Abstract
The pollution of polycyclic aromatic hydrocarbons was serious in sediments of the Pearl River estuary, China. A fluorene-degrading bacterium, strain A2-3, was isolated from hydrocarbon contaminated sediment of this estuary and identified as Rhodococcus sp. based on the analyses of 16S rRNA gene sequence and morphology. Rhodococcus sp. A2-3 can take naphthalene, p-Teropheny, fluorene, pyrene, salicylic acid, citric acid, acetic acid, diethyletheranhydrous, methanol or 4,4′-dibromodiphenyl ether as sole carbon source. 100% of 100 mg/L fluorene or 89% of 400 mg/L fluorene was removed in 7 days by strain A2-3 at 30 °C and pH 7.5. The strain A2-3 showed a high degradation efficiency of fluorene when pH values ranged from 5.5 to 8.5. The proposed pathway of fluorene catabolism by strain A2-3 was initially attacked by 3,4 dioxygenation. Our results suggested Rhodococcus sp. A2-3 can degrade PAHs under aerobic conditions and can function in bioremediation, particularly for weakly acid environment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The rapid urbanization and industrialization in the delta region of the Pearl River estuary causes increasingly accumulation of toxic organic compounds and heavy metals in the Pearl River estuary, which is one of the largest river system in China (Chau 2006; Ye et al. 2012). Because the sediment can react both as an important pollutant sink and as a carrier and potential future source of contaminants, the discussion about pollution status in sediments of this estuary is very important and necessary (Chau 2006). Polycyclic aromatic hydrocarbons (PAHs) are a series of ubiquitous hydrophobic organic pollutants, showing toxic, mutagenic, or carcinogenic properties (DeBruyn et al. 2007; Zhou et al. 2006). The PAHs pollution is serious in the Pearl River estuary, and total PAHs concentrations vary from 323 to 21,324 ng/g dry weights in surface sediments of the Pearl River estuary (Mai et al. 2001). Furthermore, Guangzhou channel locating in the upstream of the Pearl River estuary shows the highest concentrations of PAHs in sediments because Guangzhou city releases many urban/industrial waste (Mai et al. 2001; Zhang et al. 2015). In addition, the percentage of 2–3 rings PAHs shows a declined tendency from upstream to downstream of the Pearl River estuary (Mai et al. 2001).
Great efforts are taken to remove PAHs in environment. However, bacterial degradation of PAHs is considered as the most cost-effective option to cleanup PAH-contaminated sites (Margesin and Schinner 1997; Ling et al. 2011; Jeon and Madsen 2013). A high diversity of bacterial species can degrade PAHs, including the common genera of Sphingomonas, Pseudomonas, Cycloclasticus, Mycobacterium, Bacillus and Rhodococcus (Dean-Ross et al. 2002; Finkelstein et al. 2003; Lu et al. 2011; Khanna et al. 2012; Kim et al. 2015). Moreover, our previous study demonstrated higher diversity of PAHs-metabolizing bacteria was found in the Guangzhou channel through culture-independent methods targeting the bacterial PAHs ring-hydroxylating dioxygenase gene (Wu et al. 2014). Nevertheless, up to now, no PAHs-degrading bacterium is reported to isolated from the Pearl River estuary.
Fluorene, a 3-ring PAH with similar structure to several carcinogenic PAHs, is often used as a model low molecular weight (LMW) PAH for biodegradation. Furthermore, the US Environmental Protection Agency has taken fluorene as a priority pollutant (Finkelstein et al. 2003; Chupungars et al. 2009). Considering the high amount of LMW PAHs in Guangzhou channel, the purpose of the present study was to isolate and purify fluorene-degrading bacteria from the Pearl River estuary, and to discuss the PAHs-degradation capability.
Materials and methods
Chemicals and media
Phenanthrene, fluorene and pyrene were bought from Sigma–Aldrich company with the purity of 97–99%. Individual PAH stock solutions were prepared in acetone (10 g/L). All other chemicals and solvents in this study belong to analytical grade or better. Mineral salt medium (MSM) contained per liter of deionized water: (NH4)2SO4, 1.0 g; MgSO4·7H2O, 0.2 g; Na2HPO4, 0.8 g; Ca(NO3)2·4H2O, 0.05 g; KH2PO4, 0.2 g and trace elements made up of FeCl3·3H2O, 5 mg; (NH4)6Mo7O24·4H2O, 1 mg; MnCl2, 0.2 mg; CoCl2, 0.02 mg; CuSO4, 0.02 mg. Modified Luria-Bertani (LB/5) medium contained peptone 2 g/L, NaCl 2 g/L and yeast extract 1 g/L (pH 7.5).
