Abstract
The sequential extraction procedure of the European Community Bureau of Reference (BCR) was applied for the fractionation of Cd, Cr, Cu, Ni, Pb, Zn, Fe, Mn, and V in the Serbian river sediments. The aim of this paper was to describe a new approach in detection of anthropogenic elements in sediments, related to the Serbian river courses in order to assess their metal contamination. For sediment pollution evaluation, the risk assessment code (RAC) and the secondary phase enrichment factor (KSPEF) were used. Metal fractionation showed more easily mobilized forms predominant for copper, zinc, cadmium, manganese, and lead, which can be one of the indicators for anthropogenic source input. Chromium, nickel, iron, and vanadium found in the residual fraction indicate these metals may be an indicator for natural sources input. Based on RAC classification, results of sediments show no risk (Cr and V), low risk (Ni, Pb, and Fe), medium risk (Cu), high risk (Cd and Zn), and very high risk (Mn). The mean values of KSPEF were Cd > Cu > Zn > Ni > Mn > Pb > Fe > Cr > V, decreasing scale of no to moderately severe enrichment. The sediments were found to be contaminated by heavy metals to various extents, mostly Cd, Cu, and Zn. Research has shown the importance of KSPEF in quantifying degree of metal enrichment in sediments using results of sequential extraction. With the application of this factor, which is not frequently used in the scientific literature, the results obtained with sequential extraction can be used not only for assessment of mobility but also to quantify the metal pollution.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metal contamination is a worldwide spread environmental problem for many years now. The importance of the heavy metals studying is due to their toxicity, durability, non-degradability in the environment (Davutluoglu et al. 2011) and rapid accumulation by living organisms (Copaja et al. 2014). The aquatic sediments are characterized by their extreme complexity and diversity. Their capacities for bonding with heavy metals can be related to the parent materials and climatic conditions. After being transported into aquatic ecosystems, heavy metals can be absorbed by suspended solids and accumulated in sediments.
The reactivity or mobility of heavy metals in sediments, and thus their potential toxicity, depends on phase in which they occur in, and to which chemical and physical processes these phases are subjected to (Rao et al. 2010). Said phases are also referred to as fractions, and they include the following wide-range categories: water-soluble, exchangeable, specifically adsorbed, carbonate, Fe and Mn oxides, organic matter, sulfides, and silicates. All of these fractions may occur in a variety of structural forms (Favas et al. 2014).
From an environmental standpoint, it is important to determine under which conditions heavy metals could be released from sediments. A variety of sorption- and desorption-based procedures have been reported, using various extractants and experimental conditions (Davutluoglu et al. 2011). Sequential extraction is an important and widely applied tool that has provided considerable insights into the environmental behavior of potentially toxic elements (Sutherland 2010; Canuto et al. 2013). This was originally designed for the selective removal of trace elements bound to operationally defined sediment fractions. Although sequential extractions procedures are not specific to the fullest extent, they may provide comparative information on trace metal mobility in soils with changing environmental conditions, such as pH or redox potential, and may help to evaluate the relative contribution of mixed pollution sources to said mobility (Pueyo et al. 2008).
A few studies have determined metals in sediments using different sequential extraction procedures (Kartal et al. 2006; de Andrade Passos et al. 2010; Wang et al. 2010; Davutluoglu et al. 2011; Sakan and Ðordevic 2012, 2013; Alvarez et al. 2014; Fathollahzadeh et al. 2014; Favas et al. 2014). The three-stage sequential extraction procedure of the European Community Bureau of Reference (BCR) was developed in an attempt to standardize the various schemes (de Andrade Passos et al. 2010). This method has also been widely accepted and applied to metal fractionation in different environmental samples (Kartal et al. 2006; de Andrade Passos et al. 2010; Davutluoglu et al. 2011; Fathollahzadeh et al. 2014). Based on the results obtained from sequential extraction, in some studies, metal pollution assessment was made by using risk assessment codes (RAC) (de Andrade Passos et al. 2010; Wang et al. 2010; Favas et al. 2014) and secondary phase enrichment factor (KSPEF) (Cai et al. 2011; Yang et al. 2012). These indices provide useful information for identification of the most severe pollutant and level of sediment contamination.
