Abstract
Seasonal tissue concentrations of heavy metals, antioxidant enzymes, immunological components, and water quality parameters were investigated during 1 year in the ark clam, Barbatia decussate, from the coast of Lengeh port, located in the north of the Persian Gulf, Iran. The tissue accumulation of the heavy metals (Cd, Pb, Hg) significantly increased accumulations in late autumn and winter (P < 0.01). Theconcentrations of Ni and Cr nearly remained unchanged throughout the 1 year sampling period (P > 0.01). Seasonal changes were also observed in metal-induced biochemical components. In this regard, the malondialdehyde (MDA) levels and the activity of superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) elevated throughout spring and summer and then declined during autumn and winter (P < 0.01). Phagocytosis activity significantly decreased from December to February and then increased from March to September (P < 0.01). Total hemocyte counts decreased from October to March and then elevated until April (P < 0.01). Significant relationships were found between tissue heavy metal concentrations, water quality parameters, and biochemical components (P < 0.01). The negative correlations were O2 vs. antioxidant enzymes, phagocytosis, and total counts of the hemocytes (THCs); pH vs. SOD; salinity vs. Cr; and temperature vs. GPx and Ni. Positive correlations were O2 vs. Cd, Pb, Hg, and Ni; temperature vs. phagocytosis and THCs; and turbidity vs. phagocytosis, THCs, CAT, and GPx. The results of the present study showed a seasonal pattern in the accumulation of heavy metals, with maximum levels in winter for the ark clam, B. decussate . Furthermore, antioxidant defense and immunity of B. decussate are reduced during winter, which may make B. decussate susceptible to diseases.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Bivalves are a branch of marine mollusks, which are known as bioindicators of marine pollution due to their filter-feeding behavior (Sarkar et al. 1994; Boening 1999; Yusof et al. 2004; Nilin et al. 2012; Zuykov et al. 2013). Owing to this fact, bivalves are able to accumulate various types of pollutants such as aromatic hydrocarbons, crude oil, pesticides, and heavy metals (Sbriz et al. 1998; Boening 1999; Damásio et al. 2010; Zuykov et al. 2013). Metals due to some properties, especially toxicity, extensive resources, stability, and bioaccumulation, are considered as a serious problem in the marine environments (Ansari et al. 2004; Boran and Altınok 2010; Wang et al. 2013; Mostofi 2018). Industrial wastewater, agricultural drainage, transportation, combustion of fossil fuels, weathering, and erosion of rocks and soil are among the sources of heavy metals (HEMs), entering into the aqueous bodies (Windom 1992; Jayaraju et al. 2009; Al Naggar et al. 2018; Bat and Özkan 2019, Dmytro 2020). Several studies have reported the adverse impacts of heavy metals on sea life, in particular, bivalves (Naimo 1995; Jakimska et al. 2011). It is well recognized that the toxicity induced by heavy metals could impair immunity (Sheir 2010; Rault et al. 2013; Ivanina et al. 2016), hematology (Cheng and Sullivan 1984; Bindya Bhargavan and Mohammed Salih 2008), feeding (Anandraj et al. 2002; Liu et al. 2014), metabolism (Devi 1996; Faubelet al. 2008), and osmoregulation (Gregory et al. 1999) of bivalves.
There are some studies reporting the seasonal variations in the accumulation rate of HEMs in the bivalves (Lakshmanan and Nambisan 1983; Cain and Luoma 1986; Beldi et al. 2006; Góngora-Gómez et al. 2018). Salinity and temperature changes have been introduced as the main factors affecting the seasonal-dependent variations in metal bioaccumulations. The relationships between the seasonal accumulation of HEMs and the immune system of bivalve have been the focus of several studies (Solé et al. 1995; Sheehan and Power 1999; Wilhelm Filho et al. 2001; Lau et al. 2004; Manduzio et al. 2004; Bocchetti and Regoli 2006; Nahrgang et al. 2013). The Persian Gulf is one of the vital and high-traffic waterways in the world for oil transit (Din 1990). Massive oil spills from tankers during the Persian Gulf War, extraction and transportation of oil, shipping, the existence of industries and coastal facilities, etc. have made this waterway one of the most polluted marine areas (Mafi-Gholami et al. 2019). In this regard, the northern parts of the gulf are also more affected by pollutants due to shallow depth, limited water circulation, salinity, and high temperatures.