Isolation, purification and identification of PAHs-degrading bacteria
The collected sediments from the Guangzhou channel of the Pearl River estuary were inoculated into MSM amended with fluorene and pyrene (each 0.1 g/L) and incubated at 30 °C under shaking condition (200 rpm). 2-ml aliquots of supernatant were then weekly transferred to fresh MSM containing the same concentration of fluorene and pyrene. The transformation procedure was repeated more than four times until obvious bacteria grew in the fresh MSM. Fluorene and pyrene (each 0.1 g/L) were dissolved in acetone and sprayed on the surface of the MSM agar plates. After evaporating the solvent of acetone, conventional spread plate techniques were used to isolate and purify bacteria on MSM-PAHs agar plates. All isolates with different morphological characteristic were later confirmed with an ability to degrade PAHs in fresh liquid medium. Finally, an isolate named strain A2-3 grew best with fluorene and pyrene as the sole carbon and energy source.
The 16S rRNA gene and cell morphology of strain A2-3 was employed to identify this strain. The 16S rRNA gene of strain A2-3 was amplified and sequenced by PCR using the following primers: F27 (5′-AGAGTTTGATCCTGGCTCAG-3′) and R1492 (5′-GGTTACCTTGTTACGACTT-3′) (Edwards et al. 1989). The phylogenetic tree was constructed using MEGA 5 by the neighbor-joining method with bootstrap analyses for 1000 replicates (Tamura et al. 2011). The 16S rRNA sequence of strain A2-3 was submitted in the NCBI database with the accession number KP851854. The cell morphology of the isolate was also observed by light microscopy and scan electron microscope (SEM).
Carbon source utilization
Experiments were employed to determine whether the strain A2-3 could grow on other organics following the recommendations of Zhang et al. (2009). The purified strain A2-3 was cultivated on one of the following compounds at 0.01% as the sole carbon source at 30 °C: pyrene, fluorene, naphthalene, p-Terophenyl, salicylic acid, terephthalic acid, toluene, acetic acid, ethanol, ethanediol, lactate, diethyletheranhydrous, methanol, xylene, trichloromethane, formic acid, phthalic acid, citric acid and 4,4′-dibromodiphenyl ether. Bacterial growth was measured by the increase of the culture (OD600). All treatments were incubated in dark at 200 rpm and 30 °C.
Degradation of fluorene by strain A2-3
Cells of strain A2-3 pre-grown for 3 days in LB/5 medium were then harvested, centrifuged, washed and resuspended in MSM. Biodegradation of fluorene was performed in 50-ml sterilized Erlenmeyer flasks holding 20 ml of MSM and 0.2 ml of fluorene stock solution (10 g/L). Make sure to evaporate the solvent before the addition of 1 ml of resuspended cells. Non-inoculated flasks and flasks without substrate (with acetone only) were served as controls. All treatments were conducted in triplicate. The cultures were incubated (30 °C, 200 rpm in the dark) and removed for further analysis at various time intervals. The cell growth was measured by OD600. The rest PAHs in the liquid culture were deeply extracted by hexane with four times. The extract was dried, re-dissolved in 5 ml of hexane, and analyzed with gas chromatography/mass spectrometry (GC/MS) (N6890/5975B, Agilent, USA).
In addition, effects of different initial concentrations (100, 400, 800 mg/L), and pH (5.5, 6.5, 7.5, 8.5 and 9.5) on fluorene removal have also been investigated. All biodegradation experiments were conducted three times with 7 days in culture.
Results
Identification and classification of strain A2-3
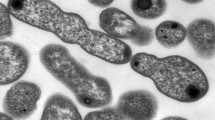
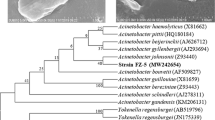
Strain A2-3 was aerobic, Gram-positive actinobacterium. On LB medium, the colonies of strain A2-3 were circular, glistening, opaque, convex, creamish pink in colour and have regular edges (Fig. 1). The SEM micrograph presented that the strain A2-3 was rod-like with diameter about 1.0 μm and length 5.0 μm (Fig. 1). The partial 16S rRNA sequence of strain A2-3 was a continuous stretch of 1359 nucleotides. Sequence alignment using a BLAST search demonstrated that strain A2-3 was 100% similarity to R. rubber Ebht1 (JF895525) and Rhodococcus rubber E10 (EU427319). Phylogenetic tree analyses also indicated that strain A2-3 was closely related to the species in genus Rhodococcus (Fig. 2).
