Abstract
Natural toxins produced by freshwater cyanobacteria, such as cylindrospermopsin, have been regarded as an emergent environmental threat. Despite the risks for food safety, the impact of these water contaminants in agriculture is not yet fully understood. Carrots (Daucus carota) are root vegetables, extensively consumed worldwide with great importance for human nourishment and economy. It is, therefore, important to evaluate the possible effects of using water contaminated with cyanotoxins on carrot cultivation. The aim of this work was to investigate cylindrospermopsin effects on D. carota grown in soil and irrigated for 30 days, with a Chrysosporum ovalisporum extract containing environmentally relevant concentrations of cylindrospermopsin (10 and 50 μg/L). The parameters evaluated were plant growth, photosynthetic capacity, and nutritional value (mineral content) in roots of carrots, as these are the edible parts of this plant crop. The results show that, exposure to cylindrospermopsin did not have a clear negative effect on growth or photosynthesis of D. carota, even leading to an increase of both parameters. However, alterations in mineral contents were detected after exposure to crude extracts of C. ovalisporum containing cylindrospermopsin. A general decline was observed for most minerals (Ca, Mg, Na, Fe, Mn, Zn, Mo, and P), although an increase was shown in the case of K and Cu, pointing to a possible interference of the cyanobacterial extract in mineral uptake. This study is the first to evaluate the effects of C. ovalisporum extracts on a root vegetable, however, more research is necessary to understand the effects of this toxin in environmentally relevant scenarios.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Toxic cyanobacterial blooms are increasing in aquatic ecosystems, potentially as a consequence of eutrophication and climate change. They can have detrimental effects on the aquatic ecosystems by altering their trophic structure and functionality (Havens 2008), causing mortality to aquatic organisms, and decreasing water quality (Quiblier et al. 2013). Among the cyanobacterial toxins, cylindrospermopsin (CYN) is the most emergent and of increased concern (Elliott 2012). Due to its multiple toxicity targets, severe impacts on health can occur, making CYN a compound of global concern in water management (De la Cruz et al. 2013).
Currently, 13 species of cyanobacteria are known to produce CYN on four of the five continents (Oceania, America, Asia, and Europe), with Chrysosporum ovalisporum being one of the main producers (Moreira et al. 2012). The presence of CYN has been detected predominantly in tropical and subtropical waters, such as lakes, rivers, ponds, and reservoirs (Pearson et al. 2010). Furthermore, the fact that CYN is highly water-soluble because of its zwitterionic structure, leads to significant extracellular levels of this toxin in waters (70–98 %) (Rücker et al. 2007; Van Apeldoorn et al. 2007). Field studies have found a wide range of CYN concentrations in waters of different countries, reaching values from 1.1 to 800 µg/L in Australia (Saker and Eaglesham 1999; Hoeger et al. 2004; Shaw et al. 2009), 97.1 µg/L in Florida (Burns 2008), 12.1 µg/L in Germany (Rücker et al. 2007), 18.4 µg/L in Italy (Bogialli et al. 2006), and 9.4 µg/L in Spain (Quesada et al. 2006). All of these concentrations exceed the guideline value proposed by Humpage and Falconer (2003) for CYN in drinking water (1 µg/L).
The mechanism of toxicity of CYN is mediated by inhibition of protein synthesis, as well as genotoxicity by DNA fragmentation (Froscio et al. 2008; Humpage 2008; Bazin et al. 2010; Žegura et al. 2011). In addition, some studies have reported the induction of oxidative stress in different plant species (Prieto et al. 2011; Freitas et al. 2015). Humans are exposed to cyanobacterial toxins through different routes, including drinking water, recreational contact, food supplements made from cyanobacteria, and through the food chain (Gutiérrez-Praena et al. 2013). Several cyanobacterial toxins have been investigated in regard to their ability to enter the food chain via food (Ibelings and Chorus 2007; Saker et al. 2004; Gutiérrez-Praena et al. 2013; Corbel et al. 2014). The use of cyanotoxin-contaminated water for agricultural purposes may represent a potential risk to food safety and security, and is of particular concern. Indeed, several studies have reported the negative impact of cyanotoxins on the yield, quality and safety of crop plants, as it was recently reviewed by De la Cruz et al. (2013).