In order to assess the general pollution of Serbian river sediment, the heavy metal contamination and the associated ecological risk in this region were determined by using: (1) the BCR sequential extraction procedure, (2) the sediment quality guidelines, (3) determination by the risk assessment code, (4) calculation of the secondary phase enrichment factor, and (5) appliance statistical analysis (Q-mode and R-mode of cluster analysis). The investigations that were the subject of this study include sediments from the international rivers the Tisa, the Sava, and the Danube, and their tributaries. As shown in paper Teodorovic (2009), as a signatory party of The Danube River Protection Convention and a contracting party of International Commission for the Protection of the Danube River (ICPDR) since August 2003, Serbia has been committed to implementation of the Danube Convention, the objective of which, among others, includes the control of accidental hazards involving hazardous substances and implementation of measures for reduction in the Danube River Basin pollution shares entering the Black Sea. Since ICPDR (2005) assuming the mandate for implementation of the European Union (EU) Water Framework Directive (WFD), although still not an EU Member State, Serbia has been undertaking measures to contribute to fulfillment of the WFD main objective—good ecological status of all European rivers by 2015. Cd, Pb, Ni, Cu, Zn, and Cr, quoted in the List of Priority Substances for the Danube River basin, are included in this research. In the previous surveys of the river sediments in Serbia, only the total content of heavy metal pollution was determined (Sakan et al. 2011, 2014). Although the determination of metal fraction that might be bioavailable was recommended for the identification of state of the sediments and river pollution (Sakan et al. 2014), until now there was no adequate research conducted. In this paper, the chemical fractionation is applied on studied sediments, and for the first time, RAC and KSPEF in this system are used for the determination of the sediment pollution.
Materials and methods
Study area and sampling localities
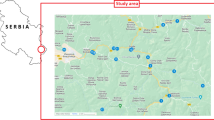
For this survey, sediments were selected from the main rivers in Serbia. Thirty-five river sediment samples from 14 rivers and one canal in Serbia were taken during the year 2008: the Danube, the Sava, the Tisa, the Ibar, the Great Morava, the West Morava, the South Morava, the Nišava, the Tamiš, the DTD canal, the Topčiderska River, the Porečka River, the Kolubara, the Pek, and the Toplica. On larger rivers, sampling was conducted at several locations (Fig. 1). The contents of the elements were determined in the granulometric fraction <63 μm of the bottom sediment samples (Sakan et al. 2014). Many studies have been carried out using this sediment fraction. A major advantage in using the <63 μm size fraction is the greater concentrations of trace elements bonded with silt/clay particles.
The sediment samples were collected by using a grab sampler from the specified sampling stations. The sampling was conducted using Van Veen grab sampler, designed to collect an accurate representative sample of the sediment. Equal volumes of sediment from nearby locations (five subsamples) were mixed for each site in order to make a composite sample to expand the area of each site represented by the sample. Immediately after sampling, the sediment was transported to the laboratory in a portable cooler. Samples were stored at 4 °C in order to prevent changes in the chemical composition of the sediments. The contents of the micro- and macro-elements in sediments were determined after air-drying for 8 days.
Sequential extraction of heavy metals
Sequential extraction was performed using the optimized BCR procedure (de Andrade Passos et al. 2010; Sutherland 2010). A description of this procedure is given below, and it is as follows:
Step 1 (fraction soluble in acid—metals exchangeable or associated with carbonates): Each 1 g of sample was added 40 mL of a 0.11 mol L−1 acetic acid solution, with agitation for 16 h at 22 °C. The extract was separated from the solid phase by centrifugation at 3000 rpm for 20 min, and the supernatant stored for later analysis.
Step 2 (reducible fraction—metals associated with oxides of Fe and Mn): Residue from stage 1 was added 40 mL of a 0.5 mol L−1 acid hydroxyl ammonium chloride solution (pH 1.5). The suspension was then agitated for 16 h at 22 °C. The extract was separated from the solid phase by centrifugation, as described for stage 1.
