Abstract
The information on concentration levels, partitioning and sources of pollutants in aquatic environment is quite necessary for pollution treatment and quality criteria. In this work, sixteen priority polycyclic aromatic hydrocarbons (PAHs) recommended by U.S. Environmental Protection Agency in the water and sediment of Yinma River Basin were firstly investigated. Among 16 individual PAHs, naphthalene was the highest average concentration in water samples as well as in sediment samples, 67.2 ng/L and 825.06 ng/g, respectively, whereas benzo(g,h,i)perylene was undetected in water samples nor in sediment samples. For three PAH compositional patterns, concentrations of light (2–3 ring) PAHs were dominant in water and sediment, accounting for 71.69 and 86.98 % respectively. The PAH partitioning in the sediment–water system was studied, results showed that PAH partitioning was in an unsteady state and tended to accumulate in the sediment. The possible sources of PAHs in water and sediment were both identified as a mixed source of petroleum and combustion. The benzo(a)pyrene equivalents (EBaP) values for PAHs in the water and sediment in some sites were relatively higher, suggesting the existence of environmental health risk.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polycyclic aromatic hydrocarbons (PAHs) are a large group of persistent pollutants with two or more (up to six) fused aromatic rings, which ubiquitously exit in the environment (Zhang et al. 2014). They are naturally originated from coal, oil and tar deposits as well as created in industrial processes and human activities (Harris et al. 2011), such as agriculture, vehicular emissions, industrial discharges (Sakulthaew et al. 2014; Ma et al. 2013). Due to their carcinogenic and mutagenic potentials (Aziz et al. 2014; Qin et al. 2014; Deng et al. 2006), the pollution of PAHs in aquatic environment draw increasing attentions from environmental protection agencies and scientific institutions (Bai et al. 2014). Especially, 16 species of PAHs have been recommended as priority pollutants by U.S. Environmental Protection Agency (USEPA) (Sereshk and Bakhtiari 2014).
Once PAHs from nature or anthropogenic activities enter aquatic environment, they may residue in the water, or bound to the sediment-associated phases due to their lipophilic characteristics and sediment adsorption (Yuan et al. 2014; Gao et al. 2011; Zhu et al. 2014). Therefore, sediment–water partitioning of PAHs is one of the dominant processes of controlling the fate of PAHs in the aquatic environment (Guo et al. 2009). The knowledge of sediment–water partitioning of PAHs would be important to develop water and sediment quality criteria, but the most studies focus on the concentration levels and pollution status of PAHs in aquatic environment (Wu et al. 2010), the information on partitioning of PAHs in sediment–water system is still limited.
Yinma River Basin spanning from 43°0′N to 45°0′N and from 124°30′E to 126°0′E, is located in the middle part of Jilin Province, China. Yinma River Basin with an area of 174,000 km2 provides domestic water with local residents and irrigates farmlands. However, in recent decades, with rapid development of urbanization, industrialization and agriculture, the pollution from industrial discharges, oil-related activities, vehicular emissions and agricultural runoffs, has increased greatly. These human activities are all possible sources of PAHs which may pose adverse effects on human body and aquatic organisms (Guo et al. 2009). Although the information on spatial distribution, partitioning and sources of PAHs in aquatic environment is important to water and sediment pollution control strategies and quality criteria, to our best knowledge, no or very few studies on this issue have been reported for this region.
Accordingly, the objectives of this study are to: (1) investigate occurrence and distribution characteristics of 16 PAHs in the water and sediment and evaluate the influences of sediment and water properties on PAH distribution, (2) illustrate partitioning of PAHs in sediment–water system, (3) identify primary sources of PAHs with the ratios of selected PAHs, and (4) assess environmental health risk of the exposure of 16 PAHs in the water and sediment.
Materials and methods
Study area and sample collection
Yinma River Basin (43°0′N–45°0′N, 124°30′E–126°0′E) located in Jilin Province of China is primary water source for domestic water supply with local residents and irrigation of farmlands in the region. In dry season, undiluted discharged industrial and domestic wastewater may induce severe contamination; therefore, dry season was selected as study period.
