Abstract
Simulated lung fluids are solutions designed to mimic the composition of human interstitial lung fluid as closely as possible. Analysis of mineral dusts using such solutions has been used to evaluate the respiratory bioaccessibility of various elements for which solubility in the lungs is a primary determinant of reactivity. The objective of this study was to employ simulated lung fluid analysis to investigate the respiratory bioaccessibility of nickel in soils. Current occupational guidelines in Australia regulate nickel compounds in terms of water solubility, though this may not be an accurate estimation of the total nickel that will dissociate in the lungs. Surface soils were collected from the city of Kalgoorlie in Western Australia, the site of an operational nickel smelter and metal mining activities. The fraction of the samples less than 10 μm was extracted from the soil, and it was this sub-10-μm fraction that was found to hold most of the total nickel present in the soil. The fine fraction was analyzed using a simulated lung fluid (modified Gamble’s solution) to isolate the nickel phases soluble in the lungs. In addition, a sequential extraction was employed to compare the bioaccessible fraction to those dissolved from different binding forms in the soil. In all samples, the simulated lung fluid extracted more nickel than the two weakest leaches of the sequential extraction combined, providing a more representative nickel bioaccessibility value than the current water leach method.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The proximity of residential communities to nickel mining and processing activities presents a potential health risk for residents in relation to inhalation of nickel-bearing particles. There is controversy over the effects of inhalation of the various compounds released by nickel-processing industries due to conflicting study results. Laboratory analyses show a linear relationship between lung cancer development in animals and airborne nickel concentration (Seilkop and Oller 2003). However, field studies conducted in nickel extraction and processing environments do not always yield statistically significant correlations (Julian and Muir 1996). Results from field studies are often disputed due to uncontrolled outdoor and indoor environments where humans are exposed to a variety of carcinogenic substances. In most cases, there is no research evaluating the interactive effects between other proven or potential carcinogens with nickel (Occupational Disease Panel 1997). Currently, nickel subsulfide, the result of roasting and smelting, is the compound of highest concern to public health, though many studies have indicated that water-soluble nickel compounds may be as toxic or more so (Grimsrud et al. 2002).
To determine the potential risks associated with inhalation of nickel-bearing particles, it is important to take into consideration all of the potential pathways through the lungs. A study conducted by Lehert (1993) found that there are three mechanisms affecting inhaled solid particles. The first mechanism is trapping of the particle in the trachea or bronchi, the second is the reaction of particles in the alveolar system, and the third can occur at any point in the lungs where phagocytes expel foreign compounds. In the first mechanism, the respiratory system will remove most of the solids using cilia in the mucous lining. This stage is not likely a cause of respiratory disease due to insoluble particle accumulation but has the potential to release a small percentage of the soluble component into epithelial cells (Kasprzak et al. 2003; Oller et al. 1997). If particles manage to pass the tracheobronchial system into the alveolar system, the remaining soluble compounds will likely dissolve into interstitial lung fluids, while the insoluble compounds will begin to accumulate as particles in the lungs (Oller et al. 1997). At any point in the process, lung phagocytes can recognize particles as foreign and will engulf them and attempt to expel them from the lungs.
Nickel toxicity is highly dependent on the solubility of the nickel compound in interstitial lung fluid, and environmental guidelines commonly regulate nickel-bearing materials according to whether they are soluble in water. For instance, the Australian Exposure Standards for Atmospheric Contaminants in the Occupational Environment lists a maximum time-weighted average over 8 h of 0.1 mg/m3 for nickel, contained within compounds soluble in water, and 1 mg/m3 “nickel metal and nickel sulfide roasting” in which the metal is within insoluble compounds (NOHSC 1995). In a 1997 study, Oller et al. demonstrated the pathways taken by the soluble versus insoluble groups, using nickel oxide and subsulfide as examples of common insoluble compounds and nickel sulfate as a common soluble compound. They determined that the compounds most likely to lead to tumor development in cells are those that are insoluble in the interstitial lung fluid (simplified to water in this case) and soluble in the acidic cell fluid. This allows the cells to absorb the nickel compounds via phagocytosis, which are released as nickel ions once inside the cell, where they can interact with the cellular DNA. On the other hand, the lungs clear soluble compounds more rapidly, after they dissociate to nickel ions. However, high concentrations of soluble compounds around epithelial cells have been observed to cause particle overload (Lehert 1993; Galle et al. 1992). Though soluble nickel compounds are relatively nontoxic at low concentrations, once dissolved, reprecipitation in the lungs can cause adverse effects. In this case, alveolar macrophages can undergo a particle overload, causing them to precipitate the metal with phosphates in the lungs and eventually leading to a hypersensitivity or allergic reaction. This process is irreversible and can permanently diminish lung function (Lehert 1990). Both soluble and insoluble compounds in lung fluids can cause inflammation, although the reaction appears to be faster for soluble compounds (Benson et al. 1986).
