stationary stage (Risaher et al. 2002), suggesting a neutral hydrogeochemical balance. A variety of natural or anthropogenic factors (e.g., climatic change, industrial activity) could upset this balance.
The pH of both rivers was similar (9.1, Table 4). In the case of lakes, Verde and El Bayo showed the lowest values, with means of 7.2 and 7.3, respectively, while the other 3 lakes recorded means slightly above 9 (Table 4). The pH of the salt flats fluctuated between 4 and 10. The lowest pH value (4.22) was recorded in Agua Amarga salt flat (Table 4). Further north, in the Antofagasta Region (20°37′-23°34′S), Rasuk et al. (2015) reported pH values between 7.6 (Llamara Salt Flat) and 10.8 (Pujsa Salt Flat), and Alpers and Whittemore (1990) reported pH values between 5.3 and 8.1 for the surface waters of the Hamburgo and Punta Negra salt flats, respectively. In the Atacama Region, Risacher et al. (1999) found values between 1 and 7.9 in some salt flats analyzed. A later study by Risacher et al. (2002) looking at the causes of acidification in two salt flats reported mean values of 3.8 for Gorbea and 3.4 for Ignorado. Those authors concluded that acidification begins with the juxtaposition of hydrothermal alteration in the basin’s volcanic rocks and high sulfur contents in its surroundings. The present study does not report pH for Ignorado Salt Flat. However, in Gorbea Salt Flat, the average pH was 6.7 (Table 4), higher than the mean of 3.7 reported by Risacher et al. (2002), who also found peak values reaching 7.4. This discrepancy suggests that pH levels in a given salt flat (Gorbea, in this case) can vary widely and indicates that water enters into the system from various sources through substrates with different chemical compositions. Risacher et al. (1999) notes that each system receives input from various sources, including hydrothermal alterations that would explain the acidity of some systems.
Of the two metals with concentrations in waters above the detection limit, arsenic had the highest values. In terms of the rivers studied, Juncalito had slightly higher concentrations of Astot and its Asdis was two orders of magnitude higher than in Lama River (Table 4). This result indicates that, unlike Juncalito, a more significant fraction of particulate As is present in Lama River.
Concentrations of As ranged from 0.5 mg L−1 in Santa Rosa to 37 mg L−1 in Negro Francisco (Table 4), whereas Laguna Verde recorded the highest concentrations of total (0.27 mg L−1) and dissolved Cu (0.25 mg L−1) (Table 4). Likewise to rivers, the highest concentrations of Astot in the lakes (Verde and Negro Francisco; Table 4) could also be explained by a higher proportion of particulate As than that found in the other systems.
In the case of the salt flats, total As values fluctuated from 0.01 mg L−1 (Gorbea) to 82.45 mg L−1 (La Laguna) (Table 4). Dissolved and total arsenic (75.23 mg L−1 and 82.25, respectively; Table 4), in La Laguna salt flat, was significantly higher than in the rest of the salt flats. Copper concentrations fluctuated from < 0.1 mg L−1 (La Isla) to 0.31 mg L−1 (Aguilar; Table 4). In Pedernales, Maricunga and Laguna Verde, Tapia et al. (2018) report average concentrations of As, Cd, and Cu in waters of 5.5 mg L−1, 0.001 mg L−1, and 0.001 mg L−1, respectively, while this study reports average values for these same basins, of 9.3 mg L−1, < 0.002 mg L−1, and 0.14 mg L−1, respectively. These results suggest a high temporal and spatial variability of the water chemistry of these aquatic systems, probably marked by seasonal variability, with the rain and insolation as the most important climatic factors influencing the water chemistry. It is important to note that only La Laguna presented values above 20 mg L−1. Further north, Rasuk et al. (2015) reported As concentrations of 0.4 mg L−1 (Coposa Salt Flat), 1.2–2 mg L−1 (Llamara Salt Flat), 5.5 mg L−1 (Atacama Salt Flat, Laguna Cejar), and 120 mg L−1 (Pujsa Salt Flat), all these systems, located in the Antofagasta Region (20°37′ − 23°34′S). In general, in northern Chile, aquatic systems from the arid region have high As concentrations, dominated by As(V) (Cáceres et al. 1992). Risacher et al. (1999) reported As concentrations between 0.002 and 10.5 mg L−1 in the salt flats of the Atacama Region. Later, Risacher et al. (2002) reported As concentrations between 0.002 and 10.5 mg L−1 in Gorbea, and between 0.01 and 0.6 mg L−1 in Ignorado. These authors did not analyze the chemical composition of the water in La Laguna Salt Flat, so we have no comparison for the high values recorded therein. The As values reports in Chilean basins are higher than those reported by Choque-Quispe et al. (2021) in some Andean basin of Peru, where the authors measured As concentration between 0.003 and 0.251 mg L−1.
Potential sources of As include primary minerals in the loess, volcanic materials, and desorption of anionic species from Fe oxides under high-pH condition (Smedley et al. 2002). The precipitation of As by complexing with Fe oxides were found to be less effective in continental waters with high pH (8.5–9.5) than in waters with a lower pH (Dzombak and Morel 1990). However, in the high-Andean systems studied herein, this trend was not observed. Indeed, the correlation values between the two variables were not significant (p-values of 0.831 for Astot and 0.92 for Asdis; p = 0.05), which indicates that pH does not significantly influence the chemical behavior of As in these systems. At the moment, we have no explanation for these extreme values, but they are undoubtedly an exception in the chemistry of salt flats in this area and could suggest extreme conditions for the development of, among others, unique microbiological communities. For example, Rasuk et al. (2015) suggest that the high As values detected in Pujsa Salt Flat (Antofagasta region) could explain, at least in part, the abundance of Cyanobacteria and Actinobacteria in this system as compared to other systems studied in the Antofagasta Region. Abundant flamingos (birds of the genus Phoenicopterus) were observed feeding directly in La Laguna Salt Flat during water sample collection. This observation suggests two characteristics; despite the high As content, there is an important microcrustacean community (Artemia probably) that serves as food for the flamingo population (Yohannes et al. 2014), and these flamingos probably have physiological adaptations that allow them to tolerate high levels of As.
Sediment chemistry
The order of abundance for metals in the river sediments was: Fe > Al > Cu > As > V > Zn > Mo > Pb > Cd > Ni. Lama River had the highest concentrations of Cu, Pb, Cd, Fe, and Al, whereas Juncalito River had higher concentrations of Ni, Mo, Zn, and V (Table 5). Arsenic contents were similar in both systems (Table 5). The order of abundance for metals in the lagoons was: Fe > Al > Cu > As > V > Zn > Pb > Mo > Cd > Ni. Laguna Verde had the lowest concentrations of Cu, As, Mo, and V, but the highest values of Zn, Pb, Ni, and Cd (Table 5). Finally, the order of abundance of metals in salt flats was: Fe > Al > Cu > V > Mo > As > Z > Pb > Cd > Ni. Agua Amarga had the highest Cu concentration, and the Pb concentration in Parinas was noticeably higher than in the rest of the salt flats (Table 5).