Utilization of carbon source
Nineteen kinds of carbon sources were selected and tested for strain A2-3 to investigate the carbon substrate utilization. This strain showed capability to degrade aromatic compounds containing 2–4 rings of phenyl (naphthalene, p-Teropheny, fluorene and pyrene). Salicylic acid (the key metabolic intermediate of PAHs) and citric acid (the intermediate of tricarboxylic acid cycle) can be utilized for strain A2-3 as the sole carbon and energy source (Table 1). Strain A2-3 can also use acetic acid, diethyletheranhydrous, methanol, while this strain cannot take terephthalic acid, toluene, ethanol, ethanediol, lactate, xylene, trichloromethane, formic acid and phthalic acid as carbon source (Table 1). In addition, 4, 4′-dibromodiphenyl ether, a kind of persistent organic pollutant utilized as flame retardant, can be degraded by strain A2-3.
Fluorene degradation
The time-courses of bacterial growth and fluorene degradation (initial concentration: 100 mg/L) were determined under the condition at 30 °C and 200 rpm in the dark. Fluorene was rapidly removed, and an obvious growth of strain A2-3 corresponding to the decline of fluorene was founded in this study (Fig. 3). The concentration of fluorene was reduced to only 8 mg/L after four days of incubation. Within 5 days, the initial amount of fluorene was completely utilized, and the highest cell density was reached with a culture turbidity of 0.25 at OD600 (Fig. 3). However, no growth was observed in the controls (Fig. 3).
The GC-MS analysis of the fluorene degradation process showed that the peak at retention time (Rt) 11.0009 min in the beginning (day 0) was the fluorene based on the published mass spectra (Fig. 4a). After incubation for 9 days, the peak of fluorene disappeared, and some new peaks occurred (Fig. 4b). However, no typical metabolic intermediate of fluorene was observed by GC-MS.
Effect of initial concentrations and pH on fluorene degradation
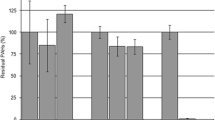
The fluorene biodegradation was carried out at different initial concentrations of fluorene and various pH values (Fig. 5). As shown in Fig. 5a, strain A2-3 can completely degrade the 100 mg/L fluorene in 7 days, and this strain can remove nearly 89% of fluorene in 7 days with an original concentration of 400 mg/L. However, about 36% of fluorene were removed in 7 days with an initial concentration of 800 mg/L.
Strain A2-3 can utilize almost 100% of 100 mg/L fluorene at pH ranged from 5.5 to 8.5 in 7 days. Meanwhile, only 10% of fluorene were degraded at pH 9.5 and slight growth of strain A2-3 was observed (OD600 = 0.02) (Fig. 5b).
Discussion
Important role of the genus Rhodococcus in PAHs degradation
Bacteria from the genus Rhodococcus were widespread in nature and played a key role in the detoxification of persistent pollutants (Finkelstein et al. 2003). Up to now, many PAHs-degrading bacteria belonging to this genus were isolated and identified from the natural environment. Fluorene, one of the 16 most hazardous PAHs, was often recognized as a model PAH for biodegradation (Finkelstein et al. 2003; Chupungars et al. 2009). Finkelstein et al. (2003) investigated four Rhodococcus species (R. opacus 4a, R. rhodochrous 172, R. opacus 557 and R. rhodnii 135) to degrade fluorene and found that the first three strains can completely transform fluorene when fluorene was treated as the sole source of carbon in the culture at concentration of 12–25 mg/L. However, when a fluorene concentration in the medium ranged from 50 to 100 mg/L, three strains transformed 50% of fluorene for 14 days incubation. In this study, within 7 days, Rhodococcus sp. A2-3 can absolutely remove the 100 mg/L fluorene and can degrade about 89% of 400 mg l−1 fluorene (Fig. 5a). Therefore, strain A2-3 showed a higher efficiency to transform fluorene.