The objective of this laboratory-based approach, as a part of the risk evaluation of inhaled particles, is to determine the proportion of nickel-bearing compounds that can react with lung cells and potentially cause adverse effects. This is termed the “bioaccessibility” of nickel and refers to the proportion of nickel ions that are made accessible for uptake by cells, in comparison with the total nickel concentration of the material analyzed. Bioaccessibility differs from bioavailability, which is the proportion of nickel ions that is actually taken up by cells. Bioaccessibility tests can be done “in vitro” using selected simulated lung fluids, while bioavailability tests are conducted “in vitro” using cell lines or “in vivo,” by exposing laboratory animals to concentrations of airborne nickel compounds above guideline values, and evaluating the reactions using live test subjects. The main advantage of the in vivo method is the ability to directly observe the reactions of living organisms with complicated body systems exposed to high concentrations of toxic compounds. However, in the case of nickel, some laboratory results have demonstrated significant differences in tolerance between different laboratory animals, and it is also difficult to determine much more than relative toxicities of compounds in humans by using animal models (Kasprzak et al. 2003). Previous in vivo work includes exposing laboratory animals to individual compounds of concern, including nickel oxide, nickel sulfate, and nickel subsulfide. Results indicate that exposure to nickel at high concentrations bound in any form will cause respiratory cancer (Grimsrud et al. 2002). Benson et al. (1996) found that nickel subsulfide is more toxic than both water-soluble compounds and nickel oxides. They also determined that the water-soluble nickel compounds were more toxic than most of the insoluble compounds, even though their residence time in the lungs is significantly shorter.
The most common in vitro method for determining the toxicity of nickel compounds is through the use of cell lines. Cell lines are cultured human cells dosed with the compounds of concern to determine the percent nickel that remains in the cell, potentially causing damage including DNA lesions. The use of cell lines can be advantageous because it is the only method that utilizes human tissue when evaluating the toxicity of a compound. Cell lines are complicated to work with given that the growing conditions (including pH, proximity of cells to one another, type of cell used, and temperature) can drastically affect the results. Previous work with cell lines has indicated that the bioavailability of water-soluble nickel compounds (such as nickel chloride) is significantly higher than that of insoluble compounds (such as nickel oxide). However, once in the cell, a higher percentage of the nickel oxide reaches the nucleus (Schwerdtle and Hartwig 2006).
Simulated lung fluid
A simulated lung solution—known as Gamble’s solution—was first developed in the 1940s as an in vitro means of determining compound toxicity in the lungs. Subsequently, various studies have employed the method for determining the bioaccessibility of radioactive compounds (Damon et al. 1984). Davies and Feddah (2003) showed that simulated lung fluid considered in their study is identical to fluid sampled from human lungs in terms of major components. Though the use of simulated lung fluids is relatively uncommon, researchers have used the technique to evaluate the bioaccessibility of industrial and artificial compounds, including cadmium (Koshi 1979), silica (Scholze and Conradt 1987), and aerosol inhaler products (Davies and Feddah 2003). The method has also been applied to soils and dusts, such as radioactive uranium particles (Garger et al. 2003) and lead in soils (Wragg and Klinck 2007). The original Gamble’s solution was a mixture of water and inorganic salts including chlorides, carbonates, and phosphates, but the solution has been modified to include proteins and other organic components present in lung fluid (Takaya et al. 2006). However, addition of proteins can affect experimental results and reproducibility due to degradation (Takaya et al. 2006). The mixture employed by Takaya et al. contains the inorganic compounds from the original Gamble’s solution, combined with selected organic acids to mimic the organic compounds present in lung fluids, but no proteins. It is necessary to take lung residence times for particles into account when evaluating bioaccessibility using any simulated lung fluid. Wragg and Klinck (2007) found that tests performed for a minimum of 100 h provide a reasonable and conservative estimate of metal bioaccessibility in the lungs. Current occupational regulations for several metals, including nickel, are divided according to particle size and the solubility of the compound in water (NOHSC 1995). The influence of particle size has been documented in a study conducted by Bright et al. in 2006. The study concluded that particles smaller than 10 μm are not only more likely to remain airborne for longer periods of time than the >10 μm fraction, but this is also considered the tracheobronchial fraction, or the fraction that is most likely to enter the trachea. The application of a simulated lung fluid technique in place of water to determine nickel bioaccessibility could provide a more accurate representation of the bioaccessible nickel fraction in a material.