Step 3 (oxidizable fraction—metals associated with organic matter and sulfides): Residue from stage 2 was added 10 mL of 8.8 mol L−1 H2O2 solution (pH 2–3), and the mixture left at room temperature for 1 h. It was then heated to 85 °C for 1 h in a water bath, and then the volume was reduced to 2–3 mL by the further heating in a water bath. Another 10 mL portion of 8.8 mol L−1 H2O2 solution was added, and the mixture heated to dryness at 85 °C for 1 h. After cooling, 50 mL of a 1.0 mol L−1 solution of ammonium acetate (pH 2) was added to the residue, followed by agitation for 16 h at 22 °C. The extract was separated from the solid phase by centrifugation, as above.
After the first three extraction steps, extract was separated from solid residue by centrifugation at 3000×g for 10 min, decant supernatant, diluted to 50 mL with 1 M HNO3, and stored in a polyethylene bottle at 4 °C until metal analysis. The residue was washed with 20 mL deionized water and shaken 15 min, followed by centrifugation for 10 min at 3000×g. The supernatant was decanted and discarded, acting cautiously not to discard any solid residues. In this way, the residue was prepared for the next BCR step (Sutherland 2010; Relić et al. 2013).
Step 4 (residual fraction–metals strongly associated with the crystalline structure of minerals): The stage 3 residue was digested using a mixture of the acids (8 mL aqua regia, 3:1, v/v, HCl–HNO3) on water bath for 1 h at 85 °C, until the volume was reduced to 2–3 mL. Another 8 mL portion of aqua regia was added, and the mixture heated to dryness at 85 °C for 1 h. The final residue was dissolved in 1 M HNO3 and diluted to 50 mL and stored in a polyethylene bottle at 4 °C until metal analysis. Digestion of the residual material is not specified in the BCR protocol.
Determination of element concentrations and quality control
Element concentrations in solutions obtained at each step were determined using an atomic emission spectrometer with an inductively coupled plasma iCAP-6500 Duo (Thermo Scientific, United Kingdom). The analytical data quality was controlled by using laboratory quality assurance and quality control methods, including the use of standard operating procedures, calibration with standards, and analysis of both reagent blanks and replicates (Sakan et al. 2015). The blank solutions were prepared in the same way as samples during the extraction procedure. Used wavelengths (nm) were as follows: Cd 214.4, Cr 266.6, Pb 220.3, Mn 257.6, Ni 231.6, Zn 213.8, Cu 217.8, V 290.8, and Fe 261.1. Sediment data in this study are reported on a dry weight mg kg−1 basis.
Acceptable accuracy and precision of metals were achieved for all steps of sequential extraction. The accuracy of the obtained results was checked by analyzing sediment reference material (BCR-701) for three-step sequential extraction. Accuracy was determined by comparing the measured concentration with the certified value and then expressed in percentage. The percentage recovery of each element was determined as:
Table 1 shows the recovery rates for the heavy metals in the standard reference material BCR-701. The average recovery values for heavy metals in the standard reference material were in the range of 87.7–107.5 %.
Duplicate samples were used to measure the precision of the method. In this research, duplicate analysis was performed for seven sediment samples for three-step sequential extraction. The precision is expressed as the relative standard deviations. The relative standard deviations of the means of duplicate measurement were less than 10 % (from 0.4 to 6.9 %).
Risk assessment code (RAC)
To estimate the environmental hazard due to metal concentrations in sampling areas, the sediment samples were classified according to a risk assessment code (RAC), based on the strength of the bond between the metals and the different geochemical fractions in the sediments. The RAC is defined by taking the ratio of the sum of exchangeable and carbonic fractions (fraction 1) to the total concentration of elements in sediments and is expressed as a percentage (Favas et al. 2014). The RAC (Table 2) was determined based on the metal content that was found in the first fraction where binding is weak and the metals pose greater risk to the environment (de Andrade Passos et al. 2010).