Mountain aquatic sediments contain distinct types of metals in highly variable concentrations. Yang et al. (2002) reported metal concentration between 100–360 mg kg−1 (Pb), 0.3–1.9 mg kg−1 (Cd), 39–180 mg kg−1 (Zn), and 8–25 mg kg−1 (Cu), in Scottish mountain lake sediments. In northern Chile, Urrutia et al. (2002) reported average concentrations of 4.1 mg kg−1 (Pb), 0.13 mg kg−1 (Cd), 21.51 mg kg−1 (Cu), and 40.61 mg kg−1 (Zn) in surface sediments from Lake Chungará (18.3°S, 4517 MASL). Further north in central Peru, Custodio et al. (2022) reported average concentrations of 4.95 mg kg−1 (As), 0.175 mg kg−1 (Cd), 34.23 mg kg−1 (Zn), 0.16 mg kg−1 (V), 8.3 mg kg−1 (Cu), 6.8 mg kg−1 (Pb), and 8.89 mg Kg−1 (Ni), in three lagoons used for fish farming. However, aquatic systems in the high-Andean Atacama averaged different metal concentrations: 25.4 mg kg−1 (Pb), 11 mg kg−1 (Cd), 57.5 mg kg−1 (Cu), and 26.7 mg kg−1 (Zn). Tapia et al. (2018) studied the metal content in sediments of Pedernales, Maricunga, and Laguna Verde, and reports average concentrations of As, Cd, Cu, and Mo of 170 mg kg−1, 1.3 mg kg−1,178 mg kg−1, and 37 mg kg−1, respectively, while this study reports average values for the three same basins, of 37.3 mg kg−1, 13.3 mg kg−1, 34.4 mg kg−1, and 28.5 mg kg−1, respectively. Spatial differences in the geological and chemical properties of the drainage basins, as well as temporal variability associated to sampling collection, could explain these differences.
Considering that Pb is a volatile pollutant that is easily transported by ascending air masses and deposited by cold condensation in high-altitude environments (Urrutia et al. 2002), the source of the metal content such as Pb, at least partially, could originate in the intense industrial activity carried out in the Atacama Region’s central valley. Other trace metals released into the atmosphere such as Cd, Cu, and Zn can be transported over long distances with aerosols or particulate forms (Hur et al. 2007; Grgic 2008; Spiro et al. 2013). For these reasons, the contaminants can accumulate in remote environments such as oceans (Chance et al. 2015; Birch 2017), mountain areas (Yang et al. 2010; Bacardit et al. 2012; Rose et al. 2012; Bing et al. 2016), and polar regions (Shotyk et al. 2003; Hur et al. 2007). A similar explanation was applied in central Chile, in the case of volatile organic pollutants found in higher concentration in Andean lakes than in coastal lakes (Barra et al. 2001; Pozo et al. 2007). Likewise, As is a relevant component of atmospheric dust in the desert (Risacher et al. 2003), but it is also a pollutant released from the numerous copper-processing plants in northern Chile (Gidhagen et al. 2002). Thus, these causes could, at least in part, explain the As content found in systems of the high-Andean Atacama.
Copper mining is the primary industry in northern Chile and, due to this, it is possible that at least a fraction of the Cu recorded in sediments represents the contribution of this activity. Previous research performed in central lakes of Chile by von Gunten et al. (2009) supports the idea that a detectable signal of excess atmospheric Cu deposition recorded in a sediment core taken in an Andean lake coincides with the commissioning of the first industrial smelters in AD 1907/1909. Also, the substantial Cu deposition increase, recorded in the sediments from the 1960s onwards, is related to the growth of copper mining in central Chile. Similar evidence was found in Inca Coya lake (22°20.3′S; 68°35.9′W), Antofagasta Region), where a sediment core showed a significant increase of Cu content along the twentieth century, in concordance with the evolution of massive copper mining activities in the Altiplano zone (Cerda et al. 2019).
Multivariate analysis
In the case of lakes, the dendrogram showed that the chemical properties of Negro Francisco, Jilguero, Santa Rosa, and Bravas lakes are very similar, while Verde and Bayo lakes showed particular chemical characteristics that differentiate them from the former group (Fig. 3). Altitudinal differences between the lakes can explain this result, because Verde and Bayo are located in the top of the altitudinal range of the high-Andean aquatic systems of the Atacama region (Fig. 1). Particularly, high contents of Cu in waters, and Pb, Zn, and Cd in the sediments of Laguna Verde can explain its uniqueness (Tables 4 and 5). It is essential to mention that, of the six lakes studied; only Laguna Verde presented the upwelling of thermal waters, which indicates an influence of volcanic activity on the chemistry of its waters. This observation correlates to the fact that this lagoon had the lowest pH values of all those studied (Table 6), which may be evidence of hydrothermal alteration of volcanic origin (Risacher et al. 1999). In the case of salt flats, dendrogram analysis identified two groups and one solitary system. La Laguna Salt Flat constitutes an independent group, as it differs significantly from the other salt flats (Fig. 3), with lower V and Al contents in its sediments (Table 5) and higher As contents in its water (Table 4). In particular, As is six times higher than the average of the other systems.
Water and sediment quality guidelines
Water
In a desert area such as the Atacama, high-Andean basins are a valuable source of water for human consumption and industrial activities. However, their use should be defined by quality standards that establish what types of use are allowed. Thus, Chilean legislation establishes maximum levels of various substances (including metals) allowed for purposes of irrigation and human consumption (MOP 1978, 2005). The concentrations measured in the waters of the salt flats, rivers, and lakes were mostly below the detection limit and, therefore, below the maximum levels allowed by the regulations. Nonetheless, this was not the case for total As and Cu contents. Concentrations of both were measurable in all studied systems, meaning that these two metals can be used with greater precision to evaluate water quality.
The graph in Fig. 4 classifies the systems under the regulatory compliance for Cu and As simultaneously. The results show that only Gorbea Salt Flat meets the standard for irrigation, and none comply with the standard for human consumption. However, this interpretation should be taken with caution because, although we found pH values between 6.70 and 6.80 in Gorbea (Table 4), Risacher et al. (1999) report pH values between 1 and 7 (mean of 3.7) in the same salt flat. Those values are beyond the ranges established by the water quality standards (5.5 to 9.0) for both irrigation and human consumption (MOP 1978, 2005). It should be noted that the water samples from this study were collected in a different sector than those used by Risacher et al. (1999). Those authors indicate a wide variety of water types in this salt flat due to different water sources and substrate types. For instance, of the four water sources sampled in Gorbea Salt Flat, three had As concentrations exceeding the standards for human consumption. However, these waters can be used for mining activities. In fact, a variety of mining facilities in the area have permits to extract water for their purposes, which are not always easy to audit.