The ark clam, Barbatia decussate, is one of the filter-feeder sessile bivalves of the Persian Gulf that lives on the rocky beds of intertidal zones. In the present work, the seasonal changes of heavy metal accumulations and immunity components (antioxidant enzymes, hemocyte counts, and phagocytosis) in the ark clam, Barbatia decussate, as well as water quality parameters were investigated to identify the heavy metal–induced biomarkers and their changes with heavy metal accumulation rate and environmental conditions. For the first time, the results of this study can provide physiologically and ecologically useful information about the seasonal change pattern of heavy metal accumulation and environmental conditions with biochemical changes in the ark clam.
Materials and methods
Collection of clam specimens
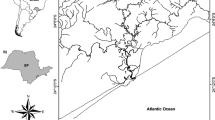
The ark clam (B. decussate) specimens were collected monthly from tidal regions of the 4 stations along the coast of Lengeh port, located in the north of the Persian Gulf, for 1 year. The sampling stations were station 1 (Kong) (26° 36′ 12″ N, 54° 56′ 46″ E), station 2 (Lengeh) (26° 33′ 29″ N, 54° 53′ 12″ E), station 3 (Shenas) (26° 31′ 27″ N, 54° 47′ 37″ E), and station 4 (Bustaneh) (26° 30′ 30″ N, 54° 39′ 42″ E) (Fig. 1). From each station, the sampling was conducted according to the method described by Zeinalipour et al. (2015). In total, 240 ark clams (mean shell length 26 ± 5.5 mm) were collected from each station with a monthly average of 20 clams per month. The clams were transferred to the lab in plastic bags labeled with the date and place of collection. In the lab, the clams were placed in seawater for 24 h to fully evacuate their digestive tract and to remove wastes from the shells as much as possible. Then, the biometric characteristics (length and weight) were measured, and the specimens were dissected to remove the gill, soft tissue, and digestive gland. The tissues were kept at − 20 °C till chemical analysis.
In addition, the water quality parameters were monthly measured in each sampling station as follows: pH and temperature by a pH meter (Model 6 APX15/C, WTW-330i), salinity by a salinometer (DEMETRA model TM-30D, Japan), dissolved oxygen by an oxygen meter (Model OxyGouard, Water Management Technologies, Inc., USA), and turbidity by a Secchi disk.
Heavy metal assays
The metal concentration was assayed in soft tissue samples of the clams. The assay was conducted according to the method of Vinodhini and Narayanan (2008) with little modifications. In this regard, 1-g tissue samples were chemically digested in ultrapure concentrated nitric acid (HNO3) and hydrogen peroxide (H2O2) (1:1 v/v) within 25-mL flasks, heated to 130 °C, cooled for 2 h, diluted by distilled water, and finally filtered by a 0.45-µm nitrocellulose membrane filter. All metals (Cd, Pb, Ni, Hg, Cr) were assayed by atomic absorption spectrophotometer (Shimadzu AA 6200). All reagents were provided by Sigma-Aldrich Company (USA), and the assay procedures were conducted according to the manufacturer’s instructions.
Biochemical assays
The antioxidant enzymes including superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) were assayed in the digestive gland of the clams. Also, the gill tissues were sampled to determine the malondialdehyde(MDA) levels.
For antioxidant enzyme assays, the digestive glands were pre-prepared, as described by Niyogi et al. (2001) with some modifications. Briefly, the tissue samples (2 g) were immediately homogenized in 4 volumes of ice-cold 10 mM Tris_HCl buffer (pH 7.6) and centrifuged (13,000 g for 25 min at 4 °C) to collect the supernatants. The supernatants were filtered (Buchner funnel), the resulting liquids were centrifuged (10,000 g for 5 min) again, and the supernatants were finally stored at − 20 °C till enzyme assays.