Secondary phase enrichment factor (KSPEF)
The KSPEF was developed to evaluate the heavy metal contamination using chemical form data usually obtained by sequential extraction analysis (Cai et al. 2011; Yang et al. 2012). The KSPEF is calculated according to the following equation:
where labile fraction is the sum of concentrations for a given element for fractions 1–3 extracted using BCR sequential extraction procedure, and the residual component, environmentally inert (fraction 4). The enrichment degree of heavy metals can be classified into the following categories: no enrichment: KSPEF ≤ 1; minor enrichment: 1 < KSPEF ≤ 3; moderate enrichment: 3 < KSPEF ≤ 5; moderately severe enrichment: 5 < KSPEF ≤ 10; and severe enrichment: KSPEF > 10. The labile/residual ratio for elements content in the DTD canal (station in Vrbas) was chosen as the reference values in this research. The element contents in the DTD canal (station in Vrbas) were chosen as the background values for elements in this research because there are no significant anthropogenic sources of toxic elements at this locality and the sediment samples are similar to the other investigated river sediments in geochemical characteristics and composition (Sakan et al. 2014).
Statistical analysis
The statistical analyses performed in this study include cluster analysis (CA) and correlation analysis (CrA). The purpose of cluster analysis is to identify groups or clusters of similar sites on the basis of similarities within a class and dissimilarities between different classes (Chabuhdhara and Nema 2012). Cluster analysis can be run in the Q-mode, when clusters of samples are sought, or in the R-mode, when clusters of variables are desired. The hierarchical clustering joining of the most similar observations, and successively of the next most similar observations, was employed in the presented research. Pearson’s correlation coefficient was used to determine the interrelationship between heavy metals and their KSPEF values. The statistical analyses were performed using IBM SPSS Statistics—Statistical Package for the Social Sciences (SPSS) (version 21.0).
Results and discussion
Total content and fractionation of studied elements
Distributions of element contents by fractions are given in Table 3. The results are presented in the form of arithmetic mean ± standard deviation and range for each extraction fraction. The percentage of extracted metal was calculated from the ratio between the element concentration in each fraction and the sum of concentrations in all fractions. The results are shown in Fig. 2.
Distribution of element content by fractions (percentage distribution, calculated as the average content of the extracted element in each fraction with respect to the total content of the extracted element). Explanation: step 1 fraction soluble in acid—metals exchangeable or associated with carbonates, step 2 reducible fraction—metals associated with oxides of Fe and Mn, step 3 oxidizable fraction—metals associated with organic matter and sulfides, and step 4 residual fraction
The results of the fractionation suggest the following ranking in order of decreasing metal mobility in the exchangeable/acid-soluble fraction: Mn > Cd > Zn > Cu > Ni > Pb > Fe = Cr > V. This fraction is the most labile and generally considered as representative of the bioavailable portion of the metals. The exchangeable fraction of Mn, Cd, and Zn was rather high, implying that these metals were mobile in the environment. There was possibility that significant part of Mn2+ in sediments precipitates as MnCO3 (Gharibreza et al. 2013). The high percentage of Mn, Cd, and Zn in weakly bound fractions indicate that the considerable amounts of these elements may be released into water following non-exchange process and dissociation of Mn carbonate phase if basins are to become more acid (Copaja et al. 2014). Similar distribution of Cd and Zn was observed in urban river sediments (Pearl River Delta) (Cai et al.2011). Lead, zinc, and copper were primarily associated with Fe–Mn oxides, and this is conformance with findings of several studies (Copaja et al. 2014; Favas et al. 2014). These elements may be released from sediment, if there is a change of oxidation state of Fe and Mn and thus may pose a long-term source of contamination. Fractionation of Cr, Fe, V, and Ni showed that the major portion of these elements was in the residual fraction. The elements are retained within the crystal lattice of minerals and in well-crystallized oxides, and could be used as an indicator of natural sources input (Favas et al. 2014).
Supplementary Figure 1 displays the chemical fractionation of studied elements in different river sediments. In most rivers, the elements showed similar fraction distribution.