To date, the effects of exposure of edible plants to cyanotoxins have been described for rice exposed to microcystins (MC) and CYN (Prieto et al. 2011; Azevedo et al. 2014), tomatoes and carrots exposed to MC alone (Gutiérrez-Praena et al. 2014; Machado 2014), and lettuce contaminated with microcystin-LR (MC-LR), with leucine (L) and arginine (R) as variable L-amino acids in the chemical structure of the toxin, CYN and a mixture of both toxins (Freitas et al. 2015). Although the effects of CYN on plants have been studied to a much lesser extent than other cyanotoxins such as MC, this toxin is expected to become increasingly recurrent and thus enhancing the knowledge of its impact on crop plants is necessary. Moreover, MC are predominantly intracellular and CYN extracellular due to its high water-solubility; this fact may also make the effects of these toxins very different.
Few studies have indicated so far several effects on plant species exposed to CYN. White mustard (Sinapis alba) experienced growth inhibition (Vasas et al. 2002), with reduced fresh mass and the length of cotyledons (M-Hamvas et al. 2010) after exposure to concentrations of CYN between 5 and 10 µg/mL. Alterations in growth were also shown in the macrophyte Phragmites australis (Beyer et al. 2009), as well as reduction in pollen germination in tobacco plants (Nicotiana tabacum) (Metcalf et al. 2004), and the induction of oxidative stress in rice (Oryza sativa) (Prieto et al. 2011). In addition, exposure of Hydrilla verticillata to Cylindrospermopsis raciborskii whole-cell extracts (25–400 µg/L) resulted in decreases in chlorophyll and changes in the chlorophyll a:b ratio, indicating complex effects that extracts might be exerting on photosynthesis (Kinnear et al. 2008). Moreover, chromatin alterations, chromosomal aberrations and mitotic delay with short-term exposures have been reported in Vicia faba cells (Garda et al. 2015).
However, the uptake of toxins by plants may induce not only morphological and physiological changes leading to a loss of productivity, but it could also have considerable implications in their nutritional value, as well as in toxin bioaccumulation. Recently, Kittler et al. (2012) reported that treatment of Brassica oleracea var. sabellica, Brassica juncea, and S. alba under varying experimental conditions showed significant CYN uptake, with CYN levels ranging from 10 to 21 % in the leaves compared to the CYN concentration applied to the roots (18–35 µg/L). This work demonstrates that crop plants irrigated with CYN-containing waters may represent an important source of this toxin within the food chain, especially when the cyanotoxins are present in high concentrations in the irrigation water (Pflugmacher et al. 2006). The plant root system is usually more exposed via soil irrigation, and therefore root vegetables are more susceptible to contamination.
Minerals are essential to plant growth and development; they are intrinsic components for normal structure, metabolism and function (Pinto and Ferreira 2015). Interestingly, Saqrane et al. (2009) have reported that exposure of Triticum durum, Zea mays, Pisum sativum, and Lens esculenta plants to MC-containing extracts resulted in changes in the mineral content in roots in a concentration-dependent manner. More recently, El Khalloufi et al. (2012) and Lahrouni et al. (2013) demonstrated that cyanobacterial bloom extracts containing MC induced changes in mineral assimilation and content in tomato (Lycopersicon esculentum) and faba bean (V. faba), respectively. According to our knowledge, only the study developed by Freitas et al. (2015) reported effects of CYN on the mineral content of plants. Therefore, because carrots are root vegetables used for animal and direct human consumption, and taking into account human health and agricultural economics, it is important to evaluate the possible effects of using water contaminated with CYN for irrigation of this crop.
