Abstract
Geophagy among orangutans is the most poorly documented in contrast to the knowledge of soil-eating practices of other great ape species. Observations of soil consumption by orangutans in the Sungai Wain Forest Preserve (Wanariset) of Borneo are presented, along with physico-mineral–chemical analyses of the ingested soil in an effort to understand what might stimulate the activity. The consumed soils are: light colored, not excessively weathered by normal standards, higher in the clay size fraction relative to controls, and are comprised of a mix of clay minerals without any specificity of 1:1, 2:1 and/or 2:1:1 (Si:Al) species. The geophagic soils contain chlorides below detection limits, effectively eliminating salt as a stimulus. Soil chemical and geochemical analyses confirm that orangutans prefer soils with pH levels near or above 4.0, while controls are consistently lower (pH = 3.5–4.0), a considerable difference in acidity for at least four out of six soils consumed. Geochemical analysis shows Al, Fe and K are high in the consumed vs control samples; higher Al follows from higher clay percentages in the consumed earth. Iron and K may play physiological roles, but Fe is mostly in the ferrous form (Fe+2) and may not be readily taken up by the animals. The preferential choice of consumed samples, with pH above 4.0 and higher clay contents, may promote a more beneficial intestinal environment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Geophagy, the ingestion of natural earths, is a non-exclusive behavior practised by animals such as birds, carnivores, elephants, primates and ungulates (see, Wrangham et al. 1991; Ke 1999; Khrishnamani and Mahaney 2000; Mahaney and Krishnamani 2003). Proposed geophagic factors include dietary supplementation (e.g., Setz et al. 1999), nutrition regulation (e.g., Mahaney and Krishnamani 2003), aid in digestion (e.g., Kreulen 1985), and/or pharmaceutical treatment (e.g., Krishnamani and Mahaney 2000; Klein et al. 2008). Among the Hominidae (great apes) known to consume soils, humans have also used soils as a famine food (e.g., Abrahams and Parsons 1997; Aufreiter et al. 1997; Mahaney et al. 1990, 1995a, b, 1996, 1997, 2000). Geophagy has been observed and studied among numerous primate species; among great apes, in both gorillas and chimpanzees (Oates 1978; Fossey 1983; Goodall 1986; Wrangham et al. 1991; Mahaney 1993; Plumptre et al. 1994; Mahaney et al. 1995b, 1996, 1997, 1999, 2005; Mahaney et al. 1996; Reynolds et al. 1998; Krishnamani and Mahaney 2000; Aufreiter et al. 2001; Ketch et al. 2001; Tweheyo and Obua 2001; Klein et al. 2008).
To some extent, the health of animal populations may well be maintained through the learned behaviors of soil ingestion that provide a means of overcoming endemic malaises of one kind or another, and variations in the chemical composition of ingested plant or animal matter that may inhibit physiological responses. Possible benefits and negative effects of soil consumption among humans have been reviewed by numerous authors, some of whom have cautioned of possible adverse effects. However, the survival of this practice among humans into modern times and its continued observation among free-ranging animals as well as some captives suggest that the benefits far outweigh the possible hazards. Indeed, geophagy is so common a behavior in vertebrates including primates that it is likely to provide a significant survival advantage for which the mechanism may well hinge on the chemical composition of the eaten soils. Krishnamani and Mahaney (2000) addressed the possible reasons for which primates engage in geophagy, and later Mahaney and Krishnamani (2003) outlined major problems with sampling site selection where animals had been observed engaging in geophagy, with a focus on the collection and standardized analysis of control samples, as well as on the age of the soils/paleosols consumed.
Free-ranging orangutans are classified as frugivores, but since ripe fruits may not always be available, they consume chiefly plant materials, in which they are notably particular in separating specific plant parts of each variety for consumption, including buds, barks, flowers, fruits, leaves, as well as wood, insects, small animals and soils (Knott 1998; Hamilton and Galdikas 1994). Their foods contain a greater proportion of cellulose and cell wall constituents than do human diets (Dierenfeld 1997). The digestive systems of orangutans are similar to those of the other great apes, with a simple stomach and a relatively long small intestine; however, in keeping with the high proportion of plant materials and fiber in their diet, the cecum and colon are large and haustrated (Caton et al. 1999). The large proximal colon is the principal site of fermentation of the large proportion of fibrous plant materials in the orangutan diet. Although arboreal, orangutans, among the great apes, show an extremely low rate of energy use (Pontzer et al. 2010). Microbial fermentation of these plant residues is a significant source of energy (Schmidt et al. 2005). The rate of digesta retention in the GI tract of orangutans is relatively long, confirming their reliance on colon fermentation as a strategy for obtaining energy (Caton et al. 1999).