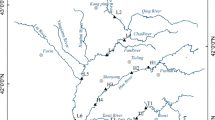
In November (dry season), 2014, 17 paired sets of water and sediment samples (as shown in Fig. 1) were collected. Six samples (YM 1 to YM 6) were collected from Yinma River. At the same time, two samples were, respectively, collected from Wukai River and Ganwuhai River to calculate contributions of PAH pollution to Yinma River. Shitou Koumen Reservoir is fed by Yinma River, Chalu River and Shuangyang River; three samples were, respectively, collected from YM 2, CL 2 and SY 2 to calculate the contributions of PAH pollution to the reservoir. Five samples (YT 1 to YT 5) were collected from Yitong River. A sample (YT 3) was collected from the state monitoring section which receives municipal sewage from Changchun City. Sample of YT 5 was collected from the state monitoring section receiving sewage from livestock farms.
Each sample was mixed by three subsamples collected from two sides and middle of sampling Sect. Twenty liters of each water samples was collected. The water samples were filtered with 0.45-μm glass fiber filters (baked at 450 °C for 4 h), stored in dark glasses, respectively, and analyzed within 24 h. Sediment samples were collected with a grab sampler and separately stored in dark glass jars. Sediment samples were freeze-dried, ground in a mortar and passed through a 100-mesh sieve.
Extraction methods
Sixteen PAHs recommended as priority pollutants by the United States Environmental Protection Agency (USEPA) were analyzed: naphthalene (Nap), acenaphthene (Ace), acenaphthylene (Acy), fluorene (Flo), phenanthrene (Phe), anthracene (Ant), fluoranthene (Fla), pyrene (Pyr), benz(a)anthracene (BaA), chrysene (Chr), benzo(b)fluoranthene (BbF), benzo(k)fluoranthene (BkF), benzo(a)pyrene (BaP), dibenz(a,h)anthracene (DahA), indeno(1,2,3-cd)pyrene (IcdP) and benzo(g,h,i)perylene (BghiP). All solvents for PAH analyses were of chromatographic grade.
Sixteen PAHs in water samples were extracted with a solid phase extraction (SPE) system (ASE-12, Tianjin Automatic Science Instrument Co., China). A filtered water sample (2 L) passed through a cartridge (Cleanert S C18-SPE, Agela Technologies Inc., USA) which was prewashed with 5 mL of dichloromethane, 5 mL of methanol and 5 mL of deionized water successively, at a constant rate of 6 mL/min. Then, the cartridge was eluted with 10 mL of dichloromethane and the elute was collected. The collected elute was reduced to nearly dryness with a rotary vacuum evaporation apparatus (RE-52AA, Shanghai Yarong Inc., China) and adjusted to about 1 mL with dichloromethane for gas chromatography (GC) analysis (Liu et al. 2014).
The sediment samples were all dry weight in the analyses. The extraction method was followed as a published method (Yang et al. 2014). Two grams of each sediment sample were mixed with anhydrous sodium sulfate (1:1/w:w) and then extracted in an ultrasonic bath with 20 mL of n-hexane/dichloromethane mixture (1:1 v:v) for 20 min (repeated 3 times). The extract was reduced to 1–2 mL with a rotary vacuum evaporation apparatus and passed through a silica/anhydrous sodium sulfate cartridge (Cleanert Florisil 1000 mg/6 mL cartridge, Agela Technologies Inc., the USA). The cartridge was eluted with 10 mL of n-hexane to remove non-PAH compounds and eluted with 30 mL of n-hexane/dichloromethane mixtures (1:1 v/v). The elutes containing PAHs were collected and concentrated to 1–2 mL for GC analysis.
