Abstract
This research aimed to characterize and compare the subchronic impacts of Cu to a Cu, Cd, and Zn mixture in early life stages of rainbow trout (Oncorhynchus mykiss) by examining uptake, survival, growth, development, and histopathology parameters. To accomplish this, rainbow trout were exposed for 31 days from eyed embryos to the swim-up fry life stage to waterborne Cu (31, 47, 70, and 104 μg/L) individually or as mixture containing Cd (4.1, 6.2, 9.3, and 14 μg/L) and Zn (385, 578, 867, and 1300 μg/L). Exposures elicited pronounced effects on survival when Cu was administered as a mixture (LC25 = 32.9 μg/L Cu) versus individually (LC25 = 46.3 μg/L Cu). Mixtures of Cu, Cd, and Zn also elicited more pronounced sublethal toxicity relative to equivalent Cu treatments with respect to reduced yolk sac resorption and increased incidence and/or severity of gill, liver, and kidney lesions. Our findings of reduced body weight (EC10, Cu = 55.0 μg/L Cu; EC10, Cu+Cd+Zn = 58.9 μg/L Cu), yolk sac resorption (LOECCu = 70 μg/L Cu; LOECCu+Cd+Zn = 70 μg/L Cu), coelomic fat (LOECCu = 47 μg/L Cu; LOECCu+Cd+Zn = 70 μg/L Cu), and increased hepatocellular cytoplasmic vacuolation (LOECCu = 70 μg/L Cu; LOECCu+Cd+Zn = 47 μg/L Cu) collectively indicate a complicated metabolic interference by metals in exposed fish. These lethal and sublethal effects observed in the laboratory could translate to reduced survival and fitness of wild salmonid populations inhabiting waterbodies receiving wastewater or runoff containing multiple metals at elevated concentrations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Metal pollution is a global issue that is mainly caused by anthropogenic activities, most notably associated with urbanization and industry, especially metal mining (Tchounwou et al. 2012; Jones 2018). Although some metals (e.g., Cu, Zn, Fe, Mo, Mn, Se, and Co) are essential for biological processes in animals in trace amounts, metals can bioaccumulate, are not biodegradable, and are a health concern for aquatic biota inhabiting waterbodies contaminated with metals (Byrne et al. 2012; Taslima et al. 2022). Regulations and legislation exist for some metals in the form of concentration limits in environmental matrices for the protection of ecosystems (e.g., Canadian water quality guidelines; Canadian Council of Ministers of the Environment (CCME) 2007) which are primarily derived from laboratory-based animal toxicity tests with individual metals. However, aquatic organisms are most often exposed to multiple metals simultaneously in the natural environment, yet the toxic effects of metal mixtures to most aquatic wildlife is poorly understood (Saibu et al. 2018).
In prolonged exposure scenarios (e.g., subchronic or chronic), it has been demonstrated that individual administration of metals results in systemic distribution via circulation, and tissue accumulation beyond manageable thresholds causes a plethora of adverse health effects in fish that manifest below acutely lethal concentrations (Farrell et al. 2011; Paul and Small 2021; Brix et al. 2022). Although the mechanisms underlying the toxic effects of individual metals are not fully elucidated, a large body of evidence reinforces the notion that labile metals, including Cu, Cd, and Zn (Ratn et al. 2018; Santos et al. 2019; Shekh et al. 2019), interact with cellular targets and cause oxidative stress (e.g., lipid peroxidation, protein carbonylation, DNA damage, and general damage to cellular functions) which is likely to affect the physical condition of fish in the long-term (Baldwin et al. 2003; Farrell et al. 2011, 2012; Lushchak 2011; Al-Bairuty et al. 2013; Heydarnejad et al. 2013; Cunningham and McGeer 2016; Santos et al. 2019).
Although decades of research have been devoted to investigating the effects of multi-metal mixtures in aquatic biota, there is a need to study the toxicokinetic and toxicodynamic interactions that occur in the presence of multiple metals more thoroughly, especially the sublethal effects of low concentration, chronic exposures. Indeed, a substantial number of studies have examined the uptake/accumulation and toxic effects of metal mixtures (see reviews: Norwood et al. 2003; Vijver et al. 2011; Meyer et al. 2015), but most of these studies report acutely toxic effects and definitive patterns regarding interactive metal toxicity have not emerged. It is unclear whether the discrepancies between the conclusions drawn from controlled laboratory experiments are due to the metal combination, exposure duration, species, developmental stage, or endpoint assessed (Meyer et al. 2015; Van Genderen et al. 2015). For example, for mixtures containing Cu, Cd, and/or Zn, there is evidence of increased toxicity (Brinkman and Woodling 2014; Song et al. 2014; Driessnack et al. 2016), but also evidence of decreased toxicity (Chouchene et al. 2011, 2016) and no interactions (Marr et al. 1995; Dethloff et al. 1999) when administered in binary mixtures versus single metal constituents in fish. Nevertheless, with several studies demonstrating pronounced subchronic toxicity of Cu-Zn and Cu-Cd binary mixtures in fish (i.e., reduced survival and growth in brown trout (Salmo trutta; 126-day Cu-Zn exposure; Brinkman and Woodling 2014), impaired reproduction in fathead minnows (Pimephales promelas; 21-day Cu-Cd exposure; Driessnack et al. 2016), altered body condition and lipid metabolism in javelin gobies (Synechogobius hasta; 30-day Cu-Cd exposure; Song et al. 2014)), and histopathological changes concomitant with an increased oxidative stress in rare minnows (Gobiocypris rarus; 7-day Cu-Cd exposure; Wu et al. 2019), it is evident that Cu, Cd, and/or Zn pose increased risks to fish health when administered as mixtures compared to individual metals. Profound ecosystem-level impacts due to metal mixture exposures observed downstream of existing and especially decommissioned metal mines in Canada (e.g., Britannia Mine, BC, Canada; Barry et al. 2000; Zis et al. 2004; Levings et al. 2005) are evident based on decades of studies reporting fish population declines and poor fish health in biomonitoring surveys (e.g., reduced fish survival, growth, and reproductive success; Environment Canada 2012; Environment and Climate Change Canada 2015). However, methodologies aimed at multi-metal mixture risk assessments are not adequately established in regulatory frameworks (Van Genderen et al. 2015). Ultimately, toxicity data evaluating the adverse effects of metal mixtures in laboratory and field scenarios using consistent/standardized methods on a range of fish species, and beyond acute duration, is necessary to more accurately determine the sublethal effect thresholds of metal mixtures that translate into significant risks to fish populations.
The co-occurrence and concentrations of metals (e.g., Cd, Pb, Cr, Hg, Zn, Cu, Ni, Al, Fe, Mn, As, and Co) have reportedly increased over time on a global scale via numerous anthropogenic sources (Zhou et al. 2020). For example, Cu, Cd, and Zn have increased greater than twenty-fold over the last five decades based on assessments of polluted freshwater waterbodies across five continents, with mean concentrations reported to increase from 5.9 μg/L Cu, 0.8 μg/L Cd, and 52.2 μg/L Zn in the 1970s to 120.0 μg/L Cu, 25.3 μg/L Cd, and 1180.1 μg/L Zn in 2017 (Zhou et al. 2020). In British Columbia, background concentrations of Cu, Cd, and Zn were reported as high as 71 μg/L Cu, 8.6 μg/L Cd, and 20 μg/L Zn in highly mineralized pristine freshwater environments (CCME 2014, 2018, 2021; B.C. MOE 2019, 2022). One study in North America reported metal concentrations downstream of a decommissioned metal mine ranging from 52–4600 μg/L Cu, 1.2–10.7 μg/L Cd, and 140–1471 μg/L Zn depending on distance from a source of acid-mine drainage (Vermont, USA; Balistrieri et al. 2007). Another study conducted within an urban Canadian setting (Greater Vancouver, British Columbia) reported that streams receiving urban runoff contained Cu, Cd, and Zn at concentrations as high as 404 μg/L Cu, 10 μg/L Cd, and 1250 μg/L Zn (Huang and Gergel 2022). Indeed, metals often exist as mixtures at concentrations that frequently exceed environmental quality guidelines which are conversely derived on an individual-metal basis. With the paucity of toxicity data available with respect to controlled experiments examining environmentally relevant metal mixture exposure scenarios, the adverse health risks to aquatic wildlife inhabiting these waters are largely uncertain.
Salmonids are a primary concern with respect to the environmental impacts of metal contamination in North America because their habitat often overlaps accessible mineral reserves and mining tenures, in addition to urban and industrial areas (Sergeant et al. 2022). Among 70 naturally occurring metals, Cu is one of the most ubiquitous metal contaminants of concern, and its large-scale extraction combined with frequent anthropogenic inputs into North American waterways justifies extensive past efforts and continued research to better characterize this metal’s toxicity, with and without common metal co-contaminants such as Cd and Zn (Vijver et al. 2011; Van Genderen et al. 2015; B.C. MOE 2019). In Canada, under the Fisheries Act as of 2012 (https://laws-lois.justice.gc.ca/eng/acts/f-14/), metal and diamond mines are required to conduct environmental effects monitoring studies to determine the potential impacts of mine effluents discharged into the aquatic environment. These field-based studies report on the toxic effects of whole mine effluents in fish, invertebrates, and plants that frequently manifest as reductions in survival, growth, and reproductive success (Environment Canada 2012; Environment and Climate Change Canada 2015), though uncertainty remains as to how metals interact to modulate organism responses and this makes a priori assessments of metal mixture toxicity an ongoing challenge (Meyer et al. 2015). Interestingly, relatively few studies have investigated the comparative subchronic toxicity of metal mixtures in fish using concurrent metal mixture and single metal exposure experiments (Ouellet et al. 2013; Song et al. 2014; Driessnack et al. 2016, 2017), and very few studies have discretely examined the subchronic effects of Cu, Cd, and Zn mixtures in sensitive early developmental stages of salmonids (Brinkman and Woodling 2014). Considering the projected increases in the global production of metals, including Cu, Cd, and Zn (Jasansky et al. 2023), and the ubiquitous occurrence of multiple metals contaminating aquatic ecosystems worldwide, studies aimed at further characterizing the plethora of toxic effects of metal mixtures are necessary steps to ensure accurate risk assessments and appropriately derive protective environmental quality criteria and standards. Therefore, the objective of the present study was to compare and characterize the subchronic lethal and sublethal toxic effects of Cu and a mixture of Cu, Cd, and Zn in sensitive early life stages of rainbow trout by examining survival, growth, development, and tissue pathology.
Material and methods
Test organism
All-female triploid rainbow trout (Oncorhynchus mykiss) eyed embryos (Jumper strain, Kamloops, BC) were obtained from Troutlodge (Bonney Lake, WA, USA). Eggs were fertilized by the supplier on April 10, 2019, and embryos were received at Simon Fraser University (Burnaby, BC) on May 10, 2019. The accumulated thermal units (ATUs; mean daily temperature (°C) multiplied by days post fertilization (dpf)) of eyed embryos were approximately 300°-days (30 dpf) at the initiation of the exposure. All live organism work adhered to the Simon Fraser University Animal Care Protocol 1290B-18 and Simon Fraser University is accredited by the Canadian Council on Animal Care; moribund fish or fish exhibiting signs of pain and distress were euthanized accordingly.