The SOD was determined through reacting xanthine with WST-1 [(2–(4-iodophenyl)-3–(4-nitrophenyl)-5–(2,4-disulfophenyl)-2Htetrazolium, monosodium salt)] and subsequent reduction of superoxide anion (O2−). The red formazan dye generated during the reaction was measured at 505 nm, and the one unit of SOD activity was estimated as the amount of enzyme that caused a 50% decrease in the rate of WST-1 reduction (Sigma, St. Louis, MO, USA).
The GPX levels were assayed through the oxidation of NADPH in the presence of glutathione reductase and cumene hydroperoxide (as a substrate). The decrease of absorbance at 340 nm was attributed to the reduction of oxidized glutathione by NADPH, H+. One unit GPx activity corresponded to the oxidation of 1 μmol of NADPH per min.
The activity of CAT was determined by estimating the degradation rate of H2O2 at 240 nm; 1 mmol of H2O2 degraded per minute was considered as one unit of catalase activity.
A thiobarbituric acid method was used to determine the levels of MDA, an indicator of lipid peroxidation. In this method, gill tissue samples were lyophilized in liquid nitrogen, homogenized (1:4 v/v) in 50 mM potassium phosphate buffer (pH 7.0 at 20 °C), sonicated for 6 s, and centrifuged for 4 min at 13,000 × g at 4 °C to separate the supernatants (SPNs). Thiobarbituric acid (0.375%) and butylated hydroxytoluene (2%) were added to SPNs and all reagents (blanks and standards). Then, the solutions were heated for 15 min at 100 °C and centrifuged at 13,000 × g for 5 min at room temperature. The reaction between MDA and thiobarbituric acid created a pink chromagen, which was read spectrophotometrically at 532 nm. The MDA levels were expressed in µmol g−1 wet weight.
The immunological parameters, including the total counts of the hemocytes (THCs) and the phagocytosis activity of the hemocytes, were assayed in hemolymph samples extracted from the posterior adductor muscle of the clams. The hemolymph was sampled using a sterile 1-mL syringe, filtered using a 1-mm2 mesh sterile gauze to remove the debris, and pooled in 50-mL Falcon tubes at 4 °C.
THCs were estimated based on the method of Zhu et al. (2011) and Mackenzie et al. (2014). Briefly, 100 mL hemolymph was diluted in 800 mL phosphate-buffered saline(PBS) and fixed by adding 100 mL 2.5% glutaraldehyde. A wet mount of the fixed hemolymph was examined with a Neubauer hemocytometer (XB-K-25, Anxin Optical Instrument) and observed under a Nikon Eclipse E600 microscopy (magnification × 1000).
Phagocytosis activity of the hemocytes was evaluated according to the methods described by Su et al. (2017). For this purpose, 20-µL yeast suspension (7 mg yeast in 1000 mL 0.1 M PBS) was added to 100 µL hemolymph mixed in 100 µL Alsever’s solution (ALS, Noble Ryder). The mixture was left for 30 min at room temperature and then incubated in a cool water bath at 4 °C for 60 min. Finally, the phagocytosis activity was ceased using 2.5% glutaraldehyde, and the blood smears were prepared and stained with Wright’s stain. The phagocytic activity was examined under a microscope (Nikon Eclipse E600 light microscope, × 1000).
Data analysis
Analysis of data was performed using the SPSS software. The normality of data was examined before the analysis of variance using the Kolmogorov–Smirnov test. After a one-way analysis of variance, the means were compared using Tukey’s test. In addition, the bivariate Pearson correlation was used to determine the relationships between the parameters.
Results
Water quality parameters
The physico-chemical parameters of water showed seasonal patterns during 1 year sampling period. In this regard, the water temperature showed significant decreases from October to February and then increased until May (Fig. 2A, P < 0.01). Water salinity decreased in June and August, return to initial values in September and October, decreased again in November, and nearly remained unchanged until May (Fig. 2B, P < 0.01). Except for an increase in November and March, water pH showed a declining trend throughout the sampling period (Fig. 2C, P < 0.01). The oxygen content of the water significantly increased during August, remained unchanged until March, and then decreased during April and May (Fig. 2D, P < 0.01). The water turbidity significantly decreased in November, nearly remained unchanged until March, and apparently increased in April and May (Fig. 2E, P < 0.01).
