Abstract
The laterite Ni ore smelting operations in Niquelândia and Barro Alto (Goiás State, Brazil) have produced large amounts of fine-grained smelting wastes, which have been stockpiled on dumps and in settling ponds. We investigated granulated slag dusts (n = 5) and fly ash samples (n = 4) with a special focus on their leaching behaviour in deionised water and on the in vitro bioaccessibility in a simulated gastric fluid, to assess the potential exposure risk for humans. Bulk chemical analyses indicated that both wastes contained significant amounts of contaminants: up to 2.6 wt% Ni, 7580 mg/kg Cr, and 508 mg/kg Co. In only one fly ash sample, after 24 h of leaching in deionised water, the concentrations of leached Ni exceeded the limit for hazardous waste according to EU legislation, whereas the other dusts were classified as inert wastes. Bioaccessible fractions (BAF) of the major contaminants (Ni, Co, and Cr) were quite low for the slag dusts and accounted for less than 2 % of total concentrations. In contrast, BAF values were significantly higher for fly ash materials, which reached 13 % for Ni and 19 % for Co. Daily intakes via oral exposure, calculated for an adult (70 kg, dust ingestion rate of 50 mg/day), exceeded neither the tolerable daily intake (TDI) nor the background exposure limits for all of the studied contaminants. Only if a higher ingestion rate is assumed (e.g. 100 mg dust per day for workers in the smelter), the TDI limit for Ni recently defined by European Food Safety Authority (196 µg/day) was exceeded (324 µg/day) for one fly ash sample. Our data indicate that there is only a limited risk to human health related to the ingestion of dust materials generated by laterite Ni ore smelting operations if appropriate safety measures are adopted at the waste disposal sites and within the smelter facility.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Identified land-based resources averaging ≥1 % Ni contain at least 130 Mt of Ni, with about 60 % of it bound in Ni laterites and 40 % in sulphide deposits (USGS 2016). Currently, about 60 % of the world’s Ni production comes from Ni sulphides, and the remaining 40 % comes from Ni laterites (Crundwell et al. 2011). In the future, it is expected that the production of Ni from laterite ores via hydrometallurgical and pyrometallurgical technologies will become of greater importance (Warner et al. 2006).
Environmental compartments such as soils, dust, water, and biota (plants, fish) in the vicinity of smelters processing laterite Ni ores and producing ferronickel (FeNi) have been studied at several sites in order to monitor the impacts of these industrial operations (e.g. Bačeva et al. 2012; Bačeva Andonovska et al. 2015; Oliveira-Filho et al. 2010, 2013; Ratié et al. 2016; Zelano et al. 2013). Surprisingly, only limited information about the mineralogical and geochemical compositions of the waste materials from laterite Ni ore smelting (slags, fly ash) is available in the literature (Ettler et al. 2016; Kierczak et al. 2009; Ratié et al. 2016). The bioaccessibility of Ni and associated contaminants (Cr, Co, Zn) have often been studied in soils developed on mafic/ultramafic bedrock (Cox et al. 2013; Palmer et al. 2013, 2014), in soils and dusts from industrial urban areas, and sites near mines and smelters (Bačeva Andonovska et al. 2015; Drysdale et al. 2012; Gbefa et al. 2011; Reis et al. 2014a, b; Vasiluk et al. 2011). However, such information is virtually unavailable for industrial dusts produced by Ni smelters (except for limited data reported in Henderson et al. 2012a and Oliveira-Filho et al. 2010, 2013). Our previous investigation indicated that contaminant leaching from slag and fly ash, generated by laterite Ni ore smelting, is highly dependent on pH and on solid speciation of inorganic contaminants in the individual materials. Thus, whereas leaching of Ni, Cr, and Co from slags was rather low (<6 % of total concentrations), a high release of contaminants (e.g. in the range 15–23 % of total Ni and Co) was observed for fly ash between pH 3 and 5.1 (this being the natural pH of a fly ash–water suspension) (Ettler et al. 2016). Following in the footsteps of our previous research, the aim of this paper was to verify the extent of contaminant leaching on a larger set of waste dust samples from a ferronickel smelter (granulated slag and fly ash materials) and to assess the contaminants’ oral bioaccessibility in simulated gastric fluid (SGF), coupled to calculated estimates of the potential exposure risk for human health.
Materials and methods
Slag and fly ash samples
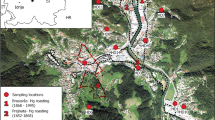
The Niquelândia and Barro Alto Ni laterite deposits are located in Goiás State (Brazil) (Fig. 1). The ore has been mined and smelted since 1979 in Niquelândia (Codemin plant) and since 2011 in Barro Alto (Barro Alto plant). The Anglo American Company currently operates the open-pit mining activities and metallurgical plants at both sites. The smelting technology is based on the following steps: (1) drying of the laterite ore (with up to 2.5 % Ni) in a rotary dryer at 800 °C to decrease the water content from 35 to 25 %; (2) calcination/pre-reduction in rotary kilns at ~800 °C, with heavy oil as the fuel and woodchips or coal as the reductant (dried ore is charged at 120 °C; the temperature of the calcine at the discharge is 900 °C; flue gas cleaning using electrostatic precipitators is connected to the upper part of the rotary kiln); (3) smelting of the calcined laterite ore in electric-arc induction furnaces at ~1500 °C (2000 t per day per furnace) to produce crude ferronickel (~70 % Fe, ~30 % Ni), which is tapped at a temperature of 1450 °C; the slag is tapped off at 1550 °C, after which it is water-quenched (granulated) and stockpiled on the dumps; and (4) refining of the FeNi to remove carbon and other impurities (sulphur, phosphorus) (Crundwell et al. 2011; Moore 2012). More information about the smelting technology and capacity of both smelters was given in our previous papers (Ettler et al. 2016; Ratié et al. 2016).