According to Risacher et al. (1999), the carbonated and sulfated pathways produce the best-quality waters. Those authors indicate that, in the southern Atacama, waters from carbonated pathways are abundant. The more diluted these waters are, the more significant is their potential for use as a resource for humans. However, the present study showed that the chemical properties of the systems studied (including those from the southern Atacama) exceed the limits established by the water quality standard for human use. Nonetheless, groundwaters may not present the same chemical characteristics as surface waters. Such is the case of Azufreras salt flat, where Risacher et al. (1999) report that the quality of the groundwater, unlike that of the surface water, is adequate for both human consumption and irrigation.
The fact that the waters in high-Andean systems are not suitable for consumption by humans does not negate their ecological value. These waters support a complex and unique ecosystem in which the richness of plant and animal species is directly related to the existence of water resources. Besides, the high variability of microbiological communities in high-Andean systems like those found, for example, in the lakes and salt flats of the Antofagasta Region (Rasuk et al. 2015), constitute a biotechnological potential that has yet to be evaluated in the study area.
Sediment
Sediments are the final deposit of all substances present in a water body and those incorporated by natural and anthropogenic processes. Despite their importance, unlike other developed countries (i.e., Canada, Australia, New Zealand), Chile has no aquatic sediment quality guidelines. Besides, a fair sediment management should be in force that balances between ecological risk, economic losses and the benefits associated with setting chemical-specific sediment and water quality thresholds (Iovanna and Griffiths 2006).
The presence of metals and other inorganic and organic substances in aquatic systems is the result of two sources; the natural one represented by the background levels, and the anthropogenic contributions that may increase the concentration of metals. The knowledge of the geochemical background of hazardous elements is crucial for defining pollution, identifying the source of contamination, and for establishing reliable ecological risk management (Galuszka and Migaszewski 2011). Thus, the contamination level of metals can be defined as the difference between total and natural background concentrations (Apitz et al. 2009).
The background concept assumes that metals present in aquatic systems are the result of erosion of lithogenic material of the basin, their transport, and deposition in the water body. The concentrations of metals in the geological formation surrounding lakes, and other aquatic systems, are usually used as background values to evaluate the enrichment into the systems. However, the natural sedimentation process could be altered by anthropogenic activities developed within the basin, increasing the input of material (and metals therein), above the natural rate, to the water systems. For this reason, the background values derived from the geological formation are only useful when they are established before the beginning of anthropic activities, which is not always possible.
Reimann et al. (2005) and Reimann and Garrett (2005) made an exhaustive review of the meaning of the “background level” concept and referred to the average concentrations of metals found in environments without any anthropogenic intervention. The authors suggest that one of the ways to establish these intrinsic values is through the analysis of pollution-free zones with similar characteristics to the areas under scrutiny.
Several methods of approximation such as the geoaccumulation index (Igeo) and the contamination index (CI) are frequently used to evaluate the environmental significance of the presence of metals in aquatic systems (Cheng et al. 2015; Tapia et al. 2018). These indices assume a base concentration value that reflects a natural condition. In both cases, the concentrations of metals in geological formations established, for example, by Rudnick and Gao (2003), Gałuszka et al. (2015), and Kelepertzis et al. (2010), have generally been used as background values. However, other authors propose to use local reference values that explain the natural presence of metals in the aquatic environment (e.g., Riemann and Garret, 2005). For instance, Tapia et al. (2018) used background concentrations of local sediments of Oruro, Bolivia, to evaluate the environmental condition of an Andean river, which in the cases of As and Sb, are distinct from values of the upper continental crust reported by Rudnick and Gao (2014).
Unfortunately, the information concerning the chemical composition of geological formations of each Andean basin of the Atacama Region is still scarce. For this reason, in this study three background reference values were used; one of them corresponding to mean concentrations found in aquatic systems without human intervention, the second corresponds to the mean concentration of some geological formations of high-Andean basins, and finally content of metals in UCC (Rudnick and Gao 2014) were used for this evaluation (Table 2).
The Igeo of Atacama Andean water systems showed different results depending on the background used. Only V showed values indicating no pollution problem by this metal in all systems, using the three background values (Fig. 5; Table III, Supplementary material). The use of background values of UCC formation to calculate the Igeo indicates notorious problem of contamination only in case of Mo and Cd (Igeo > 3) (Fig. 5; Table III, Supplementary material). When unpolluted systems are used as background values, no system has notorious contamination problems (Igeo < 1), (Fig. 5; Table III, Supplementary material). On the other hand, when local geological formation is used as background, Cd showed values above 6, which indicate a heavily polluted condition in all systems. In the case of Cu, only Azufreras, La Isla and Parinas present an unpolluted condition (Igeo < 1), while in the case of Pb, only Ignorados does not show pollution problem. In the case of Igeo results, when UCC backgrounds are used, all systems present different degrees of contamination by Cd, Mo and As (Fig. 5; Table III, Supplementary material). In general, all the metals show noticeable differences in Igeo depending on the background values used (Fig. 5). Of the three Igeo results, the use of background of unpolluted systems seems to be more conservative and similar between all metals (Fig. 5). On the contrary, the use of UCC and geological formation background provided more variable values suggesting that metals like Cu, Pb, Cd, and Mo have noticeable problems of contamination, even in those systems without historical or current record of intense industrial activities in the high-Andean zone (Fig. 5; Table III, Supplementary material).
Igeo based on three background values for metals measured in high-Andean aquatic systems. Reference dashed lines indicate categories of pollution (see Table 2). 1 corresponds to background of geological formations, 2 corresponds to background of unpolluted systems, 3 corresponds to UCC (Rudnick and Gao 2014)
As in the case of Igeo, CI of aquatic systems showed differences depending on the background values considered. All the systems showed the highest CI in the case of Cu and Pb, when geological formation values were used as background, while Gorbea, Isla, Parinas, and Ignorados showed the highest CI of Ni, with the same background (Fig. 6; Table IV, Supplementary material). All these systems showed CI above 6 for these metals, which suggests very high contamination (Table 1), result that is clearly different to CI obtained with the others two backgrounds (Fig. 6; Table IV, Supplementary material), which suggest low or moderate contamination (Table 3). Cadmium was the only metal that showed CI above 6 (very high contamination, Table 3) for all systems evaluated, using both geological formation and UCC background values (Fig. 6; Table IV, Supplementary material). Of 8 metals evaluated, V, Ni, and Zn showed no contamination or low contamination (Table 1) in most of the systems, using the three background reference values (Fig. 6; Table IV, Supplementary material). In general, the use of unpolluted systems as background seems to be more conservative resulting in similar CI between all metals (Fig. 6) and systems analyzed (Fig. 6; Table IV, Supplementary material).