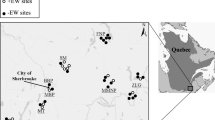
Geophagy practiced among Orangutan populations (Wanariset, Fig. 1) appears to be a routine behavior where soils are occasionally sampled en route as the animals travel through the forest. The importance of water use by wild Bornean orangutans to geophagy has been discussed by Kempf (2009) and the effects of fire and drought on orangutans in the Sungai Wain Protection Forest by Russon and Susilo (1999). The use of natural licks by orangutans in tropical rain forests has been explored by Matsubayashi et al. (2006). Study of Orangutan geophagy in neighboring areas of Sumatra (Ketambe, Fig. 1), is limited to Stambolic-Robb (1997), who found that the animals (Pongo abeli) preferentially chose soils with high concentrations of kaolinite. Voros (2000) identified key possible dietary and nutritional benefits to orangutans who engage in geophagy in Sungai Wain Forest, Borneo (the same area from which our sediment samples were collected). Captive orangutans have been observed to engage in geophagy, ingesting very soft, wet and friable soils, the only ones easily available in their enclosure (Yamazaki et al. 2010).
The Bornean orangutans in our study were released into the Sungai Wain Protection Forest from the Wanariset Orangutan Reintroduction Project (ORP) (Fig. 1), after appropriate medical and social rehabilitation to provide sufficient skills to survive in the wild. Earlier studies confirmed that these great apes engage in geophagy on an occasional basis (Russon 2002; Russon et al. 2009). Little is known about the composition of the consumed materials and whether or not it is possible to target minerals or chemical elements that might be a benefit to the species. Could geophagy provide a component deficient in their diet and thus amount to a dietary supplement that is missing in their food supply? Might soil contain a pharmacologically active ingredient beneficial to the animal? These are some of the questions we seek to explore here through analysis of consumed versus control soils. Orangutans are notoriously solitary and observing them in the forest is more or less an on-again, off-again operation; hence, the few six eaten samples with nine controls, the latter collected in the immediate vicinity of the consumed earths.
Materials and methods
Field work
Our fifteen sediment samples (six samples consumed by orangutans and nine controls) were stored in sterile sample bags and were analyzed within 6 weeks of collection. Samples were collected at identified feeding sites and control samples were collected at a later time when they could be identified at leisure. Approximately 300 g bulk samples from each feeding and control sites were collected for later physical, mineral, and chemical analyses. While there is difficulty in observing geophagic behavior, it appears that subject orangutans pause briefly (1–2 min) to observe/smell candidate sites and consume small quantities (~30–50 g) of soil.
Laboratory work
Particle size determinations of all samples follow methods outlined by Day (1965). Samples were treated with 30 % H2O2 to oxidize organic matter and standard stock solution of Na-pyrophosphate (50 g/L) to avoid flocculation of clays. The water content of the samples was determined using pressure membrane equipment and heating to determine hygroscopic water content. Moisture factors have been determined, and the air dry equivalent weight of oven-dry soil has been subjected to particle size analysis by hydrometer (Mahaney 1990).
The samples were wet-sieved to separate sands from clay plus silt. Sand grade sizes were determined by direct weight, whereas finer fractions were calculated by hydrometer. All samples have been subjected to conductivity analysis, to determine total salt content, and pH electrode (Bower and Wilcox 1965). The clay fraction was analyzed by X-ray diffraction to determine the primary and secondary mineral composition of the <2-μm-size material (Whittig 1965).
The geochemistry of the <2 mm bulk fraction was determined by instrumental neutron activation analysis (INAA) at the SLOWPOKE Nuclear Reactor at the University of Toronto, using appropriate standards (e.g., Hancock 1984; Harrison and Hancock 2005).
To determine the composition and state of weathering of the source minerals, each sample was subjected to intensive microscopic investigation, using the light microscope, on randomly counted out 300–400 grains per sample, for more detailed analysis. Out of this population, we selected a subpopulation of grains for more intensive investigation by field-emission scanning electron microscope (FESEM) and energy-dispersive spectrometry (EDS). FESEM delivers ultra-high resolutions down to 1 nm (nm) for the most demanding electron microscope applications. The ultra-high-resolution FESEM (Department of Materials Science, University of Toronto) was used to operate over the complete voltage range with probe currents up to 20 nA. For additional information on FESEM and EDS, see Mahaney (2002).
The light microscopic investigations for each sample using a Leica SAO-80 instrument were repeated to insure that the grain counts were reproducible. Subsamples of each bulk sand sample were then subjected to analysis by electron microscope to obtain imagery and determine the chemistry of grains consumed or grains avoided by the orangutans.
Results
Geophagic samples and controls
The geophagic and control samples were studied for their physical characteristics, including field texture, presence of organics, and color, both moist and dry (Fig. 2a, b). The samples were collected where the orangutans collected and consumed soil, and these sites range from fine-grained stream cuts in fluvial deposits to coarse-textured slope deposits. The presence of pebbles in discrete layers, from the top down in slope sections, suggests colluviation in many instances, a mix of different grain sizes in slope deposits, particularly for the control samples. The color data (Oyama and Takehara 1970) suggest that some weathering is restricted to a young age, possibly no more than a few millennia. Pebbles and finer material lack the red patinas common to tropical soils and common also to samples usually selected for geophagy (Mahaney et al. 1999; 2005).