An assessment of metal pollution levels in studied sediments is made by comparing obtained values of metal contents with the freshwater sediment’s Quality Guidelines published by Republic of Serbia (1994), Environment Canada (2002), and Ingersoll and MacDonald (2002). The results of a comparison of the total metal contents (defined as the sum of the four steps, Table 3) in the studied river sediments with the Quality Guidelines for freshwater sediments (Table 4) published by CSST—Consensus-based sediment quality guidelines recommendations for use and application developed (Ingersoll and MacDonald 2002) and Environment Canada (2002), show that at some localities, contents of Cd, Cu, Pb, Ni, Zn, and Mn exceeded the criteria PEL (probable effect level) and PECs (probable effect concentrations), above which harmful effects are likely to be observed. The probable effect level (PEL) represents the lowest limit range of chemical concentrations that are usually or always associated with adverse biological effects. The PECs are SQGs (Summary of existing Canadian Sediment Quality Guidelines—freshwater sediment; Environment Canada 2002) were established as concentrations of chemicals in sediment above which adverse biological effects are likely to occur (Ingersoll and MacDonald 2002). Content of Cd, Cu, Ni, Pb, and Zn exceeds the MAQ values (maximum allowed quantity) proposed by soil standards for Serbia (Republic of Serbia 1994), which indicated that the most of the sediments were with heavy metal contamination.
The observation that the content of heavy metals varies depending on location (Supplementary Figure 1) can be attributed to the variability in terrestrial inputs, hydrodynamic process, and deposition conditions (Yang et al. 2012). These results indicate the existence of anthropogenic sources of these elements in the studied area. In relation to total element content, Supplementary Figure 1 shows similar levels through the basins for Fe and V only, indicating lithogenic characteristics for these elements.
Risk assessment code
In the presented investigation, the risk assessment code has been used to evaluate the risk of heavy metals content in surface river sediments. The results of the risk assessment codes, with values given as percentages of the F1 fraction are illustrated in Table 5. In general, the sediments showed no to very high risk for all metals. The code as applied to the present study revealed that 7–70 % of Cd, 0–3 % Cr, 1–40 % Cu, 0–5 % Fe, 30–81 % Mn, 0–31 % Ni, 0–12 % Pb, 0–3 % V, and 8–54 % Zn were present in exchangeable fractions. Cd showed low–very high risk; Cr, no–low risk; Cu, no–high risk; Fe, no–low risk; Mn, medium–very high risk; Ni, no–medium risk; Pb, no–medium risk; V, no–low risk; and Zn, low–very high risk to local environment. The more easily mobile and bioavailable forms were predominant for copper, zinc, cadmium, manganese, and lead. However, the hazard assessment based on those elements suggested that a potentially dangerous situation in this area can derive mainly from Cd, Zn, and Cu, due to their toxicity. Therefore, a significant remediation must be applied for Cd, Zn, and Cu mobilization at the earliest as they might enter in the food chain and pose threat in the study area. This is important because these weakly bonded elements could equilibrate with the aqueous phase and thus become more rapidly bioavailable.
Secondary phase enrichment factor (KSPEF)
The secondary phase enrichment factor could reveal more information about the identification of the most common pollutant and degree of contamination. The KSPEF values for studied elements are presented in Supplementary Figure 2. The obtained results revealed significant differences in distribution among different metals and rivers. The maximum value was in the river Pek (for Cu, 27.0), and the minimum was in sediment from the Danube (for V, 0.5). The KSPEF values for elements were in the order: Cd (no–severe enrichment) > Cu (minor–severe enrichment) > Zn (no–severe enrichment) > Ni (no–severe enrichment) > Mn (no–moderately severe) > Pb (no–moderate enrichment) > Fe (no–minor enrichment) > Cr (no–minor enrichment) > V (no–minor enrichment).
Correlation analysis is performed between heavy metals and their KSPEF values. Based on results, the significantly high positive correlation was observed for Cd (r = 0.63), Cu (r = 0.92), Mn (r = 0.73), Pb (r = 0.68), and Zn (r = 0.91) (correlation is significant at p < 0.01 level). Positive correlation was also observed for Fe (r = 0.36) and V (r = 0.42), but values of Pearson correlation coefficients are lower and correlation is significant at p < 0.05 level for these elements. These positive correlations point to fact that Fe and V may have mixed origin (anthropogenic and natural); i.e., its contribution can be from industrial or urban wastewater discharges or from parent rocks. Negative correlation is observed for Ni (r = −0.34, at p < 0.05 level), which indicates that Ni in studied sediments mainly derived from natural sources. The absence of correlation between Cr content and its KSPEF suggested that the increased content of Cr at some localities may be due to geochemical enrichment, but also may be due to the existence of anthropogenic sources of this element at some localities.