Regarding the effects of cyanotoxins on plants, most works were based on short-term toxicological studies (5 days) with high toxin concentrations or were conducted with other toxins different from CYN: 1500–20,000 µg/L in Mitrovic et al. (2005), 5000–10,000 µg/L in M-Hamvas et al. (2010), and 2220–22,240 µg/L in El Khalloufi et al. (2011, 2012), and almost all plants were tested in early stages of development. Recently, two studies took into account environmentally relevant cyanotoxin concentrations for carrots exposed to MC (Machado 2014) and lettuce plants exposed to MC-LR and CYN (Freitas et al. 2015). However, the adverse effects of CYN exposure on carrot plants have not been previously investigated.
In this context, the aim of the present study was to assess the effects of CYN on growth, photosynthetic capacity and nutritional value (mineral content) of carrot plants (Daucus carota), at the stage of the growth and differentiation of the taproot (carrot), in soil in natural conditions, and irrigated every 2 days with non-contaminated water or with a C. ovalisporum extract containing environmentally relevant concentrations of CYN (10 and 50 μg/L).
Materials and methods
Chrysosporum ovalisporum culture
Chrysosporum ovalisporum (LEGE X-001) cyanobacterial CYN-producing strain (CYN+) was isolated from Lake Kinneret, Israel (Banker et al. 1997) and was grown for biomass production in the Interdisciplinary Centre of Marine and Environmental Research, CIIMAR (Porto, Portugal) as described in Guzmán-Guillén et al. (2014).
CYN extraction, purification, and quantification
The extraction and purification of CYN from the culture was performed according to Guzmán-Guillén et al. (2012) with modifications. Briefly, the lyophilized biomass (0.5 g) was extracted with 100 mL of MilliQ water, sonicated (15 min), stirred (1 h), and sonicated again (15 min). The resulting mixture was centrifuged (4500 rpm, 10 min), after which the supernatant was collected and 0.1 % TFA was added. The purified CYN fractions were then quantified in an HPLC system on an Atlantis® HILIC phase column (250 × 4.6 mm, 5 μm) from Waters (Milford, USA) maintained at 40 °C. The PDA range was 210–400 nm with a fixed wavelength of 262 nm. The isocratic elution was also a 5 % MeOH solution containing 2 mM sodium 1-heptanesulfonate monohydrate (99 %) with a flow rate of 0.9 mL/min and an injection volume of 10 μL. The system was calibrated using a set of seven dilutions of the CYN standard (25, 20, 10, 5, 2, 1, and 0.5 μg/mL) in ultrapure water. Each vial was injected in duplicate, and every HPLC run consisting of a series of 10 samples included a blank and two different standard concentrations. The chromatographic purity of CYN was 98 %. The Empower 2 Chromatography Data Software was used for the calculation and reporting the peak information. The retention time of the CYN peak was 7.35 min, and the concentration obtained was 27.2 μg CYN/mL.
Plant material and exposure to CYN
Carrot plants (Daucus carota cv. Soprano), approximately 1-month-old and starting the growth and differentiation of the taproots, were acquired from a nursery, taken to the laboratory, transplanted into clear plastic cups (350 mL) with soil, and placed on black trays. Plants were placed in outside conditions, in July 2014, and allowed to acclimate for 1 week before the beginning of the experiment to the natural light, temperature, and air humidity levels. The soil used was Siro® Plant Universal Substrate and its main physicochemical properties were: pH (CaCl2), 5.5–6.5; soil moisture content, 50–60 %; conductivity, 0.6–1.2 dS/m; N, 150–250 mg/L; P2O5, 150–250 mg/L; K2O, 300–500 mg/L; organic material, >70 %. During the test (30 days, in July), carrots were submitted three times a week to irrigation with 40 mL of the crude extract of C. ovalisporum diluted in water to obtain different concentrations of CYN (10 and 50 µg CYN/L), which are considered environmentally relevant. The toxin in the crude extract was quantified on days that it was administered, obtaining an average content of 27.36 μg CYN/mL (levels of CYN in the C. ovalisporum extract were maintained generally constant with no noticeable variations, in the range 27.11–27.79 μg CYN/mL). Thus, dilutions were accurately prepared to always achieve the same concentration of 10 and 50 µg CYN/L. Additional irrigation was applied to keep the soil moist during the experiment, with similar volume of clean water (free of toxin) to each plant. A soil moisture sensor (Leroy Merlin, Ref.14785393, Lille, France) with 4 intensity levels (A to D, where A corresponds to dry soil and D to water saturated soil) was used to control soil moisture. In the experiment, moisture levels in the soil were between levels B and C.