a Fresh, moist soil with a loam or clay loam texture sampled immediately after an orangutan ingested it. The moist color keys out at (10YR 4/6, 5/6), a brown to yellowish brown (see Oyama and Takehara 1970); b control soil with considerably greater amount of organic material in it
The moist field color is considerably darker than dry colors taken later in the laboratory, and all field hues key out in the 10YR 4–5 range indicating some minor weathering and minor release of Fe. The dry colors (Table 1) are significantly lighter in hue with values ranging from 10YR 6 and 8 for the ingested samples to 10YR 5–8 for the controls, indicating some overlap in terms of color and weathering strength. On the basis of field color value and chroma designations, it is reasonable to expect oxide/hydroxide release and clay mineral genesis that might stimulate geophagic behavior. However, given the 10YR hues for these samples in a tropical rainforest environment, materials collected in stream cuts/colluvial aprons, all are most probably young and of Holocene age (<10 kyr), and hence, have not lost much of their soluble constituents.
Particle size
Particle size curves have been obtained, clearly demarcating sediment source and degree of weathering and differentiating geophagic samples from controls (see Fig. 3a, b). First, the control samples with near-parabolic curves (Fig. 3a) show various degrees of sorting suggestive of aqueous/slope transport, whereas the geophagic group, which may also have had a fluvial/colluvial origin, depicts with flattened, linear curves more highly weathered sediment, and hence, higher clay content. The consumed samples contain more than 30 % clay size up to 46 %, while the controls are averaging considerably less than 30 % and in some cases 15–<10 % clay size material. Shown perhaps more clearly on the ternary diagram (Fig. 3b), soil grade size classes depict minor overlap but with generally clear-cut differences between the ingested and control suites of samples. From these data, the orangutans show a preference for finer size material, a preference that correlates well with previous work on orangutans (Voros et al. 2001; Stambolic-Robb 1997) in other areas of Indonesia.
Particle size distributions: a particle size curves for consumed and control samples (read % sand from the 63 μm line; silt plus sand from the 1.95 μ line and clay—subtract % at the 1.95 μm line from 100). Consumed samples are 1, 3, 6, 7, 8, and 14, remainder are controls. Control samples are generally less than 30 % clay, ingested >30 % clay size material. Some controls nearly merge with ingested samples at 30 % clay content; b ternary diagram showing grain size differences between ingested (filled cirlce) and control (filled square) samples. With some overlap, the ingested group is 5–10 % heavier with clay than the controls
Scanning electron microscopy
SEM analysis was carried out on a selection of collected consumed and control samples in an effort to further distinguish the two. Closer study revealed a large proportion of clear to opaque fresh quartz, (Figs. 4a, 5a) with only minor clay coatings in some cases (Fig. 4b). Iron coatings, evidence of a weathering history, were expected on the grains as a result of the high potential for oxidation in the region, but were not found. While Fe deficiencies proved prevalent in past research (Voros et al. 2001; Stambolic-Robb 1997), it is clear that iron as a stimulus to geophagy cannot always be determined from physical or microscopic analysis.
Imagery of control samples. a Fresh quartz grain, uncoated. b Decaying plant material (center); c unidentified organic material; d filaments of bacteria (arrow-right) and fungi (arrow-left) based on size—~2–4 µm (bacteria) and 8–10 µm (fungi). No hyphae are present for fungi indicating these are artifacts preserved in this instance on a plagioclase mineral
It may be that a part of the answer to the question as to why certain samples are chosen lies in the loosely cemented clay accumulations on quartz grains [Fig. 4c (arrow), d] observed in the consumed samples. The spectra confirmed 1:1 Si:Al in the clay coatings, as well as very minor Mg, which signals either kaolinite or metahalloysite clay minerals. In light of the possible medicinal uses of clay minerals, the presence of 1:1 clay minerals as a means of treating diarrhea may contribute to geophagic behavior. Leaf-eating great apes, including orangutans, are prone to diarrhea which is a near constant ailment that may be controlled if not partially alleviated by ingesting soil rich in Si:Al (1:1) clay minerals such as kaolinite, halloysite, and metahalloysite. Pharmaceutical kaolinite or Kaopectate™ is used by humans to treat diarrhea, and it is quite likely great ape species learned to consume certain soils/earths to achieve similar effects (see Mahaney et al. 1990). While we cannot be certain that orangutans can detect kaolinite or its mineral affinities in soil, we can state that in this case they are consuming soils rich either in kaolinite and/or metahalloysite. On the other hand, given the data in Table 2, we can also state that they could obtain either mineral in control as well as consumed soils.
Another clear difference between consumed (ingested) or control samples lies in the elevated levels of organics found in the control samples. While some isolated instances are present in the consumed samples, data collected from the control samples reveal decaying wood fragments (Fig. 5b) with mineralized as well as active bacteria (Fig. 5c, d). This wet region is rife with the latter which may well noticeably decrease the pH of the material, possibly affecting the taste of the soil.
The presence of fungal filaments (Fig. 5d) of unknown species suggests the possibility of self-treatment on the part of the orangutans who may obtain pharmacological benefits from the consumption of particular microbes, i.e., a source of Penicillin-like substances. Ketch et al. (2001) described such substances in soils consumed by chimpanzees in Tanzania.