Chemicals
All chemicals and reagents were obtained from ThermoFisher Scientific (Burnaby, BC, Canada) unless otherwise stated. Exposure water was prepared using dechlorinated municipal tap water amended with technical grade salts to achieve moderately hard water between 80 and 100 mg/L CaCO3 with a slightly alkaline pH (Environment Canada 2007). Concentrated metal stock solutions were prepared with stoichiometric equivalents of metal salts (CuSO4, CdCl2, and ZnSO4) for the highest test concentration. These solutions were subsequently used to make 1.5-fold serial dilutions to achieve stocks for the lower metal test concentrations. Stock solutions were stored in the dark at 4–6 °C. Fish exposure water was then prepared by adding 5 mL of the concentrated stock metal solution to 20 L of moderately hard dilution water. The waterborne metal exposure experiments included the following treatments: a moderately hard water control; a Cu only concentration series (31, 47, 70, and 104 μg/L Cu); and a metal mixture concentration series comprised of Cu (31, 47, 70, and 104 μg/L), Cd (4.1, 6.2, 9.3, and 14 μg/L), and Zn (385, 578, 867, and 1300 μg/L). Test concentrations were selected to reflect an environmentally relevant range that were expected to cause adverse apical responses spanning no effects, sublethal effects, and lethal effects (Wang et al. 2014). While test concentrations were above long-term Canadian Water Quality Guidelines (CCME 2014; 0.8 μg/L Cu, 0.14 μg/L Cd, and 10 μg/L Zn based on water chemistry measured in the present study; i.e., 86.6 mg/L CaCO3 water hardness, 8.11 pH, 1.31 mg/L dissolved organic carbon, 15.7 mg/L Ca, 11.7 mg/L Mg, 28.4 mg/L Na, 2.56 mg/L K, 4.54 mg/L Cl, 70 mg/L CaCO3 alkalinity), the lowest test concentrations do reflect natural metal concentration ranges reported in Canadian freshwater systems. In addition, the high test concentrations were near the maximum allowable effluent discharge limits specified in the Metal and Diamond Mining Effluent Regulations (MDMER, Schedule 4; monthly mean concentrations for mines operational before and after June, 2021, respectively, are 100 and 300 μg/L for Cu and 400 and 500 μg/L for Zn) and common discharge limits set under the B.C. Environmental Management Act (SBC 2003, c 53 EMA; e.g., see B.C. Gazette Authority 2016).
Rainbow trout laboratory exposure to metals
The metal exposure experiments were initiated when rainbow trout were at the eyed embryo life stage and continued for 31 days until ~90% of the control fish had reached the swim-up fry development stage (i.e., when visual inspection indicated complete yolk sac reabsorption; 61 dpf or 678°-days). These waterborne exposure experiments were conducted with an experimental design adapted from the Environment Canada protocol Biological Test Method: Toxicity Tests Using Early Life Stages of Salmonid Fish (Rainbow Trout) (Environment Canada 1998). Metal treatments were administered in quadruplicate 10 L glass test vessels filled with 6 L of exposure water. Thirty eyed embryos were randomly allocated to each replicate vessel. The temperature was regulated at 12 °C by circulating chilled water through a four-chamber water bath system assembled in a randomized complete block design. Test vessels were supplied with constant aeration and a period of complete darkness was implemented until one week after the control fish hatched, at which point a 16:8-hour light (100–500 lux) to dark photoperiod was implemented. For each replicate, the overall health, hatching, and survival of individuals were visually monitored twice daily. Water changes were completed three-times-weekly by renewing 80% of the volume with freshly prepared control water or metal solutions. Water quality, including dissolved oxygen (DO), conductivity, temperature, and pH, was measured before and after water renewals in a single replicate vessel within each treatment group. Ammonia was measured weekly using the Salicylate Method (Hach Canada, London, Ontario; Catalog No. 2668000-CA). To analytically verify nominal metal concentrations and water hardness (as CaCO3) for all treatment groups including the control, 60 mL of water was collected immediately after water renewal from a single replicate test vessel per treatment group and submitted to ALS Environmental (Burnaby, BC, Canada) for analysis. Inductively coupled plasma mass spectrometry (ICP-MS) was performed on unfiltered and filtered (0.45 μm syringe filters) water samples to measure total and dissolved metals, respectively (Martin et al. 1994; EPA 1998).
Fish were sacrificed with a lethal dose of tricaine methanesulphonate (MS222; Western Chemical, Ferndale, WA) buffered with sodium bicarbonate to pH 7. Wet weight (wwt), fork length, deformities, and developmental stage were recorded for individual fish. Development was evaluated based on visual external survey of yolk sac resorption and categorized as having: 1, a completely resorbed yolk sac where the abdomen was completely covered by a fully formed epidermis; 2, a lack of epidermis joining at the mid-ventral longitudinal plane of the fish and some visible yellow or orange yolk; or 3, a lack of epidermis joining at the mid-ventral longitudinal plane of the fish and prominent protruding yellow or orange yolk sac, as depicted in Fig. S1 (Hegeman and Marlatt 2021). Two fish per replicate vessel were pooled as a single composite sample (n = 4 samples per treatment) and then submitted to ALS Environmental (Burnaby, BC) for whole-body metals analysis by inductively coupled plasma mass spectrometry (EPA 1998). An additional two fish per replicate test vessel (n = 8 per treatment) were submitted for tissue processing. Due to high mortality and insufficient tissue quantities, the highest metal mixture treatment was excluded from microscopic analysis and both the highest Cu only and metal mixture treatments were excluded from tissue metal analyses.
Histopathology
In a subset of two euthanized fish per replicate test vessel (n = 8 per treatment; excluding 104 + 14 + 1300 μg/L Cu+Cd+Zn treated fish), an incision was made along the ventral body wall, 10% neutral buffered formalin (NBF) was directly applied to the gills via a syringe, and then the whole fish were immersed in 10% NBF. Formalin-fixed fish were dehydrated, embedded in paraffin wax, step sectioned along the sagittal plane (5 μm thickness), and stained with hematoxylin and eosin (H&E) by Wax-It Histology Services Inc. (Vancouver, BC). Glass slides containing multiple step sections through the whole bodies of 64 fish were evaluated microscopically by a board-certified veterinary pathologist. For every examined fish, the severity of each microscopic finding was scored as follows: absent (0), minimal (1), mild (2), moderate (3), or marked (4). When a microscopic finding was observed in more than one step section in the same fish, the most severe lesion was scored. Pertinent microscopic findings in the gills, liver, kidney, and coelomic cavity are presented and discussed.
Data analysis
All statistical analyses used to evaluate the lethal and sublethal toxicity of metal treatments in early life stage rainbow trout were performed in the R Studio Environment (R Core Team 2022). Data were initially analyzed by plotting the individual responses as a function of treatment concentration; outliers were evaluated by Tukey’s outlier rule and only omitted if deemed sufficiently unusual based on biological grounds (OECD 2016). Data for each endpoint was tested for normality and equal variances using Shapiro-Wilk and Levene’s homogeneity of variance procedures. Data that did not satisfy parametric assumptions were log-transformed and retested. Where relevant, model diagnostics and validation were performed with the R package DHARMa (Hartig 2021) using residual plots, quantile-quantile plots, Kolmogorov–Smirnov tests for normality, and Levene’s test for equal variance. Evidence of an effect was accepted at the 5% level for all statistical analyses (p < 0.05).
Generalized linear mixed models (GLMM; binomial distribution, logit link) were used to test if metal treatments affected cumulative survival (R package lme4; Bolker et al. 2009; Bates et al. 2015). Linear mixed models (LMM; lme4) were performed on the weight, length, and Fulton’s condition factor (weight × 100 ÷ length3; Fulton 1904) for non-deformed individuals. Application of GLMMs and LMMs followed procedures outlined by Bolker et al. (2009) and Bates et al. (2015) with the general syntax: response ~ treatment + block + (1|tank), where the non-independence of observations within replicate vessels was preserved with a tank random effect and block was included as a fixed effect given the small number of levels (n = 4). The median lethal concentration (LCx), sublethal effect concentration (ECx), time to mortality (LTx), and time to hatch (Hx) were estimated by fitting Weibull type 2 models using the R package drc (Ritz et al. 2015). To evaluate whether treatments affected development, a generalized estimating equation (GEE; R package geepack; Halekoh et al. 2006) was fit to proportion yolk sac resorption score data with a binomial family, logit link, and clustering parameter to account for non-independent observations as described in Hothorn (2016). Differences in the mean whole-body rainbow trout concentrations of exposure metals (Cu, Cd, and Zn) and electrolytes (Ca2+, Mg2+, K+, and Na+) were evaluated with a one-way analysis of variance (ANOVA); an analysis of covariance (ANCOVA) tested if there was a difference in linear relationship between aqueous and whole-body Cu concentration when fish were exposed to Cu only versus as a mixture of Cu with Cd and Zn. The Rao-Scott Cochran-Armitage by Slices (RSCABS) procedure, first described by Green et al. (2014) and endorsed by the Organisation for Economic Co-operation and Development (OECD 2015; Elmore et al. 2017), was developed specifically to analyze concentration-response relationships of histopathology endpoints in toxicological studies. The RSCABS procedure was therefore applied to evaluate differences in the incidences and severity of lesions within each of the Cu only and metal mixture categories (R package RSCABS; Swintek 2018).
Results
Water quality and fish tissue chemistry
The water quality measured routinely throughout the experiment (i.e., temperature, pH, dissolved oxygen, conductivity, and ammonia) met the validity criteria prescribed by Environment Canada (1998) for this in vivo rainbow trout bioassay (Table 1). Analytical measurements for total Cu, Cd, and Zn in metal exposure treatments collected from test vessels immediately after water renewals were between 91–98, 89–98, and 93–101% their nominal targets, respectively. Relative to total values, Cu was 86–92% dissolved in Cu only and Cu, Cd, and Zn mixture treatments; Cd was 96–100% dissolved and Zn was 90–98% dissolved in metal mixture treatments (Table 2). Trace concentrations of Cu, Cd, and Zn were detected in the dechlorinated municipal tap water control and reflect background concentrations of the water source. The hardness (as CaCO3) was similar between all treatment groups with a mean of 86.6 and a standard deviation (SD) of 1.3 mg/L CaCO3 (range = 83.8–87.9).
Median whole-body Cu and Zn concentrations (95% CI) in control fish were measured to be 1.00 mg/kg (0.76–1.33) and 17.6 mg/kg (15.1–20.5), respectively, whereas Cd was near or below its detection limit (DL = 0.0020 mg/kg). The untransformed whole-body Cu, Cd, and Zn concentrations are presented with respect to dissolved exposure concentrations for Cu only and the metal mixture treatments (Fig. 1). Whole-body metal accumulation was concentration dependent such that there was a positive linear relationship between dissolved metal concentrations and log-transformed whole-body metal concentrations for Cu (r2 Cu only = 0.86 and r2 metal mixture = 0.85; p < 0.0001), Cd (r2 metal mixture = 0.99; p < 0.0001), and Zn (r2 metal mixture = 0.92; p < 0.0001). Cu was significantly elevated in all metal exposed fish relative to the control (p < 0.0001; Fig. 1a), equating to median fold increases of 2.3, 2.5, and 4.4 in treatments of 31, 47, and 70 μg/L Cu only and 1.9, 3.9, and 4.3 in metal mixtures containing nominally equivalent amounts of Cu, respectively. There was no evidence of an interaction between treatments of Cu only and the metal mixture on whole-body Cu content (p = 0.3537 for non-parallel slopes) and thus no indication that Cd and Zn influenced whole-body Cu accumulation. Fish exposed to the first three concentrations of the metal mixture did however experience significant, concentration-dependent whole-body Cd (fold increases of 174–230) and Zn (fold increases of 2.0–2.9) accumulation relative to unexposed controls (p < 0.0001; Fig. 1b, c).