Quantifications of PAHs
Quantifications of 16 PAHs in the water and sediment samples were performed on a GC equipped with a flame ionization detector (Clarus 680, PerkinElmer Inc., the USA). A 30 m × 0.25 mm i.d. HP-5 capillary column with a 0.25-μm film thickness was used to separate target compounds. The detector was set at temperature of 300 °C. Helium (99.999 %) was used as carrier gas and set at a constant flow of 1 mL/min. Pre-treated samples (1 μL) were injected in splitless mode under temperature of 250 °C. The column temperature was started at 80 °C and maintained for 1 min, then ramped to 255 °C at 15 °C/min and held for 1 min, after that increased to 265 °C at 2.5 °C/min and stayed for 1 min, finally raised to 300 °C at 2.5 °C/min and held for 5 min.
Analytical quality controls
All the analyses were subjected to rigorous quality assurance. Mixed standard solution of 16 PAHs (each at 100 μg/mL in dichloromethane) obtained from National Standard Material Center (China) was used to perform method validation and quality control. Blank and duplicate analyses were conducted for quality control. Quantification of 16 PAHs was performed by five-point external standard method, and the correlation coefficients for calibration curves were all above 0.998. Recovery efficiencies were checked by spiking blank samples with mixed standard solutions. The results of six parallel experiments for recovery efficiencies showed that recovery efficiencies for 16 PAHs in water samples were 70–110 % and relative standard deviations (RSDs) ranged from 4.2 to 13.6 %; recovery efficiencies for 16 PAHs in sediment samples were 65–125 %, and RSDs were 2.1–12.9 %. Detection limits for this method were 0.82–15.78 ng/L for water samples and 2.8–10.19 ng/g for sediment samples.
Other analyses
The water physicochemical parameters, pH and temperature, were recorded in situ during the survey. The values of pH were measured by digital pH meter (PHS-3C, Shanghai INESA Scientific Instrument Co., China). The contents of dissolved organic carbon (DOC) in water samples and total organic carbon (TOC) in sediment samples were measured by a total organic carbon analyzer (TOC-VCPN, Shimadzu Corp., Japan).
Results and discussion
Distribution of PAHs in water and sediment
PAH distribution characteristics
Seventeen paired sets of water and sediment samples collected from Yinma River Basin were analyzed for 16 PAHs. As shown in Table S1, 12 of 16 investigated PAHs were detected in water samples, 11 of 16 PAHs were detected in sediment samples. Eleven of sixteen PAHs were both found in water and sediment samples. Among 16 PAHs, Nap was the highest average concentration in water samples as well as in sediment samples, 67.2 ng/L and 825.06 ng/g, respectively, whereas BghiP was undetected in water samples nor in sediment samples. Coefficients of variation (C.V) for detectable PAHs in the water and sediment samples all exceeded 50 %, suggesting that anthropogenic activities involved in PAH pollution. It should be noted that BaP as the most concerned carcinogen PAH (Hu et al. 2007) was found in 41.2 % of water samples. The BaP concentrations beyond Environmental Quality Standard for Surface Water in China (2.8 ng/L, GB3838-2002) (MHPRC 2007) were found in Yitong River near Changchun City (YT 3) and Yinma River near Dehui City (YM 5) (Table S1 and Table S2).
To further elucidate PAH distribution, PAH compositional patterns were studied. Sixteen PAHs can be classified into three groups according to the number of rings, they are light (2–3 rings), 4-ring and heavy (5–6 rings) PAHs (Jiang et al. 2011). The proportions for PAH compositional patterns in water and sediment samples were found to be similar (Fig. 2a, b). Light PAHs was both dominant in water samples and sediment samples, accounting for 71.69 and 86.98 %, respectively. The dominance of light PAHs in the water may be due to their relatively high vapor pressure and water solubility (Sun et al. 2009).