Clay mineralogy
The clay mineralogy was analyzed to determine whether preference for clay size material translates also into a preference for clay mineral species of one kind over another. The data (Table 1) show a considerable range of 1:1 (Si:Al = 1:1) clay minerals such as kaolinite and metahalloysite among both the consumed and control samples. If the orangutans sought 1:1 clay minerals, they might just as well eat the control samples as the ones they actually foraged on. Similarly, among the 2:1 clay minerals (Si:Al = 2:1), including illite, randomly interstratified illite–smectite and vermiculite, there is little preference indicated when comparing the consumed versus control samples. Minor amounts of chlorite, a 2:1:1 clay mineral that may have a soil weathering or hydrothermal metamorphic origin, appear sporadically within the orangutan’s foraging range, but outside of its Fe content, it probably has little significance to geophagy.
Among the primary minerals of quartz and plagioclase feldspar, the consumed samples are equivalent in concentration to the control sample group, a distributional pattern which parallels closely the secondary minerals summarized above. The primary rock-forming minerals would have to be dissolved and absorbed across the intestinal epithelium to supply nutritional benefits beyond providing a dispersing matrix for food particles, and their geophagic significance is unknown. One element in each primary mineral with potential nutritional significance is Ca, supplied in the plagioclase minerals if it can be hydrolyzed in the GI tract and absorbed. As indicated in the geochemistry discussed below, the total concentration of Ca is below detection limits, so while little Ca is available to the orangutans from the inorganic mineral complex, sufficient Ca may be obtained from local vegetation.
Soil chemistry
The pH of the samples was analyzed to determine the hydrogen ion (H+) content among the consumed and control samples. The pH of the ingested and control samples range from 3.6 to 4.6 while the control group alone registers as more acidic, whereas two-thirds of the consumed group is less acidic than the control group. However, a comparison of total salts in the consumed group versus the control group shows a poor correlation with pH trends, as concentrations range from 40 to 203 µS/cm in the former and 40–153 µS/cm in the latter. Hence, as with the clay minerals, if the orangutans can distinguish samples by taste/smell, and total salts determine ingestion of the soil, consumed or control samples could be chosen with similar benefit. On the basis of soil pH composition, it appears the animals prefer less acidic material. With Cl below analytical detection limits throughout both sample groups, only variations of nitrates and sulfates could account for the anomalous high conductivity. While exact organic carbon content is unknown, soil color values and chroma suggest low concentrations of organic matter in the consumed group, leaving S as the likely salt source, possibly derived from weathering of pyrite frequently seen during microscopic observations. There is approximately 5,000 times more H+ in the control samples relative to two-thirds of the consumed group. As acidity most likely impacts the microbial content, a tentative working hypothesis is that the animals may have learned to sample soils low in certain groups of microorganisms that might have negative health effects for them. Alternatively, their choice of soils may simply be associated with a preference for a less acidic stomach or intestinal environment.
Geochemistry
Analysis by INAA of the geochemistry of the two groups of samples shows near doubling of the total Al content of 1.6–4.2 % in the control group to 4.4–6.7 % in the consumed group, an increase that parallels the increase in clay percentages from the control to the consumed suite of samples (Fig. 3a). Increased Al and Fe in the consumed groups relative to the control group is clearly shown in Fig. 6a, although with very minor overlap, which suggests the orangutans may prefer Fe in addition to clay-rich material.
Total Fe in the consumed group of samples ranges from 1.1 to 3.6 %, whereas in the control group, the range is 0.4–2.2 %, with some overlap. The soils selected for ingestion by the orangutans are occasionally 1.0 % higher in total Fe compared with the control group and given the colors previously documented most Fe appears to be in the ferrous (Fe+2) state. The amount of secondary Fe (Fe+3—hematite, goethite, ferrihydrite minerals) that might be more easily absorbed by the consuming organisms is unknown. If Fe+3 is important in this instance as a stimulus to geophagy, it would have to be in a bioavailable form, i.e., absorbed across the intestine (see Mahaney et al. 2005, with reference to chimpanzees). While INAA does not measure P, the presence of elevated Ce (Fig. 6b) in the consumed samples relative to controls suggests the presence of monazite a P-rich mineral that may play a role in orangutan physiology.
Analysis of the rare earth elements (REEs), including La, Eu and Lu, indicates an elevation of these elements in the consumed group of samples, an increased concentration resulting from elevated clay content as indicated above.
The average ratio of REEs in consumed relative to control soils is 1.39 ± 0.22. This implies more diluting material (mainly organics) in the control sediments. As hypothesized previously (Mahaney et al. 2005), consumption of organics may introduce bacteria with other than beneficial results.
An important indicator in the above data set is that the average Hf concentrations are similar in both consumed and control sediments. Since Hf tends to be associated with Zr in zircon-rich quartz, the similar Hf concentrations imply that the control sediments are richer than the consumed ones in organics and free quartz. Reversing this argument, it implies that the consumed sediments are richer in clay size material, as already shown above in the particle size database. Considering the solubility of Cs, higher concentrations in the consumed soils, as shown in Fig. 6c, strongly indicate that the ingested samples are in colluvial landforms on slopes above frequent overbank water action on the floodplains. While there is no known physiological importance for Sc, it is likely this REE is a free metal sojourning within the clay complex.