Fly ash from the calcination furnaces is currently collected at both smelting sites, mixed with the ore, and because of its high Ni content is recycled in the smelting process (7.3–27.6 g/kg; Ratié et al. 2016). However, high quantities of fly ash have been deposited in the settling ponds at Codemin in the past. For the current assessment, historical and recent slags (n = 5) and fly ash materials (n = 4) were collected as grab dust samples at waste disposal sites (dumps, settling ponds), or obtained directly from the Anglo-American staff. Descriptions of the studied samples are given in Table 1, and the GPS locations of individual sampling points are reported in Table S1 in Supplementary Material.
Each dust sample was homogenised and prepared for further analysis. The granulometry of the two types of materials was assessed by sieving; the majority of slag particles were determined to be <2 mm, with less than 16 % of the particles below 0.5 mm (Table S3 in Supplementary Material). In contrast, the fly ash samples were fine-grained; whereas NFA1 contained some larger particles above 0.5 mm (48.1 %), all the other fly ash samples were finer than 0.5 mm. The granulometry of the <0.5 mm fraction of fly ash materials was determined using a Sympatec particle size analyser equipped with a HELOS laser diffraction sensor and an ultrasound sample treatment (Sympatec GmbH, Germany). This indicated that the largest proportion of finer particles in sample NFA1 were below ~30 µm (90 % of the particles), compared with other fly ash samples, which all exhibited similar size-distribution patterns to each other with 90 % of particles below 100 µm (Figure S1).
The bulk chemical composition of each sample was determined after digestion in mineral acids (HClO4, HF, HNO3) and/or alkali sintering according to Ettler et al. (2009). Gravimetric and volumetric analyses as well as photometry were used to determine major elements, whereas the trace elements were analysed by inductively coupled plasma optical emission spectrometry (ICP-OES, ThermoScientific iCAP 6500 radial, UK). Total sulphur and total carbon content was determined using an ELTRA CS 530 (combustion with infrared detection analyser; ELTRA, Germany). The accuracy of the digestion procedure and subsequent analytical determinations were controlled by a parallel analysis of the certified reference material SU-1b (nickel–copper–cobalt ore), certified by CANMET within the framework of the Canadian Certified Reference Materials Project (CCRMP). The quality control/quality assurance (QC/QA) results are reported in Table S2 in Supplementary Material, and they indicate good agreement between the measured and certified values.
The phase composition of the samples was assessed by X-ray powder diffraction analysis (XRPD) using a PANalytical X’Pert Pro diffractometer with a X’Celerator detector (PANalytical, the Netherlands) (analytical conditions: CuKα radiation at 40 kV and 30 mA, 2-theta range 2°–80°, step 0.02°, counting time 150 s per step). X’Pert HighScore Plus 3.0 software coupled to the Crystallography Open Database (COD) (Gražulis et al. 2012) was used for analysis of the XRD patterns. Waste dust specimens prepared as polished sections were examined under a Leica DM LP polarising microscope (Leica, Germany) and subsequently studied by a scanning electron microscope (SEM; TESCAN VEGA3 XM, Czech Republic) equipped with an energy-dispersive spectrometer (EDS; Quantax 200 X-Flash 5010, Bruker, Germany).
Leaching experiments
The smelter waste samples were subjected to a 24-h leaching test in deionised water at a liquid-to-solid (L/S) ratio of 10, according to European standard EN 12457-2 (2002), which is used for the assessment of the hazardous properties of the studied materials according to EU legislation (EU 1999, 2003). At the end of the leaching experiments, the suspended solids were allowed to settle for about 10 min. Then, the physical–chemical parameters (pH, Eh, specific conductivity) were immediately measured in the supernatant using Schott multimeters (Schott Gerätte, Germany), and the leachates were filtered to 0.45 µm (Millipore®). All the experiments were performed in duplicate and with procedural blanks.
Leachate samples from each experiment were diluted in 2 % HNO3 (v/v) and analysed for As, Ba, Cd, Co, Cr, Cu, Ni, Pb, Sb, and Zn by quadrupole-based inductively coupled plasma mass spectrometer (ICP-MS; ThermoScientific XseriesII, UK). The accuracy of the analyses of metals and metalloids in the leachates was controlled by NIST 1643d (trace elements in water) standard reference material (SRM), and these were in good agreement with respect to the certified values (Table S2).
Bioaccessibility testing and exposure assessment
Oral bioaccessibility testing was performed on both types of samples without any grain size reduction by sieving despite the fact that granulated slag samples were relatively coarse-grained materials (see “Results” section and Table S3). For the sake of consistency with the previously performed protocol of leaching in deionised water (EN 12457-2), where 95 % of particles of the tested material must be smaller than 4 mm, we used slag samples as they were even for the bioaccessibility testing. However, Ruby et al. (1999) clearly demonstrated that the contaminant bioaccessibility in metal(loid)-bearing materials increases with decreasing size of grains. It is recognised that in a real-life scenario, only the smaller grain size fraction would potentially be ingested, and we are aware that especially for slags the bioaccessible fractions could be underestimated, despite the fact that handling and transport of slags between the smelter and the dumps can potentially lead to re-suspension of coarser particles.
To investigate the amounts of contaminants that can be extracted in simulated gastric fluid (SGF), we used the US EPA’s (2007) protocol representing the “worst-case” scenario, and simulating conditions corresponding to a fasting human. This test was selected mainly due to its simplicity and reliability with in vivo tests (e.g. Deshommes et al. 2012). The extraction fluid contained 0.4 M glycine (30.028 g glycine dissolved in 800 mL of deionised water), adjusted to pH 1.5 ± 0.05 by reagent grade HCl (Merck, Germany), and finalised by diluting to 1 L with deionised water (MilliQ+, Millipore Academic, USA) and pH verification. A solid-to-fluid ratio of 1/100 was used for the extraction. A mass corresponding to 0.4 g of the original dust sample was placed in polypropylene centrifuge tubes (P-Lab, Czech Republic), 40 mL of extraction fluid was then added, and the mixture was agitated for 2 h at 37 °C in a GFL 3032 shaking incubator (GFL, Germany). All extractions were performed in duplicate with procedural blanks. After the extraction procedures, each extract was filtered through a 0.45-µm nitrocellulose membrane filter (Millipore®, USA), diluted, and analysed for As, Ba, Cd, Co, Cu, Cr, Ni, Pb, Sb, and Zn by ICP-MS. The accuracy of the measurements was also determined by the use of NIST 1643d SRM (Table S2). Subsequently, the bioaccessible concentrations of the inorganic contaminants were expressed in mg/kg and converted to the bioaccessible fractions (BAFs) expressed as a % of the total contents.