There were 9 trays, with 17 carrot plants per tray for a total of 153 plants (51 plants for each of the control group, 10 and 50 μg CYN/L). A 30-days exposure was performed outside under natural light, temperature, and humidity conditions, allowing plants to develop enlarged and orange taproots (carrots). At the end of the exposure experiment, plants were analyzed with respect to the photosynthetic capacity (n = 20 per condition), growth (n = 37 per condition), and mineral content (n = 4 per condition).
Determination of photosynthetic capacity
Twenty randomly harvested carrots per condition (control, 10, and 50 µg CYN/L) were selected to nondestructively measure the photosynthetic capacity of the leaves by pulse amplitude modulation (PAM) using PAM 2000 (Walz, Effeltrich, Germany) on day 30. Plants were transferred to a dark room where they remained for 30 min. After the adjustment period, the leaves were illuminated with a saturating light pulse and the emitted fluorescence was immediately recorded by the instrument. This procedure allows measuring the maximum efficiency (Fv/Fm), which is directly related to the functional state of photosystem II (PSII) protein complex and photosynthetic efficiency of the plants (Maxwell and Johnson 2000).
Determination of plant growth
After 30 days of CYN exposure, carrot plants were harvested, and roots and leaves separated. The fresh weight (fw) of roots of 37 randomly selected plants per condition (control, 10, and 50 µg CYN/L) was determined on a semi-analytical balance. Root growth was expressed as the mean fresh weight (fw) ± standard deviation (SD) of n = 37.
Determination of mineral content in carrots
At the harvest, four randomly selected plants per exposure condition were taken for determination of mineral content (n = 4; 10 g/plant). The root system of each plant, constituted mainly by the differentiated and enlarged taproot (carrot), was harvested and washed with deionized water to remove soil contaminants, weighed, frozen at −80 °C and lyophilized (Telstar Lyoquest) for 1 week. Carrot samples were ground and homogenized in a blade homogenizer (GRINDOMIX GM 200, Retsch, Haan, Germany). The microwave-assisted acid digestion and the elemental analysis were performed according to Pinto et al. (2014a). For the microwave-assisted acid digestion, 200 mg of lyophilized sample were weighed in polytetrafluoroethylene (PTFE) vessels and 3 mL of HNO3 (≥69 %, w/w) plus 1 mL of H2O2 (30 %, v/v) were added. Then, the PTFE vessels were placed in the microwave digestion unit (MLS 1200 Mega, Milestone, Sorisole, Italy), which was equipped with a rotor HPR-1000/10 S, and subjected to the following digestion program: 250 W for 1 min, 0 W for 2 min, 250 W for 5 min, 400 W for 5 min, and 600 W for 5 min. Once digested, the sample solutions were transferred to 25 mL volumetric flasks, and the volume was made up with ultrapure water. This procedure was performed for all samples, as well as for the certified reference material. In each digestion, a blank was included, consisting of the digestion process in the absence of biological material.
Sample solutions were analyzed for their content of Ca, Mg, Na, and K by a 3100 flame (air-acetylene) atomic absorption spectrometer (Perkin Elmer, Überlingen, Germany). Cathode lamps (Perkin Elmer, Überlingen, Germany) were used as a radiation source. The multi-element calibration standards were prepared from 1000 mg/L standard solutions of one element of Ca, Mg, Na, and K (Sigma, St. Louis MO, USA). A lanthanum solution (10 % w/v) was used in the determination of Ca and Mg to eliminate chemical interferences.