Bromine and iodine are both likely present in all of the sediments from decayed vegetation, and often their concentrations were not measurable, so are not included in the consumed–control ratios. The low concentration of calcium, nearly unvarying between the two groups of samples, is expected given the felsic (acidic rock) composition of the material. Sodium, while slightly higher in the control group, is in short supply along with Cl (below detection limits); thus chlorides are definitely not a stimulus. All other microelements, including Ti [slightly elevated at 0.3 % (control) to 0.4 % (consumed)], are within the normal limits of granitic terrain and have ill-defined or unknown correlation to geophagy.
Discussion
This study is unique in that sample collection was carefully instrumented, and two clear groups of material, consumed and controls, were identified for soils available to free-living orangutans, a species very difficult to observe in the wild and monitor for geophagic behavior. After rigorous analysis of the two groups of soils, some differences were determined, though none as glaring as initially estimated. Clay content remains a key potential stimulus, as was found in similar research conducted on other great ape species (Mahaney et al. 1990; Mahaney and Krishnamani 2003). Though the benefits are unclear, the orangutans show a definite preference for clay-rich soil, similar in texture to soil long used among humans as medication (Aufreiter et al. 1997). For instance, kaolinite and metahalloysite in these samples, two different mineralogies but with similar chemistries [Al2(Si2O5)(OH4)], aid in the regulation of digestive upsets.
Na salt at least is not a motivator as shown in Table 3, implying that the animals must be receiving sufficient amounts from their diet. Sodium total concentrations are in the low hundred ppm range, although Cl varies from 30 to 52 ppm in the control samples to detection limits in the eaten soil group. Whether or not the orangutans can detect such low quantities of Na is problematical, but variations in concentrations are real.
Among the elements determined, calcium totals, which might be considered a nutrient supplement, are below detection limits in all samples, and thus an unlikely stimulus from the mineral complex. Iron, Co, As, Mn and V, of nutritional interest, are all slightly elevated in the eaten soils, and Fe especially may be a relevant stimulus to engage in geophagy provided sufficient concentrations are in a bioavailable form.
The REEs in the consumed soil may be of interest. Within the REEs, an occasional slight elevation of Ce in the eaten samples may argue for an increase in monazite, and hence, phosphorus. Although the amount of P is negligible, its overall stimulus value at low concentration is unknown but may be related to the concentration of REEs (Wang and Liang 2014). Other rare earths [lanthanum (La), cerium (Ce), neodynium (Nd) and samarium (Sm)] have been investigated in monkeys (Ji and Cui 1988) with inconclusive results. While only Ce and La are reported here, both elements are higher in the consumed samples. Studies in humans (Zhang et al. 2000) have found REEs to have negative effects on cell mitochondria in males which are irreversible while in females effects are reversible. Cerium, and other lanthanides, have been studied as antioxidants and anticarcinogens in humans (Hanahan and Weinberg 2000; Palizban et al. 2010). As reported in these publications, lanthanides may act to attract free radicals and protect cells/tissues from oxidation stress, thus inhibiting cancer cell growth. Because orangutans, like all great apes, are close to humans in physiology, and likely subject to similar toxicity thresholds, it is of interest to monitor and study chemical uptake from soils and plants, including REEs and other trace elements.
Whereas the effects of REEs on plant growth (Diatloff et al. 2004; Turra et al. 2011) is gaining attention among researchers, the overall importance of REEs in human nutrition is sadly neglected (Wei et al. 2009). The physiological effects of REE concentrations in soils may have toxic effects although the importance of such in orangutan physiology is unknown.
The prevalent minerals of quartz and feldspar, while not directly medicinally/chemically active, may aid in breakdown of bark and other more difficult to digest components of the orangutan diet.
It seems possible since site choices are relatively random that the consuming organisms are able to collect organic-free, clay-rich material, potentially having medicinal or nutritional value. The answer is most likely found in the high content of decomposing organics within the control group, a material the animals preferentially avoid (Voros 2000). The slightly lower pH values of the control material may be important, as well as the presence of greater percentages of bacteria, both active and mineralized, possibly as a function of the decay. It is probable that the animals are avoiding sites with high organics, to prevent health or digestive issues associated with organic residue content or bacteria active in organic decomposition.
As clearly indicated from the field samples and the microscopy, the control group contains a high frequency of residual organics, complete with a residue of lignin and cellulose, both of which contain a reservoir of bacteria and fungi with unknown, undocumented and possibly deleterious physiological effects. The orangutans show a clear preference to avoid these contaminants despite the fact they pick up samples literally “on the fly” with little apparent previous site study, other than brief stops of 1–2 min to observe and presumably smell earth that might be consumed. This precision is consistent with the feeding behavior of orangutans in the wild, who while foraging in a challenging environment where many plants produce defensive toxins, show great care in choosing which parts of available food plants are ingested, thus avoiding some toxins. Since much of this material is in the form of non-starch polysaccharides, the products of fermentation of plant fiber in the capacious orangutan colon are thought to be their main source of energy. The choice of soils high in finely divided clay particles for ingestion may be a strategy effectively extending the retention time of digesta in the colon, increasing the time available for fermentation to take place, since particulates show a longer retention time than liquids (Caton et al. 1999). The small amounts of clay repeatedly ingested also likely hold any moisture present for longer in the intestinal tract, aiding in fermentation.