Whole-body (a) Cu, (b) Cd, and (c) Zn concentrations (wet weight) in non-feeding Oncorhynchus mykiss swim-up fry exposed to treatments of Cu only or Cu, Cd, and Zn mixtures for 31 days from the eyed embryo developmental stage. The mean whole-body metal concentration and 95% confidence intervals are presented for four samples per treatment (n = 4), where one sample refers to a composite sample of two fish and treatment is expressed as measured dissolved metal concentrations in the exposure water. Significant differences in metal concentrations between treatments are indicated by different letters (one-way ANOVA performed on the log-scale followed by a Tukey’s post hoc test; p < 0.05). There was no evidence that Cu uptake differed between Cu only treatments and Cu, Cd, and Zn mixture treatments containing nominally equivalent concentrations of Cu (ANCOVA; p = 0.3537 for non-parallel slopes)
Significant treatment-related differences in whole-body concentrations of Mg2+ (p = 0.0117) and Ca2+ (p = 0.0001), but not Na+ (p = 0.746) and K+ (p = 0.951), were associated with high concentration metal treatments (data not shown). Fish reared in the 70 μg/L Cu and 70 + 9.3 + 867 μg/L Cu+Cd+Zn treatments had 30 and 34% reductions in the mean Ca2+ whole-body concentrations compared to levels measured in unexposed control fish (2032 mg/kg 95% CI 1797–2268; data not shown). A 21% increase in mean whole-body Mg2+ was observed in the 70 + 9.3 + 867 μg/L Cu+Cd+Zn treatment relative to the control fish (246 mg/kg 95% CI 225 – 270), but Mg2+ content was not significantly influenced in other treatments (data not shown).
Survival
The mean survival of rainbow trout reared in the control group (mean proportion survival = 0.96, 95% CI = 0.90 – 0.98) was well above the Environment Canada (1998) toxicity test method validity criteria upon termination of the exposure. Treatments of 31 μg/L Cu, 47 μg/L Cu, and 31 + 4.1 + 385 μg/L Cu+Cd+Zn did not significantly affect survival. All other treatments were associated with significantly reduced survival after 31 days of exposure (p = <0.0001; Fig. 2a; Supplementary Fig. S1 and Table S1) with mean proportions (95% CIs) of surviving individuals as follows: 70 μg/L Cu = 0.65 (0.55–0.74) and 104 μg/L Cu = 0.38 (0.29–0.49); 47 + 6.2 + 578 μg/L Cu+Cd+Zn = 0.85 (0.76–0.91), 70 + 9.3 + 867 μg/L Cu+Cd+Zn = 0.38 (0.29–0.49), and 104 + 14 + 1300 μg/L Cu+Cd+Zn = 0.06 (0.03–0.12). The effects of treatments on survival were dependent on metal concentration such that there was evidence that the concentration-mortality curves for Cu only and metal mixture treatments were dissimilar (p = 0.0016; Fig. 2a). In relation to dissolved Cu in the exposure water, the lethal effect concentrations (LCx) were lower for metal mixture treatments than for Cu only treatments (p = 0.0067; Table 3). Reduced survival was driven by spikes in mortality coinciding with hatching on exposure day 4 through exposure day 10 (Fig. 2b, c). Metal mixture treatments significantly reduced the median time to mortality (LTx) compared to equivalent concentrations of Cu only (p < 0.0001; pairwise differences reflected by non-overlapping confidence intervals adjusted for simultaneous inference; Table 4), for instance, at the 25% effect level (LT25): 70 μg/L Cu = 29.0 days (25.9 – 32.1) versus 70 + 9.3 + 867 μg/L Cu+Cd+Zn = 6.8 days (6.1–7.4); 104 μg/L Cu = 9.2 days (8.4–10.1) versus 104 + 14 + 1300 μg/L Cu+Cd+Zn = 3.9 days (3.6 – 4.2).
Survival of Oncorhynchus mykiss from eyed embryo life stage to swim-up fry life stage exposed to waterborne treatments of Cu only or a Cu, Cd, and Zn mixture (n = 4 replicate test vessels per treatment; 30 fish per vessel), where each treatment contained 120 fish at day 0 of the experiment. a Fitted concentration-response curves and data points depicting the mean cumulative proportion of dead fish and 95% confidence intervals based on measured dissolved Cu concentrations (Weibull type 2 model). There was evidence that the concentration-mortality curves for Cu only and metal mixture treatments were dissimilar (one-way analysis of variance; p < 0.05); different letters denote significant differences in proportion mortality between treatment groups (GLMM; p < 0.05). b Cu only and c Cu, Cd, and Zn mixture daily mean proportion of surviving fish and 95% confidence intervals for nominal treatment groups. Significant reductions in survival for fish reared in treatments of Cu (70 and 104 μg/L Cu) and Cu, Cd, and Zn mixture (47 + 6.2 + 578 μg/L Cu+Cd+Zn, 70 + 9.3 + 867 μg/L Cu+Cd+Zn, and 104 + 14 + 1300 μg/L Cu+Cd+Zn) relative to the control occurred by days 5–7 and remained significant until termination on exposure day 31 (GLMM; p < 0.05)
Growth
Rainbow trout reared in the control treatment had a mean (95% CI) wet weight, length, and Fulton’s condition factor of 0.145 g (0.141–0.149), 28.4 mm (27.2–29.6), and 0.64 K (0.464–0.816), respectively. Effects on rainbow trout growth were concentration dependent but there was no evidence the concentration-response curves and effect concentration parameters (ECx) were different for weight and length endpoints between treatments of Cu only and the Cu, Cd, and Zn mixture (ANOVA, p > 0.05; Table 3). A significant effect of treatment on weight was observed at concentrations as low as 47 μg/L Cu and 47 + 6.2 + 578 μg/L Cu+Cd+Zn (p = <0.0001; Fig. 3a; Table S1). Decreased weight of fish reared in the 47 μg/L, 70 μg/L, and 104 μg/L Cu only treatments equated to respective percent differences (95% CI) of 7.3% (3.9–10.7), 17.9% (14.1–21.6), and 26.8% (22.6–31.1) as compared to unexposed control fish; and percent differences in 47 + 6.2 + 578 μg/L, 70 + 9.3 + 867 μg/L, and 104 + 14 + 1300 μg/L Cu+Cd+Zn metal mixture groups were 4.2% (0.6–7.7), 14.4% (10.0–18.7), and 32.0% (21.3–42.6), respectively, as compared to unexposed control fish (Fig. S1). The mean length was significantly reduced at concentrations as low as 70 μg/L Cu and 70 + 9.3 + 867 μg/L Cu+Cd+Zn (p = <0.0001; Fig. 3b; Supplementary Table S1), while a significant effect on K was only observed in the highest test concentrations of Cu only and the Cu, Cd, and Zn mixture (p = 0.0143; data not shown).
Weight (a) and length (b) of Oncorhynchus mykiss swim-up fry following a 31-day exposure to laboratory water and nominal treatments of Cu only or a Cu, Cd, and Zn mixture, each seeded with 30 eyed embryos in 4 replicate test vessels (120 fish in total) at test initiation. Box plots depict the medians (horizontal line), lower and upper 25th and 75th percentiles (boundaries of the box), minimum and maximum values (whiskers), and outliers (data points outside the whiskers) for individual wet weight and fork length measurements made on surviving fish (data points) in 4 replicate vessels per treatment (except 104 + 14 + 1300 μg/L Cu+Cd+Zn: 3 replicate vessels). Different letters denote significant differences between treatment groups (LMM; p < 0.05)
Development
The proportion of embryos that successfully hatched is depicted as a function of time for each treatment group (Fig. 4). The hatch curves were dissimilar with respect to the control, Cu only, and the metal mixture treatments (p < 0.0001). Embryos began hatching on exposure day 4 or 5 (33–34 dpf) regardless of treatment but there was a significant interaction between treatment and time on the proportion of embryos hatched (p < 0.0001). For example, the estimated time to reach 90% hatch (H90 with 95% CIs adjusted for simultaneous inference; Table S2) was approximately 1 day earlier for fish exposed to 104 μg/L Cu (6.1 95% CI 6.9 – 7.2) and 1 to 2 days later for fish exposed to all metal mixture treatments (31 + 4.1 + 385 μg/L Cu+Cd+Zn = 8.5 95% CI 8.3–8.7; 47 + 6.2 + 578 μg/L Cu+Cd+Zn = 8.4 ± 8.2–8.6; 70 + 9.3 + 867 μg/L Cu+Cd+Zn = 8.2 95% CI 8.0–8.4; 104 + 14 + 1300 μg/L Cu+Cd+Zn = 8.9 95% CI 8.6–9.2) compared to the control (7.0 95% CI 6.9–7.2). Fish in the control and Cu only treatments were completely hatched by exposure days 8 or 9 (37 or 38 dpf). However, the mean proportion of embryos hatched on day 5 in the 104 μg/L Cu treatment (0.48 95% CI 0.42–0.54), and on day 6 in the 70 μg/L Cu (0.53 95% 0.46 – 0.59) and 104 μg/L Cu (0.90 95% CI 0.84–0.96) treatments, was significantly higher than the control treatment (0.28 95% CI 0.22–0.35 and 0.34 95% CI 0.28–0.40) on day 5 and 6, respectively; Fig. 4a; (p < 0.05). The hatch period for all metal mixture treatments was prolonged to exposure days 11–13 (40–42 dpf), resulting in significantly decreased proportions of embryos hatched relative to the control from exposure day 5 through day 8 (Fig. 4b; p < 0.05).
Hatching as a function of time (exposure day) for Oncorhynchus mykiss exposed to waterborne treatments of (a) Cu only or (b) a Cu, Cd, and Zn mixture. Embryos were 30 days post-fertilization (accumulated thermal units = 300°-days) at the time the experiment was initiated. Curves and 95% confidence intervals were fitted using four-parameter Weibull type 2 models across the first 13 days of the experiment. Data points represent the mean proportion hatched of 4 replicate test vessels (30 fish per replicate, 120 fish in total) for each treatment. Relative to the control, treatments of Cu only (70 μg/L Cu and 104 μg/L Cu) significantly increased the proportion of embryos hatched on exposure days 5-6 (p < 0.05). All Cu, Cd, and Zn mixture treatments significantly decreased the proportion of embryos hatched on days 5–8 (p < 0.05)
Swim-up fry development assessed via visual inspection of the degree of yolk sac resorption indicated 98% (95% CI 95–99%; Fig. 5; Supplementary Table S3) of control fish had reached the target developmental index at the end of the exposure (e.g., a yolk sac score of 1 indicating a fully formed epidermis and no visible yolk). Fish reared in the two lowest test concentrations of Cu only and metal mixture treatments demonstrated patterns of development comparable to the control, but higher metal concentrations elicited significant concentration-dependent effects on development via an inhibition of yolk sac resorption (p < 0.0001). The frequency of fish having a fully formed epidermis and no visible yolk was reduced to 78% (55–91%) and 73% (58–85%) in 70 μg/L Cu and 70 + 9.3 + 867 μg/L Cu+Cd+Zn treatments, respectively, with concordant 20–22% increases in the number of fish presenting with slight epidermal separation at the mid-ventral longitudinal plane and some visible yolk (yolk sac score 2; Fig. 5). Development impairments were more pronounced in the 104 μg/L Cu only treatment group such that 88% of fish were underdeveloped on average (range of 78–94%; Fig. 5), of which 40% exhibited a yolk sac score of 2 and 46% had a score of 3 (e.g., lack of epidermal joining at the mid-ventral longitudinal plane and a protruding yellow or orange yolk sac). In the 104 + 14 + 1300 μg/L Cu+Cd+Zn treatment, 39% (11–77%) and 60% (23–88%) had a yolk sac score of 2 and 3, while no survivors had reached the target development (Fig. 5). Thus, there is some evidence of intensified effects by Cd and/or Zn on Cu-induced developmental impairments at high concentrations but not at low concentrations.