Heavy metal accumulations
The accumulation of the heavy metals including Cd, Pb, and Hg showed seasonal changes with maximum accumulations in late autumn and winter (Table 1, P < 0.01). During spring and summer, the levels of these metals significantly decreased (Table 1, P < 0.01). The concentrations of Ni and Cr nearly remained unchanged throughout the experiment period (Table 1, P < 0.01).
Metal-induced biochemical biomarkers
Seasonal changes were observed in metal-induced biochemical components including antioxidant enzymes (SOD, CAT, GPx) and immunological components (phagocytosis activity, THCs) (Fig. 3, P < 0.01). In this regard, the MDA levels (Fig. 3A, P < 0.01) and the activity of SOD (Fig. 3B, P < 0.01), CAT (Fig. 3C, P < 0.01), and GPx (Fig. 3D, P < 0.01) increased throughout April to August and then decreased during September to February (P < 0.01). The Phagocytosis activity (Fig. 3E, P < 0.01) showed significant decreases from December to February, while it increased from March to September (P < 0.01). THCs showed significant decreases from October to March and then elevated gently until April (Fig. 3F, P < 0.01).
Correlations
According to the analysis of Pearson, significant correlations were observed between tissue heavy metal concentrations, water quality parameters, and biochemical components (Table 2, P < 0.01). Negative correlations were O2 vs. antioxidant enzymes, phagocytosis, and THCs; pH vs. SOD; salinity vs. Cr; and temperature vs. GPx and Ni. Positive correlations were O2 vs. Cd, Pb, Hg, and Ni; temperature vs. phagocytosis and THCs; turbidity vs. phagocytosis, THCs, CAT, and GPx (Tables 3 and 4).
Discussion
In the present study, seasonal variations were observed in the accumulation of heavy metals in the ark clam, Barbatia decussate, as reported previously in many studies with bivalves. In wedge clam, Donax trunculus, the highest accumulation of heavy metals in summer was attributed to the lower marine currents and also reproduction of the bivalve in this season (Beldi et al. 2006). In Mytilus galloprovincialis, the higher accumulations occurred in winter for Zn, Cu, and Cd, while Pb showed higher accumulation in summer (Rouane-Hacene et al. 2015). These results might be due to the seasonal variations in water quality parameters, animal metabolism rate, food availability, and entrance of land and air-based pollutions to the sea (Rouane-Hacene et al. 2015). In the study of Góngora-Gómez et al. (2018), a seasonality was observed in concentrations of heavy metals in soft tissue and muscle of the pen shell Atrina Maura in the dry season (winter and spring), which was related to the upwelling currents and anthropogenic activities such as agriculture and aquaculture. In C. glaucum, the highest metal accumulations occurred during the winter and autumn seasons, while less accumulation was found during spring and summer. Similar to the above studies, seasonal variations were observed in tissue concentration of heavy metals of B. decussate. The maximum accumulations were found in late autumn and winter, which may be due to the decreased metabolism of the B. decussate and consequently its weak ability to excrete the metals to medium. In addition, the water quality parameters may affect the metal accumulations, because these parameters showed some significant correlations with tissue metal content. In this regard, water temperature and salinity had negative correlations with tissue concentrations of Ni and Cr, respectively. In contrast, water dissolved oxygen showed positive correlations with tissue concentrations of Cd, Pb, Hg, and Ni.