The oral exposure was calculated using the bioaccessible concentrations, with a dust ingestion rate corresponding to 50 mg/day, which is a general ingestion rate for an adult in this kind of exposure assessment (Bierkens et al. 2011). Adults, particularly workers, have been suggested to be the most exposed targets in and near the smelter facilities. The obtained daily intakes of contaminants calculated for an adult weighing 70 kg were then compared with the background exposure (BE) and the tolerable daily intake (TDI) limits taken from Baars et al. (2001), and, if available, more recent TDI limits set by European Food Safety Authority (EFSA) or Scientific Committee on Health and Environmental Risks (SCHER). Tolerable daily intake has been recently revised for Cd (tolerable weekly intake [TWI] was established to 2.5 µg/kg body weight per day; EFSA 2009), Cr(III) (TDI of 0.3 mg/kg body weight per day; EFSA 2014), Ni (TDI of 2.8 µg/kg body weight per day; EFSA 2015) and Ba (0.2 mg/kg body weight per day; SCHER 2012). However, EFSA recently concluded that provisional TWI limits for Pb (15 µg/kg body weight) and As (15 µg/kg body weight) are no longer appropriate (EFSA 2010, 2014b) and TDI or TWI limits for the remaining contaminant studied are not available.
Results
Chemical and mineralogical compositions of slag and fly ash samples
Table 2 reports the bulk chemical compositions of the waste materials from laterite Ni ore smelting. Both types of wastes studied are rich in Si, Fe, Mg, and Al; but slags exhibit higher concentrations of Si (up to 23.7 wt%). In addition, the Fe(III)/Fe(II) ratio was much lower for slags due to iron reduction in the electric-arc furnace (0.12–0.33) compared with fly ash (2.7–8.5) (Table 2). By comparison with slags, fly ash also contained higher levels of Ctot (up to 8.54 wt%; Table 2), probably due to residues from fuel (coal, oil, woodchips) used in the charge to the rotary kiln. There is a major difference in the chemical compositions between the two types of waste in the minor and trace elements. Some slags were slightly richer in Cr (slags: 6000–7580 mg/kg, fly ash: 1870–6750 mg/kg), but were impoverished in Co (slag: 73.3–96.9 mg/kg; fly ash: 488–679 mg/kg), Ni (slag: 779–963 mg/kg; fly ash: 25,100–27,100 mg/kg), Cu (slag: <5–9.45 mg/kg; fly ash: 36.9–92.5 mg/kg) and Zn (slag: 87.4–224; fly ash: 281–462 mg/kg) (Table 2). Concentrations of other contaminants were comparable for the two types of waste and ranged between tens to lower hundreds of mg/kg (Table 2).
The XRPD analyses and SEM observations indicated that the slags were mainly composed of olivine [(Mg,Fe)2SiO4], silicate glass, pyroxene [(Mg,Fe)2Si2O6]; and for some of the samples, trace amounts of quartz (SiO2) and melilite [Ca2(Mg,Al)(Si,Al)2O7] were also detected (Figure S2). The presence of glass, skeletal crystals, dendrites of silicates, and symplectite textures in metallic inclusions indicated that the slag melt was rapidly quenched during water granulation (Fig. 1a–c). Whereas Cr was detected by EDS in the slag glass, olivine, and pyroxene (up to 2.54 wt% as Cr2O3) (Fig. 2a, b), Ni was mainly bound in the Ni–Fe alloys (Fig. 2c) and to a minor extent in olivine (up to 0.13 wt% as NiO; Fig. 2a).
Scanning electron micrographs in backscattered electrons (BSE) of the studied waste dusts: a skeletal crystals and dendrites of olivine (Ol) in glassy matrix (granulated slag sample NSO1); b skeletal crystals of pyroxene (Px) and pyroxene dendrites in glassy matrix (slag sample NSO1); c spherical inclusion composed of symplectite of Ni–Fe and Ni–Fe–S alloys trapped within the glass (slag sample NSO1); d vesicular glassy particles associated with spinel-phase (Spl) inclusions and smaller spinel droplets (fly ash sample NFA1); e spherical silicate particle composed of spinel, olivine, residual glass, and smaller fragments of glass-like phases and/or Ni-rich silicates corresponding to dehydrated serpentines (fly ash sample NFA1); f Cr-spinel inclusion within a magnetite (Mag) euhedral grain associated with silicate glass-like phase forming a rim (fly ash sample NFA1). The Ni and Cr concentrations obtained by EDS are expressed in wt% of NiO and Cr2O3, respectively
The phase composition of the fly ash samples determined by XRPD was dominated by high-temperature silicates (olivine, pyroxene), glass, serpentine–lizardite [(Mg,Fe,Al)2Si2O5(OH)4], spinel (mainly magnetite, Fe2+Fe2 3+O4), and ubiquitous traces of quartz; additionally, for some of the samples haematite (Fe2O3), analcime (NaAlSi2O6·H2O), diaspor (AlOOH), and hexagonal carbon (C) have also been encountered (Figure S3). In agreement with our previous investigations, the serpentine-like phase seems to be partly dehydrated (due to the calcination process in rotary kilns) and partly transformed to glass (Ettler et al. 2016). Glass formed either spherical or vesicular particles and exhibited high concentrations of Ni (up to 6.30 wt% as NiO) (Fig. 2d–f). Spinel-type phases occurred either as spherical particles, often less than 10 µm in size, probably formed during the calcination process in rotary kilns or as euhedral residual grains of the primary ore materials (concentrations of Ni and Cr were up to 3.44 wt% as NiO, and 35.6 wt% as Cr2O3, respectively) (Fig. 2d–f). Olivine occurred as skeletal crystals in spherical silicate particles and also exhibited high concentrations of Ni (up to 3.98 wt% as NiO) (Fig. 2e). Concentrations of Co and other relevant trace metal(loid)s have not been detected in any of the phases analysed by SEM/EDS.