Figure 3 shows variation of KSPEF in the studied sediments using the box plot. For Cd, Cu, and Zn, the mean value is slightly elevated and has a wide distribution of coefficients, while for others had equal distribution, which suggests a slight increase in Cd, Cu, and Zn contamination. Outlier and extreme values were observed in the Tisa (Cd, Mn, and Ni), the Ibar (Cd and Zn), the West Morava (Cu), the Pek (Cu, Ni, and Zn), and the Nišava (Ni), suggesting that these are the most critical sites of metal contamination. Pollution of said rivers is mainly caused by permanent and accidental pollution from industrial plants and mines located in the basins of these rivers: Trepca mining complex—Pb and Zn (Ibar), mining complex in Majdanpek—Cu (Pek) and other basins, agricultural products, mainly due to the use of manganese-containing products, such as fertilizer and fungicide.
Cluster analysis (CA)
Cluster analysis (Pearson method) was performed to group the stations based on similarity in the F1 fractions (environmentally risky fraction) of the heavy metals (Figs. 4 and 5). Two different cluster analyses were done: R-mode and Q-mode clustering.
R-mode cluster analysis revealed three distinct clusters (Fig. 4). Cd, Zn, and Pb have good similarity and are clustered in one group; Fe, Ni, Cu, Cr, and Mn are clustered into another group, while V is individual. Strong similarity between Cd, Zn, and Pb showed that bond of these metals comes from the same origin. Since these elements are mainly pollution indicators, strong similarity of Cd, Zn, and Pb indicated strong anthropogenic input. Fe, Ni, Cu, Cr, and Mn are mainly derived from lithogenous source. Iron and manganese oxides have been well recognized for their abilities to adsorb and enrich other metallic elements such as Cr, Cu, and Ni. They play an important role to the extent that their deposition occurred in the bed sediments (Gharibreza et al. 2013). In general, these groups may be an indication of common sources of these metals and their similar geochemical characteristic. Explanation for V in the third group may be its origin from different sources and its chemical behavior in sediments. V is mainly associated with Fe (hydr)oxides, clay minerals, and organic matter, and it can also occur as discrete mineral phases such as carnotite [K2(UO2)2(VO4)2·3H2O] and vanadinite [Pb5(VO4)3Cl] (Cappuyns and Slabbinck 2012). There was a possibility that the role of clay minerals as well as organic acids may be more significant for vanadium bonding than the V fraction adsorbed by Fe oxides.
Q-mode cluster analysis (Fig. 5) revealed three different groups depending on the enrichment of elements in the first fraction. The sites in groups have similar features and anthropogenic/natural background source types (Sundray et al. 2011). Group I included the less contaminated sites, which represent the regions with medium level pollutes. Group II and III may be characterized by anthropogenic impacts. The pollution in those stations arises from the effluent discharge of the industrial facilities and factories, as well as mining activities.
Conclusion
Heavy metal pollution impact on the quality of the river sediments in Serbia was evaluated using the BCR sequential extraction procedure, comparison of the metal contents with the sediment quality guidelines, determination by the risk assessment code, and calculation of the secondary phase enrichment factor. The fractionation studies indicate that chromium, nickel, vanadium, and iron were mainly present in the inert fraction, and their concentration can be taken as a measure of the contribution by natural sources. The high mobility of Mn, Cu, Zn, Pb, and Cd in sediments is based on their origin from human activity. These elements could be readily available to aquatic organisms and may be a potential threat to water environment safety. The results of sequential extraction were corroborated by the results of risk assessment for selected elements and suggest that high mobility of Cu, Zn, Pb, and Cd in river sediments may be an indicator for the existence of significant anthropogenic sources of these elements.