In the present study, seasonality was also observed in the values of immunological and antioxidant components, as previously reported for other bivalves (Sheehan and Power 1999). A El-Saidy et al. (2020) found seasonality in metal accumulations, antioxidant enzymes (CAT, GPx), and MDA levels in the bivalve C. glaucum, where the activity of these enzymes was lower during autumn and winter compared to other seasons. However, in the same study, the MDA levels were higher during autumn and winter, as observed during winter in the present study. Seasonal variations in antioxidant enzymes were also observed in M. galloprovincialis, which its pattern was similar to tissue concentrations of PAHs, PCBs, DDTs, and lindane (Solé et al. 1995). The activity of antioxidant enzyme (SOD, GPx) decreased during winter in M. edulis, which was attributed to decreased antioxidant defense, as a result of lowered temperature and food availability (Manduzio et al. 2004). As a biomarker of oxidative stress (Hajirezaee et al. 2019), the MDA levels and also the activity of SOD, CAT, and GPx increased throughout the spring, summer, and mid-autumn and then decreased during late autumn and whole winter. It was recognized that temperature and food availability induce oxygen consumption and cellular oxyradical generation, which are compensated by enhancing antioxidant defenses (Sheehan and Power 1999; Manduzio et al. 2004). Therefore, the increased activity of ANEs during spring and summer may be a result of increases in temperature and food availability during these seasons. In addition, we found negative relationships between metal accumulations and activity of the antioxidant enzymes, which may be due to the suppressing effects of metals toxicity on the antioxidant defense of B. decussate. THCs and phagocytosis activity are known as the main components of immunity in Mollusca. There are some studies regarding the seasonal alternations in the immune-related components of bivalves. In the study of Hong et al. (2020), the decrease in the THCs was attributed to the reduced immunity of the giant honeycomb oyster, Hyotissa hyotis, after reproduction in spring. Flye-Sainte-Marie et al. (2009) showed that seasonal fluctuations in temperature control the THC in Ruditapes philippinarum.
In the present study, the phagocytosis activity and THCs decreased during December to February and November to March, respectively. In addition, negative correlations were found between immunological parameters and tissue concentration of heavy metals. These results may indicate the immunosuppressive effects of these metals on the ark clam immunity, which may make this species susceptible to diseases during winter.
Conclusion
The results of the present study revealed a close relationship between heavy metal accumulations in ark clam, water quality parameters, and the clam’s immune components. In this regard, decreased water oxygen levels and temperature significantly reduced the immune-related components including antioxidant enzymes, phagocytosis, and THCs. In contrast, an increase in temperature and turbidity was accompanied by a considerable elevation in phagocytosis, THCs, CAT, and GPx activity. In general, the above results show that the temperature and oxygen level of water adversely affects the immunity of the ark clam. However, more studies, especially laboratory studies, are needed in the future to examine the relationships more closely.
Data availability
The datasets used during the current study are available from the corresponding author on reasonable request.
References
Al Naggar Y, Khalil MS, Ghorab MA (2018) Environmental pollution by heavy metals in the aquatic ecosystems of Egypt. Open Acc J Toxicol 3:555603
El-Saidy AS, El-Khodary MG, Omran EN, Abd El-Aziz KK, Mona HM (2020) Seasonal variations of heavy metals in the marine bivalve Cerastoderma glaucum (Bruguière, 1789) from Temsah Lake, Ismailia, Egypt, and their relation to antioxidant enzymes. Egypt J Aquat Biol Fish 24(3):257–270
Anandraj A, Marshall DJ, Gregory MA, McClurg TP (2002) Metal accumulation, filtration and O2 uptake rates in the mussel Perna perna (Mollusca: Bivalvia) exposed to Hg2+, Cu2+ and Zn2+. Comp Biochem Physiol C Toxicol Pharmacol 132(3):355–363
Ansari TM, Marr IL, Tariq N (2004) Heavy metals in marine pollution perspective-a mini review. J Appl Sci 4(1):1–20