Spatial distribution of PAHs in water and sediment
The concentrations of 16 total PAHs in the water and sediment at different sampling sites are, respectively, shown in Fig. 3a, b. The water in Yinma River Basin contained about 175 to 325 ng/L of PAHs, the relatively higher concentrations were found at YM 1, YM 2 and YM6, indicating the existence of potential pollution source for Yinma River, Shitou Koumen Reservoir and Secondary Songhua River. The PAH concentrations of water at YM 2 and SY 2 were around 325 and 250 ng/L, respectively, suggesting that the water in Shitou Koumen Reservoir might be contaminated, but the concentrations of PAHs in Shitou Koumen Reservoir was relatively lower (below 200 ng/L), this may be attributed to the role of constructed wetland in wastewater treatment. For Yitong River, PAH concentrations of water at all the sampling sites were relatively lower, ranging from about 175 to 200 ng/L, except YT 3 (about 325 ng/L). The municipal sewage discharged from Changchun City led to higher concentrations of PAHs at YT 3. The concentrations of PAHs in sediment ranged from around 1000 to 5750 ng/g. It was not surprisingly that the extremely high PAH concentration was found at YM 1, because the sample was collected from downstream of domestic sewage drain. However, except YM 1, the spatial variation of PAHs in sediment was not great, the PAH concentrations ranged from about 1000 to 2500 ng/g. The concentrations of PAHs in water had no relation to those in sediment; this may be due to disequilibrium partitioning between sediments and water.
Effects of water and sediment properties on PAH distribution
Pearson correlation analysis was applied to study relationship between PAH distribution and properties of water and sediment. The properties including temperature, pH, and DOC in the water and TOC in the sediments were studied.
The water properties including pH, and temperature have been frequently reported to influence behavior and fate of PAHs in the water (Maruya et al. 1996). As shown in Table 1, no significant correlations between pH and PAHs in water were observed. Sixteen PAHs, except BaP, in the water were not significantly correlated with temperature. DOC is generally considered as one of the most important factors controlling fate and behavior of PAHs in the water because the most PAHs are hydrophobic, low-soluble, and tend to bind onto dissolved organic matters (Tremblay et al. 2005), and TOC has been reported to have a strong correlation with PAHs in the sediment (Zhang et al. 2004). However, in this study, the results indicated that PAHs in water had a weak correlation with DOC, except Ant having a positive correlation with DOC (r = 0.672, p < 0.01), and PAHs in sediment showed a weak correlation with TOC except Acy (r = 0.645, p < 0.01) and Pyr (r = 0.493, p < 0.05). This results may be due to the interferences of various inorganic matrices such as clay minerals in the field samples, small number of samples and inaccurate field measurement (Shi et al. 2007).
Sediment–water partitioning of PAHs in the sediment–water system
The octanol/water partitioning coefficient (Kow) closely related to distribution coefficient can describe the fate of chemicals in the environment (Guo et al. 2009). The value of Kow for a chemical is higher, the tendency for chemical to partition to organic phase is greater (Chen et al. 2004). Organic carbon normalized partitioning coefficients (Koc) can evaluate a chemical’s relative tendency to adsorb onto solid phases and partitioning degree (Guo et al. 2009). To illustrate PAH partitioning in the sediment–water system, organic carbon normalized partition coefficients (Koc) were employed (Shi et al. 2007), and the values of Koc were calculated as Eq. (1) (Fairbairn et al. 2015):
where Cs (ng/g) and Cw (ng/L) are monitoring PAH concentrations, respectively, in the sediment and water, TOC (unitless) is organic carbon fraction in the sediment, K′oc(sed–w) (unitless) is equilibrium partitioning coefficient for sediment–water system (Sun et al. 2014).
The values of log K’oc for PAHs are presented in Table 1. The mean values of log K′oc ranging from 0.59 to 2.22, indicated that PAH partitioning behavior was various in different areas, which may be due to the differences in PAH distribution and contents of organic carbon in different areas (Qin et al. 2014). The mean values of log K′oc for PAHs were all lower than their corresponding values of log Koc and log Kow, suggesting that PAHs preferably tended to adsorb onto sediment, rather than exchanged with water phase. The differences between values of log’Koc and their corresponding values of log Koc ranged from 1.11 to 5.42, indicating that PAHs in the sediment–water system were in an unsteady state. The differences between log’Koc and log’Koc for PAHs with high molecular weights were relatively greater, suggesting their greater degree of unsteady state in the sediment–water system.