Contamination Index based on three background values for metals measured in high-Andean aquatic systems. Reference dashed lines indicate categories of pollution (see Table 3). 1 corresponds to background of geological formations, 2 corresponds to background of unpolluted systems, 3 corresponds to UCC (Runnick and Gao, 2014)
Geoaccumulation index and contamination index are two methods used to evaluate the contamination degree of aquatic systems. However, even when both indexes use the same background values, the results are not always similar. In the case of high-Andean systems, Fig. 7 summarizes the cross values of both indexes, using three background reference values. When using both indexes, with the same background, the same conclusion is expected, which is graphically represented by all systems located in the same interpretation area (light blue area in Fig. 7A, B). In the case of indexes calculated with background of geological formation, only Zn, V, and Ni indicate low or moderate contamination, while Cu, Pb, and Cd indicate the maximum degrees of contamination, in the case of CI (> 6, Table 1; Fig. 7A; Table IV, Supplementary material), but not the same in the case of Igeo (< 4; Fig. 7A; Table III, Supplementary material). On the other hand, when unpolluted systems values are used as background, both indexes indicate moderate contamination for all metals measured in the aquatic systems (Fig. 7B; Tables II and III, Supplementary material). Finally, when UCC backgrounds are used, both CI and Igeo suggest moderate contamination in the case of Ni, Zn, V, Pb, and Cu, while the other metals exhibit different degrees of contamination (Fig. 7C; Tables III and IV, Supplementary material).
Igeo versus CI using three different background values. A Geological formation background, B unpolluted systems background, and C UCC background (Rudnick and Gao 2014)
These results represent a serious concern to the definition of the best strategy to evaluate the environmental condition of high-Andean aquatic systems, because not only different background values generate different results for a same environmental index, but similar environmental indexes, like CI and Igeo, provide contradictory interpretations even when the same background values are used. However, the combined analysis of the environmental indexes and background values suggests that local background of unpolluted systems seems to be a better alternative to evaluate the environmental condition of the high-Andean aquatic systems of Atacama region. This conclusion agrees with the advice of some authors (Reimann and Garrett, 2005) and environmental agencies (ANZECC 2000), who indicate that a local background is a better base of evaluation of aquatic systems.
The potential ecological risk (E), as a diagnostic tool for water pollution control purposes, is used, i.e., to sort out which lakes/basins and substances should be specially considered (Häkanson 1980). Even when the E of all metals showed noticeable differences depending on the background used (Table V, Supplementary material), most aquatic systems present low or moderate risk for the metals analyzed, except in the case of Cd and As. Cd showed extremely high risk, in all systems, when geological formation and UCC background were used, while the use of unpolluted system as background shows low risk for the systems studied. On the other hand, As showed high risk in 11 of 21 systems, when UCC was used as background (Table V, Supplementary material). In general, and with the exception of Cd and As, a different background does not change the ecological risk condition of the high-Andean aquatic systems for the metals analyzed (Table 5). Cd is a special case, because the differences are notoriously dependent on the background used to calculate ecological risk. In this case, background of geological formation and UCC provided results forty and one hundred times higher than unpolluted background, respectively (Table I, Supplementary material). However, the final ecological risk condition in the case of background of geological formation and UCC does not change, being of extremely high risk for all the systems (Table V, Supplementary material). In the case of As, the UCC background suggests that 14 systems have moderate or higher risk, while the unpolluted system background suggests that all the systems show low ecological risk (Table V, Supplementary material).
CI and E need to be used carefully by considering geochemical background, the toxicity of metals, specific physical–chemical conditions, and the organisms’ response to the environment for a given lagoon, which may generate uncertainties in estimating the ecological risk (Smedley et al. 2002). For example, the CI evaluates the proportion of metal that exceeds the background level defined by geological formation of some basins of high-Andean Atacama region, and not necessarily indicate an anthropogenic origin of this excess of metal. Various factors, like the climatic and geologic events, as well as human activities, should increase the metal concentration in aquatic systems.
Potential ecological risk index (PERI) evaluates the risk condition of an aquatic system combining the effect of individual E (Häkanson 1980), and is usually used as a strong environmental tool to infer the health of marine and continental ecosystems (Saravanan et al. 2018; Williams and Antoine 2020; Xia et al. 2020; Qi et al. 2020). In high-Andean aquatic systems, the PERI based on background of geological formation and UCC was higher than 300 (mostly than 600) for all the systems, which suggests considerable and very high ecological risk. On the contrary, the use of unpolluted systems as background indicates low ecological risk for all the systems (Table 6).
Table 7 summarizes the SQG results and shows the values proposed by MacDonald et al. (2000) and the average concentrations and ranges of the parameters measured in the high-Andean Atacama systems. Ni, Zn, and Pb measurements are below the TEC suggesting no adverse effect for organisms, while Cu showed values mostly between TEC and PEC, indicating that adverse effects may or may not occur. Finally, both Cd and As exceed the PEC limit proposed by MacDonald et al. (2000) for lagoon systems, which could represent an obvious risk for the communities of aquatic organisms that inhabit these systems. However, it is likely that the particular physic-chemical conditions of the high-Andean Atacama water bodies require unique reference values based on local data in order to identify base concentrations (no anthropogenic intervention). For example, Cheng et al. (2015) suggest that if SQGs values are below local background values, then they should be replaced by this last value. This is the case of the aquatic systems of Andean region, where local background values of Cu, Cd, and As, measured in unpolluted systems are higher (Table 2) than TEC proposed by MacDonald et al. (2000; Table 3). In this regard, the data reported herein constitute a first approach for establishing reference values aimed at evaluating eventual contamination processes that endanger the health of the communities of organisms inhabiting high-Andean Atacama systems.
Regardless of the background value used to calculate environmental indexes, there was no coincidence in the case of both indexes of ecological risk used in this study. Only in the case of Cd and As, using geological formation and UCC as background, the E showed similar results to the SQG. For the rest of the metals analyzed, there is no risk in case of E, but SQG suggests a different degree of risk for biological communities. This, regardless of the background value used.
This situation suggests, preliminarily, that a background of unpolluted systems could be a more realistic reference value to evaluate environmental conditions of high-Andean aquatic systems of Atacama Region, Chile. However, new studies are necessary in order to increase the temporal and spatial representativity of the chemical composition of water and sediments of high-Andean systems of this extreme environment, particularly to define carefully the background levels of metals that represent the geological condition of each basin.