Leaching tests
Leaching in deionised water using the EN12457-2 test indicated that the steady-state pH of the waste-water suspensions after 24 h of equilibration was rather variable. The pH for slag leachates varied in the range of 5.80–8.41 and for fly ash leachates in the range of 5.07–9.32 (Table S4). Eh ranged between 315 and 489 mV and indicated oxidising conditions during the test (Table S4). Whereas specific conductivities for the slag leachates were rather low (11–52 µS/cm), indicating low amounts of total dissolved salts, the fly ash leachates exhibited some higher conductivities and ranged from 39 to 5340 µS/cm. Leached concentrations of the measured contaminants are reported in Table S5. Moreover, the leaching of major contaminants occurring in the highest concentrations in the wastes (Cr, Ni, Zn) and comparisons with EU limits for inert, non-hazardous, and hazardous wastes are plotted in Fig. 3. With the exception of fly ash sample NFA1, all other samples exhibited extremely low levels of contaminant leaching and can be classified as inert wastes. Leached Ni for fly ash NFA1 was 4250 mg/kg, exceeding by 106 times the limit for hazardous waste. For the same sample, leached Cd exceeded 13 times and Zn exceeded 5.6 times the limits for inert waste (0.04 mg/kg, and 4 mg/kg, respectively). Cobalt leaching was also relatively high (72.8 mg/kg), but this metal has not been regulated by EU waste legislation (EU 2003). The remaining contaminants leached from sample NFA1 at concentrations similar to those found in the other samples studied and were well below EU limits (Table S5).
Boxplots for leached concentrations of Cr, Ni, and Zn (being the major contaminants) in slags and fly ash (FA) materials. The results were obtained by the EU normalised leaching test EN 12457-2. Comparisons with EU legislation limits for inert waste (IW), non-hazardous waste (NHW), and hazardous waste (HW) are indicated
Bioaccessibility testing and exposure assessment
The bioaccessible concentrations of individual contaminants obtained by leaching in SGF and corresponding BAFs are reported in Table 3. Whereas more Cr has been released from slags (probably due to higher bulk concentration), generally, other contaminants have been leached to a greater extent from the fly ash samples (Table 3). The differences in BAF values between the two waste types were most pronounced for the contaminants being present in highest concentrations in the studied materials (Co, Cr, Ni, Zn). Slag exhibited the following BAF values: Co (0.89–1.7 %), Cr (0.18–1.6 %), Ni (0.55–2.0 %), and Zn (1.0–8.0 %). In contrast, except for Cr (0.10–1.2 %), generally higher BAFs were found for the fly ash samples: Co (7.1–19.1 %), Ni (2.8–12.9 %), and Zn (3.4–8.5 %). The higher BAF value for Ni reported for sample NFA1 might be related to the higher proportion of a relatively soluble serpentine-like phase (lizardite) compared with the other studied fly ash samples (Table 3) and higher proportion of smaller particles in the <0.5 mm fraction. It is important to note that BAFs for other contaminants can also reach high values (e.g. As: 36.3 %, Ba: 40.7 %), but due to their low total concentrations in the material, their absolute bioaccessible concentrations are very low (Table 3) (see chapter on exposure estimates).
Despite higher contaminant bioaccessibilities in the fly ash materials compared with slags, calculated daily intakes of individual contaminants for an adult person (presumably a worker in the Ni refinery), assuming a dust ingestion rate of 50 mg/day, did not exceed either the TDI or BE values for any of the studied contaminants (Table 4). For Co, Cr, and Ni, the estimated maximum daily intake was much lower than the BE limits defined by Baars et al. (2001): 4.5 times, 13 times, and 1.7 times, respectively. For all other contaminant elements, the estimated maximum intake was far lower, by a factor of 100 s to 1000 s of times (Table 4). Nevertheless, it is important to remind that TDI for Ni recently defined by EFSA (2015) dropped to 2.8 µg/kg body weight, which is below the BE limit previously defined by Baars et al. (2001) (4 µg/kg body weight).
Discussion
Contaminant leaching in water and SGF
The extent of contaminant leaching from solid wastes is affected by a number of parameters; granulometry of the material, pH of the leaching solution, and solid speciation of contaminants (mineralogy) being among the most important. Our results indicate that the two waste types studied have contrasting granulometry and mineralogy, whereas in each subset of samples, a significant variability in the steady-state leachate pH has been observed. Except for Cr, the contaminant leaching was up to several orders of magnitude higher for fly ash materials than for slags (Table S5, Fig. 2), which might be explained by their significantly smaller grain size. Nevertheless, one fly ash sample (NFA1) exhibited exceptionally high leaching of Ni, Co, and Zn compared with the other fly ash samples (Table S5, Fig. 2). This phenomenon can be partly related to the higher proportion of finer particles in the <0.5 mm fraction (Figure S1), but also to the lower steady-state pH of the leachate (5.07) with respect to the other fly ash samples. Previous pH-dependent leaching experiments conducted on a fly ash from laterite Ni ore smelting indicated extremely high Ni leaching in the pH range 3–6 (5750–3880 mg/kg), followed by a substantial drop at pH 7 (1140 mg/kg), pH 8 (56.1 mg/kg), and pH 9 (5.78 mg/kg) (Ettler et al. 2016). For this reason, we hypothesise that higher steady-state pH values of the leachates of the other fly ash samples (6.67–9.32) may be responsible for the significantly lower leaching of Ni and other contaminants (Tables S4 and S5).