Proportion of Oncorhynchus mykiss swim-up fry at various stages of development at the termination of 31-day waterborne Cu only or a Cu, Cd, and Zn mixture exposure experiments. Experiments were initiated when fish were eyed embryos (n = 4 replicate test vessels per treatment; 30 fish per vessel; 120 fish in total) and terminated when control fish had completely absorbed their yolk sacs upon visual inspection. Developmental scores correspond to the degree of yolk sac resorption: 1, a completely resorbed yolk sac where the abdomen is completely covered by a fully formed epidermis; 2, a lack of epidermis joining at the mid-ventral longitudinal plane of the fish and some visible yellow or orange yolk; or 3, a lack of epidermis joining at the mid-ventral longitudinal plane of the fish and a prominent protruding yellow or orange yolk sac. The bars represent the mean proportion of surviving individuals of 4 replicate test vessels per treatment (except 104 + 14 + 1300 μg/L Cu+Cd+Zn: n = 3 replicate vessels) scored as 1, 2, or 3 for yolk sac reabsorption. Letters denote significant differences between treatments in the mean proportion yolk sac score 1 (generalized estimating equation; p < 0.05)
Histopathology
Microscopic examination of the whole-body of 64 rainbow trout swim-up fry (n = 8 fish per treatment, excluding 104 + 14 + 1300 μg/L Cu+Cd+Zn treated fish) revealed lesions in the gills, kidney, and liver. Incidence and severity data for major findings are depicted in Fig. 6 and summarized below with respect to the statistical analysis of concentration-response trends. A more complete histological assessment and the summary statistics are provided in Supplementary Tables S4–S9.
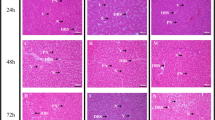
Gill, kidney, and liver lesions in Oncorhynchus mykiss swim-up fry exposed to 31 days of waterborne treatments of Cu only or a Cu, Cd, and Zn mixture. a Branchial cavity, gill arch, and/or gill raker epithelial hyperplasia (BGH); (b) gill filament fusion (GFF); (c) increased karyorrhexis in gill filaments and/or lamellae (GFK); (d) gill lamellar epithelial hyperplasia and/or hypertrophy (GLH); (e) renal tubular epithelium cytoplasmic hyaline droplets (RTH); and (f) hepatocellular cytoplasmic vacuoles (VAC). Two fish from each of 4 replicate test vessels per treatment were collected, sectioned, and stained for whole-fish microscopic examination. The bars depict the proportions of individuals within each treatment exhibiting different lesion severity scores (n = 8 for all lesions/treatment groups except: nVAC = 7 for 31 and 104 μg/L Cu only; nRTH = 6 for 104 μg/L Cu only). Differences in the incidences of lesion severity scores were evaluated with the Rao-Scott Cochran-Armitage adjusted trend test By Slices (RSCABS). Significantly different treatment groups are denoted by * (p < 0.05)
Multiple gill lesions were observed in swim-up fry exposed to Cu only and Cu, Cd, and Zn mixture treatments. Gill lesions consisted of hyperplasia of epithelial cells lining the branchial cavities, gill arches and/or gill rakers (BGH), gill filament fusion (GFF), gill lamellar fusion (GLF), increased karyorrhexis in gill filaments and/or lamellae (GFK), gill lamellar epithelial hyperplasia and/or hypertrophy (GLH), and gill lamellar mucous cell hyperplasia (GMH). The incidence and/or severity of BGH, GFF, GFK, and GLH increased with increasing Cu concentrations as low as 47 μg/L in Cu only or Cu, Cd, and Zn mixture treatments relative to the control (Fig. 6a–d; Table S4); there was evidence of a concentration-response, as indicated by the RSCABS analysis (p < 0.05; Table S7). These lesions were absent in the control fish (Fig. 7a) and in the lowest metal treatments, apart from one fish treated with 31 μg/L Cu presenting with minimal lamellar mucous cell hyperplasia. A severely compromised gill of a fish treated with 70 + 9.3 + 867 μg/L Cu+Cd+Zn is depicted in Fig. 7b, showing gill lamellar epithelial cell hyperplasia and hypertrophy with gill lamellar fusion, gill filament fusion, and mucous cell hyperplasia. Although the 104 μg/L Cu only treatment elicited the most pronounced effects on the gills (note that 104 + 14 + 1300 μg/L Cu+Cd+Zn treated fish were not assessed), fish reared in the 70 + 9.3 + 867 μg/L Cu+Cd+Zn exhibited a higher incidence and severity of GLH, GLF, and GFF compared to 70 μg/L Cu only (Fig. 6a–d; Table S4 and Table S7) indicating that Cu-induced effects on gill histology were more pronounced when fish were co-treated with Cd and Zn.
Histopathologic findings in the gills, livers, and kidney (H&E staining) of Oncorhynchus mykiss swim-up fry following 31-day waterborne exposures to treatments of Cu only or a mixture of Cu, Cd, and Zn in the laboratory. a Control gill for comparison (red arrows point to gill filaments; 10x). b 70 + 9.3 + 867 μg/L Cu+Cd+Zn treated gill: diffuse gill lamellar epithelial cell hyperplasia and hypertrophy with lamellar fusion (black arrow); filament fusion (red arrows point to the tips of adjacent filaments that are partially fused together proximally), and multifocal mucous cell hyperplasia (blue arrow; 20x). c 70 + 9.3 + 867 μg/L Cu+Cd+Zn treated kidney: tubular epithelial cytoplasmic hyaline droplets (black arrows; 40x) and tubular epithelial hydropic degeneration (red arrows); these renal lesions were absent in the kidneys of control fish. d Control liver for comparison (black arrow points to a hepatocellular cytoplasmic vacuole; 20x). e 104 μg/L Cu treated liver: increased number of hepatocellular cytoplasmic vacuoles (black arrows; 40x)
Renal changes were observed in fish exposed to metal treatments. Specifically, renal tubular epithelial cytoplasmic hyaline droplets (RTH) and renal tubular epithelial hydropic degeneration (RTD) were identified in varying numbers of fish in the Cu only and metal mixture treatments (Table S5). Two 104 μg/L Cu treated fish with RTH exhibited ruptured yolk sacs (data not shown) and these fish were not included in the statistical analyses for RTH since RTH was considered a sequela to the ruptured yolk sacs. The prevalence of minimal RTH in 70 + 9.3 + 867 μg/L Cu+Cd+Zn treated fish was significantly increased relative to the control and all other treatment groups based on the RSCABS analysis (p < 0.05; Fig. 6e; Table S8). RTH was often accompanied by RTD as shown in Fig. 7c. Despite no clear relationship between treatment concentration and the incidence and severity of RTH and RTD in exposed fish, neither of these lesions were observed in the renal tubules in any control fish.
Hepatocellular cytoplasmic vacuoles were evident in all microscopically examined livers (n = 7 for 31 and 104 μg/L Cu treatments; all other treatments n = 8; Table S6). All fish reared in the control treatment had minimal liver vacuolation (Fig. 7d). In contrast, fish exposed to Cu only treatments containing 70 μg/L and 104 μg/L Cu and mixture treatments containing 70 + 9.3 + 867 μg/L Cu+Cd+Zn, but not lower concentration treatments, experienced mild to moderate levels of liver vacuolation (Fig. 7e), which were significantly increased as compared to the control (p < 0.05; Fig. 6f; Table S9). However, there was no evidence indicating increased liver vacuolation was concentration dependent.
The amount of adipose tissue that was visible microscopically in the coelomic cavity was scored in each fish (Table S6). Sixty-three percent of control fish had mild amounts of fat in the coelomic cavity, while the remaining control fish had minimal or no fat. Fish treated with 31 μg/L Cu, 31 + 4.1 + 385 μg/L Cu+Cd+Zn, or 47 + 6.2 + 578 μg/L Cu+Cd+Zn did not exhibit a significant reduction in coelomic fat as compared to the control. In contrast, the amount of coelomic fat in fish treated with 47, 70, 104 μg/L Cu, and 70 + 9.3 + 867 μg/L Cu+Cd+Zn was significantly reduced relative to the control such that fat for fish reared in these treatments was either absent or minimal (p < 0.05; Table S9).
Other notable findings that were observed in the 104 μg/L Cu treatment group included a ruptured yolk sac in two fish (n = 2; data not shown), one of which also exhibited ventricular myocardial hypertrophy with hydropericardium and a unilateral cataract (n = 1; data not shown).
Discussion
The present study employed an eyed embryo to swim-up fry standardized toxicity test procedure to characterize and compare the subchronic effects of Cu to a Cu-Cd-Zn mixture in rainbow trout by examining survival, growth, development, and histopathology. The findings of the present study demonstrated significant mortality following exposure to ≥70 μg/L Cu only (31-day LC25 = 46.3 μg/L dissolved Cu) and ≥47 μg/L Cu when tested as a mixture (LC25 = 32.9 μg/L dissolved Cu) containing ≥6.2 ug/L Cd and ≥578 Zn. Body weight reductions were more sensitive than lethality but only when Cu was administered individually. Interestingly, there was no evidence of a difference between Cu only and metal mixture treatments for any of the growth parameters assessed (e.g., body weight EC10, Cu = 55.0 μg/L Cu versus EC10, Cu+Cd+Zn = 58.9 μg/L Cu). Treatments of Cu only and the metal mixtures that contained ≥70 μg/L Cu impaired development via reduced yolk sac resorption with more pronounced effects of the mixture at the highest concentrations tested. Overall, the most sensitive endpoint was hatching rate, whereby hatching delayed in all metal mixture treatments but none of the Cu only treatments. Fish exhibited an increased incidence and/or severity of lesions in multiple tissues, in some instances at lower concentrations than those causing significant lethality. Gill lesions were notably severe in high concentration treatments, and often more severe in fish treated with mixtures relative to equivalent Cu treatments. The present study presents novel data characterizing the lethal and sublethal toxicity thresholds for three commonly co-occurring metal pollutants (e.g., Cu and a Cu-Cd-Zn mixture) in terms of water and whole-body concentrations in a non-feeding early life stage salmonid. The comparative toxicity of Cu to a mixture of Cu, Cd, and Zn on multi-organ histopathology and development has not been investigated in early life stage salmonids; by linking these effects to survival and growth indices considered by regulators, this information is applicable to environmental monitoring and risk assessments of fish-bearing aquatic environments polluted with metals.
There was a strong positive relationship between dissolved metal concentrations in exposure water and metal concentrations in the whole bodies of non-feeding early life stage rainbow trout following this 31-day waterborne exposure, and the presence of Cd and Zn in exposure water did not alter whole-body Cu bioconcentration. Consistent with this observation, Driessnack et al. (2016; 2017) reported no evidence of an interaction between Cu-Cd (75 μg/L and 5 μg/L) and Cd-Zn (75 μg/L and 5 μg/L) mixtures on whole-body accumulation in adult fathead minnow after 21 days of exposure, although Cd-Zn mixture exposures reduced Cd accumulation in both the gill and liver tissue and Cu-Cd mixture exposures reduced Cu accumulation in liver but not gill tissue (Driessnack et al. 2016, 2017). These fathead minnow studies by Driessnack et al. (2016; 2017) are consistent with competitive Cd-Zn and non-competitive Cu-Cd uptake behaviors on the gill surface (e.g., Cu via epithelial Na+ channels; Cd and Zn via apical epithelial calcium channels; Farrell et al. 2011, 2012) and additionally suggest that metal-metal interactions are limiting hepatic Cu and Cd accumulation. Here, whole-body thresholds corresponding to significant weight and survival reductions were similar to those reported in rainbow trout feeding fry exposed to Cu for 56 days (e.g., 2- and 3-fold whole-body Cu increases corresponding to reduced weight and survival, respectively; Hansen et al. 2002). While interactions between Cu, Cd, and/or Zn may influence the accumulation of each metal within target tissues and thus modulate tissue-specific and linked whole-organism effects (Song et al. 2014; Driessnack et al. 2016, 2017), the present study demonstrated a relationship between intensified effects on survival, development, and tissue-level histology and Cu accumulation that was unimpeded by co-uptake of Cd and Zn. These findings are therefore relevant to future environmental effects monitoring studies measuring field collected whole-body concentrations of Cu, Zn, and Cd in early life stage rainbow trout in that lethal and sublethal toxicity thresholds were identified and associated with water and whole-body Cu, Zn, and Cd metal concentrations.