Identification of PAH sources
Molecular diagnostic ratios of isomeric PAHs can be applied to identify possible sources of PAHs as they equally distribute in the environment and dilute to a similar extent (Aziz et al. 2014). In order to identify the possible sources of PAHs in Yinma River Basin 1, molecular diagnostic ratios of isomeric PAHs were applied. The ratio of Ant/(Ant + Phe) is generally used to distinguish between petroleum and combustion sources. The ratio above 0.1 is considered as a dominant source of combustion while the ratio below 0.1 is considered as a dominant source of petroleum (Wang et al. 2009; Fang et al. 2007). The ratio of Fla/(Fla + Pyr) is generally applied to provide a clear pattern in PAH sources. The ratio below 0.4 is considered as petroleum input; the ratio between 0.4 and 0.5 is regarded as liquid fossil fuel combustion (vehicle and crude oil); the ratio above 0.5 is taken as combustion of grass, wood or coal (Zhang et al. 2006; Sany et al. 2014). The results of the ratios for Ant/(Ant + Phe) and Fla/(Fla + Pyr) are shown in Fig. 4.
The sources of PAHs are wide-spread (An et al. 2011); the possible sources of PAHs in study region were identified based on the reality. As shown in Fig. 4, PAHs in the water and sediment were both originated from a mixed source of petroleum and combustion. Petroleum sources are regarded as being originated from petro-chemical products; PAHs formed in combustion process are considered as being derived from industrial discharges (Guo et al. 2009). In Yinma River Basin, PAHs formed in combustion process were probably derived from sewage and emissions discharged by plants located in Changchun City, Dehui City and Jiutai City. The contamination of PAHs originated from unburned products might be due to oil-related activities in oil foundations located in Changchun City, and oil spills produced by fishery activities. Furthermore, according to the results of ratios for Fla/(Fla + Pyr), the sources of PAHs in the water in all the sampling sites, except YT 2 and YM 6, were originated from petroleum input. YT 2 and YM 6 were exposed to PAH contamination derived from vehicular emissions and crude oil. It could be concluded that possible sources of PAHs in the water were mainly derived from oil-related activities in oil foundations and oil spills produced by fishery activities, whereas the sources of PAHs in the sediment showed a different pattern. The sources of PAHs in sediment at all the sampling sites, except YM 3, were derived from grass, wood and coal combustion. Actually, local farmers burn agricultural wastes in October and November which result in PAH pollution in sediment. Also, coal combustion by industrial plants located in Yinma River Basin contributed to PAH contamination in sediment. The PAH contamination at YM 3 was originated from vehicular emissions and crude oil. According to the reality, vehicular emissions from roads around Shitou Koumen Reservoir and oil spills produced by fishery activities and cruise ships might result in PAH contamination in sediment at YM 3.
Environmental health risk assessment
Environmental health risk of PAHs in aquatic environment has often been calculated on the basis of BaP concentration as BaP is well-characterized toxicologically (Lang et al. 2012). In this study, BaP equivalents (EBaP) was employed to assess environmental health risk of PAHs in the water and sediment from Yinma River Basin, which was calculated by the following equation (Lv et al. 2014):
Figure 5a presents EBaP values for 16 PAHs in the water from Yinma River Basin. The EBaP values ranged from 0.3 to 28.13 ng/L with a mean value of 15.33 ng/L. For YM 5 and YT 4, the EBaP values were greater than Environmental Quality Standard for Surface Water in China (2.8 ng/L, GB3838-2002) (Ministry of Health of the People’s Republic of China (MHPRC) 2007), suggesting the existence of environmental health risk in YM 5 and YT 4. According to the results of source identification, YM 5 and YT 4 were exposed to PAH contamination derived from oil-related activities and oil spills. Therefore, efforts from local authorities should be taken to control oil-related activities and oil spills for reducing PAH concentrations in the water.