Conclusions
Laboratory analyses carried out on these two sample suites point to a preference for soils with considerably higher clay size contents and a lower silica to aluminum ratio, i.e., with a kaolinitic composition similar to Kaopectate™, a mix with good potential for adsorbing toxins. Along with kaolinite, there are also copious amounts of metahalloysite present in the consumed samples, a companion 1:1 clay mineral, with a similar chemical composition to kaolinite, but with a capacity to retain moisture. The equal or near-equal amount of kaolinite and metahalloysite present within the control group of samples suggests that if the chemistry of the clays is a significant stimulus, the orangutans could equally benefit from ingesting either the control soils or the consumed soils. If increasing clay content is important the orangutans clearly benefit from the samples consumed. The presence of bacteria, observed in coatings on sands, further suggests that with orangutans, strategic avoidance and amelioration of microbiological pestilence is a subject warranting additional investigation.
A unique aspect of this preparatory work points to the orangutans selecting samples with a higher REE composition (chemical elements lanthanum, europium and lutetium) residing in the clay size material. A feature of the selected material is that iron is seen as a possible minor stimulus only, a conclusion that differs from many previous studies (see Krishnamani and Mahaney 2000) of animal geophagy. Finally, the pH of the consumed material in four out of six samples analyzed is less acidic than two-thirds of samples in the control group suggesting that future studies focus on the relationship between pH and total salts.
References
Abrahams, P. W., & Parsons, J. A. (1997). Geophagy in the tropics: An appraisal of three geophagical materials. Environmental Geochemistry and Health, 19, 19–22.
Aufreiter, S., Mahaney, W. C., Hancock, R. G. V., & Stambolic, A. (1997). Geochemistry and mineralogy of soils eaten by humans. International Journal of Food Sciences and Nutrition, 40, 447–460.
Aufreiter, S., Mahaney, W. C., Milner, M. W., Hancock, R. G. V., Huffman, M., Wink, M., et al. (2001). Mineralogical and chemical interactions of soils eaten by chimpanzees of the Mahale and Gombe Stream National Parks, Tanzania. Journal of Chemical Ecology, 27, 285–311.
Bower, C. A., & Wilcox, L. V. (1965). Soluble salts. In C. A. Black (Ed.), Methods of soil analysis, part 2 (pp. 933–951). Madison, WI: American Society Agronomy.
Caton, J. M., Hume, I. D., Hill, D. M., & Harper, P. (1999). Digesta retention in the Gastro-intestinal tract of the Orang Utan (Pongo pygmaeus). Primates, 40(4), 551–558.
Day, P. E. (1965). Particle fractionation and particle size analysis. In C. A. Black (Ed.), Methods of soil analysis (pp. 545–567). Madison, WI: American Society of Agronomy.
Diatloff, E., Roberts, M., Sanders, D., & Roberts, S. K. (2004). Characterization of anion channels in the plasma membrane of Arabidopsis epidermal root cells and the identification of a citrate-permeable channel induced by phosphate starvation. Plant Physiology,. doi:10.1104/pp.104.046995.
Dierenfeld, E. S. (1997). Orangutan nutrition. In C. Sodaro (Ed.), Orangutan SSP husbandry manual. Brookfield, IL: Orangutan SSP and Brookfield Zoo.
Fossey, D. (1983). Gorillas in the mist. Boston: Houghton Mifflin.
Goodall, J. (1986). The chimpanzee of Gombe. Patterns of behaviour. Cambridge, MA: The Belknap Press of Harvard University Press.
Hamilton, R. A., & Galdikas, B. M. F. (1994). A preliminary study of food selection by the orang-utan in relation to plant quality. Primates, 35(3), 255–263.
Hanahan, D., & Weinberg, R. A. (2000). The hallmarks of cancer. Cell, 100, 57–70.
Hancock, R. G. V. (1984). On the source of clay used for Cologne Roman pottery. Archaeometry, 26, 210–217.
Harrison, T. P., & Hancock, R. G. V. (2005). Geochemical analysis and sociocultural complexity: A case study from early Iron Age Megiddo (Israel). Archaeometry, 47, 705–722.
Ji, Y. J., & Cui, M. Z. (1988). Toxicological studies on safety of rare earths used in agriculture. Biomedical Environment Sciences, 1(3), 270–276.
Ke, (1999). Geophagy in the golden-faced saki monkey (Pithecia pithecia chrysocephala) in the Central Amazon. Journal Zoological Society London, 277, 91–103.
Kempf, E. (2009). Patterns of water use in primates. Folia Primatologica, 80, 275–294.