The inductively coupled plasma-mass spectrometry (ICP-MS) equipment used for the analysis of Fe, Mn, Zn, Mo, Cu, and P was a VG Elemental, PlasmaQuad 3 model (Winsford, UK). A Minipuls 3 peristaltic pump (Gilson, Villiers le Bel, France) was used for sample introduction. Argon of 99.9997 % purity (Gasin II, Leça da Palmeira, Portugal) was used as the plasma source. The ICP-MS analysis was performed under the following conditions: argon flow rate (13 L/min); auxiliary argon flow rate (0.7 L/min); nebulizer flow rate (0.8 L/min); RF power (1350 W); scan regions dwell time (100 ms); and detection mode (pulse counting). The elemental isotopes (m/z ratios) 31P, 55Mn, 56Fe, 65Cu, 66Zn and 95Mo were monitored for analytical determination; 45Sc, 89Y, 115In, 159Tb and 209Bi were used as internal standards. The instrument was tuned daily for maximum signal sensitivity and stability using 115In as the target isotope. For this analysis, calibration standards were prepared from the AccuStandard® (New Haven, CT) 10 μg/mL multi-element ICP-MS standard solution (ICP-MS-200.8-CAL1-1) and the 1000 μg/mL single-element standard solutions (P ion chromatography standard). Results were expressed on a fresh weight basis.
All materials used in the preparation of the samples were decontaminated with HNO3 (10 % v/v) and washed 3 times with ultrapure water. In the preparation of the solutions, all reagents used were analytical grade: HNO3 (≥69 % w/w, TraceSELECT® Ultra Fluka, L’Isle d’Abeau Chesnes, France) and H2O2 (30 % v/v TraceSELECT® Fluka, Seelze, Germany). Ultrapure water (18.2 MΩ cm, 25 °C) was obtained from a Sartorius Arium® pro water purification system (Goettingen, Germany). A certified reference material (BCR 679, white cabbage, provided by the EC Institute for Reference Materials and Measurements, Geel, Belgium) was used to verify the precision and accuracy of the analytical procedures. Recovery, calculated as the determined concentration for a given element divided by its certified value, ranged between 97.2 and 103.6 % for all the analyzed elements (Online Resource 1).
Statistical analysis
All results were subjected to one-way analysis of variance (ANOVA) (GraphPad Instat Software, La Jolla, USA). Differences in mean values between groups were assessed by the Tukey’s test and were considered statistically different at p < 0.05.
Results and discussion
Effects of CYN on photosynthetic capacity
Regarding photosynthetic capacity, we observed an increase in the Fv/Fm of the plants irrigated with 10 and 50 µg CYN/L with respect to the control group (Fig. 1). An Fv/Fm between 800 and 830 is considered an optimal value for this parameter and values lower than this may indicate a decrease in the photosynthetic capacity of plants caused by some kind of stress (Maxwell and Johnson 2000). In this work, contrary to what was expected, an increase in Fv/Fm was observed and in the case of 50 µg CYN/L treatment was statistically significant. However, increased Fv/Fm is rarely observed and is poorly characterized in the existing literature at the physiological and plant performance levels (Machado 2014; Madhana Sekhar et al. 2014; Li et al. 2014). This may reflect a hormesis effect, showing an active metabolism in an early stage for low doses of toxicants (such as 10 and 50 µg CYN/L), but it may be possible that for higher CYN concentrations, an inhibition of photosynthesis occurs, as reported in other plant species exposed to different toxic agents (Dai et al. 2010; Capelo et al. 2012; Erofeeva 2014; Jia et al. 2015).
Fluorescence measures in leaves from carrots (Daucus carota) grown for 30 days with different concentrations of a Chrysosporum ovalisporum extract containing CYN (10 or 50 µg CYN/L). Values are expressed as mean ± SD (n = 20). The significance levels observed are **p < 0.01 when comparing the control and CYN-exposed plants (10 or 50 µg CYN/L)
With regard to the relationship between Fv/Fm and photosynthesis, Madhana Sekhar et al. (2014) demonstrated that elevated CO2 grown mulberry plants showed a persistent stimulation of photosynthesis, avoiding photosynthetic acclimation, based on increased Fv/Fm and other parameters. The authors suggested this is perhaps due to efficient sink capacity, which implies a positive regulation of the photosynthesis by an efficient utilization and/or storage of the photosynthetic assimilates (produced in the source organs, leaves) in the sink organs (roots, flowers, seeds, fruits, etc.). By contrast, ineffective sink capacity would lead to an accumulation of photosynthetic assimilates in the leaves and subsequent inhibition of photosynthesis and production of photosynthetic assimilates. Moreover, photosynthetic activity (Fv/Fm) was improved in Sedum alfredii by elevated CO2, facilitating the plant to keep normal physiological functions under cadmium stress and therefore alleviating metal toxicity to the plant (Li et al. 2014). These authors also demonstrated a positive correlation between plant growth promotion and increased photosynthetic rate at elevated CO2, in agreement with our results. According to these findings, it may be possible that carrot plants are reinforcing their photosynthetic mechanism in order to confront the higher energetic cost needed to fight the toxic effects of CYN.