As represented in Fig. 5b, EBaP values for PAHs in sediment ranged from 33.14 to 190.71 ng/g with a mean value of 85.51 ng/g. The highest EBaP value was found at CL 2, which is 1.5 times higher than the second highest one. The second highest EBaP values were found at YM 2 and WK 1, both about 120 ng/g. Based on the results of source identification, burning agricultural wastes by local farmers and coal combustion by industrial plants were identified as the primary sources of PAHs in sediment of those areas; therefore, local authorities should take steps to control these activities for reducing discharges of PAHs into river environment.
Conclusions
In this work, 16 priority PAHs were analyzed in 17 paired sets of water and sediment samples collected from Yinma River Basin. For 16 individual PAHs, Nap was the highest average concentration in water and sediment samples, 67.2 ng/L and 825.06 ng/g, respectively, whereas BghiP undetected in any water samples nor in sediment samples was the lowest. Among three PAH compositional patterns, light PAHs were both dominant in the water and sediments, accounting for 71.69 and 86.98 %, respectively. PAH partitioning in the sediment–water system was studied; PAHs in the sediment–water system were in an unsteady state and preferably tended to adsorb onto the sediment. The primary sources of PAHs in the water were identified as oil-related activities; the sources of PAHs in the sediment were identified as combustion of agricultural wastes and coal. The results of EBaP suggested the existence of environmental health risk in some sites; local authorities should take efforts to reduce discharge of PAHs into river environment.
References
An, T. C., Qiao, M., Li, G. Y., Sun, H. W., Zeng, X. Y., & Fu, J. M. (2011). Distribution, sources, and potential toxicological significance of PAHs in drinking water resources within the Pearl River Delta. Journal of Environmental Monitoring, 13(5), 1457–1463.
Aziz, F., Syed, J. H., Malik, R. N., Katsoyiannis, A., Mahmood, A., Li, J., et al. (2014). Occurrence of polycyclic aromatic hydrocarbons in the Soan River, Pakistan: Insights into distribution, composition, sources and ecological risk assessment. Ecotoxicology and Environmental Safety, 109, 77–80.
Bai, Y. W., Meng, W., Xu, J., Zhang, Y., Guo, C. S., Lv, J. P., & Wan, J. (2014). Occurrence, distribution, environmental risk assessment and source apportionment of polycyclic aromatic hydrocarbons (PAHs) in water and sediments of the Liaohe River Basin, China. Bulletin of Environmental Contamination and Toxicology, 93, 744–747.
Chen, B. L., Xuan, X. D., Zhu, L. Z., Wang, J., Gao, Y. Z., Yang, K., et al. (2004). Distributions of polycyclic aromatic hydrocarbons in surface waters, sediments and soils of Hangzhou City, China. Water Research, 38, 3559–3565.
Deng, H. M., Peng, P. A., Huang, W. L., & Song, J. Z. (2006). Distribution and loadings of polycyclic aromatic hydrocarbons in the Xijiang River in Guangdong, South China. Chemosphere, 64, 1402–1408.
Fairbairn, D. J., Karpuzcu, M. E., Arnold, W. A., Barber, B. L., Kaufenberg, E. F., Koskinen, W. C., et al. (2015). Sediment–water distribution of contaminants of emerging concern in a mixed use watershed. Science of the Total Environment, 505, 897–901.
Fang, M. D., Hsieh, P. C., Ko, F. C., Baker, J. E., & Lee, C. L. (2007). Sources and distribution of polycyclic aromatic hydrocarbons in the sediments of Kaoping river and submarine canyon system, Taiwan. Marine Pollution Bulletin, 54, 1184–1187.
Gao, P., Feng, Y. J., Zhang, Z. H., Liu, J. F., & Ren, N. Q. (2011). Comparison of competitive and synergetic adsorption of three phenolic compounds on river sediment. Environmental Pollution, 159, 2876–2879.