However, the effect of pH change on leaching cannot be considered in bioaccessibility testing using SGF, where the pH is monitored, and should not drift by more than 0.5 pH units (US EPA 2007; the final pH of all our leachates being ~1.9). Thus, granulometry and mineralogy must be the key factors affecting contaminant leaching in SGF. Morrison and Gulson (2007) and Morrison et al. (2016) have recently demonstrated how bioaccessible concentrations increase with a decrease in the grain size fractions of slags. We are aware that our slag samples are too coarse for being ingested in real exposure scenarios; only small size fractions could potentially be windblown from the dumps (Ziskind 2006) and become accessible for humans via ingestion or inhalation. Despite the fact that the bioaccessible fractions of contaminants are very low for slags (<2 % of total concentrations; Table 3), it is possible that finer slag fractions could be enriched in contaminants compared with the coarser fractions, which would increase their potential bioaccessibility (Morrison and Gulson 2007; Morrison et al. 2016). As a result, bioaccessible testing should also be carried out on finer fractions of Ni smelter slags, because finer particles are more reactive due to greater surface area (Ruby et al. 1999); thus, we hypothesise that the obtained BAFs may be slightly underestimated for the slags studied. On the other side, our SEM observations (see also microphotographs in Ettler et al. 2016) indicated that fine grains of granulated slags seem to have a similar mineralogical composition; therefore, we do not expect any effects of mineralogical composition on bioaccessible leaching on fine-grained fractions compared with the coarse-grained slag materials.
Interestingly, a recent work dealing with slag dusts and flue gas cleaning dusts from Cu smelters in Zambia failed to ascertain the effect of granulometry on contaminant leaching in SGF and underlined the importance of mineralogy (Ettler et al. 2014). However, Ettler et al. (2016) demonstrated that no significant change occurred in the mineralogical composition of Ni slag after leaching at pH 3. On the contrary, some high-temperature silicate phases (olivine, pyroxene) disappeared from XRPD patterns after leaching of fly ash at pH 3, and the proportion of serpentine-like phase also decreased in the leached residue. This was also confirmed by extended X-ray absorption fine structures (EXAFS) spectroscopy, which indicated that in the serpentine-like phase (being the dominant Ni-bearing phase), glass and silicates partially dissolved during the fly ash leaching at pH 3 (Ettler et al. 2016), a similar phenomenon being suggested for the dissolution during the bioaccessibility tests presented in this paper.
Our leaching tests in SGF indicate that BAFs for the relevant contaminants (Co, Cr, Ni, Zn) are relatively low (Table 4). The obtained data are difficult to compare with the results from the literature, because bioaccessibility testing for these elements has only been performed on attic dusts or soils. Bačeva Andonovska et al. (2015) studied attic dusts collected in the vicinity of a ferronickel smelter, using 0.1 M HCl solution as SGF and a carbonate buffer as a simulated lung fluid (SLF). They found that none of the contaminants could be detected in the SLF extract, and only up to 9.2 % Co, Cr 3.5 %, 7.6 % Ni, and 31 % Zn were extracted by SGF. Quite low (<3 %) extractabilities of Ni in SLF were reported by Drysdale et al. (2012) in the <10 µm fraction of Ni smelter-affected soils. However, despite generally low bioaccessibilities, the Ni release increased as a function of time, and the authors suggested that Ni can be leached from particles trapped in the lungs over the longer term. In soils developed on volcanic rocks and naturally enriched in Ni and Cr, BAF values for Ni (1.4–43.8 % of total concentration) are generally higher than those for Cr (0.4–5.4 %) (Palmer et al. 2014). The authors explained this finding with previously published data, indicating that Ni can be bound in the more soluble primary phases derived from basaltic rocks (e.g. olivine) than Cr, which is mostly bound in less soluble spinels (Cox et al. 2013). Similar differences between BAF values for Ni and Cr were also observed in industrially polluted urban soils (up to 1.83 % for Ni, versus 0.3 % for Cr in the gastric phase; Gbefa et al. 2011). Vasiluk et al. (2011) studied Ni bioaccessibility and bioavailability in two Ni smelter-affected soils and found that BAF values ranged between 3.88 and 17.2 %, and were surprisingly higher for the 150–250 µm fraction than for the <70 µm, despite the higher bulk Ni concentrations of the latter. Similarly, in vivo investigations performed with rats after oral administration of the soil fractions indicated that higher amounts of Ni (12–22 %) were absorbed by the animals from the coarse-grained fractions; whereas Ni bioavailabilities from the finer soil fraction were negligible (Vasiluk et al. 2011). The authors concluded that the observed differences between in vivo estimates of bioavailable Ni and in vitro estimates of bioaccessible Ni are still not fully understood, although others have suggested the results showing that finer soil fractions have a lower bioavailability and bioaccessibility could be related to differences in Ni mineralogy or a combination of other factors (surface area, soil buffering capacity, absorption processes, etc.) (Vasiluk et al. 2011). Henderson et al. (2012b) recently carried out bioaccessibility testing on pure Ni substances (Ni acetate, Ni chloride, Ni fluoride, Ni hydroxycarbonate, Ni oxide, Ni dihydroxide, Ni sulphamate, Ni subsulphide, and Ni sulphide) in SGF; they found that insoluble Ni substances (Ni sulphides, Ni (hydr)oxides) exhibited BAFs varying from <LOQ (limit of quantification) to 29.6 %, whereas soluble Ni substances (e.g. sulphates, chlorides) had BAFs >82.4 %.