To date, no studies concerning the effects of CYN on photosynthesis on edible plants have been reported, so there are no data available for comparison. Nevertheless, Kinnear et al. (2008) conducted a study in the aquatic plant Hydrilla verticillata exposed to whole-cell extracts of C. raciborskii containing 25–400 µg CYN/L, remarking that these might exert some adverse effects on photosynthesis.
In relation to other cyanotoxins, such as MC, contradictory results have been obtained depending on the plant species, concentration and exposure length to the toxin. Similar to our study, the findings by Machado (2014) in carrots exposed to MC-LR for 14–28 days show that this parameter increased from the concentration of 10 µg MC-LR/L and with 50 µg MC-LR/L. However, other studies showed inhibition of the photosynthetic process in wheat, maize, peas, lentils, tomatoes, and rice exposed to MC (Saqrane et al. 2009; El Khalloufi et al. 2012; Gutiérrez-Praena et al. 2014; Azevedo et al. 2014). These studies proved that MC impaired the photosynthetic activity, partially due to a damage of PSII reaction centers, deleterious effects on PSII activity and changes in the function of various proteins involved in the photosynthetic process related to the chloroplast ATP synthesis, carbon fixation, photosynthesis, and metabolism of carbohydrates. However, most of the above-mentioned research was carried out with MC, a cyanotoxin which is chemically and functionally very distinct from CYN. Thus, the effects observed in other works may be not necessarily reproduced in our study with CYN, as can be seen in the present work where the toxin may even have contrasting effects. Taking into account the results obtained in our study, more research will be needed to understand if the high Fv/Fm value observed in plants exposed to 50 μg CYN/L can be translated into an increased photosynthetic efficiency, or if, conversely, the Fv/Fm value has no physiological significance with CYN.
Effects of CYN on carrot growth
Exposure of carrot plants to 10 and 50 µg CYN/L for 30 days led to a significant increase in the fresh weight of roots (Fig. 2). This was similar to the increase in root growth of Hydrilla verticillata observed by Kinnear et al. (2008) after 14 days of exposure to C. raciborskii extracts containing 400 µg CYN/L. Moreover, Prieto et al. (2011) found significant increases in the fresh weight of roots from rice plants exposed for 48 h to C. ovalisporum cell extracts containing 2.5 µg CYN/L, however, no changes were observed upon a 9-day exposure to the same extracts. The growth of lettuce roots was stimulated after 10 days of exposure to 1, 10, and 100 µg CYN/L, whereas the leaf fresh weight decreased only at 100 µg CYN/L (Freitas et al. 2015). Similarly, Garda et al. (2015) detected transient stimulation of root growth in V. faba seedlings at 6 days of treatment with 0.1 μg CYN/mL. According to these authors, growth responses of vascular plants to CYN are variable, showing growth stimulation at low concentrations (0.1–0.4 μg/mL). Kinnear et al. (2008) reported that some benefits could result from an increased root production, such as the potential production of exudates, allelochemicals or phytochelatins, which might scavenge CYN, preventing its uptake by plant cells. By contrast, Vasas et al. (2002) reported the growth inhibition of etiolated Sinapis alba seedlings exposed to CYN isolated from C. ovalisporum. Moreover, developmental alterations (reduction of fresh mass and length of cotyledons) have been observed in seedlings of S. alba by exposure to this toxin (M-Hamvas et al. 2010). The reduction in growth reported by those authors may be attributed to the high toxin concentrations employed (18,200 and 5000–10,000 µg CYN/L, respectively). Beyer et al. (2009) showed that concentrations of CYN between 500 and 40,000 µg/L decreases root and shoot elongation in the macrophyte Phragmites australis. Regarding carrot plants exposed to MC, Machado (2014) observed a decrease in root growth of carrots exposed to MC-LR (50 µg/L) for 28 days, corroborating the results reported by El Khalloufi et al. (2012) in tomato plants (Lycopersicon esculentum). Several factors may explain the differences between those studies and our results, such as the plant species and their stage of development, as well as the time and dose of exposure.