Ketch, L. A., Malloch, D., Mahaney, W. C., & Huffman, M. (2001). Comparative microbial analysis and clay mineralogy of soils eaten by chimpanzees (Pan troglodytes schweinfurthii) in Tanzania. Soil Biology and Biochemistry, 33, 199–203.
Klein, N., Fruhlich, F., & Krief, S. (2008). Geophagy: Soil consumption enhances the bioactivities of plants eaten by chimpanzees. Naturwissenschaften, 95, 325–331.
Knott, C. D. (1998). Changes in orangutan caloric intake, energy balance and ketones in response to fluctuating fruit availability. International Journal of Primatology, 19, 1061–1079.
Kreulen, D. A. (1985). Lick use by large herbivores: A review of benefits and banes of soil consumption. Mammal Review, 15, 107–123.
Krishnamani, R., & Mahaney, W. C. (2000). Geophagy among primates; adaptive significance and ecological consequences. Animal Behaviour, 59, 899–915.
Mahaney, W. C. (1990). Ice on the Equator. Ellison Bay, WI: W.M Caxton Ltd.
Mahaney, W. C. (1993). Scanning electron microscopy of earth mined and eaten by mountain gorillas in the Virunga Mountains, Rwanda. Primates, 34(3), 311–319.
Mahaney, W. C. (2002). Atlas of sand grain surface textures and applications. Oxford: Oxford University Press.
Mahaney, W. C., Aufreiter, S., & Hancock, R. G. V. (1995a). Mountain gorilla geophagy: A possible seasonal behavior for dealing with the effects of dietary changes. International Journal of Primatology, 16(3), 475–488.
Mahaney, W. C., Hancock, R. G. V., Aufreiter, S., & Huffman, M. A. (1996). Geochemistry and clay mineralogy of termite mound soil and the role of geophagy in chimpanzees of the Mahale Mountains, Tanzania. Primates, 37(2), 121–134.
Mahaney, W. C., & Krishnamani, R. (2003). Understanding geophagy in animals: Standard procedures of sampling soils. Journal of Chemical Ecology, 29, 1477–1499.
Mahaney, W. C., Milner, M. W., Aufreiter, S., Hancock, R. G. V., Wrangham, R., & Campbell, S. (2005). Geophagy soils consumed by chimpanzees of the Kanyawara community in the Kibale Forest, Uganda. International Journal of Primatology, 26, 1375–1397.
Mahaney, W. C., Milner, M. W., Mulyono, H., Hancock, R. G. V., Aufreiter, S., Reich, M., & Wink, M. (2000). Mineral and chemical analyses of soils eaten by humans in Indonesia. International Journal of Environmental Health Research, 10, 93–109.
Mahaney, W. C., Milner, M. W., Sanmugadas, K., Hancock, R. G. V., Aufreiter, S., Wrangham, R., & Pier, H. W. (1997). Geophagy amongst chimpanzees in Kibale forest, Uganda, [Analysis of geophagy soils in Kibale Forest, Uganda]. Primates, 38(2), 159–176.
Mahaney, W. C., Stambolic, A., Knezevich, M., Hancock, R. G. V., Aufreiter, S., Sanmugadas, K., et al. (1995b). Geophagy amongst rhesus macaques on Cayo Santiago, Puerto Rico. Primates, 36(3), 323–333.
Mahaney, W. C., Watts, D. P., & Hancock, R. G. V. (1990). Geophagia by mountain gorillas (Gorilla gorilla beringei) in the Virunga Mountains, Rwanda. Primates, 31(1), 113–120.
Mahaney, W. C., Zippin, J., Milner, M. W., Sanmugadas, K., Hancock, R. G. V., Aufreiter, S., et al. (1999). Chemistry, mineralogy and microbiology of termite mound soil eaten by chimpanzees of the Mahale Mountains, Western Tanzania. Journal of Tropical Ecology, 15, 565–588.
Matsubayashi, H., Lagan, P., Majalap, N., Tanagah, J., Sukor, J. R. A., & Kitayama, K. (2006). Importance of natural licks for the mammals in Bornean inland tropical rain forests. Ecological Research,. doi:10.1007/s11284-006-0313-4.
Oates, J. F. (1978). Water-plant and soil consumption by guereza monkeys (Colobus guereza): A relationship with minerals and toxins in the diet? Biotropica, 10, 241–253.
Oyama, M., & Takehara, H. (1970). Standard soil color charts. Forestry and Fisheries: Japan Research Council for Agriculture.
Palizban, A. A., Sadeghi-Aliabadi, H., & Abdollahpour, F. (2010). Effect of cerium lanthanide on Hela and MCF-7 cancer cell growth in the presence of transferring. Research in Pharmaceutical Sciences, 5(2), 119–125.
Plumptre, A. J., Reynolds, V. & Bakuneeta, C. (1994). The contribution of fruit eating primates to seed dispersal and natural regeneration after selective logging. ODA project R 4738 report.
Pontzer, H., Raichen, D. A., Shumacher, R. W., Ocobock, C., & Wich, S. A. (2010). Metabolic adaptation for low energy throughput in orangutans. Proceedings of the National Academy of Sciences of the United States of America, 107(32), 14048–14052.