Bacteria from the genus Rhodococcus can degrade not only fluorene but also other PAHs. Tongpim and Pickard (1996) showed that the isolated Rhodococcus sp. S1 can mineralized anthracene, but not phenanthrene or naphthalene. Di Gennaro et al. (2001) found Rhodococcus opacus R7 isolated from a PAHs contaminated soil can grow on 1 g/L naphthalene as the exclusive carbon and energy source. R. rhodnii 135 and R. opacus 412 were adapted to mineralize phenanthrene (Leneva et al. 2009). Pasternak et al. (2011) invetisgated the biodegradation of coal tar performed by R. erythropolis B10 and found this strain had the capacity to utilize 2–3 rings of PAHs. Song et al. (2011) demonstrated that Rhodococcus sp. P14 can utilize phenanthrene, pyrene, and benzo[a]pyrene as a sole carbon and energy source. After 30 days of cultivation with 50 mg/L of following PAHs, strain P14 consumed 43% phenanthrene, 34% pyrene and 30% benzo[a]pyrene. R. wratislaviensis strain 9 can degrade high-molecular weight PAHs. Within 7 days, 40% of 50 μM pyrene or 28% of 40 μM benzo[a]pyrene (BaP) was degraded by R. wratislaviensis strain 9 (Subashchandrabose et al. 2019). Rhodococcus sp. NJ2 can degraded 78% of 1000 ppm anthracene supplemented in minimal salt medium, within 10 days (Jauhari et al. 2020). In this study, strain A2-3 can utilize naphthalene, fluorene and pyrene (Table 1). Thus, results from this study further imply that the genus Rhodococcus should be one of the important and common genera to degrade the PAHs.
Environmental conditions controlling PAHs degradation
Environmental conditions can affect the bacterial process of PAHs degradation, while pH and initial concentration are common factors to control PAHs degradation (Zhang et al. 2009; Lu et al. 2011). To investigate the degradation capacity of fluorene by strain A2-3, a series of different concentration of fluorene was set. When the original concentration of fluorene was below 400 mg/L, the high degradation efficiency was shown (Fig. 5a). But, the degradation capacity was suppressed by the higher concentration of fluorene (800 mg/L) (Fig. 5a). The reason was probably due to the toxicity of fluorene on bacterial reproduction. This was consistent with previous studies which demonstrated the toxicity effect of high PAHs concentration on bacterial community (Zhang et al. 2009; Ling et al. 2011).
Previous studies suggest that many bacteria exhibit optimal degradation of PAHs at neutral pH (Lu et al. 2011). In addition, there are several reports suggest that slightly alkaline values are benefit for bacteria to transform PAHs. Hambrick et al. (1980) demonstrated that soil bacteria can degrade PAHs preferring to alkaline condition rather than acid condition. Zhao et al. (2009) found that a highly efficient degradation of phenanthrene by Pseudomonas stutzeri ZP2 was observed when pH at 8.0. Feng et al. (2012) tested the ability of phenanthrene biodegradation by Martelella sp. AD-3 at pH 6.0–10.0 and found the 100% depletion of phenanthrene when the initial pH value was 9.0. However, strain A2-3 reached a high degradation efficiency of fluorene when pH values belong to weakly acid. 100 mg/L fluorene was completely transformed by strain A2-3 at pH 5.5–7.5 after 7 days (Fig. 5b). Except for the factors mentioned above, surfactant, co-substrate and nutrient can also exert influence on bacterial degradation of PAHs (Haritash and Kaushik 2009; Lu et al. 2011).
The proposed pathway of fluorene catabolism by strain A2-3
Fluorene catabolism can be transformed by three alternative pathways (Casellas et al. 1997; Habe et al. 2004). Two of these pathways started with a dioxygenation at the 1,2-C or 3,4-C positions. The third pathway was initiated via a monooxygenation at the C-9 position to give 9-fluorenol, which can be then transformed to phthalic acid (Casellas et al. 1997; Habe et al. 2004). The pathway by dioxygenase at 3,4 positions can be further metabolized, yielding salicylic acid (Casellas et al. 1997; Habe et al. 2004). Carbon source utilization indicated that strain A2-3 can take salicylic acid as the sole carbon source, but not with phthalic acid (Table 1). Therefore, the analysis of carbon source utilization and the GC-MS analysis of the fluorene degradation pathway suggest that strain A2-3 complete utilize the fluorene molecules initially attacked by 3, 4 dioxygenation (Fig. 6).