Average fresh weight of Daucus carota taproots (carrots) grown for 30 days with different concentrations of a Chrysosporum ovalisporum extract containing CYN (10 or 50 µg CYN/L). Values are expressed as mean ± SD (n = 37). The significance levels observed are *p < 0.05 when comparing the control and CYN-exposed plants (10 or 50 µg CYN/L)
Hence, our results seem to indicate that chronic exposure of carrots to low concentrations of CYN does not impair photosynthesis and plant growth. Among the causes of this tolerance could be the expression of several molecular and specialized tissues that protect adult plants against pathogens and soil-borne toxins. Several of these defense mechanisms are present in roots and are constituted by the root exudates (allelochemicals or phytochelatins) and other secondary metabolites used in defense against pathogenic microorganisms and toxins (Baetz and Martinoia 2014). Other defense mechanisms include the root border cells that engineer the chemical and physical properties of the external environment (Hawes et al. 2000), and the suberized tissues of the epidermis and the endodermis with a casparian band, that function as barriers to the entry of external chemicals and pathogen invasion (Kosma et al. 2015; Enstone et al. 2002). On the other hand, the stimulation of root growth is in line with the stimulation of photosynthesis and the probable increase of photosynthetic assimilates that are translocated and accumulated in storage organs as the carrots. The potential beneficial effects of the cyanobacterial extract nutrients seem unlikely, since these were highly diluted in the irrigation water used in the experiment (dilution rates of around 2700 and 550 to obtain 10 and 50 μg CYN/L, respectively).
The fact that we did not observe serious damages induced by CYN on photosynthetic capacity and growth of carrot plants even after 30 days of exposure, does not exclude the potential hazard of this toxin in other scenarios of prolonged exposure. If we consider that the accumulation of toxin may increase with the length of exposure (Kittler et al. 2012), there is the possibility that toxic CYN concentrations in carrots may be reached after 30 days of exposure, and with the transpose of the defense barriers of the roots. Therefore in terms of risk assessment there is the need to clarify the exposure length effects of CYN in crops (effects on yield, contamination, physiology) and monitor the effects of CYN in the whole life cycle of crops.
Effects of CYN on mineral content in carrot roots
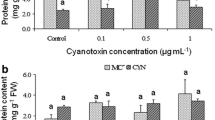
A significant decline in the content of almost all minerals (Ca, Mg, Na, K, Fe, Zn, Mo, and P) in carrot roots exposed to CYN, especially with 50 µg CYN/L was observed (Figs. 3 and 4). Only Mn and Cu were significantly increased after exposure to CYN, although for Cu this effect was only observed with 10 µg CYN/L (Fig. 4a, b). These results demonstrate that the exposure concentration is an important factor for the toxic effects of CYN on carrots. Contrary to our results, Machado (2014) and Freitas et al. (2015) obtained a general concentration-dependent enhancement of the mineral content in carrot roots exposed to MC and lettuce leaves exposed to CYN, respectively. However, Cu and P followed the same trend (increasing and decreasing, respectively) in the present and the above-mentioned studies, whatever the cyanotoxin was (CYN or MC).