Bat L, Özkan EY (2019) Heavy metal levels in sediment of the Turkish Black Sea coast. Oceanography and Coastal Informatics: Breakthroughs in Research and Practice, 86–107
Beldi H, Gimbert F, Maas S, Scheifler R, Soltani N (2006) Seasonal variations of Cd, Cu, Pb and Zn in the edible mollusc Donax trunculus (Mollusca, Bivalvia) from the gulf of Annaba, Algeria. Afr J Agric Res 1(4):85–90
Bindya Bhargavan PV, Mohammed Salih KY (2008) Haematological responses of green mussel Perna viridis (Linnaeus) to heavy metals copper and mercury. Doctoral dissertation, Cochin University of Science and Technology
Bocchetti R, Regoli F (2006) Seasonal variability of oxidative biomarkers, lysosomal parameters, metallothioneins and peroxisomal enzymes in the Mediterranean mussel Mytilus galloprovincialis from Adriatic Sea. Chemosphere 65(6):913–921
Boening DW (1999) An evaluation of bivalves as biomonitors of heavy metals pollution in marine waters. Environ Monit Assess 55(3):459–470
Boran M, Altınok I (2010) A review of heavy metals in water, sediment and living organisms in the Black Sea. Turk J Fish Aquat Sci 10(4):565–572
Cain DJ, Luoma SN (1986) Effect of seasonally changing tissue weight on trace metal concentrations in the bivalve Macoma balthica in San Francisco Bay. Mar Ecol Prog Ser 28:209–217
Cheng TC, Sullivan JT (1984) Effects of heavy metals on phagocytosis by molluscan hemocytes. Mar Environ Res 14(1–4):305–315
Damásio J, Navarro-Ortega A, Tauler R, Lacorte S, Barceló D, Soares AM, … Barata C (2010) Identifying major pesticides affecting bivalve species exposed to agricultural pollution using multi-biomarker and multivariate methods. Ecotoxicology 19(6):1084–1094
Devi VU (1996) Bioaccumulation and metabolic effects of cadmium on marine fouling dressinid bivalve, Mytilopsis sallei (Recluz). Arch Environ Contam Toxicol 31(1):47–53
Din MAE (1990) The transport importance of the Arabian (Persian) Gulf. Transp Rev 10(2):127–148
Dmytro STROIANOVSKYI (2020) The study of welding requirements during construction and installation of seismic-resistant steel structures. J Res Sci Eng Technol 8(2)
Faubel D, Lopes-Lima M, Freitas S, Pereira L, Andrade J, Checa A, Machado J (2008) Effects of Cd2+ on the calcium metabolism and shell mineralization of bivalve Anodonta cygnea. Mar Freshw Behav Physiol 41(2):131–146
Flye-Sainte-Marie J, Soudant P, Lambert C, Le Goïc N, Goncalvez M, Travers MA, … Jean F (2009) Variability of the hemocyte parameters of Ruditapes philippinarum in the field during an annual cycle. J Exp Mar Biol Ecol 377(1):1–11
Góngora-Gómez AM, Domínguez-Orozco AL, Villanueva-Fonseca BP, Muñoz-Sevilla NP, García-Ulloa M (2018) Seasonal levels of heavy metals in soft tissue and muscle of the pen shell Atrina maura (Sowerby, 1835) (Bivalvia: Pinnidae) from a farm in the southeastern coast of the Gulf of California, Mexico. Revista Internacional De Contaminación Ambiental 34(1):57–68
Gregory MA, George RC, Marshall DJ, Anandraj A, Mcclurg TP (1999) The effects of mercury exposure on the surface morphology of gill filaments in Perna perna (Mollusca: Bivalvia). Mar Pollut Bull 39(1–12):116–121
Hajirezaee S, Rafieepour A, Shafiei S, Rahimi R (2019) Immunostimulating effects of Ginkgo biloba extract against toxicity induced by organophosphate pesticide, diazinon in rainbow trout, Oncorhynchus mykiss: innate immunity components and immune-related genes. Environ Sci Pollut Res 26(9):8798–8807
Hong HK, Jeung HD, Kang HS, Choi KS (2020) Seasonal variations in the hemocyte parameters, gonad development, energy storage and utilization of the giant honeycomb oyster Hyotissa hyotis (Linnaeus 1758) in Jeju Island off the south coast of Korea. Aquac Rep 17:100299
Ivanina AV, Hawkins C, Sokolova IM (2016) Interactive effects of copper exposure and environmental hypercapnia on immune functions of marine bivalves Crassostrea virginica and Mercenaria mercenaria. Fish Shellfish Immunol 49:54–65
Jakimska A, Konieczka P, Skóra K, Namieśnik J (2011) Bioaccumulation of metals in tissues of marine animals, part I: the role and impact of heavy metals on organisms. Polish J Environ Stud 20(5)
Jayaraju N, Sundara Raja Reddy BC, Reddy KR (2009) Heavy metal pollution in reef corals of Tuticorin Coast, southeast coast of India. Soil Sediment Contam 18(4):445–454
Lakshmanan PT, Nambisan PNK (1983) Seasonal variations in trace metal content in bivalve molluscs, Villorita cyprinoides var. cochinensis (Hanley), Meretrix casta (Chemnitz) and Perna viridis (Linnaeus).