Conclusions
A high effective fluorene degrading strain named A2-3 was isolated from hydrocarbon contaminated sediment of the Pearl River estuary, China. The strain was identified belonging to the genus Rhodococcus based on the analyses of 16S rRNA gene sequence and morphology. Rhodococcus sp. strain A2-3 can utilize 2-4 rings of phenyl, salicylic acid, citric acid, acetic acid, diethyletheranhydrous, methanol or 4,4′′-dibromodiphenyl ether as sole carbon source. Strain A2-3 can absolutely remove the 100 mg/L fluorene and can degrade about 89% of 400 mg/L fluorene within 7 days at 30 °C and pH 7.5. The analysis of carbon source utilization and the GC-MS analysis of the fluorene biodegradation indicated that strain A2-3 transformed fluorene by a dioxygenase at 3, 4 positions. This strain showed a high degradation efficiency of fluorene when pH values ranged from 5.5 to 8.5. Our results further indicated that the genus Rhodococcus was one of the important and common genera to degrade the PAHs.
References
Casellas M, Grifoll M, Bayona JM, Solanas AM (1997) New metabolites in the degradation of fluorene by Arthrobacter sp. strain F101. Appl Environ Microbiol 63:819–826
Chau KW (2006) Persistent organic pollution characterization of sediments in Pearl River estuary. Chemosphere 64:1545–1549
Chupungars K, Rerngsamran P, Thaniyavarn S (2009) Polycyclic aromatic hydrocarbons degradation by Agrocybe sp. CU-43 and its fluorene transformation. Int Biodeter Biodegr 63(1):93–99
Dean-Ross D, Moody J, Cerniglia CE (2002) Utilization of mixtures of polycyclic aromatic hydrocarbons by bacteria isolated from contaminated sediment. FEMS Microbiol Ecol 41(1):1–7
DeBruyn JM, Chewning CS, Sayler GS (2007) Comparative quantitative prevalence of Mycobacteria and functionally abundant nidA, nahAc, and nagAc dioxygenase genes in coal tar contaminated sediments. Environ Sci Technol 41:5426–5432
Di Gennaro P, Rescalli E, Galli E, Sello G, Bestetti G (2001) Characterization of Rhodococcus opacus R7, a strain able to degrade naphthalene and o-xylene isolated from a polycyclic aromatic hydrocarbon-contaminated soil. Res Microbiol 152:641–651
Edwards U, Rogall T, Bloecker H, Emde M, Boettger EC (1989) Isolation and direct complete nucleotide determination of entire genes: characterization of a gene coding for 16S ribosomal RNA. Nucleic Acids Res 17:7843–7853
Feng TC, Cui CZ, Dong F, Feng YY, Liu YD, Yang XM (2012) Phenanthrene biodegradation by halophilic Martelella sp. AD-3. J Appl Microbiol 113:779–789
Finkelstein ZI, Baskunov BP, Golovlev EL, Vervoort J, Rietjens IMCM, Baboshin MA, Golovleva LA (2003) Fluorene transformation by bacteria of the genus Rhodococcus. Microbiology 72:660–665
Habe H, Chung JS, Kato H, Ayabe Y, Kasuga K, Yoshida T, Nojiri H, Yamane H, Omori T (2004) Characterization of the upper pathway genes for fluorene metabolism in Terrabacter sp. strain DBF63. J Bacteriol 186:5938–5944
Hambrick GA, Delaune RD, Patrick WH (1980) Effect of estuarine sediment pH and oxidation-reduction potential on microbial hydrocarbon degradation. Appl Environ Microbiol 40:365–369
Haritash AK, Kaushik CP (2009) Biodegradation aspects of polycyclic aromatic hydrocarbons (PAHs): a review. J Hazard Mater 169:1–15
Jauhari N, Mishra S, Kumari B, Singh SN, Chauhan PS, Upreti DK (2020) Bacteria induced degradation of anthracene mediated by catabolic enzymes. Polycycl Aromat Comp 40(2):313–325
Jeon CO, Madsen EL (2013) In situ microbial metabolism of aromatic-hydrocarbon environmental pollutants. Curr Opin Biotech 24(3):474–481
Khanna P, Goyal D, Khanna S (2012) Characterization of pyrene utilizing Bacillus spp. from crude oil contaminated soil. Braz J Microbiol 43(2):606–617
Kim SJ, Kweon O, Sutherland JB, Kim HL, Jones RC, Burback BL, Graves SW, Psurny E, Cerniglia CE (2015) Dynamic response of Mycobacterium vanbaalenii PYR-1 to BP Deepwater Horizon crude oil. Appl Environ Microb 81(13):4263–4276