Guo, W., He, M. C., Yang, Z. F., Lin, C. Y., Quan, X. C., & Men, B. (2009). Distribution, partitioning and sources of polycyclic aromatic hydrocarbons in Daliao River water system in dry season, China. Journal of Hazardous Materials, 164, 1379–1380.
Harris, K. A., Yunker, M. B., Dangerfield, N., & Ross, P. S. (2011). Sediment-associated aliphatic and aromatic hydrocarbons in coastal British Columbia, Canada: Concentrations, composition, and associated risks to protected sea otters. Environmental Pollution, 159, 2666–2670.
Hu, Y. D., Bai, Z. P., Zhang, L. W., Wang, X., Zhang, L., & Yu, Q. C. (2007). Health risk assessment for traffic policemen exposed to polycyclic aromatic hydrocarbons (PAHs) in Tianjin, China. Science of the Total Environment, 382, 240–250.
Jiang, Y. F., Wang, X. T., Wu, M. H., Sheng, G. Y., & Fu, J. M. (2011). Contamination, source identification, and risk assessment of polycyclic aromatic hydrocarbons in agricultural soil of Shanghai, China. Environmental Monitoring and Assessment, 183, 142–144.
Lang, Y. H., Wang, N. N., Gao, H. W., & Bai, J. (2012). Distribution and risk assessment of polycyclic aromatic hydrocarbons (PAHs) from Liaohe estuarine wetland soils. Environmental Monitoring and Assessment, 184, 5547–5551.
Liu, F., Liu, Y., Jiang, D. S., Zhang, R. F., Cui, Y. B., & Li, M. (2014). Health risk assessment of semivolatile organic pollutants in Lhasa River China. Ecotoxicology, 23, 567–572.
Lv, J. P., Xu, J., Guo, C. S., Zhang, Y., Bai, Y. W., & Meng, W. (2014). Spatial and temporal distribution of polycyclic aromatic hydrocarbons (PAHs) in surface water from Liaohe River Basin, northeast China. Environmental Science and Pollution Research, 21, 7090–7094.
Ma, W. L., Liu, L. Y., Qi, H., Zhang, Z. F., Song, W. W., Shen, J. M., et al. (2013). Polycyclic aromatic hydrocarbons in water, sediment and soil of the Songhua River Basin, China. Environmental Monitoring and Assessment, 185, 8399–8404.
Maruya, K. A., Risebrough, R. W., & Home, A. J. (1996). Partitioning of polycyclic aromatic hydrocarbons between sediments from San Francisco Bay and their porewater. Environmental Science and Technology, 30, 2942–2947.
Ministry of Health of the People’s Republic of China (MHPRC). (2007). Annual report of China health statistics. http://www.moh.gov.cn/publicfiles/business/htmlfiles/zwgkzt/ptjnj/200807/37168.htm.
Qin, N., He, W., Kong, X. Z., Liu, W. X., He, Q. S., Yang, B., et al. (2014). Distribution, partitioning and sources of polycyclic aromatic hydrocarbons in the water–SPM–sediment systemof Lake Chaohu, China. Science of the Total Environment, 496, 414–419.
Sakulthaew, C., Comfort, S., Chokejaroenrat, C., Harris, C., & Li, X. (2014). A combined chemical and biological approach to transforming and mineralizing PAHs in runoff water. Chemosphere, 117, 2–7.
Sany, S. B. T., Hashim, R., Salleh, A., Safari, O., Mehdinia, A., & Rezayi, M. (2014). Risk assessment of polycyclic aromatic hydrocarbons in the West Port semi-enclosed basin (Malaysia). Environmental Earth Sciences, 71, 4323–4328.
Sereshk, Z. H., & Bakhtiari, A. R. (2014). Distribution patterns of PAHs in different tissues of annulated sea snake (Hydrophis cyanocinctus) and short sea snake (Lapemis curtus) from the Hara Protected Areaon the North Coast of the Persian Gulf, Iran. Ecotoxicology and Environmental Safety, 109, 117–120.