Lau PS, Wong HL, Garrigues P (2004) Seasonal variation in antioxidative responses and acetylcholinesterase activity in Perna viridis in eastern oceanic and western estuarine waters of Hong Kong. Cont Shelf Res 24(16):1969–1987
Liu GX, Shu MA, Chai XL, Shao YQ, Wu HX, Sun CS, Yang SB (2014) Effect of chronic sublethal exposure of major heavy metals on filtration rate, sex ratio, and gonad development of a bivalve species. Bull Environ Contam Toxicol 92(1):71–74
Mackenzie CL, Lynch SA, Culloty SC, Malham SK (2014) Future oceanic warming and acidification alter immune response and disease status in a commercial shellfish species, Mytilus edulis L. PLoS One 9(6):e99712
Mafi-Gholami D, Zenner EK, Jaafari A, Bakhtyari HRR, Bui DT (2019) Multi-hazards vulnerability assessment of southern coasts of Iran. J Environ Manag 252:109628
Manduzio H, Monsinjon T, Galap C, Leboulenger F, Rocher B (2004) Seasonal variations in antioxidant defences in blue mussels Mytilus edulis collected from a polluted area: major contributions in gills of an inducible isoform of Cu/Zn-superoxide dismutase and of glutathione S-transferase. Aquat Toxicol 70(1):83–93
Mostofi F (2018) Heavy metal contamination of zinc and lead in region 1 and 2 of the main city of Ardabil. J Res Sci Eng Technol 6(04):14–20
Nahrgang J et al (2013) Seasonal variation in biomarkers in blue mussel (Mytilus edulis), Icelandic scallop (Chlamys islandica) and Atlantic cod (Gadus morhua)—implications for environmental monitoring in the Barents Sea. Aquat Toxicol 127:21–35
Naimo TJ (1995) A review of the effects of heavy metals on freshwater mussels. Ecotoxicology 4(6):341–362
Nilin J, Pestana JLT, Ferreira NG, Loureiro S, Costa-Lotufo LV, Soares AM (2012) Physiological responses of the European cockle Cerastoderma edule (Bivalvia: Cardidae) as indicators of coastal lagoon pollution. Sci Total Environ 435:44–52
Niyogi S, Biswas S, Sarker S, Datta AG (2001) Antioxidant enzymes in brackishwater oyster, Saccostrea cucullata as potential biomarkers of polyaromatic hydrocarbon pollution in Hooghly Estuary (India): seasonality and its consequences. Sci Total Environ 281(1–3):237–246
Rault P, Fortier M, Pédelucq J, Lacaze E, Brousseau P, Auffret M, Fournier M (2013) Immunotoxicity of heavy metals (silver, cadmium, mercury and lead) on marine bivalve Mytilus edulis: in vitro exposure of hemocytes. J Xenobiotics e8–e8
Rouane-Hacene O, Boutiba Z, Belhaouari B, Guibbolini-Sabatier ME, Francour P, Risso-de Faverney C (2015) Seasonal assessment of biological indices, bioaccumulation and bioavailability of heavy metals in mussels Mytilus galloprovincialis from Algerian west coast, applied to environmental monitoring. Oceanologia 57(4):362–374
Sarkar SK, Bhattacharya B, Debnath S (1994) The suitability of tropical marine bivalves as biomonitors of heavy metals in deltaic Sundarbans, north-east India. Chemosphere 29(4):759–770
Sbriz L, Aquino MR, Fowler SW, Sericano JL (1998) Levels of chlorinated hydrocarbons and trace metals in bivalves and nearshore sediments from the Dominican Republic. Mar Pollut Bull 36(12):971–979
Sheehan D, Power A (1999) Effects of seasonality on xenobiotic and antioxidant defence mechanisms of bivalve molluscs. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol 123(3):193–199
Sheir SKH (2010) Effects of trace metals on cellular immune responses, tissue injury and gene expression in the mussell, Mytilus Edulis: implications for biological monitoring of marine pollution.