Leneva NA, Kolomytseva MP, Baskunov BP, Golovleva LA (2009) Phenanthrene and anthracene degradation by microorganisms of the genus Rhodococcus. Appl Biochem Microbiol 45:169–175
Ling J, Zhang G, Sun H, Fan Y, Ju J, Zhang C (2011) Isolation and characterization of a novel pyrene-degrading Bacillus vallismortis strain JY3A. Sci Total Environ 409:1994–2000
Lu X, Zhang T, Fang HH (2011) Bacteria-mediated PAH degradation in soil and sediment. Appl Microbiol Biot 89:1357–1371
Mai BX, Fu JM, Zhang G, Lin Z, Min YS, Sheng GY, Wang XM (2001) Polycyclic aromatic hydrocarbons in sediments from the Pearl river and estuary, China: spatial and temporal distribution and sources. Appl Geochem 16:1429–1445
Margesin R, Schinner F (1997) Bioremediation of diesel-oil contaminated alpine soils at low temperatures. Appl Microbiol Biot 47:462–468
Pasternak G, Rutkowski P, Sliwka E, Kolwzan B, Rybak J (2011) Broad coal tar biodegradative potential of Rhodococcus erythropolis B10 strain isolated from former gasworks site. Water Air Soil Poll 214:599–608
Song X, Xu Y, Li G, Zhang Y, Huang T, Hu Z (2011) Isolation, characterization of Rhodococcus sp. P14 capable of degrading high-molecular-weight polycyclic aromatic hydrocarbons and aliphatic hydrocarbons. Mar Pollut Bull 62:2122–2128
Subashchandrabose SR, Venkateswarlu K, Naidua R, Megharaj M (2019) Biodegradation of high-molecular weight PAHs by Rhodococcus wratislaviensis strain 9: overexpression of amidohydrolase induced by pyrene and BaP. Sci Total Environ 651:813–821
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739
Tongpim S, Pickard MA (1996) Growth of Rhodococcus S1 on anthracene. Can J Microbiol 42:289–294
Wu P, wang YS, Sun FL, Wu ML, Peng YL (2014) Bacterial polycyclic aromatic hydrocarbon ring-hydroxylating dioxygenases in sediments from the Pearl River estuary. China. Appl Microbiol Biot 98:875–884
Ye F, Huang XP, Zhang DW, Tian L, Zeng YY (2012) Distribution of heavy metals in sediments of the Pearl River Estuary, Southern China: implications for sources and historical changes. J Environ Sci 24:579–588
Zhang GY, Ling JY, Sun HB, Luo J, Fan YY, Cui ZJ (2009) Isolation and characterization of a newly isolated polycyclic aromatic hydrocarbons-degrading Janibacter anophelis strain JY11. J Hazard Mater 172:580–586
Zhang JD, Wang YS, Cheng H, Jiang ZY, Sun CC, Wu ML (2015) Distribution and sources of the polycyclic aromatic hydrocarbons in the sediments of the Pearl River estuary, China. Ecotoxicology 24(7-8):1643–1649
Zhao H, Wu Q, Wang L, Zhao X, Gao H (2009) Degradation of phenanthrene by bacterial strain isolated from soil in oil refinery fields in Shanghai China. J Hazard Mater 164:863–869
Zhou HW, Guo CL, Wong YS, Tam NFY (2006) Genetic diversity of dioxygenase genes in polycyclic aromatic hydrocarbon-degrading bacteria isolated from mangrove sediments FEMS Microbiol Lett 262(2):148–157
Acknowledgements
This research was supported by the National Natural Science Foundation of China (No. U1901211, No. 41876126), the National Key Research and Development Plan (No. 2017FY100700), Key Special Project for Introduced Talents Team of Southern Marine Science and Engineering Guangdong Laboratory (Guangzhou) (No. GML2019ZD0305), Innovation Academy of South China Sea Ecology and Environmental Engineering, Chinese Academy of Sciences (No. ISEE2019ZR02 and No. ISEE2018ZD02), the International Partnership Program of Chinese Academy of Sciences (No. 133244KYSB20180012) and the Strategic Priority Research Program of the Chinese Academy of Sciences (No. XDA23050200, No. XDA13010500 and No. XDA13020503).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wu, P., Wang, YS. Fluorene degradation by Rhodococcus sp. A2-3 isolated from hydrocarbon contaminated sediment of the Pearl River estuary, China. Ecotoxicology 30, 929–935 (2021). https://doi.org/10.1007/s10646-021-02379-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-021-02379-5