Materials and method
Site description
The Western Australian city of Kalgoorlie with a population of around 30,000 is located approximately 600 km east-northeast of the capital city of Perth. The soils in Kalgoorlie are extremely fine-grained and easily made airborne due to persistently high winds and a lack of ground vegetation. An epidemiological study named mining towns in the state, such as Kalgoorlie, “asthma hot spots,” prompting further investigation into individual potentially harmful elements (Lee et al. 2006). However, asthma is not the only respiratory disease of concern. Compared with the rest of the state average, citizens of Kalgoorlie have higher rates of bronchitis (Holman et al. 1987) and respiratory cancer (HDC 2004). Given the widespread presence of metallic ore bodies, the surface soils in Kalgoorlie have elevated concentrations of various metals. Many of these are potentially harmful to the respiratory system. One element of particular concern in the region is nickel, which is both allergenic and carcinogenic when inhaled. The region’s primary nickel smelter, the Kalgoorlie Nickel Smelter, is located 10 km south of the city center. Though smelter emissions are controlled, storage and transport of nickel-bearing ore, matte, and slag present a potential source of airborne nickel to the town’s residents.
Sample collection and preparation
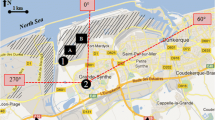
Soil samples were collected from four locations within the urban area of Kalgoorlie and three sites outside the town to allow comparison of soil composition and to estimate a background soil level for nickel in the general area (Fig. 1). The urban soil samples were collected from undeveloped land plots lacking vegetation near residential parks or suburban areas where citizens of Kalgoorlie may be regularly exposed to the dust generated from the soil. Samples were also collected from three locations downwind (east–northeast) of the nickel smelter outside the city in order to determine the effect of the smelter on surrounding soils. Two evaporite encrusted dry lake beds or “playas” were sampled to assess any influence on bioaccessibility from the crusts. All samplings were conducted at sites accessible to the public.
Soil samples were collected using a plastic trowel to prevent metal contamination. They consisted of 2–3 kg of the top 5 cm of soil, given that this is the portion most likely to become airborne. Three samples were taken at each location to elucidate site-scale variability. The soil samples were air-dried overnight in the laboratory and then sieved using a graduated set of sieves down to 63 μm to determine soil particle size distribution fractionation. After the samples were dried, we mixed composites for each sample site by mixing equal weights of each size fraction to increase sample size for collection of the respirable fraction. The <63 μm fraction of each composite sample was kneaded gently through a 10-μm vinyl mesh sieve by hand for 2–3 h until 1 g of the respirable fraction of each sample was isolated. The resulting 1 g sample was split into two 0.5 g samples, one of which was analyzed using the sequential extraction technique, and the other was subjected to the simulated lung fluid analysis using the modified Gamble’s solution.
General characteristics
pH was measured in deionized water according to the Australian guidelines for potentially contaminated soils (NEPC 1999). Soil composition was determined by polarized light microscopy and electron microprobe analysis using an ARL-SEMQ electron microprobe with an energy dispersive detector. The solid-phase nickel concentration of the <2 mm fraction was determined using nitric acid (HNO3) and hydrogen peroxide (H2O2) leach and analyzed using ICP-MS (Perkin Elmer Elan 6000) at the School of Earth and Environment at the University of Western Australia, Perth.