Reynolds, V., Plumptre., A. J., Greenham, J., & Harborne, J. (1998). Condensed tanning and sugars in the diet of chimpanzees (Pan troglodytes schweinfurthii) in the Budongo Forest, Uganda. Oecologia, 115, 331–336.
Russon, A. E. (2002). Return of the native: Cognition and site-specific expertise in orangutan rehabilitation. International Journal of Primatology, 23(3), 461–478.
Russon, A. E. & Susilo, A. (1999). The effects of the 1997–98 droughts and fires on orangutans in Sungai Wain Protection Forest, E. Kalimantan, Indonesia. In: H. Suhartoyo & T. Toma (Eds.), Impacts of fire and human activities on forest ecosystems in the tropics: Proceedings of the third international symposium on Asian tropical forest management (pp. 348–372). Samarinda, Indonesia: Tropical Forest Research Center, Mulawarman University and Japan International Cooperation Agency.
Russon, A. E., et al. (2009). Geographic variation in orangutan diet. In S. Wich, S. Utami Atmoko, T. Mitra Setia, & C. van Schaik (Eds.), Orangutans: Geographic variation in behavioral ecology and conservation. Oxford: Oxford University Press.
Schmidt, D. A., Kerley, M. S., Dampsey, J. L., Porton, I. J., Porter, J. H., Griffin, M. E., et al. (2005). Fiber digestibility by the Orangutan (Pongo abelii): In vitro and in vivo. Journal of Zoo and Wildlife Medicine, 36(4), 571–580.
Setz, E. Z. F., Enzweiler, J., Solferini, V. N., Amendola, M. P., & Berton, R. S. (1999). Geophagy in the golden-faced saki monkey (Pithecia pithecia chrysocephala) in the Central Amazon. Journal Zoological Society London, 277, 91–103.
Stambolic-Robb, A. (1997). Geophagy amongst free-ranging Orangutans (Pongo pygmaeus abelii) of Gunung Leuser National Park and rehabilitated Borneo Orangutans (Pongo pygmaeus pygmaeus) of Sungai Wain Forest, Indonesia. MSc. Thesis, York University, pp. 117.
Turra, C., Fernandes, E. A. N., & Bacchi, M. A. (2011). Evaluation on rare earth elements of Brazilian agricultural supplies. Journal of Environmental Chemistry and Ecotoxicology, 3(4), 86–92.
Tweheyo, M., & Obua, J. (2001). Feeding habits of chimpanzees (Pan troglodytes), red tailed monkeys (Cercopithecus ascanius schmidti) and blue monkeys (Cercopithecus mitis stuhlmanii) on figs in Budongo Forest Reserve, Uganda. African Journal of Ecology, 39, 1–7.
Voros, J., (2000). Geophagy by rehabilitated Orangutans (Pongo pygmaeus pygmaeus) in Sungai Wain Forest, Indonesian Borneo, MSc. Thesis, Dept. of Geography, York University, Toronto.
Voros, J., Mahaney, W. C., Krishnamani, R. K., Aufreiter, S., Hancock, R. G. V., & Milner, M. W. (2001). Geophagy by the Bonnet macaques (Macaca radiata) of Southern India: A preliminary analysis. Primates, 42, 327–344.
Wang, L., & Liang, T. (2014). Effects of exogenous rare earth elements on phosphorus adsorption and desorption in different types of soils. Chemosphere, 103, 148–155.
Wei, Z. L., Rui, Y. K. & Tian, Z. H. (2009). Content of rare earth elements in wild Hypericum japonicum Thunb. Published by NIH, US National Library of Medicine (on line only).
Whittig, L. D. (1965). X-ray diffraction techniques for numerical identification and mineralogical composition. In C. A. Black (Ed.), Methods of soil analysis (pp. 671–696). Madison WI: American Society Agronomy.
Wrangham, R. W., Conklin, N. L., Chapman, C. A., & Hunt, K. D. (1991). The significance of fibrous foods for Kibale forest chimpanzees. Philosophical Transactions of the Royal Society of London. Series B: Biological Science, 334(171), 178.
Yamazaki, S., Takeda, S., Torii, E., Suzuki, S., Shimizu, M., & Kurotori, H. (2010). Pedological analysis of geophagic behaviours in captive Borneo Orangutan (Pongo pygmaeus). Primate Research, 26(1), 59–66.
Zhang, H., Feng, J., Shu, W., Liu, C., Xu, S., Wu, D., et al. (2000). Chronic toxicity of rare-earth elements on human beings: Implications of blood biochemical indices in REE-high regions, South Jiangxi. Biological Trace Element Research, 73, 1–17.
Acknowledgments
This research was carried out with funding from Quaternary Surveys, Toronto and a minor research grant from York University to WCM. We thank Anne Russon (York University) for collecting the samples and making them available to us for analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mahaney, W.C., Hancock, R.G.V., Aufreiter, S. et al. Bornean orangutan geophagy: analysis of ingested and control soils. Environ Geochem Health 38, 51–64 (2016). https://doi.org/10.1007/s10653-015-9678-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-015-9678-z