The cumulative mortality and the rate of mortality was lower when Cu was administered alone versus in a mixture with Cd and Zn based on significantly different LCx, LOEC, and LTx values in the present study, which generally corroborates a subchronic study with brown trout and an acute study with rainbow trout. In continuous subchronic exposure experiments ( > 67 days, from embryos to swim-up fry) with brown trout, Brinkman and Woodling (2014) found that a Cu-Zn binary mixture (15 μg/L and 400 μg/L), but not a Cd-Zn binary mixture (1 μg/L and 400 μg/L), was more lethal than either metal individually at the same concentration with most of the mortality occurring after the embryos had hatched (Brinkman and Woodling 2014). Sharp declines in survival within the first four to five days after hatching in the present study suggests an acutely lethal effect by high concentration treatments. Likewise in single-metal exposures, Wang et al. (2014) observed significantly reduced survival of 1 day post-hatch (dph) rainbow trout during the first four days of exposure to Cu (96-hour LC50 = 60 μg/L Cu) and only small differences between the LC20s reported after 21 days (LC20 = 43 μg/L Cu) and 52 days (LC20 = 36 μg/L Cu) indicate these estimates were driven by mortality occurring within an acute timeframe; whereas Cd reduced survival steadily over time (e.g., 21- and 56-day LC20s of 12 and 5.3 μg/L Cd, respectively) and Zn was not lethal at the highest concentration tested (e.g., 56-day LC20 = > 755 μg/L Zn; Wang et al. 2014). Although it was beyond the scope of the present study to discern if additive, antagonistic, or synergistic effects of Cu, Cd, and Zn occurred, the intensified effects on cumulative survival may represent a less than additive effect given the ~30% lower 31-day LCx values for co-treatments of Cu, Cd, and Zn (e.g., LC25, Cu = 46.3 μg/L Cu versus LC25, Cu+Cd+Zn = 32.9 μg/L Cu). Supporting this, Naddy et al. (2015) explicitly examined Cu, Cd, and Zn additivity in acute experiments with rainbow trout and found that these metals elicited a less than additive effect of 2.3 toxic units (TUs; where 1 TU = 96-hour LC50s of 91.2 μg/L Cu, 6.28 μg/L Cd, 304 μg/L Zn), representing a 23% reduction in magnitude relative to a strictly additive response (Altenburger 2015). Overall, the findings of the present study reflect previous data showing the relatively high sensitivity of early alevin life stages compared to embryonic life stages to the toxic effects of Cu, Cd, and Zn and their mixtures, emphasizing that this critical period of development warrants careful consideration when assessing the risks of metals.
The metal mixture did not elicit pronounced reductions in growth relative to when Cu was administered alone in the present study despite the increased frequency of mortality when Cu was administered with Cd and Zn. Nevertheless, with significant weight reductions occurring at equivalent or lower concentrations affecting survival, these data provide useful effect sizes for this eyed embryo to swim-up fry standardized procedure and indicate that the magnitude of effect is significant with respect to thresholds deemed high environmental risk (e.g., 25% survival and 20% weight reductions; Environment Canada 2012). One mechanism ascribed to the growth impairments induced by metals and other toxicants is the reallocation of energy expenditures away from growth and development towards detoxification/protective processes (e.g., MT induction; Sevcikova et al. 2011). For example, exposures of brown trout from embryos to swim-up fry ( > 67 days) to co-treatments of 7.5 μg/L Cu and 200 μg/L Zn, but not 7.5 μg/L Cu alone, caused transient acute tolerance in subsequent 96-hour exposures at the expense of reduced body weight (e.g., 30% weight reduction translating to 1.9-fold LC50 increase; Brinkman and Woodling 2014). The degree of acute tolerance acquired by juvenile brown trout and rainbow trout was positively correlated to hepatic MT protein levels stimulated by previous exposures to sublethal concentrations of a metal mixture (24 μg/L Cu, 0.4 μg/L Cd, 46 μg/L Zn, and 0.64 μg/L Pb) for 3–5 weeks; the relatively high tolerance of brown trout (4-fold increase) occurred at the detriment of reduced weight, while the lower tolerance of rainbow trout (1.5-fold increase) did not translate to reduced weight (Marr et al. 1995). The attenuated acclimation response and lack of growth effects in rainbow trout could be attributed to the fact that, compared to brown trout, Cu and Zn were accumulated to a lesser degree and Cd did not accumulate to a measurable extent in the liver (Marr et al. 1995). Having not measured hepatic MT or tissue-specific metal accumulation in rainbow trout in the present study, the physiological basis underlying comparable Cu and metal mixture growth impairments that appear to be driven predominantly by Cu warrants further investigation.
The observed developmental impairments in rainbow trout in the present study corroborate several single metal toxicity studies in other salmonids that also report abnormal time to hatch and reduced yolk sac resorption. For example, in brook trout, Cu as low as 35 μg/L expedited hatching and decreased the rate of yolk sac reabsorption (McKim and Benoit 1971). Cd as low as 2 μg/L decreased the rate of Atlantic Salmon (Salmo salar) yolk sac resorption (Peterson et al. 1983) and conversely delayed the rate of rainbow trout hatching (Lizardo-Daudt and Kennedy 2008). The mechanism by which metals affect the hatching process is suspected to involve the egg envelope dissolution enzyme chorionase, whereby ionic imbalances caused by Cd and Cu can have an inhibitory or slightly stimulatory effect, respectively (Yamagami 1973; Santos et al. 2019), as observed in metal mixture (containing Cd) and Cu only treatments in the present study. Prior to exogenous feeding, embryonic and larval life stages of lecithotrophic fish species access energy-rich stores in their yolk to sustain somatic growth and development (Tocher 2003). This process involving intricate remodeling and transport of lipids from yolk to body is poorly understood from a toxicological perspective for metals as well as non-metal contaminants (Fraher et al. 2016). However, toxicants can interfere with transcriptional networks regulating the conversion of fish yolk to usable forms (Fraher et al. 2016; Sant and Timme-Laragy 2018), for example, by activating peroxisome proliferator-activated receptor gamma (PPARγ). Indeed, PPARγ activation was proposed as a pathway underpinning yolk sac retention in zebrafish embryos exposed to bisphenol A (Martínez et al. 2020) and posited to contribute to Cu-induced lipogenesis in fish livers (Zhong et al. 2022). It is therefore possible that reduced yolk sac resorption due to exposure to metal treatments in the present study was a manifestation of interference with similar yolk lipid remodeling or transport pathways upon which Cu, Cd, and/or Zn may disrupt.
The gill was the most severely affected tissue in the rainbow trout swim-up fry in the present study with several lesions exhibiting concentration-dependent increases in incidence and/or severity (e.g., as low as 47 μg/L Cu) that were often intensified by the metal mixture treatments compared to Cu individually. Toxic substances, such as metals, cause a nonspecific response to the gill that typically involves hyperplasia and hypertrophy of epithelial cells that line the surfaces of the gill. This is sometimes accompanied by increased numbers of mucous cells, chloride cells, and leukocytes and, in advanced cases of epithelial hyperplasia, the filling of lamellar/filament sulci by proliferating epithelial cells, which can lead to lamellar/filament fusion (Wolf et al. 2015). In the present study, the response of the gills to Cu, with and without Cd and Zn, was consistent with that described by Wolf et al. (2015) and with lesions reported in fish exposed to Cu (20 – 5500 μg/L; Mazon et al. 2002; Vutukuru et al. 2005; Schjolden et al. 2007; Al-Bairuty et al. 2013; Kumar et al. 2015; Naz et al. 2021), Cd (4500–7790 μg/L; Thophon et al. 2003; Naz et al. 2021), and Zn (1600–7500 μg/L Zn; Hemalatha and Banerjee 1997; Köck and Bucher 1997). In juvenile rainbow trout, for example, exposure to 20 μg/L Cu for 10 days caused lamellar epithelial hyperplasia and edema, lamellar fusion, clubbed tips, mucocyte hypertrophy, and telangiectasis and, after 4 days of exposure to 100 μg/L Cu, near complete mortality was consistent with increased severity of these lesions (Al-Bairuty et al. 2013). In 7-day experiments with adult rare minnow (Gobiocypris rarus), gill lesions induced by Cu (19.6 μg/L) and Cd (149.8 μg/L) were largely the same type (e.g., hypertrophy, hyperplasia, lamellar fusion) and, as a binary mixture at half the concentration, these metals exacerbated hyperplastic lesions concomitant with an enhanced oxidative stress response (Wu et al. 2019). The general paradigm is that, if the stressor is removed and ample recovery time is provided, proliferative gill lesions are reversible (Roberts 2012; Wolf et al. 2015) and partial or complete resolution of lamellar fusion and filament fusion is possible (Goldes et al. 1988; Cerqueira and Fernandes 2002; Velcheva et al. 2013). Nevertheless, increased oxygen diffusion distance and loss of respiratory epithelium associated with these structural alterations impart osmorespiratory impairments and increase energy expenditures which can lead to heavy mortality, particularly when oxygen levels are low and metabolic demands are high (Roberts 2012). Given that Cu exposure has been reported to induce hypoxia and increase energy expenditures in the gills (van Heerden et al. 2004), it is probable that compromised gills contributed to short-term mortality and most likely contributed to the long-term metabolic deficits experienced by early life stage rainbow trout in the present study. The gill pathology profiles obtained here are consistent with previous studies and may prove useful in future environmental monitoring studies involving fish by including non-lethal gill biopsy sampling methods for histopathologic analyses.
Rainbow trout swim-up fry treated with Cu only or metal mixture treatments in the present study exhibited reductions in coelomic fat content, an increased number of hepatocellular cytoplasmic vacuoles, and increased incidence or severity of renal tubular epithelial cytoplasmic hyaline droplets. Reduced growth captured in Cu only and metal mixture treatments corroborates microscopic findings of reduced coelomic body fat content. In fish, as with most vertebrates, moderate amounts of excess fat are stored in the liver short-term, while long-term storage primarily occurs in mesenteric/coelomic adipose tissue; conversely, mesenteric fat reserves can be mobilized when energy requirements exceed the amounts immediately available for metabolically costly biological processes (Tocher 2003). The reductions in body fat in exposed fish in the present study are presumed to be a product of increased mobilization, further supporting the hypothesis that bioenergetics were shifted towards detoxification/protective mechanisms in these fish.