Exposure estimates and implications for human health
Major contaminants leached from waste dusts generated by the laterite Ni ore smelting (Co, Cr, Ni, Zn; Table 3) all have complex functions in biological systems. Cobalt is bound within vitamin B12, with the recommended daily intake in an adult diet set to 0.1 g/day; however, at higher exposures it can cause neurotoxicological disorders, genotoxicity, and even cancer (Gál et al. 2008 and references therein). Trivalent Cr is essential for maintaining normal glucose metabolism; however, hexavalent Cr has been found to cause adverse effects, and exposure to chromate dust can increase the incidence of lung cancer (Arita and Costa 2009; Costa and Klein 2006; Goldhaber 2003). Zinc is an essential component of a wide variety of enzymes (Goldhaber 2003), and its importance in biological functions in organisms is also reflected by relatively high values of BE (300 µg/kg body weight/day) and TDI (500 µg/kg body weight/day) (Baars et al. 2001). The essentiality and toxicity of Ni are quite complicated. Despite the fact that Ni-containing enzymes are known in the bacterial world, and humans are constantly exposed to this ubiquitous element (having a relatively high abundance in the Earth’s crust), the essential nature of Ni to higher organisms is questionable (Denkhaus and Salnikow 2002). Exposure to high doses of Ni seems to provoke skin allergies, lung fibrosis, variable degrees of kidney and cardiovascular fibrosis, DNA damage, as well as changes in DNA methylation that can lead to development of cancer (Denkhaus and Salnikow 2002; Arita and Costa 2009 and references therein). Older epidemiological studies, which reported the development of respiratory tract and nasal cancers in workers of Ni refineries, and these being related to the occupational exposures to soluble Ni compounds, have recently been questioned (Heller et al. 2009). Based on the analysis of historical data sets and available in vivo animal inhalation studies, Heller et al. (2009) concluded that the designation of soluble Ni compounds as carcinogenic should be reconsidered and that the true causes of lung cancer risk at Ni refineries lie in other exposures including insoluble Ni compounds, arsenic, or sulphuric acid mists. Interestingly, based on in vivo studies, it has been suggested that insoluble Ni compounds (Ni sulphides, NiO) are stronger carcinogens because they are not easily cleared from the tissues, whereas soluble Ni compounds (Ni sulphates) should present no risk of carcinogenicity due to their rapid clearance and absence of any efficient delivery of Ni ions to the target sites in the cell (nucleus) (Oller et al. 1997; Denkhaus and Salnikow 2002 and references therein). In contrast, more recent in vivo testing of acute oral toxicity of various Ni substances with Sprague–Dawley rats indicated that median lethal dose concentrations (LD50) for individual materials varied from 310 to >11,000 mg Ni/kg (Henderson et al. 2012a), with the highest acute toxicity observed for soluble Ni compounds (Ni sulphate, Ni chloride) and lowest for insoluble Ni compounds (Ni sulphide, Ni oxide) and complex Ni-bearing materials (Ni ash and Ni matte).
The calculated daily intakes of contaminants based on bioaccessibility leaching in SGF are very low, far below the BE and TDI values (Table 4). Thus, our exposure estimates are indicating that for the given scenario (dust ingestion rate of 50 mg/kg), there will be no risk for human health. We are nevertheless aware that despite the safety measures taken in the mining and smelting areas of Niquelândia and Barro Alto (filters, masks) our calculation might underestimate the occupational oral exposure of workers and that the ingestion rate of 50 mg/day may be too conservative in real life. If the assumed ingestion rates were doubled (100 mg/day), the TDI value recently defined for Ni by European Food Safety Authority (EFSA 2015; 196 µg/day) is exceeded (324 µg/day) for only one of the fly ash samples (Table 4).
Our findings correlate well with the low Ni bioavailability from Ni ash and Ni matte recently determined by in vivo acute toxicity tests by Henderson et al. (2012a). Moreover, Oliveira-Filho et al. (2010) used similar fly ash from calcination in a ferronickel plant to spike water at 5, 10, and 50 % for in vivo tests with Oreochromis niloticus fish and found absence of acute toxicity, cytotoxicity, and genotoxicity. Subsequent bioavailability assessment in the gastrointestinal tract of O. niloticus indicated that after 96 h of exposure to the dust particles and after 15–30 days of stay in clean water, both gills and gastrointestinal tract were nearly devoid of particles and metal concentrations in the fish body returned to baseline levels (Oliveira-Filho et al. 2013).
Conclusions
Fine-grained smelting wastes (granulated slags, fly ash materials) from laterite Ni ore smelting in Goiás State, Brazil, contained significant amounts of contaminants, with up to 2.6 wt% Ni, 7580 mg/kg Cr, and 508 mg/kg Co. However, when assessed using a standardised EU leaching procedure (EN 12457-2 test), the overall contaminant leaching was very low, and only one fly ash sample exceeded the limit for hazardous waste according to EU legislation, whereas other materials were classified as inert wastes. In vitro bioaccessibility leaching behaviour in simulated gastric fluid indicated that bioaccessible fractions of major contaminants (Ni, Co, Cr) were quite low for the slag dusts (<2 % of the total concentrations), and for the fly ash materials reached 13 % for Ni and 19 % for Co. The observed differences in bioaccessibilities are related to differences in the granulometry (slags were coarse-grained than fly ash materials) and in the mineralogical compositions. We calculated the daily intakes assuming an oral exposure pathway, an adult of 70 kg, and a dust ingestion rate of 50 mg/day. Given these assumptions, none of the contaminant values exceeded either the tolerable daily intake (TDI) limit or the background exposure (BE). For this reason, we assume a limited risk for human health related to ingestion of dust materials generated by laterite Ni ore smelting operations. Nevertheless, for some of the areas in and near the smelter, dust ingestion rates can be higher than 50 mg/day, but safety measures (filters, masks) currently adopted by the mining/smelting company should efficiently prevent any exposure risks.
References
Arita, A., & Costa, M. (2009). Epigenetics in metal carcinogenesis: Nickel, arsenic, chromium and cadmium. Metallomics, 1, 222–228.
Baars, A. J., Theelen, R. M. C., Janssen, P. J. C. M., Hesse, J. M., van Apeldoorn, M. E., Meijerink, M. C. M., et al. (2001). Re-evaluation of human-toxicological maximum permissible risk levels. RIVM report 711701025, Bilthoven, the Netherlands.
Bačeva Andonovska, K., Stafilov, T., & Karadjova, I. (2015). Assessment of trace elements bioavailability – ingestion of toxic elements from the attic dust collected from the vicinity of the ferro-nickel smelter plant. Contributions, Section of Natural, Mathematical and Biotechnical Sciences, MASA, 36, 93–104.