Sequential extraction
Sequential extraction involves the use of various solutions of increasing strength to dissolve elements bound within different phases in a medium. The Tessier sequential extraction (Tessier et al. 1979) involves six separate steps where the first two steps are designed to isolate the weight percent nickel present in the water-soluble and exchangeable phases in the soil, respectively. Each sample was weighed to 0.5 g in a 15-mL plastic centrifuge tube, and the different solutions were added sequentially to the samples. After each dissolution procedure, the samples were centrifuged for 20 min at 5,000 rpm and the supernatant collected for metal analysis, whereby the next solution was added to the sample. In order to isolate the water-soluble fraction, 7.5 mL of deionized water was added to the sample and agitated for 2 h at room temperature in a shaker water bath. In the second step, 4 mL of 1 M magnesium chloride [MgCl2] (pH = 7) was added to the sample and agitated for 1 h at room temperature in order to dissolve the exchangeable fraction. The third step aimed to dissolve nickel bound to carbonates by agitating for 5 h in 4-mL solution containing 8.203 g of sodium acetate [C2H3NaO2] dissolved in 100 mL of deionized water and brought to pH 5 using 1 mL of concentrated acetic acid [C2H4O2]. The oxide-bound fraction was dissolved with 10 mL of a 0.04 M hydroxylamine hydrochloride [NH2OH.HCl] in 25% HOAc solution and set in a water bath at 96°C. The organic-bound fraction involved mixing the samples with 20 mL of concentrated nitric acid [HNO3] and left to reflux to 5 mL, after which 1-mL aliquots of hydrogen peroxide [H2O2] were added until effervescence was minimal. The residual fraction was dissolved in a heated mixture of hydrofluoric acid [HF] and perchloric acid [HCLO4]. The samples were taken to near dryness and then redissolved in 5% hydrochloric acid [HCl] and made up to 20 mL in a volumetric flask. The sequential extraction was performed at the EIGG laboratory at Curtin University in Perth, WA. The supernatants were collected after each step and analyzed for nickel by ICP-MS (Perkin Elmer Elan 6000) at the School of Earth and Environment at the University of Western Australia, Perth.
Simulated lung fluid extraction
The lung fluid was prepared as listed in Table 1. About 0.25 g of the <10 μm fraction of the composite soil sample was placed in a plastic centrifuge tube to which 10 mL of the simulated lung fluid was added. The sample and fluid were thoroughly mixed using a vortex mixer and placed in a water bath at 37°C with occasional agitation. Takaya et al. (2006) noted that the pH of the solution tended to rise gradually above 7.4 in their experiments; however, no pH buffering was necessary in this case as the pH remained stable between 7.1 and 7.4 throughout the experiment. After 1 h in the water bath, the sample was centrifuged for 20 min at 5,000 rpm, and the supernatant was poured into a separate centrifuge tube for nickel analysis. The solid sample was resuspended using 10 mL of fresh modified Gamble’s solution, and the procedure was repeated with further removal of the solution after 1 day and then again after 7 days. All extracted solutions were analyzed for nickel using ICP-MS as above.
Quality control and calculations
One blank and one duplicate sample from each step of the sequential extraction were analyzed together with one blank for every ten samples, and one duplicate for each sample extracted with simulated lung fluid. For the total analysis, the blanks constituted 0–2% nickel compared to the average concentration in the samples. For the sequential extraction, the blanks held 0–1% nickel compared to the average of the samples for all extractions except the first (deionized water) and the second step (MgCl) where the blanks held 12 and 5% of the average concentration of the samples, respectively. For the lung fluid extractions, the blanks held 7–11% of the average nickel concentrations in the samples that increased with time, indicating some contamination with time. All blank values were subtracted from that measured in the samples for each step prior to further evaluation. Duplicates of each sample were tested using the simulated lung fluid, and standard error was <15% of the average. In the case of the simulated lung fluid, the method detection limit was 0.09 ppm Ni, well below the lowest concentration recorded in each extraction.
The nickel bioaccessibility was calculated as a percentage of the total nickel concentration calculated as the total nickel concentration dissolved from all steps in the sequential extraction. Since the last step of the sequential extraction involved the dissolution of the sample in HF, the total of all steps should represent the total Ni in the sample. For the assessment of bioaccessibility after 1 day, the dissolved concentration in the simulated lung fluid was added to the concentration after 1 h, and for the bioaccessibility assessment after 1 week, the concentrations after all three time points were added in order to provide the total bioaccessibility after 1 week.