Hepatocellular cytoplasmic vacuolation was increased in Cu only ( ≥ 47 μg/L Cu) and metal mixture treatments (70 + 9.3 + 867 μg/L Cu+Cd+Zn); the hepatocellular vacuolation was morphologically consistent with lipid. Individual administration of Cu and Cd for subchronic durations was reported to cause increased hepatic steatosis via increased lipogenic and decreased lipolytic enzyme activities in adult zebrafish (Pan et al. 2019), juvenile yellow catfish (Pelteobagrus fulvidraco; Zhong et al. 2022), and juvenile javelin goby (Synechogobius hasta; Liu et al. 2010; Liu et al. 2011). When administered as a binary mixture, Cu and Cd enhanced these effects on lipid metabolism enzyme activities and intensified steatosis in the liver of the javelin goby after 30 days of exposure (Song et al. 2014), which is consistent with our findings of increased hepatocellular vacuolization by Cu-Cd-Zn treatments. It is suggested that Cu-mediated triglyceride synthesis and accumulation in the liver involves transcriptional interferences as evidenced in zebrafish and yellow catfish primary hepatocyte in vitro experiments (Pan et al. 2019; Zhong et al. 2022). For example, lipid deposition was ascribed to an oxidative stress pathway involving Nrf2 recruitment to the PPARγ promoter (Zhong et al. 2022). Indeed, many environmental contaminants, including Cu, Cd, and Zn, have interactions with the PPARγ gene (Comparative Toxicogenomics Database, http://ctdbase.org/; Dreier et al. 2020), which has led to several adverse outcome pathways positing PPARγ activation as the molecular initiating event resulting in adipogenesis (https://aopwiki.org/; Tsakovska et al. 2014). Our findings of increased liver vacuoles concomitant with reduced mesenteric fat stores appear to reflect metabolic disruption by metals in non-feeding fry that ultimately manifested in reduced body growth and development.
Significantly increased incidences of renal tubular epithelial cytoplasmic hyaline droplets observed in 50% of fish reared in the 70 + 9.3 + 867 μg/L Cu+Cd+Zn treatment in the present study was in accordance with previous reports of this lesion developing in fish exposed to Cu (Sawsan et al. 2017) and Cd (Gill et al. 1989; Thophon et al. 2003). Less frequent incidences (13–25%) of hyaline droplets, as well as renal tubular epithelial hydropic degeneration, were observed in fish exposed to Cu only treatments (31, 47, 70, and 104 μg/L Cu) and the low-concentration metal mixture treatment (31 + 4.1 + 385 μg/L Cu+Cd+Zn) but not any control fish examined. These findings are evidence that renal changes were treatment related. One mechanism attributed to hyaline droplet formation is increased endogenous protein production and subsequent deposition in the renal epithelial cells (Kondera et al. 2014). In contrast, the primary pathogenic mechanism underlying renal tubular epithelial hydropic degeneration involves an interference with ion transport pumps (e.g., hypoxia or chemically induced Na+/K+-ATPase inhibition) leading to cellular ionic imbalances and the influx of water (Roberts 2012; Miller and Zachary 2017). In the present study, the hyaline droplets may have been comprised of MT-metal aggregates (Kondera et al. 2014) and a buildup of free Cu and/or Cd (i.e., due to insufficient renal MT synthesis; Gill et al. 1989) may have been responsible the observed degenerative changes. In conjunction with the pathological findings in other tissues and whole organism growth and development effects, hyaline droplet formation and hydropic degeneration in the renal tubular epithelial cells likely contributed to the declining health of early life stage rainbow trout in the present study.
Waterborne concentrations of Cu, Cd, and Zn derived from various sources can vary widely by geographic location and additional factors need to be considered when assessing ecological impacts of metal pollution (e.g., other metals and combinations, water chemistry, species and life stage, and endpoint sensitivity). A series of recent studies also investigated apical effects in several teleosts (Atlantic salmon, rainbow trout, European perch (Perca fluviatilis), and common roach (Rutilus rutilus)) exposed to a six-metal mixture at concentrations pertinent to European inland waters (5 μg/L Cd, 10 μg/L Cr, 10 μg/L Cu, 10 μg/L Ni, 5 μg/L Pb, and 100 μg/L Zn as a whole mixture and by reducing one of six metals by 10-fold), but additionally measured changes in tissue-specific accumulation, genotoxicity and cytotoxicity in erythrocytes in peripheral blood, gill, kidney, and liver tissue (Stankevičiūtė et al. 2017, 2018, 2021; Sauliutė et al. 2020). While condition factor (Stankevičiūtė et al. 2021) and hepatic and renal MT (Sauliutė et al. 2020) were not significantly changed, the authors demonstrated complex patterns of metal accumulation and significant genotoxic/cytotoxic responses that were dependent on fish species, tissue, metal, and varying metal concentrations. The genotoxic/cytotoxic effects evident in all fish species (including juvenile rainbow trout) exposed to the Cd-Cr-Cu-Ni-Pb-Zn mixture (Stankevičiūtė et al. 2017, 2018, 2021; Sauliutė et al. 2020) occurred at concentrations lower than Cu-Cd-Zn effect thresholds identified in rainbow trout swim-up fry in the present study. These data indicate that metal-induced erythrocyte nuclear alterations observed in multiple tissues of several teleosts are more sensitive indicators than the apical endpoints accepted by regulators to gauge fish health and ultimately assess the ecological impacts of metal mines in Canada (Environment Canada 2012). In light of numerous studies reporting the interaction of metals with various cellular targets and inducing oxidative stress leading to DNA damage, lipid peroxidation, protein carbonylation, etc. (Baldwin et al. 2003; Farrell et al. 2011, 2012; Lushchak 2011; Al-Bairuty et al. 2013; Heydarnejad et al. 2013; Cunningham and McGeer 2016; Santos et al. 2019), future studies incorporating molecular biomarkers such as DNA damage are warranted. Indeed, DNA damage can translate into significant organism-level adverse outcomes (e.g., cancer, teratogenicity, embryotoxicity, and reduced reproductive capacity) and, if DNA damage is in fact a key toxic effect of metals, it merits intensive investigation as a potential biomarker of metal exposure.
Conclusions
This study presents data on the subchronic adverse effects of waterborne Cu and Cu, Cd, and Zn mixture laboratory exposures to early life stage rainbow trout, including decreased survival, reduced growth, altered energy usage and storage, impaired yolk sac resorption, altered hatch timing, and gill, liver, kidney, and mesenteric histopathologic changes. Mixtures of Cu, Cd, and Zn elicited more pronounced toxicity with respect to survival, metabolic, developmental, and pathological effects compared to Cu on its own. These lethal and sublethal effects could translate to reduced survival and fitness of wild salmonid populations inhabiting waterbodies receiving industrial wastewater inputs or runoff that typically contain multiple metals at elevated concentrations. Since metals are predominantly regulated on a single-metal basis, there is a need to develop multi-metal assessment tools and integrate them into policy to mitigate harm to wild fishes. Collectively, the present findings can be applied to evaluate the risk of adverse effects to a model freshwater salmonid during critical early life stages by measuring concentrations of Cu, Cd, and Zn in environmental water samples or whole swim-up fry residing in waterbodies downstream of mining, urban, and industrial areas. Future studies examining additional metals in various mixture combinations can expand upon this potential such that a broader knowledge of the adverse impact of metal exposure scenarios could be used with a more comprehensive suite of health diagnostics. For instance, the risk assessment regime would greatly benefit from the development of reliable molecular biomarkers of exposure/effect for both metal mixtures and individual metals. Since molecular events precede higher order toxic outcomes, the application of molecular biomarkers in an environmental monitoring framework may lead to the earlier detection of adverse effects in exposed fish or facilitate the prioritization of individual metals within mixtures that are most concerning for fish health. This study is particularly complimentary to the standard early life stage rainbow trout lab and field based in situ bioassays currently prescribed by Environment and Climate Change Canada (e.g., Environment Canada 1998) to assess the impacts of metal pollution.
References
Al-Bairuty GA, Shaw BJ, Handy RD, Henry TB(2013) Histopathological effects of waterborne copper nanoparticles and copper sulphate on the organs of rainbow trout (Oncorhynchus mykiss) Aquatic Toxicol 126:104–115. https://doi.org/10.1016/j.aquatox.2012.10.005
Altenburger R (2015) Understanding combined effects for metal co-exposure in ecotoxicology. In: Metal Ions in Toxicology: Effects, Interactions, Interdependencies. De Gruyter, Berlin, Boston, pp 1–26
Baldwin DH, Sandahl JF, Labenia JS, Scholz NL (2003) Sublethal effects of copper on coho salmon: impacts on nonoverlapping receptor pathways in the peripheral olfactory nervous system. Environ Toxicol Chem 22:2266–2274. https://doi.org/10.1897/02-428
Balistrieri LS, Seal RR, Piatak NM, Paul B (2007) Assessing the concentration, speciation, and toxicity of dissolved metals during mixing of acid-mine drainage and ambient river water downstream of the Elizabeth Copper Mine, Vermont, USA. Appl Geochem 22:930–952. https://doi.org/10.1016/j.apgeochem.2007.02.005
Barry KL, Grout JA, Levings CD et al. (2000) Impacts of acid mine drainage on juvenile salmonids in an estuary near Britannia Beach in Howe Sound, British Columbia. Canadian journal of fisheries and aquatic sciences 57:2032–2043. https://doi.org/10.1139/f00-157
Bates D, Mächler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48. https://doi.org/10.18637/jss.v067.i01
B.C. Gazette Authority (2016) Environmental Protection Notice: Volume CLVI, No. 51
B.C. Ministry of Environment and Climate Change Strategy (2019) Copper water quality guideline for the protection of freshwater aquatic life - technical report. water quality guideline series. WQG-03-1, Victoria, BC
B.C. Ministry of Environment and Climate Change Strategy (2022) Zinc water quality guidelines (reformatted from: British Columbia Ministry of Environments, Land and Parks, 1997. Ambient water quality criteria for zinc). Water Quality Guideline Series, WQG-19, Victoria, BC
Bolker BM, Brooks ME, Clark CJ et al. (2009) Generalized linear mixed models: a practical guide for ecology and evolution. Trends Ecol Evolut 24:127–135. https://doi.org/10.1016/j.tree.2008.10.008
Brinkman SF, Woodling JD (2014) Acclimation and deacclimation of brown trout (Salmo trutta) to zinc and copper singly and in combination with cadmium or copper. Arch Environ Contam Toxicol 67:214–223. https://doi.org/10.1007/s00244-014-0026-6
Brix KV, De Boeck G, Baken S, Fort DJ (2022) Adverse outcome pathways for chronic copper toxicity to fish and amphibians. Environ Toxicol Chem 41:2911–2927. https://doi.org/10.1002/etc.5483