Bačeva, K., Stafilov, T., Šajn, R., & Tanaselia, C. (2012). Moss biomonitoring of air pollution with heavy metals in the vicinity of a ferronickel smelter plant. Journal of Environmental Science and Health, Part A, 47, 645–656.
Bierkens, J., Van Holderbeke, M., Cornelis, C., & Torfs, R. (2011). Exposure through soil and dust ingestion. In F. A. Swartjes (Ed.), Dealing with contaminated sites (pp. 261–286). Berlin: Springer.
Costa, M., & Klein, C. B. (2006). Toxicity and carcinogenicity of chromium compounds in humans. Critical Reviews in Toxicology, 36, 155–163.
Cox, S. F., Chelliah, M. C. M., McKinley, J. M., Palmer, S., Ofterdinger, U., Young, M. E., et al. (2013). The importance of solid-phase distribution on the oral bioaccessibility of Ni and Cr in soils overlying Palaeogene basalt lavas, Northern Ireland. Environmental Geochemistry and Health, 35, 553–567.
Crundwell, F. K., Moats, M. S., Ramachandran, V., Robinson, T. G., & Davenport, W. G. (2011). Extractive metallurgy of nickel, cobalt and platinum-group metals. Amsterdam: Elsevier.
Denkhaus, E., & Salnikow, K. (2002). Nickel essentiality, toxicity, and carcinogenicity. Critical Reviews in Oncology/Hematology, 42, 35–56.
Deshommes, E., Tardif, R., Edwards, M., Sauvé, S., & Prévost, M. (2012). Experimental determination of oral bioavailability and bioaccessibility of lead particles. Chemistry Central Journal, 6, 138.
Drysdale, M., Bjorklund, K. L., Jamieson, H. E., Weinstein, P., Cook, A., & Watkins, R. T. (2012). Evaluating the respiratory bioaccessibility of nickel in soil through the use of a simulated lung fluid. Environmental Geochemistry and Health, 34, 279–288.
EFSA. (2009). Scientific opinion of the Panel on Contaminants in the Food Chain on a request from the European Commission on cadmium in food. EFSA Journal, 2009(980), 1–139.
EFSA. (2010). Scientific opinion on lead in food. EFSA Journal, 8(4), 1570.
EFSA. (2014a). Scientific opinion on the risks to public health related to the presence of chromium in food and drinking water. EFSA Journal, 12(3), 3595.
EFSA. (2014b). Dietary exposure to inorganic arsenic in the European population. EFSA Journal, 12(3), 3597.
EFSA. (2015). Scientific opinion on the risks to public health related to the presence of nickel in food and drinking water. EFSA Journal, 13(2), 4002.
EN 12457–2. (2002). Characterisation of waste-leaching—Compliance test for leaching of granular waste materials and sludges, part 2. Brussels: CEN.
Ettler, V., Johan, Z., Kříbek, B., Šebek, O., & Mihaljevič, M. (2009). Mineralogy and environmental stability of slags from the Tsumeb smelter, Namibia. Applied Geochemistry, 24, 1–15.
Ettler, V., Kvapil, J., Šebek, O., Johan, Z., Mihaljevič, M., Ratié, G., et al. (2016). Leaching behaviour of slag and fly ash from laterite nickel ore smelting (Niquelândia, Brazil). Applied Geochemistry, 64, 118–127.
Ettler, V., Vítková, M., Mihaljevič, M., Šebek, O., Klementová, M., Veselovský, F., et al. (2014). Dust from Zambian smelters: Mineralogy and contaminant bioaccessibility. Environmental Geochemistry and Health, 36, 919–933.
EU. (1999). Council Directive 99/31/EC of 26 April 1999 on the landfill of waste. Official Journal of European Communities, L182, 1–19.
EU. (2003). Council decision of 19 December 2002 establishing criteria and procedures for the acceptance of waste at landfills pursuant to Article 16 of and Annex II to Directive 1999/31/EC. Official Journal of European Communities, L11, 27–49.
Gál, J., Hursthouse, A., Tatner, P., Stewart, F., & Welton, R. (2008). Cobalt and secondary poisoning in the terrestrial food chain: Data review and research gaps to support risk assessment. Environment International, 34, 821–838.
Gbefa, B. K., Entwhistle, J. A., & Dean, J. R. (2011). Oral bioaccessibility of metals in an urban catchment, Newcastle upon Tyne. Environmental Geochemistry and Health, 33, 167–181.
Goldhaber, S. B. (2003). Trace element risk assessment: Essentiality vs. toxicity. Regulatory Toxicology and Pharmacology, 38, 232–242.
Gražulis, S., Daškevič, A., Merkys, A., Chateigner, D., Lutterotti, L., Quirós, M., et al. (2012). Crystallography Open Database (COD): An open-access collection of crystal structures and platform for world-wide collaboration. Nucleic Acids Research, 40, D420–D427.
Heller, J. G., Thornhill, P. G., & Conard, B. R. (2009). New views on the hypothesis of respiratory cancer risk from soluble nickel exposure; and consideration of this risk’s historical sources in nickel refineries. Journal of Occupational Medicine and Toxicology, 4, 23.
Henderson, R. G., Cappellini, D., Seilkop, S. K., Bates, H. K., & Oller, A. R. (2012a). Oral bioaccessibility testing and read-across hazard assessment of nickel compounds. Regulatory Toxicology and Pharmacology, 63, 20–28.
Henderson, R. G., Durnado, J., Oller, A. R., Merkel, D. J., Marone, P. A., & Bates, H. K. (2012b). Acute oral toxicity of nickel compounds. Regulatory Toxicology and Pharmacology, 62, 425–432.
Kierczak, J., Néel, C., Puziewicz, J., & Bril, H. (2009). The mineralogy and weathering of slag produced by the smelting of lateritic Ni ores, Szklary, Southwestern Poland. Canadian Mineralogist, 47, 557–572.
Moore, P. (2012). Anglo’s new nickel. International Mining, March 2012, 20–24.