Conclusions
Salinity allowed the clearest classification of the three types of systems found in the study area. On average, salinity was lowest in rivers, intermediate in lakes, and highest in salt flats. The chemical composition of waters and sediments revealed a wide range of concentrations for various metals. In particular, the average concentration of As was three times higher in La Laguna Salt Flat than in the other systems. The presence of some metals like Cu, As, and Pb in the waters and sediments of these aquatic systems can be explained, at least in part, by emissions transported by dominant winds, from industrial (mainly mining) zones to the high Andes through the air. geoaccumulation index, contamination index, and potential ecological risk showed diverse threats for organisms present in aquatic systems, but there is no coincidence between them, related to which systems and/or metals represent this condition, The combined analysis of the environmental indexes and background values, suggests that local background, like unpolluted systems, seems to be a better alternative to evaluate the environmental condition of the high-Andean aquatic systems of the Atacama region. Sediment Quality Guidelines indicate that Cd and As constitute a risk for the communities of organisms that inhabit these ecosystems. Local quality standards established should be considering the inherent physicochemical characteristics. These norms would allow evaluation of how extreme ecosystems might be affected by possible modifications and processes through anthropogenic activity. Waters are not suitable for human consumption or irrigation due to high content of Cu and As, and pH values exceeding Chilean water quality guidelines. However, other studies conducted in these systems show that this resource supports abundant wildlife, constituting unique ecosystems of extreme environments and with great potential for non-consumptive use such as special interest tourism and conservation. In this last point, the chemical information and the ecological risk assessment of these Andean aquatic systems could be a contribution to the current discussion in order to increase the protected surface by the establishment of a new National Park.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Alpers C, Whittemore D (1990) Hydrogeochemistry and stable isotopes of ground and surface waters from two adjacent closed basins, Atacama desert, northern Chile. Appl Geochem 5:719–734
ANZECC (2000). Australian and New Zealand guidelines for fresh and marine water quality. Agriculture and Resource Management Council of Australia and New Zealand, Camberra, 2–1 to 2–20
Apitz S, Degetto S, Cantaluppi C (2009) The use of statistical methods to separate natural background and anthropogenic concentration of trace elements in radio-chronologically selected surface sediments of the Venice Lagoon. Mar Pollut Bull 58:402–4014
Arriagada C, Cobbold P, Roperch P (2006) Salar de Atacama basin: A record of compressional tectonics in the central Andes since the mid-Cretaceous. Tectonics 25:1–19
Bacardit M, Krachler M, Camarero L (2012) Whole-catchment inventories of trace metals in soils and sediments in mountain lake catchments in the Central Pyrenees: apportioning the anthropogenic and natural contributions. Geochim Cosmochim Acta 82:52–67
Bandowe FL, Grosjean M, Tylmann W, Mosquera P, Hampel H, Schneider T (2018) A 150-year record of polycyclic aromatic compound (PAC) deposition from high Andean Cajas National Park, southern Ecuador. Sci Total Environ 621:1652–1663
Barra R, Pozo K, Urrutia R, Cisternas M, Pacheco P, Focardi S (2001) Plaguicidas organoclorados persistentes en sedimentos de tres lagos costeros y un lago andino de Chile central. Bol Soc Ch Quim 46:149–159
Beck S, Zandt G, Myers S, Wallace T, Silver P, Drake L (1996) Crustal-thickness variations in the central Andes. Geology 24:407
Best M (2003) Igneous and metamorphic petrology, 2nd edn. Wiley-Blackwell, p 752
Bing HJYH, Zhou J, Li R, Wang J (2016) Historical trends of anthropogenic metals in Eastern Tibetan Plateau as reconstructed from alpine lake sediments over the last century. Chemosphere 148:211–219
Birch GF (2017) Determination of sediment metal background concentrations and enrichment in marine environments - a critical review. Sci Total Environ 580:813–831
Brantley S, Goldhaber M, Ragnarsdottir V (2007) Crossing disciplines and scales to understand the critical zone. Elements. 3:307–314
Brown M (1991) Comparative geochemical interpretation of Permian-Triassic plutonic complexes of the coastal range and Altiplano (25°30′ to 26°30′S), northern Chile. In: Geological Society of America special paper 265: Andean magmatism and its tectonic setting, pp 157–178
Cáceres L, Gruttner V, Contreras R (1992) Water recycling in arid regions: the Chilean case. Ambio 21(2):138–144
Cerda M, Evangelista H, Valdés J, Sifeddine A, Boucher H, Nogueira J, Ortlieb L (2019) A new 20th century lake sedimentary record from the Atacama Desert/Chile reveals persistent PDO (Pacific Decadal Oscillation) impact. J South Am Earth Sci 95:102302
Chance R, Jickells T, Baker A (2015) Atmospheric trace metal concentrations, solubility and deposition fluxes in remote marine air over the south-east Atlantic. Mar Chem 177:45–56
Cheng H, Li M, Zhao C, Yang K, Li K, Peng M, Yang Z, Liu F, Liu Y, Bai R, Cui Y, Huang Z, Li L, Liao Q, Luo J, Jia S, Pang X, Yang J, Yin G (2015) Concentrations of toxic metals and ecological risk assessment for sediments of major freshwater lakes in China. J Geochem Explor 157:15–26
Chong G (1988) The Cenozoic saline deposits of the Chilean Andes between 18°00’ and 27°00’ South Latitude. Springer Verlag, Lecture Notes Earth Sci 17:87–102
Choque-Quispe D, Froehner S, Ligarda-Samanez C, Ramos-Pacheco B, Peralta-Guevara D, Palomino-Rincón H, Choque-Quispe Y, Solano-Reynoso A, Barboza-Palomino G, Taipe-Pardo F, Zamalloa-Puma M (2021) Insights from water quality of high Andean springs for human consumption in Peru. Water 13:2650
Choque-Quispe D, Froehner S, Palomino-Rincón H, Peralta-Guevara D, Barboza-Palomino G, Kari-Ferro A, Zamalloa-Puma L, Mojo-Quisani A, Barboza-Palomino E, Zamalloa-Puma M, Martínez-Huamán E, Calla-Florez E, Aronés-Medina E, Solano-Reynoso A, Choque-Quispe Y (2022) Proposal of a water-quality index for high Andean basins: application to the Chumbao river, Andahuaylas, Peru. Water 2022(14):654