This study suggests that the metal contamination cannot be simply evaluated by examining metal concentrations alone. A complementary approach that integrates sediment standard criteria and applies different methods for metal determination in sediment should be considered in order to provide a more accurate appraisal of the outcome and transport of metals from anthropogenic sources.
References
Alvarez, M. B., Quintas, P. Y., Domini, C. E., Garrido, M., & Fernándes Band, B. S. (2014). Chemometric approach to visualize and easily interpret data from sequential extraction procedures applied to sediment samples. Journal of Hazardous Materials, 274, 455–464.
Cai, J., Cao, Y., Tan, H., Wang, Y., & Luo, J. (2011). Fractionation and ecological risk of metals in urban river sediments in Zhongshan City, Perl River Delta. Journal of Environmental Monitoring, 13, 2450–2456.
Canuto, F. A. B., Garcia, C. A. B., Alves, J. P. H., & Passos, E. A. (2013). Mobility and ecological risk assessment of trace metals in polluted estuarine sediments using a sequential extraction scheme. Environmental Monitoring and Assessment, 185, 6173–6185.
Cappuyns, V., & Slabbinck, E. (2012). Occurrence of vanadium in Belgian and European alluvial soils. Applied and Environmental Soil Science. doi:10.1155/2012/979501.
Chabuhdhara, M., & Nema, A. (2012). Assessment of heavy metal contamination in Hindon River sediments: A chemometric and geochemical approach. Chemosphere, 87, 945–953.
Copaja, S. V., Molina, X., & Tessada, R. (2014). Determination of heavy metals in Choapa river sediments using BCR sequential extraction procedure. Journal of the Chilean Chemical Society, 59(1), 2353–2358.
Davutluoglu, O. I., Seckin, G., Ersu, C. B., Yimlaz, T., & Sari, B. (2011). Heavy metal content and distribution in surface sediments of the Seyhan River, Turkey. Journal of Environmental Management, 92, 2250–2259.
de Andrade Passos, E., Alves, J. C., dos Santos, I. S., Alves, J. P. H., Garcia, C. A. B., & Costa, C. S. (2010). Assessment of trace metals contamination in estuarine sediments using a sequential extraction technique and principal component analysis. Microchemical Journal, 96, 50–57.
Environment Canada (2002). Canadian sediment quality guidelines for the protection of aquatic life: Summary tables, updated. In Canidian environmental quality Guidelines, 1999. Winnipeg, MB: Canadian council of ministers of the environment (CCME).
Fathollahzadeh, H., Kaczala, F., Bhatnagar, A., & Hogland, W. (2014). Speciation of metals in contaminated sediments from Oskarshamn Harbor, Oskarshamn, Sweden. Environmental Science and Pollution Research, 21, 2455–2464.
Favas, P. J. C., Sarkar, S. K., & Rakshit, D. (2014). Geochemical fractionation of trace elements in stream sediments contaminated by mining activity. Clean Soil, Air, Water. doi:10.1002/clean.201200718.
Gharibreza, M., Ashraf, M.A., Yusoff, I., & Raj, J.K. (2013). An evaluation of Bera Lake (Malasya) sediment contamination using sediment quality guidelines. Journal of Chemistry. 10.1155/2013/387035.
ICPDR (2005). The Danube River Basin District. River basin characteristics, impact of human activities and economic analysis required under Article 5, Annex II and Annex III, and inventory of protected areas required under Article 6, Annex IV of the EU Water Framework Directive (2000/60/EC). Part A-basin-wide overview. ICPDR, Vienna.
Ingersoll and MacDonald. (2002). http://www.epa.gov/greatlakes/sediment/Vol3.pdf.
Kartal, Ş., Aydin, Z., & Tokalioğlu, Ş. (2006). Fractionation of metals in street sediment samples by using the BCR sequential extraction procedure and multivariate statistical elucidation of the data. Journal of Hazardous Materials, 132, 80–89.
Pueyo, M., Mateu, J., Rigol, A., Vidal, M., López-Sánczez, J. F., & Rauret, G. (2008). Use of modified BCR three-step sequential extraction procedure for the study of trace element dynamics in contaminated soils. Environmental Pollution, 152, 330–341.