Results and discussion
The soil samples collected consisted primarily of quartz, with minor carbonate, clay minerals, and goethite. They were fine-grained and red in color due to the presence of iron oxide grain coatings. Figure 2 shows the relationship between nickel concentrations in the <2 mm soil fraction and the <10 μm fraction. At most sites, nickel concentrations are higher in the <10 μm fraction indicating that most nickel found in the soil is in fact bound to the finer particles (as anticipated given the fine-grained nature of the soils). The exception is the soil collected from the playas, where the <2 mm fraction holds more nickel than the <10 μm fraction, thereby indicating that the nickel is distributed in larger particles as well. However, because larger particles from the playas were aggregated and we did not intentionally break these up during soil preparation, this finding could also suggest that a sufficient proportion of the <10 μm particles were not ultimately included in the <10 μm fraction analyzed.
The pH of the soils was generally basic, ranging from 8.07 to 9.63. Table 2 shows the variation in pH, nickel bioaccessibility, and total nickel content of soil samples taken from each location. The results showed that the total nickel concentrations were significantly higher near the smelter than in any of the other sampled locations, in particular at sampling site 5. The concentrations in the urban area of Kalgoorlie were similar or slightly elevated compared to those in the sites sampled for background. One location, sampling site C3, had nickel concentrations at about twice the other urban sites. This site was situated closest to the Kalgoorlie Superpit, Australia’s largest open cut gold mine. The playa samples held concentrations within the range found at the background sites. Compared to Australian guideline values (NEPC 1999), all samples except those collected near the smelter were within the background range of 5–500 mg/kg, and also below the health investigation limit of 600 mg/kg. However, all samples were above the ecologically set guideline value of 60 mg/kg which considers toxicity to plants. It should be noted that all samples were collected where the soil surface area was bare.
For all samples, the bioaccessibility was low and increased with time. Most samples showed 0.5–1% bioaccessibility after 1 h, rising to 1–2% after 1 day and 1.5–3% after 1 week. One of the samples collected near the smelter showed a higher bioaccessibility of nickel, with 1.8% of the total nickel concentration in the <10 μm fraction being dissolved in the lung fluid after 1 h, rising to 3.3% after 1 day and to 4.2% after 1 week. All samples showed a steady increase in bioaccessibility with time, although the increase was small. In a comparable study of lead in mine tailings, the dissolution of lead in simulated lung fluid peaked after less than 100 h for each sample (Wragg and Klinck 2007). This was not the case for nickel in the present study, where the metal continued to dissolve after 1 week. Wragg and Klinck attributed the decrease in dissolved lead over time to the formation of insoluble lead phosphates due to the presence of sodium phosphate in the simulated lung fluid (Wragg and Klinck 2007). Seaman et al. (2001) found that the precipitation of phosphates decreased the solubility of other compounds, including uranium and aluminum. However, in a study investigating the stabilization of nickel-contaminated sediments with hydroxyapatite, the strength of nickel adsorption on phosphate is less than that observed with aluminum, uranium, and lead (Seaman et al. 2001), potentially allowing more nickel to dissolve continuously.
Numerous studies have used both water and salt solutions to represent interstitial lung fluid, including most of the studies involving respiratory nickel toxicology (Oller et al. 1997). The first two steps of the Tessier sequential extraction employed in this study comprise water and salt solution (water-soluble and exchangeable). In Fig. 3, results for the simulated lung fluid used in this study are plotted for extractions after 1 h, 1 day, and 1 week with results from the first two weak leaches (extracted over a total of 3 h), as well as the extracted amount after the third step. In each case, the simulated lung fluid dissolved more nickel after 1 h than the two weak leach solutions dissolve after 3 h. The amount extracted after the third sequential step was in fact more similar to the bioaccessible fraction after 1 week in most cases, but for some sites also after 1 day. In fact, for most playa and city samples, more nickel was dissolved in the lung fluid after 1-week extraction than was found in the carbonate-bound fraction.