Clarke J (2006) Antiquity of aridity in the Chilean Atacama desert. Geomorphology 73:101–114
Crane M (2003) Proposed development of Sediment Quality Guidelines under the European Water Framework Directive: a critique. Toxicology Letters 142:195/206
Custodio M, Espinoza C, Orellana E, Chanamé F, Fow A (2022) Assessment of toxic metal contamination, distribution and risk in the sediments from lagoons used for fish farming in the central region of Peru. Toxicol Rep 9:1603–1613
Davidson J, Harmon R, Wörner G (1991) The source of central Andean magmas; some considerations. In: Geological society of America special paper 265: Andean magmatism and its tectonic setting, pp 233–244
DeMets C, Gordon R, Argus D, Stein S (1990) Current plate motions. Geophys J Int 101:425–478
Deocampo D, Renault R (2016) Geochemistry of African soda lake. In: Schagerl M (ed) Soda Lakes of East Africa. Springer, pp 77–93
Dept. Hydraulic Engineering (2009) Levantamiento Hidrogeológico para el desarrollo de nuevas fuentes de agua en áreas prioritarias de la Zona Norte de Chile, Regiones XV, I, II, y III. Pontificia Universidad Católica de Chile. Informe N°:460625, 155 pp. https://snia.mop.gob.cl/sad/REH5161v4.pdf
Depto. Ingeniería Hidráulica (2009). Levantamiento hidrogeológico para el desarrollo de nuevas Fuentes de agua en áreas prioritarias de la zona norte de Chle, regions Xv, I, II y III. Ministerio de Obras Públicas, Dirección General de Aguas, Gobierno de Chile. 155 pp
DGA (1987) Isoyetas del Balance Hídrico de Chile (1987) Ministerio de Obras Públicas:62
Dzombak D, Morel F (1990) Surface complexation modeling: hydrous ferric oxide. John Wiley & Sons, New York
Escudero L, Bijman J, Chong G, Pueyo J, Demergasso C (2013) Geochemistry and microbiology in an acidic, high altitude (4,000 m) salt flat — High Andes, Northern Chile. Advanced Materials Research 825:28–32
Escudero L, Oetiker N, Gallardo K, Tebes-Cayo C, Guajardo M, Nuñez C, Davis-Belmar C, Chong PJ, G, Demergasso C. (2018) A thiotrophic microbial community in an acidic brine lake in Northern Chile. Antonie Van Leeuwenhoek 111(8):1403–1419
Escudero M, Lozano A, Hierro J, Tapia O, del Valle J, Alastuey A, Moreno T, Anzano J, Querol X (2016) Assessment of the variability of atmospheric pollution in National Parks of mainland Spain. Atmos Environ 132:332–344
Falvey M, Garreaud R (2005) Moisture variability over the South American Altiplano during the SALLJEX observing season. J Geophys Res 110:D22105. https://doi.org/10.1029/2005JD006152
Fernández P, Vilanova RM, Martínez C, Appleby P, Grimalt JO (2000) The historical record of atmospheric pyrolytic pollution over Europe registered in the sedimentary PAH from remote mountain lakes. Environ Sci Technol 34:1906–1913. https://doi.org/10.1021/es9912271
Galuszka A, Migaszewski Z (2011) Geochemical background - an environmental perspective. Mineralogia 42(1):7–17. https://doi.org/10.2478/v10002-011-0002-y
Gałuszka A, Migaszewski ZM, Dołęgowska S, Michalik A, Duczmal-Czernikiewicz A (2015) Geochemical background of potentially toxic trace elements in soils of the historic copper mining area: a case study from Miedzianka Mt., Holy Cross Mountains, south-central Poland. Environ Earth Sci 74:4589–4605. https://doi.org/10.1007/s12665-015-4395-6
Garreaud R, Vuille M, Clement A (2003) The climate of the Altiplano: observed current conditions and mechanisms of past changes. Pal Pal Pal 194:5–22
Gidhagen L, Kahelin H, Schimidt-Thomé P, Johansson C (2002) Anthropogenic and natural levels of arsenic in PM 10 in central and northern Chile. Atmos Env 36(23):3803–3817
Global Volcanism Program (2013) Volcanoes of the World, v. 4.7.6. In: Venzke E (ed) Smithsonian Institution. Downloaded 27 Feb 2019. https://doi.org/10.5479/si.GVP.VOTW4-2013
Gochfeld M, Burger J, Vyas V (2005) Statistical analysis of data sets with values below detection limits. Report for. Piscataway, New Jersey. http://cresp.org/Amchitka/Amchitka_Final_Report/finalreport/11Append_radionuclides/11F_Statisticalanalysis
Gottler R (2017) Metals. In: Baird, Eaton, Rice (eds) Standard methods for the examination of water and wastewater, 23rd edn. American public health association, American water works association, water environment federation publisher, 3–1 to 3–114
Grgic I (2008). Metals in aerosols. In Colbeck (ed) Environmental chemistry of aerosols. Wiley-Blackwell , New York, pp 117–139
Guédron S, Tolu J, Delaere C, Sabatier P, Barre J, Heredia C, Brisset E, Campilo S, Bindler R, Fritz S, Baker P, Amoroux D (2021) Reconstructing two millennia of copper and silver metallurgy in the Lake Titicaca region (Bolivia/Peru) using trace metals and lead isotopic composition. Anthropocene 34:100288
Grosjean M, Meserli B, Ammann C, Geyh M, Graf K, Jenny B, Kammer K, Nuñez L, Schreier H, Schotterer U, Schwalb A, Valero-Garcés B, Viulle M (1995) Holocene environmental changes in the Atacama Altiplano and paleoclimatic implications. Bull Inst Fr études andines 24(3):585–594
Häkanson L (1980) An Ecological Risk Index for Aquatic Pollution Control – A Sedimentological Approach. Water Res 14(8):975–1001
Helsel D (2005) Nondetects and data analysis: statistics for censored environmental data. John Wiley & Sons Eds, New York, 250 pp
Houston J, Butcher A, Ehren P, Evans K, Godfrey L (2011) The evaluation of brine prospects and the requirement for modifications to filing standards. Econ Geol 106:1225–1239
Hur S, Cunde X, Hong S, Barbante C, Gabrielli P, Lee K, Boutron C, Ming Y (2007) Seasonal patterns of heavy metal deposition to the snow onLambert Glacier basin, East Antarctica. Atmos Environ 41:8567–8578
INN (1997) Aguas residuales - Métodos de análisis - Parte 25: Determinación de metales por espectroscopía de emisión de plasma - Método de plasma acoplado inductivamente (I.C.P.). NCh2313/25. Insitituto Nacional de Normalización, p 57
Instituto Nacional de Normalización (1987). Requisitos de calidad de agua para diferentes usos, Norma Chilena Oficial. NCh 1333. Of78. 15 pp
Iovanna R, Griffiths Ch (2006) Clean water, ecological benefits, and benefits transfer:a work in progress at the U.S. EPA Ecological Economics 60:473–482