Shi, Z., Tao, S., Pan, B., Liu, W. X., & Shen, W. R. (2007). Partitioning and source diagnostics of polycyclic aromatic hydrocarbons in rivers in Tianjin, China. Environmental Pollution, 146, 494–498.
Sun, H. W., An, T. C., Li, G. Y., Qiao, M., & Wei, D. B. (2014). Distribution, possible sources, and health risk assessment of SVOC pollution in small streams in Pearl River Delta. Environmental Science and Pollution Research, 21, 10083–10095.
Sun, J. H., Wang, J. H., Chai, Y., Zhang, G., Li, J., & Feng, J. L. (2009). Distribution of polycyclic aromatic hydrocarbons (PAHs) in Henan Reach of the Yellow River, Middle China. Ecotoxicology and Environmental Safety, 72, 1616–1621.
Tremblay, L., Kohl, S. D., Rice, J. A., & Gagne, J. P. (2005). Effects of temperature, salinity, and dissolved humic substances on the sorption of polycyclic aromatic hydrocarbons to estuarine particles. Marine Chemistry, 96, 21–34.
Wang, L. L., Yang, Z. F., Niu, J. F., & Wang, J. Y. (2009). Characterization, ecological risk assessment and source diagnostics of polycyclic aromatic hydrocarbons in water column of the Yellow River Delta, one of the most plenty biodiversity zones in the world. Journal of Hazardous Materials, 169, 462–463.
Wu, B., Zhang, Y., Zhang, X. X., & Cheng, S. P. (2010). Health risk from exposure of organic pollutants through drinking water consumption in Nanjing, China. Bulletin of Environmental Contamination and Toxicology, 84, 46–48.
Yang, W., Lang, Y. H., & Li, G. L. (2014). Cancer risk of polycyclic aromatic hydrocarbons (PAHs) in the soils from Jiaozhou Bay wetland. Chemosphere, 112, 291–293.
Yuan, Z. J., Liu, G. J., Wang, R. W., & Da, C. N. (2014). Polycyclic aromatic hydrocarbons in sediments from the Old Yellow River Estuary, China: Occurrence, sources, characterization and correlation with the relocation history of the Yellow River. Ecotoxicology and Environmental Safety, 109, 169–174.
Zhang, J., Cai, L. Z., Yuan, D. X., & Chen, M. (2004). Distribution and sources of polynuclear aromatic hydrocarbons in Mangrove surficial sediments of Deep Bay, China. Marine Pollution Bulletin, 49, 479–486.
Zhang, H. B., Luo, Y. M., Wong, M. H., Zhao, Q. G., & Zhang, G. L. (2006). Distributions and Concentrations of PAHs in Hong Kong Soils. Environmental Pollution, 141, 108–112.
Zhang, G. J., Zang, X. H., Li, Z., Wang, C., & Wang, Z. (2014). Polydimethylsiloxane/metal-organic frameworks coated fiber for solid-phasemicroextraction of polycyclic aromatic hydrocarbons in river and lake water samples. Talanta, 129, 600–605.
Zhu, H. W., Cheng, P. D., Zhong, B. C., & Wang, D. Z. (2014). The mechanisms of contaminants release due to incipient motion at sediment–water interface. Physics, Mechanics & Astronomy, 8(57), 1563–1568.
Acknowledgments
This research was funded by the National Major Program of Water Pollution Control and Treatment Technology of China (2014ZX07201011) and the National Natural Science Foundation of China (No. 41371495).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All the authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sun, C., Zhang, J., Ma, Q. et al. Polycyclic aromatic hydrocarbons (PAHs) in water and sediment from a river basin: sediment–water partitioning, source identification and environmental health risk assessment. Environ Geochem Health 39, 63–74 (2017). https://doi.org/10.1007/s10653-016-9807-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-016-9807-3