Percentage of nickel dissolved in simulated lung fluid after 1 h, 1 day, and 1 week, together with the combined water-soluble and exchangeable fraction, and the combined water-soluble, exchangeable, and carbonate-bound fraction calculated as a percentage of the total nickel concentration in the <10 μm fraction. S Smelter, C City, P Playa, B Background
Current occupational regulations for exposure to nickel compounds in the workplace have set limits for water-soluble compounds (NOHSC 1995) but do not include many of the other compounds that are soluble in lung fluids and may react in a comparable way once dissolved. Various toxicological studies, including a laboratory test for nickel toxicology (Oller et al. 1997), have attempted to simulate interstitial lung fluids using only water or a salt solution containing no amino acids. However, as is evident in Fig. 3, the addition of amino acids to the solution in this study increased the solubility of nickel compounds even during a shorter period of extraction, and some soils had bioaccessible fractions more similar to that bound by carbonates. By omitting amino acids from simulated lung fluid, the respiratory bioaccessibility of nickel could be significantly underestimated. These organic compounds appear to play a major role in the dissolution of nickel compounds by acting as weak chelating agents, dissolving compounds otherwise insoluble in aqueous solutions. Previous studies have noted such a reaction, primarily by examining nickel chelation by citrate compounds, where citrate readily sequesters nickel (Baker et al. 1983). Pyruvate and lactate, both present in Gamble’s solution, are also weak chelating agents and could lead to the dissolution of water-insoluble nickel compounds (Bernaudat and Bulow 2005; Leussing 1964).
In conclusion, lung bioaccessibility tests are likely to provide a better estimation of nickel bioaccessibility, and thus its toxicity, than estimations using water and salt solutions alone. Although nickel bioaccessibility in the investigated samples was generally low, the experimental setting did not show a peak in dissolution in the simulated lung fluid, and it is possible that nickel may continue to leach out of particles trapped in the lung beyond the endpoints examined in this study.
References
Baker, E. N., Baker, H. M., Anderson, B. F., & Reeves, R. D. (1983). Chelation of nickel(II) by citrate. The crystal structure of a nickel-citrate complex, K2[Ni(C6H5O7)(H2O2)2]2·4H2O. Inorganica Chimica Acta, 78, 281–285.
Benson, J. M., Henderson, R. F., McClellan, R. O., Hanson, R. L., & Rebar, A. H. (1986). Comparative acute toxicity of four nickel compounds to F344 rat lung. Fundamental and Applied Toxicology, 7, 340–347.
Bernaudat, F., & Bulow, L. (2005). Rapid evaluation of nickel binding properties of His-tagged lactate dehydrogenases using surface plasmon resonance. Journal of Chromatography. A, 1066, 219–224.
Bright, D. A., Richardson, G. M., Dodd, M. (2006). Do current standards of practice in Canada measure what is relevant to human exposure at contaminated sites? I: A discussion of soil particle size and contaminant partitioning in soil. Human and Ecological Risk Assessment, 12, 591–605.
Damon, E. G., Eidson, A. F., Hahn, F. F., Griffith, W. C., & Guimette, R. A. (1984). Comparison of early lung clearance of yellowcake aerosols in rats with in vitro dissolution and IR analysis. Health Physics, 46, 859–866.
Davies, N. M., & Feddah, M. R. (2003). A novel method for assessing dissolution of aerosol inhaler products. International Journal of Pharmaceutics, 255, 175–187.
Eidson, A. F., & Mewhinney, J. A. (1983). In vitro dissolution of respirable aerosols of industrial uranium and plutonium mixed oxide nuclear fuels. Health Physics, 45, 1023–1037.
Galle, P., Berry, J. P., & Galle, C. (1992). Role of alveolar macrophages in precipitation of mineral elements inhaled as soluble aerosols. Environmental Health Perspectives, 97, 145–147.
Garger, E. K., Odintsov, A. A., & Sazhenyuk, A. D. (2003). Estimation of the solubility of radioactive aerosol particles in biological liquids. Radiochemistry, 45(3), 298–303.
Grimsrud, T. K., Steinar, R. B., Haldorsen, T., Andersen, A. (2002). Exposure to different forms of nickel and risk of lung cancer. American Journal of Epidemiology, 156(12). doi:10.1093/aje/kwf165.
Health Data Collections (HDC). (2004). Cancer in Western Australia, 1998–2002: Incidence and mortality by statistical local area (SLA). Perth, Western Australia: Health Information Centre, Department of Health. Statistical Series 72.