Byrne P, Wood PJ, Reid I (2012) Impairment of river systems by metal mine contamination: a review including remediation options. Crit Rev Environ Sci Technol 42:2017–2077. https://doi.org/10.1080/10643389.2011.574103
Canadian Council of Ministers of the Environment (CCME) (2007) A protocol for the derivation of water quality guidelines for the protection of aquatic life 2007. Winnipeg
Canadian Council of Ministers of the Environment (CCME) (2014) Canadian water quality guidelines for the protection of aquatic life: cadmium. In: Canadian environmental quality guidelines, 1999, Canadian Council of Ministers of the Environment, Winnipeg. Excerpt from Publication No. 1299; ISBN 1-896997-34-1. https://ccme.ca/en/res/cadmium-en-canadian-water-quality-guidelines-for-the-protection-of-aquatic-life.pdf
Canadian Council of Ministers of the Environment (CCME) (2018) Canadian water quality guidelines for the protection of aquatic life: zinc (dissolved). In: Canadian environmental quality guidelines, 1999, Canadian Council of Ministers of the Environment, Winnipeg. Excerpt from Publication No. 1299; ISBN 1-896997-34-1. https://ccme.ca/en/res/zinc-en-canadian-water-quality-guidelines-for-the-protection-of-aquatic-life.pdf
Cerqueira CCC, Fernandes MN (2002) Gill tissue recovery after copper exposure and blood parameter responses in the tropical fish Prochilodus scrofa. Ecotoxicol Environ Saf 52:83–91. https://doi.org/10.1006/eesa.2002.2164
Chouchene L, Banni M, Kerkeni A et al. (2011) Cadmium-induced ovarian pathophysiology is mediated by change in gene expression pattern of zinc transporters in zebrafish (Danio rerio). Chem Biol Interact 193:172–179. https://doi.org/10.1016/j.cbi.2011.06.010
Chouchene L, Pellegrini E, Gueguen M-M et al. (2016) Inhibitory effect of cadmium on estrogen signaling in zebrafish brain and protection by zinc. Journal of applied toxicology 36:863–871. https://doi.org/10.1002/jat.3285
Cunningham JL, McGeer JC (2016) The effects of chronic cadmium exposure on repeat swimming performance and anaerobic metabolism in brown trout (Salmo trutta) and lake whitefish (Coregonus clupeaformis). Aquatic Toxicol 173:9–18. https://doi.org/10.1016/j.aquatox.2015.12.003
Dethloff GM, Schlenk D, Hamm JT, Bailey HC (1999) Alterations in physiological parameters of rainbow trout (Oncorhynchus mykiss) with exposure to copper and copper/zinc mixtures. Ecotoxicol Environ Saf 42:253–264. https://doi.org/10.1006/eesa.1998.1757
Dreier DA, Bowden JA, Aristizabal-Henao JJ et al. (2020) Ecotoxico-lipidomics: An emerging concept to understand chemical-metabolic relationships in comparative fish models. Comp Biochem Physiol Part D Genomics Proteomics 36:100742–100742. https://doi.org/10.1016/j.cbd.2020.100742
Driessnack MK, Jamwal A, Niyogi S (2017) Effects of chronic waterborne cadmium and zinc interactions on tissue-specific metal accumulation and reproduction in fathead minnow (Pimephales promelas). Ecotoxicol Environ Saf 140:65–75. https://doi.org/10.1016/j.ecoenv.2017.02.023
Driessnack MK, Matthews AL, Raine JC, Niyogi S (2016) Interactive effects of chronic waterborne copper and cadmium exposure on tissue-specific metal accumulation and reproduction in fathead minnow (Pimephales promelas). Comp Biochem Physiol Toxicol Pharmacol 179:165–173. https://doi.org/10.1016/j.cbpc.2015.10.009
Elmore SA, Chen VS, Hayes-Bouknight S et al. (2017) Proceedings of the 2016 National Toxicology Program Satellite Symposium. Toxicol Pathol 45:11–51. https://doi.org/10.1177/0192623316672074
Environment and Climate Change Canada (ECCC) (2021) Federal environmental quality guidelines: copper. Gatineau (QC): Environment Canada, National Guidelines and Standards Office. Available from: https://www.canada.ca/en/environment-climate-change/services/evaluating-existing-substances/federal-environmental-quality-guidelines-copper.html
Environment and Climate Change Canada (2015) Third national assessment of environmental effects monitoring information from metal mines subject to the Metal Mining Effluent Regulations
Environment Canada (1998) Biological Test Method: Toxicity Tests Using Early Life Stages of Salmonid Fish (Rainbow Trout), EPS 1/RM/2. Ottawa, ON
Environment Canada (2007) Biological test method: test of reproduction and survival using the cladoceran Ceriodaphnia dubia. Ottawa, ON
Environment Canada (2012) Metal Mining Technical Guidance for Environmental Effects Monitoring. Ottawa, ON
EPA (1998) Method 6020A (SW-846): inductively coupled plasma-mass spectrometry
Farrell AP, Wood CM, Brauner CJ (2011) Homeostasis and toxicology of essential metals. Academic Press
Farrell AP, Wood CM, Brauner CJ (2012) Homeostasis and toxicology of non-essential metals Chris M., Wood, Anthony P. Farrell, Colin J. Brauner ed., 1st ed. Academic Press, an imprint of Elsevier, London; Waltham, Mass.
Fraher D, Sanigorski A, Mellett NA et al. (2016) Zebrafish embryonic lipidomic analysis reveals that the yolk cell Is metabolically active in processing lipid. Cell Rep (Cambridge) 14:1317–1329. https://doi.org/10.1016/j.celrep.2016.01.016
Fulton TW (1904) The rate of growth of fishes. In: Fisheries Board of Scotland, Annual Report 22 part 3. pp 141–241
Van Genderen E, Adams W, Dwyer R et al. (2015) Modeling and interpreting biological effects of mixtures in the environment: introduction to the metal mixture modeling evaluation project. Environ Toxicol Chem 34:721–725. https://doi.org/10.1002/etc.2750
Gill TS, Pant JC, Tewari H (1989) Cadmium nephropathy in a freshwater fish, Puntius conchonius hamilton. Ecotoxicol Environ Saf 18:165–172. https://doi.org/10.1016/0147-6513(89)90077-8
Goldes SA, Ferguson HW, Moccia RD, Daoust PY (1988) Histological effects of the inert suspended clay kaolin on the gills of juvenile rainbow trout, Salmo gairdneri Richardson. J Fish Dis 11:23–33. https://doi.org/10.1111/j.1365-2761.1988.tb00520.x
Green JW, Springer TA, Saulnier AN, Swintek J (2014) Statistical analysis of histopathological endpoints. Environ Toxicol Chem 33:1108–1116. https://doi.org/10.1002/etc.2530
Halekoh U, Højsgaard S, Yan J (2006) The R Package geepack for generalized estimating equations. J Stat Softw 15:1–11. https://doi.org/10.18637/jss.v015.i02
Hansen J, Lipton J, Welsh P et al. (2002) Relationship between exposure duration, tissue residues, growth, and mortality in rainbow trout (Oncorhynchus mykiss) juveniles sub-chronically exposed to copper. Aquatic toxicology 58:175–188. https://doi.org/10.1016/S0166-445X(01)00234-X
Hartig F (2021) DHARMa: residual diagnostics for hierarchical (multi-level/mixed) regression models
van Heerden D, Vosloo A, Nikinmaa M (2004) Effects of short-term copper exposure on gill structure, metallothionein and hypoxia-inducible factor-1α (HIF-1α) levels in rainbow trout (Oncorhynchus mykiss). Aquatic Toxicol 69:271–280. https://doi.org/10.1016/j.aquatox.2004.06.002
Hegeman KA, Marlatt VL (2021) Reproductive and thyroid endocrine axis cross-talk in rainbow trout (Oncorhynchus mykiss) alevins. Gen Comp Endocrinol 312:113855. https://doi.org/10.1016/j.ygcen.2021.113855
Hemalatha S, Banerjee T (1997) Histopathological analysis of sublethal toxicity of zinc chloride to the respiratory organs of the airbreathing catfish Heteropneustes fossilis (Bloch). Biol Res 30:11–21
Heydarnejad MS, Khosravian-Hemamai M, Nematollahi A (2013) Effects of cadmium at sub-lethal concentration on growth and biochemical parameters in rainbow trout (Oncorhynchus mykiss). Ir Vet J 66:11–11. https://doi.org/10.1186/2046-0481-66-11
Hothorn LA (2016) Statistics in Toxicology Using R. CRC Press
Huang J, Gergel SE (2022) Landscape indicators as a tool for explaining heavy metal concentrations in urban streams. Landsc Urban Plan 220:104331. https://doi.org/10.1016/j.landurbplan.2021.104331
Jasansky S, Lieber M, Giljum S, Maus V (2023) An open database on global coal and metal mine production. Sci Data 10:52–52. https://doi.org/10.1038/s41597-023-01965-y
Jones K (2018) Wayne G. Landis, Ruth M. Sofield, Ming-Ho Yu: Introduction to environmental toxicology and molecular substructures to ecological landscapes (5th edition). Chromatographia 81:1121–1121. https://doi.org/10.1007/s10337-018-3479-3
Köck G, Bucher F (1997) Accumulation of zinc in rainbow trout (Oncorhynchus mykiss) after waterborne and dietary exposure. Bull Environ Contam Toxicol 58:305–310. https://doi.org/10.1007/s001289900335
Kondera E, Ługowska K, Sarnowski P (2014) High affinity of cadmium and copper to head kidney of common carp (Cyprinus carpio L.). Fish Physiol Biochem 40:9–22. https://doi.org/10.1007/s10695-013-9819-1
Kumar M, Kumar P, Devi S (2015) To study the histopathological changes in the gills of Clarias batrachus, an air breathing teleost after short term exposure of copper sulphate. J Aquac Res Dev 6:1–4
Levings CD, Varela DE, Mehlenbacher NM et al. (2005) Effect of an acid mine drainage effluent on phytoplankton biomass and primary production at Britannia Beach, Howe Sound, British Columbia. Mar Pollut Bull 50:1585–1594. https://doi.org/10.1016/j.marpolbul.2005.06.032
Liu X-J, Luo Z, Li C-H et al. (2011) Antioxidant responses, hepatic intermediary metabolism, histology and ultrastructure in Synechogobius hasta exposed to waterborne cadmium. Ecotoxicol Environ Saf 74:1156–1163. https://doi.org/10.1016/j.ecoenv.2011.02.015
Liu XJ, Luo Z, Xiong BX et al. (2010) Effect of waterborne copper exposure on growth, hepatic enzymatic activities and histology in Synechogobius hasta. Ecotoxicol Environ Saf 73:1286–1291. https://doi.org/10.1016/j.ecoenv.2010.06.019
Lizardo-Daudt HM, Kennedy C (2008) Effects of cadmium chloride on the development of rainbow trout Oncorhynchus mykiss early life stages. J Fish Biol 73:702–718. https://doi.org/10.1111/j.1095-8649.2008.01971.x
Lushchak VI (2011) Environmentally induced oxidative stress in aquatic animals. Aquatic Toxicol 101:13–30. https://doi.org/10.1016/j.aquatox.2010.10.006
Marr JCA, Bergman HL, Lipton J, Hogstrand C (1995) Differences in relative sensitivity of naive and metals-acclimated brown and rainbow trout exposed to metals representative of the Clark Fork River, Montana. Can J Fish Aquatic Sci 52:2016–2030. https://doi.org/10.1139/f95-793
Martin TD, Creed JT, Brockhoff CA (1994) Sample preparation procedure for spectrochemcial determination of total recoverable elements: U.S. Environmental Protection Agency Report, Revision 2.8, EMMC Version
Martínez R, Navarro-Martín L, van Antro M et al. (2020) Changes in lipid profiles induced by bisphenol A (BPA) in zebrafish eleutheroembryos during the yolk sac absorption stage. Chemosphere (Oxford) 246:125704–125704. https://doi.org/10.1016/j.chemosphere.2019.125704
Mazon AF, Cerqueira CCC, Fernandes MN (2002) Gill cellular changes induced by copper exposure in the South American tropical freshwater fish Prochilodus scrofa. Environ Res 88:52–63. https://doi.org/10.1006/enrs.2001.4315
McKim JM, Benoit DA(1971) Effects of long-term exposures to copper on survival, growth, and reproduction of brook trout (Salvelinus fontinalis) Can J Fisheries Aquatic Sci 28:655–662. https://doi.org/10.1139/f71-097