Solé M, Porte C, Albaiges J (1995) Seasonal variation in the mixed-function oxygenase system and antioxidant enzymes of the mussel Mytilus galloprovincialis. Environ Toxicol Chem Int J 14(1):157–164
Su W, Zha S, Wang Y, Shi W, Xiao G, Chai X, Wu H, Liu G (2017) Benzo [a] pyrene exposure under future ocean acidification scenarios weakens the immune responses of blood clam, Tegillarca granosa. Fish & Shellfish Immunology 63:465–470
Vinodhini R, Narayanan M (2008) Bioaccumulation of heavy metals in organs of fresh water fish Cyprinus carpio (Common carp). Int J Environ Sci Technol 5(2):179–182
Wang SL, Xu XR, Sun YX, Liu JL, Li HB (2013) Heavy metal pollution in coastal areas of South China: a review. Mar Pollut Bull 76(1–2):7–15
Windom HL (1992) Contamination of the marine environment from land-based sources. Mar Pollut Bull 25(1–4):32–36
Wiesner L, Günther B, Fenske C (2001) Temporal and spatial variability in the heavy-metal content of Dreissena polymorpha (Pallas) (Mollusca: Bivalvia) from the Kleines Haff (northeastern Germany). Hydrobiologia 443(1–3):137–145
Wilhelm Filho D, Tribess T, Gaspari C, Claudio FD, Torres MA, Magalhaes ARM (2001) Seasonal changes in antioxidant defenses of the digestive gland of the brown mussel (Perna perna). Aquaculture 203(1–2):149–158
Yusof AM, Yanta NF, Wood AKH (2004) The use of bivalves as bio-indicators in the assessment of marine pollution along a coastal area. J Radioanal Nucl Chem 259(1):119–127
Zeinalipour M, Hasanzadeh KB, Shokri MR, Ashja AA (2015) Distribution of the Ark clam Barbatia decussata (Bivalvia: Arcidae) on rocky intertidal shores in the northern Persian Gulf. J Anim Environ 7:77–89
Zhu Z, Xu L, Wu X, Zhang Z, Wu L, Lou H (2011) Morphological, structural characteristics and phagocytic and enzymatic activities of haemocytes in blood clam Tegillarca granosa. J Fish China 35(10):1494–1504
Zuykov M, Pelletier E, Harper DA (2013) Bivalve mollusks in metal pollution studies: from bioaccumulation to biomonitoring. Chemosphere 93(2):201–208
Acknowledgements
The authors would like to thank all those who contributed to this research.
Funding
There is no government or organizational fund for this work.
Author information
Authors and Affiliations
Contributions
AJ as the only author of this article has made the testing, collected the data, analyzed the results, and wrote the article.
Corresponding author
Ethics declarations
Ethics approval
In this study, all stages of sampling and manipulation of animals have been performed in accordance with ethical standards.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The author declares no competing interests.
Additional information
Responsible Editor: V.V.S.S. Sarma
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Khoei, A.J. Seasonal heavy metal accumulations in the bivalve Barbatia decussate and their relationships with water quality and the metal-induced biochemical biomarkers. Environ Sci Pollut Res 29, 16103–16112 (2022). https://doi.org/10.1007/s11356-021-16893-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-16893-w