Holman, C. D., Psaila-Savona, P., Roberts, M., & McNulty, J. C. (1987). Determinants of chronic bronchitis and lung dysfunction in Western Australian gold miners. British Journal of Industrial Medicine, 44(12), 810–818.
Julian, J. A., Muir, D. C. F. (1996). A study of cancer incidences in Ontario nickel workers. Occupational Disease Panel.
Kasprzak, K. S., Sunderman, F. W., & Salnikow, K. (2003). Nickel carcinogenesis. Mutation Research, 533, 67–97.
Koshi, K. (1979). Solubility and celltoxicity of cadmium. Industrial Health, 17, 187–197.
Lee, Y. P., Cook, A., Thompson, P., & Weinstein, P. (2006). Land use classifications and community asthma burden in regional Australia (ISEE/ISEA 2006 conference abstracts supplement: poster abstracts: abstracts). Epidemiology, 17(6), 283–284.
Lehert, B. E. (1990). Alveolar macrophages in a particle “Overload” condition. Journal Aerosol Medicine, 3(S1), S9–S30.
Lehert, B. E. (1993). Defense mechanisms against inhaled particles and associated particle-cell interactions. Health effects of mineral dusts (pp. 427–469). Washington DC: Ribbe, P.H., Mineralogical Society of America.
Leussing, D. L. (1964). A numerical study of the nickel(II)-pyruvate-glycinate system using a high speed computer. Talanta, 11, 189–201.
National Environmental Protection Council, Australia (NEPC). (1999). Guideline on the laboratory analysis of potentially contaminated soils, Schedule B(3). 120 pp.
National Occupational Health and Safety Commission (NOHSC). (1995). Adopted national exposure standards for atmospheric contaminants in the occupational environment, 1003.
Occupational Disease Panel (ODP). (1997). Report to the workers’ compensation board on cancer of the larynx in workers in primary nickel production. 75 pp.
Oller, A. R., Costa, M., & Oberdörster, G. (1997). Carcinogenicity assessment of selected nickel compounds. Toxicology and Applied Pharmacology, 143, 152–166.
Scholze, H., & Conradt, R. (1987). In in vitro study of the chemical durability of siliceous fibres. Annals of Occupational Hygiene, 31, 683–692.
Seaman, J. C., Arey, S. A., & Bertsch, P. M. (2001). Immobilization of nickel and other metals in sediments. Journal of Environmental Quality, 30, 460–469.
Schwerdtle, T., Hartwig, A. (2006). Bioavailability and genotoxicity of soluble and particulate nickel compounds in cultured human lung cells. Mat.-wiss. U. Werkstofftech, 37, 521–525.
Seilkop, S. K., Oller, A. R. (2003). Respiratory cancer risks associated with low-level nickel exposure: An integrated assessment based on animal, epidemiological and mechanistic data. Regulatory Toxicology and Pharmacology, 37(2), 173–190.
Takaya, M., Shinohara, Y., Serita, F., Ono-Ogasawara, M., Otaki, N., Toya, T., et al. (2006). Dissolution of functional materials and rare earth oxides into pseudo alveolar fluid. Industrial Health, 44, 639–644.
Tessier, A., Campbell, P. G. C., & Bisson, M. (1979). Sequential extraction procedure for the speciation of particulate trace metals. Analytical Chemistry, 51(7), 844–851.
Wragg, J., & Klinck, B. (2007). The bioaccessibility of lead from Welsh mine waste using a respiratory uptake test. Journal of Environmental Science and Health Part A, 42, 1223–1231.
Acknowledgments
The authors would like to thank two anonymous reviewers whose comments helped us improve the manuscript. We are also grateful to the Environmental Inorganic Chemistry Group at Curtin University, particularly David Oldmeadow, and to Mitchell Skuce of Queen’s University for help with the figures. Funding was provided by an NSERC Discovery Grant to HEJ and by CRC Asthma, Sydney.
Conflicts of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Drysdale, M., Ljung Bjorklund, K., Jamieson, H.E. et al. Evaluating the respiratory bioaccessibility of nickel in soil through the use of a simulated lung fluid. Environ Geochem Health 34, 279–288 (2012). https://doi.org/10.1007/s10653-011-9435-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-011-9435-x