Rao, C. R. M., Sahuquillo, A., & Lopez-Sanchez, J. F. (2010). Comparison of single and sequential extraction procedures for the study of rare earth elements remobilization in different types of soils. Analytica Chimica Acta, 662, 128–146.
Relić, D., Đorđević, D., Sakan, S., Anđelković, I., Pantelić, A., Stanković, R., & Popović, A. (2013). Conventional, microwave, and ultrasound sequential extractions for the fractionation of metals in sediments within the Petrochemical Industry, Serbia. Environmental Monitoring and Assessment, 185, 7627–7645.
Republic of Serbia (1994). Official gazete of Republic Serbia, 23/1994. Rule book of allowed concentrations of dangerous and hazardous materials in soil and in water for irrigation and methods for analysis, 23.
Sakan, S., Dević, G., Relić, D., Anđelković, I., Sakan, N., & Đorđević, D. (2014). Risk assessment of trace element contamination in river sediments in Serbia using pollution indices and statistical methods: a pilot study. Environmental Earth Sciences. doi:10.1007/s12665-014-3886-1.
Sakan, S., Dević, G., Relić, D., Anđelković, I., Sakan, N., & Đorđević, D. (2015). Evaluation of sediment contamination with heavy metals: the importance of determining appropriate background content and suitable element for normalization. Environmental Geochemistry and Health, 37, 97–113.
Sakan, S., & Đorđević, D. (2012). Distinguishing between natural and anthropogenic sources of trace elements in the sediments using the methods of geochemical normalization and statistical analysis. In D. A. De Leon & P. R. Aragon (Eds.), Trace elements: Environmental sources, geochemistry and human health (pp. 117–134). New York: Nova Science Publishers, Inc.
Sakan, S., & Đorđević, D. (2013). Assessment of trace element contamination in the river and alluvial sediments using a sequential extraction technique and statistical analysis. In S. Hong-Bo (Ed.), Metal contamination: Sources, detection and environmental impact (pp. 119–155). New York: Nova Science Publishers, Inc.
Sakan, S., Đorđević, D., Dević, G., Relić, D., Anđelković, I., & Đuričić, J. (2011). A study of trace element contamination in river sediments in Serbia using microwave-assisted aqua regia digestion and multivariate statistical analysis. Microchemical Journal, 99, 492–502.
Sundray, S. K., Nayak, B. B., Lin, S., & Bhatta, D. (2011). Geochemical speciation and risk assessment of heavy metals in the river estuarine sediments-a case study: Mahanadi basin, India. Journal of Hazardous Materials, 186, 1837–1846.
Sutherland, R. A. (2010). BCR-701: A review of 10-years of sequential extraction analyses. Analytica Chimica Acta, 680, 10–20.
Teodorović, I. (2009). Ecotoxicological research and related legislation in Serbia. Environmental Science and Pollution Research, 16(Suppl 1), S123–S129.
Wang, S., Jia, Y., Wang, S., Wang, X., Wang, H., Zhao, Z., & Liu, B. (2010). Fractionation of heavy metals in shallow marine sediments from Jinzhou Bay, China. Journal of Environmental Sciences (China), 22, 23–31.
Yang, Y., Chen, F., Zhang, L., Liu, J., Wu, S., & Kang, M. (2012). Comprehensive assessment of heavy metal contamination in sediment of the Pearl River Estuary and adjacent Shelf. Marine Pollution Bulletin, 64, 1947–1955.
Acknowledgments
This study was supported by the Ministry of Education, Science and Technological Development of Serbia, Grant Nos. 172001 and 43007. In addition, we would like to thank the Republic Hydrometeorological Service of Serbia for the sediment samples.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sakan, S., Popović, A., Anđelković, I. et al. Aquatic sediments pollution estimate using the metal fractionation, secondary phase enrichment factor calculation, and used statistical methods. Environ Geochem Health 38, 855–867 (2016). https://doi.org/10.1007/s10653-015-9766-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-015-9766-0