Kay S, Coira B (2009) Shallowing and steepening subduction zones, continental lithospheric loss, magmatism, and crustal flow under the Central Andean Altiplano-Puna Plateau. In: Backbone of the Americas: Shallow Subduction, Plateau Uplift, and Ridge and Terrane Collision Geological Society of America, pp 229–259
Kay S, Mpodozis C, Tittler A, Cornejo P (1994) Tertiary magmatic evolution of the maricunga mineral belt in Chile. Int Geol Rev 36:1079–1112
Kelepertzis E, Argyraki A, Daftsis E, Ballas D (2010) Geochemical background heavy metals concentrations of stream sediments at mineralized areas of NE Chalkidiki. Hell J Geosciences. 45:153–162
Kendrick E, Bevis M, Smalley R, Brooks B, Vargas R, Lauria E, Fortes L (2003) The Nazca–South America Euler vector and its rate of change. J South Am Earth Sci. 16:125–131
Kraemer B, Adelmann D, Alten M, Schnurr W, Erpenstein K, Kiefer E, van den Bogaard P, Görler K (1999) Incorporation of the Paleogene foreland into the Neogene Puna plateau: The Salar de Antofalla area, NW Argentina. J South Am Earth Sci 12:157–182
Long E, Macdonald D, Smith S, Calder F (1995) Incidence of adverse biological effects within ranges of chemical concentrations in marine and estuarine sediments. Environ Manage 19:81–97
MacDonald D, Ingersoll C, Berger T (2000) Development and evaluation of consensus-based sediment quality guideline for freshwater ecosystems. Arch Environ Contam Toxicol 39:20–31
Matschullat J, Ottenstein R, Reimann C (2000) Geochemical background – can we calculate it? Environ Geol 39:990–1000. https://doi.org/10.1007/s002549900084
McLennan S (2001) Relationships between the trace element composition of sedimentary rocks and upper continental crust. Geochem Geophys Geosystems 2. https://doi.org/10.1029/2000GC000109
MOP (1978) Requisitos de calidad de agua para diferenes usos. Norma Chilena 1333. Instituto Nacional de Normalización:15
MOP (2005) Parte 1, Requisitos. In: Norma Chilena 409. Instituto Nacional de Normalización, p 13
Mpodozis C, Cornejo Peláez P, Kay S, Tittler A (1995) La Franja de Maricunga: síntesis de la evolución del Frente Volcánico Oligoceno-Mioceno de la zona sur de los Andes Centrales. In: La Franja Maricunga síntesis la Evol. del Frente Volcánico Oligoceno-Mioceno la Zo, vol 22. Sur los Andes Cent, pp 273–313
Mpodozis C, Kay S, Gardeweg M, Coira B (1996) Geología de la región de Ojos del Salado (Andes Centrales, 27°S): Implicancias de la migración hacia el este del frente volcánico Cenozoico Superior. XIII Congr. Geológico Argentino, Actas III:539–548
Müller G (1979) Schwermetalle in den sedimenten des Rheins—Veränderungen seit 1971. Umschau 79:778–783
Naranjo J, Hevia F, Arcos R, Polanco E (2019) Geología de las áreas Nevado Ojos del Salado y Cerro El Fraile, Región de Atacama. In: Servicio Nacional de Geología y Minería, Carta Geológica de Chile, Serie Geología Básica 204–205: 96 p. 1 mapa escala 1:100.000, Santiago
Pozo K, Urrutia R, Barra R, Mariottini M, Treutler H, Araneda A, Focardi S (2007) Records of polychlorinated biphenyls (PCBs) in sediments of four remote Chilean Andean Lakes. Chemosphere 66:1911–1921
Qi G, Jia Y, Liu W, Wei Y, Du B, Fang W, Guo Y, Guo F, Wu Y, Zou Q, Liu J (2020) Leaching behavior and potential ecological risk of heavy metals in Southwestern China soils applied with sewage sludge compost under acid precipitation based on lysimeter trials. Chemosphere 249:126212
Rasuk M, Fernández A, Kurth D, Contreras M, Novoa F, Poiré D, Farías M (2015) Bacterial diversity in microbial mats and sediments from the Atacama Desert. Microb Eco 71(1):44–56
Reimann C, Garrett R (2005) Geochemical background—concept and reality. Sci Total Eviron 350:12–27
Reimann C, Filzmoser P, Garrett RG (2005) Background and threshold: critical comparison of methods of determination. Sci Total Environ 346:1–16
Richards J, Boyce A, Pringle M (2001) Geologic evolution of the Escondida area, Northern Chile: A model for spatial and temporal localization of porphyry Cu mineralization. Econ Geol 96:271–305
Risacher F, Alonso H, Salazar C (1999). Geoquímica de aguas en cuencas cerradas: I. II y II Tegiones – Chile. S.I.T N° 51, Vol. IV, Estudio de Cuencas – Tercera Región: 1–247
Risacher F, Alonso H, Salazar C (2002) Hydrochemistry of two adjacent acid saline lakes in the Andes of northern Chile. Chem Geol 187:39–57
Risacher F, Alonso H, Salazar C (2003) The origin of brines and salts in Chilean salars: a hydrochemical review. Earth-Sci Rev 63:249–293
Rizzo A, Daga R, Arcagni M, Perez S, Bubach D, Sánchez R, Ribeiro S, Arribére M (2010) Concentraciones de metales pesados en distintos compartimentos de lagos andinos de Patagonia Norte. Ecol Austal 20:155–171
Rose NL, Yang HD, Turner SD, Simpson GL (2012) An assessment of the mechanisms for the transfer of lead andmercury fromatmospherically contaminated organic soils to lake sediments with particular reference to Scotland. UK Geochim Cosmochim Acta 82:113–135
Rudnick R, Gao S (2003) Composition of the Continental Crust. Treatise on Geochemistry 3:1–64
Rudnick RL, Gao S (2014) Composition of the continental crust. Treatise on Geochemistry 4:1–51
Saravanan P, Krishnakumar S, Pradhap D, Silva J, Arumugan K, Magesh N, Srinivasalu S (2018) Elemental concentration based potential ecological risk (PER) status of the surface sediments, Pulicat lagoon, Southeast coast of India. Mar Pollut Bull 133:107–116
Sarricolea E, Herrera-Ossandon M, Meseguer O (2017) Climatic regionalization of continental Chile. J Maps 13(2):66–73
Schnurr W, Trumbull R, Clavero J, Hahne K, Siebel W, Gardeweg M (2007) Twenty-five million years of silicic volcanism in the southern central volcanic zone of the Andes: Geochemistry and magma genesis of ignimbrites from 25 to 27 °S, 67 to 72 °W. J Volcanol Geoth Res 166:17–46
Sernageomin (2003) Mapa Geológico de Chile: versión digital. Servicio Nacional de Geología y Minería de Chile, Publicación Digital, 4:25
Shotyk W, Goodsite ME, Roos-Barraclough F, Frei R, Heinemeier J, Asmund G, Lohse C, Hansen TS (2003) Anthropogenic contributions to atmospheric Hg, Pb and As accumulation recorded by peat cores from southern Greenland and Denmark dated using the 14C “bomb pulse curve.” Geochim Cosmochim Acta 67:3991–4011