Morrison, A. L., & Gulson, B. L. (2007). Preliminary findings of chemistry and bioaccessibility in base metal smelter slags. Science of the Total Environment, 382, 30–42.
Morrison, A. L., Swierczek, Z., & Gulson, B. L. (2016). Visualisation and quantification of heavy metal accessibility in smelter slags: The influence of morphology on availability. Environmental Pollution, 210, 271–281.
Oliveira-Filho, E. C., Freitas Muniz, D. H., Ferreira, M. F. N., & Grisolia, C. K. (2010). Evaluation of acute toxicity, cytotoxicity and genotoxicity of a nickel mining waste to Oreochromis niloticus. Bulletin of Environmental Contamination and Toxicology, 85, 467–471.
Oliveira-Filho, E. C., Lima, L. S., Freitas Muniz, D. H., Ferreira, M. F. N., Malaquias, J. V., & Grisolia, C. K. (2013). Bioavailability assessment of metals from a nickel mining residue in the gastrointestinal tract of Oreochromis niloticus in vivo. Bulletin of Environmental Contamination and Toxicology, 91, 533–538.
Oller, A. R., Costa, M., & Oberdörster, G. (1997). Carcinogenicity assessment of selected nickel compounds. Toxicology and Applied Pharmacology, 143, 152–166.
Palmer, S., Cox, S. F., McKinley, J. M., & Ofterdinger, U. (2014). Soil-geochemical factors controlling the distribution and oral bioaccessibility of nickel, vanadium and chromium in soil. Applied Geochemistry, 51, 255–267.
Palmer, S., Ofterdinger, U., McKinley, J. M., Cox, S., & Barsby, A. (2013). Correlation analysis as a tool to investigate the bioaccessibility of nickel, vanadium and zinc in Northern Ireland soils. Environmental Geochemistry and Health, 35, 569–584.
Ratié, G., Quantin, C., Jouvin, D., Calmels, D., Ettler, V., Sivry, Y., et al. (2016). Nickel isotope fractionation during laterite Ni ore smelting and refining: Implications for tracing the sources of Ni in smelter-affected soils. Applied Geochemistry, 64, 136–145.
Reis, A. P., Patinha, C., Noack, Y., Robert, S., & Dias, A. C. (2014a). Assessing human exposure to aluminium, chromium and vanadium through outdoor dust ingestion in the Bassin Minier de Provence, France. Environmental Geochemistry and Health, 36, 303–317.
Reis, A. P., Patinha, C., Noack, Y., Robert, S., Dias, A. C., & Ferreira da Silva, E. (2014b). Assessing the human health risk for aluminium, zinc and lead in outdoor dusts collected in recreational sites used by children at an industrial area in the western part of the Bassin Minier de Provence, France. Journal of African Earth Sciences, 99, 724–734.
Ruby, M. V., Schoof, R., Brattin, W., Goldade, M., Post, G., Harnois, M., et al. (1999). Advances in evaluating the oral bioavailability of inorganics in soil for use in human health risk assessment. Environmental Science and Technology, 33, 3697–3705.
SCHER. (2012). Assessment of the tolerable daily intake of barium. Scientific Committee on Health and Environmental Risks. http://ec.europa.eu/health/scientific_committees/environmental_risks/docs/scher_o_161.pdf.
Tiesjema, B., & Baars, A. J. (2009). Re-evaluation of some human-toxicological maximum permissible risk levels earlier evaluated in the period 1991–2001. RIVM report 711701092, Bilthoven, the Netherlands.
US EPA. (2007). Estimation of relative bioavailability of lead in soil and soil-like materials using in vivo and in vitro methods. Office of Solid Waste and Emergency Response. OSWER 9285 (pp. 7–77). Washington, DC: US EPA.
USGS. (2016). Nickel. USGS Mineral Resources Program. http://minerals.usgs.gov/minerals/pubs/commodity/nickel/mcs-2016-nicke.pdf.
Vasiluk, L., Dutton, M. D., & Hale, B. (2011). In vitro estimates of bioaccessible nickel in field-contaminated soils, and comparison with in vivo measurement of bioavailability and identification of mineralogy. Science of the Total Environment, 409, 2700–2706.
Warner, A. E. M., Díaz, C. M., Dalvi, A. D., Mackey, P. J., & Tarasov, A. V. (2006). JOM World Nonferrous Smelter Survey, part III: Nickel: laterite. JOM Journal of the Minerals Metals and Materials Society, 58, 11–20.
Zelano, I., Sivry, Y., Quantin, C., Gélabert, A., Tharaud, M., Jouvin, D., et al. (2013). Colloids and suspended particulate matters influence on Ni availability in surface waters of impacted ultramafic systems in Brazil. Colloids and Interfaces A: Physicochemical Engineering Aspects, 435, 36–47.
Ziskind, G. (2006). Particle resuspension from surfaces: Revisited and re-evaluated. Reviews in Chemical Engineering, 22, 1–123.
Acknowledgments
This study was supported by the Czech Science Foundation project (GAČR 13-17501S) and was carried out in the framework of the Marie Curie International Research Staff Exchange Scheme Fellowship within the 7th European Community Framework Programme (NIDYFICS, no. 318123). Part of the equipment used for this study was purchased from the Operational Programme Prague—Competitiveness (Project CZ.2.16/3.1.00/21516). The staff of Anglo American at Codemin (Niquelândia) and Barro Alto provided access to the field facilities and kindly helped with the sampling. Petr Drahota helped with XRPD data acquisition, Zuzana Korbelová with SEM/EDS measurements, and Marie Fayadová with leaching and bioaccessibility tests. Peter Lemkin is thanked for revision of the manuscript. Valuable comments of two anonymous reviewers helped to improve the original version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ettler, V., Polák, L., Mihaljevič, M. et al. Oral bioaccessibility of inorganic contaminants in waste dusts generated by laterite Ni ore smelting. Environ Geochem Health 40, 1699–1712 (2018). https://doi.org/10.1007/s10653-016-9875-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-016-9875-4