Meyer JS, Farley KJ, Garman ER (2015) Metal mixtures modeling evaluation project: 1. background. Environ Toxicol Chem 34:726–740. https://doi.org/10.1002/etc.2792
Miller M, Zachary J (2017) Mechanisms and morphology of cellular injury, adaptation, and death. In: Zachary J (ed) Pathologic Basis of Veterinary Disease, 6th edn. Elsevier, St. Louis, Missouri, p 2–43
Naddy RB, Cohen AS, Stubblefield WA (2015) The interactive toxicity of cadmium, copper, and zinc to Ceriodaphnia dubia and rainbow trout (Oncorhynchus mykiss). Environ Toxicol Chem 34:809–815. https://doi.org/10.1002/etc.2870
Naz S, Hussain R, Ullah Q et al. (2021) Toxic effect of some heavy metals on hematology and histopathology of major carp (Catla catla). Environ Sci Pollut Res Int 28:6533–6539. https://doi.org/10.1007/s11356-020-10980-0
Norwood WP, Borgmann U, Dixon DG, Wallace A (2003) Effects of Metal Mixtures on Aquatic Biota: A Review of Observations and Methods. Human Ecol Risk Assessment 9:795–811. https://doi.org/10.1080/713610010
Organization for Economic Co-operation and Development (OECD) (2015) Test no. 240: medaka extended one generation reproduction test (MEOGRT), OECD guidelines for the testing of chemicals, section 2. Paris
Organization for Economic Co-operation and Development (OECD) (2016) Current approaches in the statistical analysis of ecotoxicity data: a guidance to application, OECD series on testing and assessment, no. 54
Ouellet JD, Dubé MG, Niyogi S (2013) A single metal, metal mixture, and whole-effluent approach to investigate causes of metal mine effluent effects on fathead minnows (Pimephales promelas). Water Air Soil Pollut 224:1–19. https://doi.org/10.1007/s11270-013-1462-z
Pan Y-X, Zhuo M-Q, Li D-D et al. (2019) SREBP-1 and LXRα pathways mediated Cu-induced hepatic lipid metabolism in zebrafish Danio rerio. Chemosphere (Oxford) 215:370–379. https://doi.org/10.1016/j.chemosphere.2018.10.058
Paul JS, Small BC (2021) Chronic exposure to environmental cadmium affects growth and survival, cellular stress, and glucose metabolism in juvenile channel catfish (Ictalurus punctatus). Aquatic toxicology 230:105705. https://doi.org/10.1016/j.aquatox.2020.105705
Peterson RH, Metcalfe JL, Ray S (1983) Effects of cadmium on yolk utilization, growth, and survival of Atlantic salmon alevins and newly feeding fry. [Salmo salar]. Arch Environ Contam Toxicol 12:37–44. https://doi.org/10.1007/BF01054999
R Core Team (2022) R: A language and environment for statistical computing
Ratn A, Prasad R, Awasthi Y et al. (2018) Zn2+ induced molecular responses associated with oxidative stress, DNA damage and histopathological lesions in liver and kidney of the fish, Channa punctatus (Bloch, 1793). Ecotoxicol Environ Saf 151:10–20. https://doi.org/10.1016/j.ecoenv.2017.12.058
Ritz C, Baty F, Streibig JC, Gerhard D (2015) Dose-response analysis using R. PLoS One 10:e0146021. https://doi.org/10.1371/journal.pone.0146021
Roberts RJ (2012) Fish pathology. John Wiley & Sons, Incorporated, Hoboken
Saibu Y, Jamwal A, Feng R et al. (2018) Distribution and speciation of zinc in the gills of rainbow trout (Oncorhynchus mykiss) during acute waterborne zinc exposure: Interactions with cadmium or copper. Comp Biochem Physiol Toxicol Pharmacol 206–207:23–31. https://doi.org/10.1016/j.cbpc.2018.02.004
Sant KE, Timme-Laragy AR (2018) Zebrafish as a model for toxicological perturbation of yolk and nutrition in the early embryo. Curr Environ Health Rep 5:125–133. https://doi.org/10.1007/s40572-018-0183-2
Santos SW, Cachot J, Gourves P-Y et al.(2019) Sub-lethal effects of waterborne copper in early developmental stages of rainbow trout (Oncorhynchus mykiss) Ecotoxicol Environ Saf 170:778–788. https://doi.org/10.1016/j.ecoenv.2018.12.045
Sauliutė G, Markuckas A, Stankevičiūtė M (2020) Response patterns of biomarkers in omnivorous and carnivorous fish species exposed to multicomponent metal (Cd, Cr, Cu, Ni, Pb and Zn) mixture. Part III. Ecotoxicology (London) 29:258–274. https://doi.org/10.1007/s10646-020-02170-y
Sawsan H, Amira H, Mostafa M, Nashaat A (2017) Histopathology and residues in fresh water fish exposed to acute and chronic copper and mercury toxicity. J Fish Pathol 30:115–134
Schjolden J, Sørensen J, Nilsson GE, Poléo ABS (2007) The toxicity of copper to crucian carp (Carassius carassius) in soft water. Sci Total Environ 384:239–251. https://doi.org/10.1016/j.scitotenv.2007.06.009
Sergeant CJ, Sexton EK, Moore JW et al. (2022) Risks of mining to salmonid-bearing watersheds. Sci Adv 8:eabn0929–eabn0929. https://doi.org/10.1126/sciadv.abn0929
Sevcikova M, Modra H, Slaninova A, Svobodova Z (2011) Metals as a cause of oxidative stress in fish: a review. Vet Med (Praha) 56:537–546. https://doi.org/10.17221/4272-VETMED
Shekh K, Tang S, Kodzhahinchev V et al. (2019) Species and life-stage specific differences in cadmium accumulation and cadmium induced oxidative stress, metallothionein and heat shock protein responses in white sturgeon and rainbow trout. Sci Total Environ 673:318–326. https://doi.org/10.1016/j.scitotenv.2019.04.083
Song Y-F, Luo Z, Pan Y-X et al. (2014) Effects of copper and cadmium on lipogenic metabolism and metal element composition in the Javelin Goby (Synechogobius hasta) after single and combined exposure. Arch Environ Contam Toxicol 67:167–180. https://doi.org/10.1007/s00244-014-0011-0
Stankevičiūtė M, Makaras T, Pažusienė J et al. (2021) Biological effects of multimetal (Ni, Cd, Pb, Cu, Cr, Zn) mixture in rainbow trout Oncorhynchus mykiss: Laboratory exposure and recovery study. Ecotoxicol Environ Saf 216:112202. https://doi.org/10.1016/j.ecoenv.2021.112202
Stankevičiūtė M, Sauliutė G, Makaras T et al. (2018) Responses of biomarkers in Atlantic salmon (Salmo salar) following exposure to environmentally relevant concentrations of complex metal mixture (Zn, Cu, Ni, Cr, Pb, Cd). Part II. Ecotoxicology (London) 27:1069–1086. https://doi.org/10.1007/s10646-018-1960-2
Stankevičiūtė M, Sauliutė G, Svecevičius G et al. (2017) Genotoxicity and cytotoxicity response to environmentally relevant complex metal mixture (Zn, Cu, Ni, Cr, Pb, Cd) accumulated in Atlantic salmon (Salmo salar). Part I: importance of exposure time and tissue dependence. Ecotoxicology (London) 26:1051–1064. https://doi.org/10.1007/s10646-017-1833-0
Swintek J (2018) RSCABS: an R package for performing the Rao-Scott adjusted Cochran-Armitage trend test by slices
Taslima K, Al-Emran M, Rahman MS et al. (2022) Impacts of heavy metals on early development, growth and reproduction of fish – A review. Toxicol Rep 9:858–868. https://doi.org/10.1016/j.toxrep.2022.04.013
Tchounwou PB, Yedjou CG, Patlolla AK, Sutton DJ (2012) Heavy metal toxicity and the environment. Molecular, Clinical and Environmental Toxicology 133–164. https://doi.org/10.1007/978-3-7643-8340-4_6
Thophon S, Kruatrachue M, Upatham ES et al. (2003) Histopathological alterations of white seabass, Lates calcarifer, in acute and subchronic cadmium exposure. Environmental pollution (1987) 121:307–320. https://doi.org/10.1016/S0269-7491(02)00270-1
Tocher DR (2003) Metabolism and functions of lipids and fatty acids in teleost fish. Rev Fish Sci 11:107–184. https://doi.org/10.1080/713610925
Tsakovska I, al Sharif M, Alov P et al. (2014) Molecular modelling study of the PPARγ receptor in relation to the mode of action/adverse outcome pathway framework for liver steatosis. Int J Mol Sci 15:7651–7666. https://doi.org/10.3390/ijms15057651
Velcheva I, Georgieva E, Atanassova P (2013) Gill tissue recovery after copper exposure in Carassius gibelio (Pisces: Cyprinidae) Cent Eur J Biol 8:1112–1118. https://doi.org/10.2478/s11535-013-0233-6
Vijver MG, Elliott EG, Peijnenburg WJGM, de Snoo GR (2011) Response predictions for organisms water-exposed to metal mixtures: A meta-analysis. Environ Toxicol Chem 30:1482–1487. https://doi.org/10.1002/etc.499
Vutukuru SS, Suma C, Radha Madhavi K et al. (2005) Studies on the development of potential biomarkers for rapid assessment of copper toxicity to freshwater fish using Esomus danricus as model. Int J Environ Res Public Health 2:63–73. https://doi.org/10.3390/ijerph2005010063
Wang N, Ingersoll C, Dorman R et al. (2014) Chronic sensitivity of white sturgeon (Acipenser transmontanus) and rainbow trout (Oncorhynchus mykiss) to cadmium, copper, lead, or zinc in laboratory water-only exposures. Environ Toxicol Chem 33:2246–2258. https://doi.org/10.1002/etc.2641
Wolf JC, Baumgartner WA, Blazer VS et al. (2015) Nonlesions, misdiagnoses, missed diagnoses, and other interpretive challenges in fish histopathology studies. Toxicol Pathol 43:297–325. https://doi.org/10.1177/0192623314540229
Wu L, Yu Q, Zhang G et al. (2019) Single and combined exposures of waterborne Cu and Cd induced oxidative stress responses and tissue injury in female rare minnow (Gobiocypris rarus). Comparat Biochem Physiol Toxicol Pharmacol 222:90–99. https://doi.org/10.1016/j.cbpc.2019.04.013
Yamagami K (1973) Some enzymological properties of a hatching enzyme (Chorionase) isolated from the fresh-water teleost, Oryzias latipes. Compar Biochem Physiol Part B: Compar Biochem 46:603–614. https://doi.org/10.1016/0305-0491(73)90100-4
Zhong C-C, Zhao T, Hogstrand C et al. (2022) Copper (Cu) induced changes of lipid metabolism through oxidative stress-mediated autophagy and Nrf2/PPARγ pathways. J Nutr Biochem 100:108883–108883. https://doi.org/10.1016/j.jnutbio.2021.108883
Zhou Q, Yang N, Li Y et al. (2020) Total concentrations and sources of heavy metal pollution in global river and lake water bodies from 1972 to 2017. Glob Ecol Conserv 22:e00925. https://doi.org/10.1016/j.gecco.2020.e00925
Zis T, Ronningen V, Scrosati R (2004) Minor improvement for intertidal seaweeds and invertebrates after acid mine drainage diversion at Britannia Beach, Pacific Canada. Mar Pollut Bull 48:1040–1047. https://doi.org/10.1016/j.marpolbul.2003.12.007
Acknowledgements
We are grateful to Blake Danis, Geoffery Su, Daniel King, and Yiqiu Yang (Simon Fraser University) for their assistance during the experimental exposure.
Author contributions
MM: Project administration; Methodology; Material preparation; Data collection; Formal analysis and investigation; Writing–draft preparation. VM: Funding acquisition; Project administration; Study design; Supervision. LB: Histopathologic methodology, evaluation, and reporting. All authors: Study conceptualization; Writing—review, editing, and approval.
Funding
This research was funded by Gitanyow Fisheries Authority and Genome British Columbia’s GeneSolve Program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethics approval
Animal procedures adhered to the Simon Fraser University Animal Care Protocol 1290B-18 in accordance with the Canadian Council on Animal Care Guidelines.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
McKay, M.E., Baseler, L., Beblow, J. et al. Comparative subchronic toxicity of copper and a tertiary copper mixture to early life stage rainbow trout (Oncorhynchus mykiss): impacts on growth, development, and histopathology. Ecotoxicology 33, 1–21 (2024). https://doi.org/10.1007/s10646-023-02721-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-023-02721-z