Smedley P, Nicolli D, Macdonald M, Barros A, Tullio O (2002) Hydrogeochemistry of arsenic and other inorganic constituents in groundwaters from La Pampa, Argentina. Appl Geochem 17:259–284
Spiro B, Udachin V, Williamson BJ, Purvis OW, Tessalina SG, Weiss DJ (2013) Lacustrine sediments and lichen transplants: two contrasting and complimentary environmental archives of natural and anthropogenic lead in the South Urals. Russia Aquat Sci 75:185–198
Stern C (2004) Active Andean volcanism. Rev Geol. Chile 31:161–206
Tapia J, González R, Townley B, Oliveros V, Álvarez F, Aguilar G, Menzies A, Calderón M (2018) Geology and geochemistry of the Atacama Desert. Antonie Van Leeuwenhoek 111:1273–1291
Tranvik L, Downing J, Cotner J, Loiselle S, Striegl R, Ballatore T, Dillon P, Finlay K, Fortino K, Knoll L, Kortelainen P, Kutser T, Larsen S, Laurion I, Leech D, McCallister S, McKnight D, Melack J, Overholt E, Porter J, Prairie Y, Renwick W, Roland F, Sherman B, Schindler D, Sobek S, Tremblay A, Vanni M, Verschoor A, Wachenfeldt E, Weyhenmeyer G (2009) Lakes and reservoirs as regulators of carbon cycling and climate. Limnol. Oceanogr. 54(6 part 2):2298–2314
Trolle D, Hamilton DP, Pilditch CA (2010) Evaluating the influence of lake morphology, trophic status and diagenesis on geochemical profiles in lake sediments. Appl Geochem 25:621–632
Trumbull R, Wittenbrink R, Hahne K, Emmermann R, Büsch W, Gerstenberger H, Siebel W (1999) Evidence for Late Miocene to Recent contamination of arc andesites by crustal melts in the Chilean Andes (25–26°S) and its geodynamic implications. J South Am Earth Sci. 12:135–155
Urrutia R, Yevenes M, Barra R (2002) Determinación de los niveles basales de metales traza en sedimentos de tres lagos andinos de Chile: Lagos Chungará, Laja y Castor. Bol Soc Chil Quím 47(4):457–467
Usenko S, Landers DH, Appleby PG, Simonich SL (2007) Current and historical deposition of PBDEs, pesticides, PCBs, and PAHs to Rocky Mountain National Park. Environ Sci Technol 41:7235–7241. https://doi.org/10.1021/es0710003
USEPA (1992) Sediment clasissication methods compendium. Ofice of water (WH-556). EPA 823-R-92-006. https://archive.epa.gov/water/archive/polwaste/web/pdf/classmethods.pdf
Van Drooge BL, López J, Fernández P, Grimalt JO, Stuchlík E (2011) Polycyclic aromatic hydrocarbons in lake sediments from the High Tatras. Environ Pollut 159:1234–1240. https://doi.org/10.1016/j.envpol.2011.01.035
Vilanova R, Fernández P, Martínez C, Grimalt JO (2001) Organochlorine pollutants in remote mountain lake waters. J Environ Qual 30:1286–1295. https://doi.org/10.2134/jeq2001.3041286x
Vila I, Mühlhauser H (1987) Dinámica de lagos de altura, perspectivas de investigación. Arch Biol Med Exp 20:95–103
von Gunten L, Grosjean M, Eggenberger U, Grob Ph, Urrutia R, Morales A (2009) Pollution and eutrophication history AD 1800–2005 as recorded in sediments from five lakes in Central Chile. Global Planet Change 68:198–208
Vuille M, Bradley R, Keimig F (2000) Interannual climate variability in the Central Andes and its relation to tropical Pacific and Atlantic forcing. J Geophys Re 105:12447–12460
Walker J, Moulds T, Zentilli M, Feigenson M (1991) Spatial and temporal variations in volcanics of the Andean Central Volcanic Zone (26 to 28°S). In: Geological society of America special paper 256: Andean magmatism and its tectonic setting, pp 139–156
Williams J, Antoine J (2020) Evaluation of the elemental pollution status of Jamaican surface sediments using enrichment factor, geoaccumulation index, ecological risk and potential ecological risk index. Mar Pollut Bull 157:111288
Xia P, Sun R, Yang Y, Tang X, Yan D, Lin T, Zhang Y, Yi Y (2020) Evaluation of potential ecological risk, possible sources and controlling factors of heavy metals in surface sediment of Caohai Wetland, China. Sci Total Environ 749:40231
Yang HD, Battarbee RW, Turner SD, Rose NL, Derwent RG, Wu GJ, Yang RQ (2010) Historical reconstruction of mercury pollution across the Tibetan Plateau using lake sediments. Environ Sci Technol 44:2918–2924
Yang H, Rose N, Battarbee N (2002) Distribution of some trace metals in Lochnagar, a Scottish mountain lake ecosystem and its catchment. Sci Total Environ 285(1–3):197–208
Yohannes E, Arnaud A, Béchet A (2014) Tracking variations in wetland use by breeding flamingos using stable isotope signatures of feather and blood. Estuar Coast Shelf Sci 136:11–18
YSI (2012) 6-Series. Multiparameter water quality sondes. Environmental monitoring system operation manual. YSI Incorporated Publisher, pp 379
Yuan X, Sobolev S, Kind R (2002) Moho topography in the Central Andes and its geodynamic implications. Earth Planet Sci Lett 199:389–402
Acknowledgements
Ercio Metifogo, from Puna Atacama, a tourism company, is thanked for their invaluable field support. Jorge Valdés thanks MINEDUC-UA project, cod. ANT 1999. Thanks to Antonio Serrano for his support in formatting review. Finally, we appreciate the contributions of anonymous reviewers, whose comments allowed the improvement of this manuscript.
Funding
The authors received funding from the Regional Government of Atacama through the Innovation Fund for Competitiveness (FIC).
Author information
Authors and Affiliations
Contributions
JV and YM contributed to the study conception and design. All authors contributed to material preparation, data collection and field experiment. JV and AC contributed to the analysis of samples and interpretation of data. The first draft of the manuscript was written by JV and all authors commented on previous versions of the manuscript. All authors have approved the submitted version (and any substantially modified version that involves the author’s contribution to the study), and to have agreed both to be personally accountable for the author’s own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Philippe Garrigues
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Valdés, J., Marambio-Alfaro, Y., Castillo, A. et al. Metal(oid)s content in High-Andean aquatic systems of the Atacama Desert, Chile: environmental assessment of extreme ecosystems. Environ Sci Pollut Res 30, 33018–33039 (2023). https://doi.org/10.1007/s11356-022-24294-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-24294-w