Concentration of Ca, Mg, Na, and P a and K b, (mg/kg F.W.) in Daucus carota taproots (carrots) grown for 30 days with different concentrations of a Chrysosporum ovalisporum extract containing CYN (10 or 50 µg CYN/L). Values are expressed as mean ± SD (n = 4). The significance levels observed are *p < 0.05 or **p < 0.01 when comparing the control and CYN-exposed plants (10 or 50 µg CYN/L), and #p < 0.05 among both doses of CYN
Concentration of Fe, Zn, Mn a, Mo and Cu b (mg/kg F.W.) in Daucus carota taproots (carrots) grown for 30 days with different concentrations of a Chrysosporum ovalisporum extract containing CYN (10 or 50 µg CYN/L). Values are expressed as mean ± SD (n = 4). The significance levels observed are *p < 0.05, **p < 0.01 or ***p < 0.001 when comparing the control and CYN-exposed plants (10 or 50 µg CYN/L), and #p < 0.05 among both doses of CYN
In terms of mineral concentrations, it seems that carrots are more vulnerable to CYN toxicity compared to other plant species, at the conditions assayed. In our study, a general decrease was observed in fully developed carrots (after 30 days of exposure), compared to Freitas et al. (2015), who observed a general increase in mineral content in lettuce leaves after 5 and 10 days of exposure to CYN, indicating the potential tolerance of lettuce plants to this cyanotoxin. One of the reasons for the increased susceptibility of carrots to CYN, in terms of mineral uptake, was the longer time of exposure (30 days) to the toxin, which showed to be critical to mineral uptake, as contrasting to the 5 and 10 days CYN exposure studied in Freitas et al. (2015). In this sense, it seems that the above-mentioned root defense mechanisms do not protect completely root mineral uptake from the external stressor CYN. Another reason could be that root metabolism is more affected than leaf metabolism, since roots are directly in contact with the toxin, and this is also reflected in terms of mineral content differences in the two organs. Noticeably, CYN had a more severe effect on our carrot roots than cyanotoxin MC-LR, as described by Machado (2014).
The uptake of minerals in the plant, especially micronutrients, can be affected by various factors such as the substrate where plants grow, microbial composition of the substrate, environmental factors and the presence of contaminants (Pinto et al. 2014b). In this regard, the differences observed in the mineral uptake in this work vs. Freitas et al. (2015) could also be due to differences in physiology between the hydroponically grown plants of Freitas et al. (2015) compared with our soil-grown plants. Keeping in mind that the present work was carried out by irrigating the plants with water contaminated with C. ovalisporum crude extracts, we point out the possible combined action of the toxin CYN and other secondary cyanobacterial metabolites produced by C. ovalisporum to explain the differential uptake of minerals observed in this work, compared to Freitas et al. (2015), where purified CYN was used instead. In this sense, cyanobacteria are known to produce a diverse array of secondary metabolites, including toxins, lipopolysaccharides, and other unknown metabolites that, as a whole, are known to contribute to the bioactivity and toxicity of corresponding crude extracts (Berry et al. 2009; Kinnear 2010).
To ensure the proper growth of plants it is essential that nutrients are assimilated at adequate levels, as both a deficiency and an excess of nutrients could affect plant growth by direct effects on cell metabolism (such as the biosynthesis of macromolecules or functioning of the photosynthetic system) (White and Brown 2010). In our study, the general decrease observed in nutrient concentrations does not seem sufficient to impair carrot growth. In this sense, macro and micronutrients contents evaluated in this work are generally within the ranges reported by other authors for several varieties of carrot taproots. The amount of Ca we detected was in the range of 233–298 mg/kg F.W. (for 50 µg/L and control groups, respectively), similarly to Zaccari et al. (2015) who obtained values ranging 290–370 mg/kg F.W. Warman and Havard (1997) reported 1000–1570 mg of Mg/kg F.W. and 1170–4980 mg of Na/kg F.W., compared to our values of 1145–1468 and 1142–2289 mg/kg F.W. for Mg and Na, respectively. The content of Zn in the carrots studied ranges from 3.62 to 5.53 mg/kg F.W. (for 50 µg/L and control groups, respectively), and agrees with the reported by other authors which show different ranges in the literature: 3.1–5.6 mg/kg F.W. (Scherz and Kirchhoff 2006), 1.8–3.9 mg/kg F.W. (Nicolle et al. 2004), 7–10 mg/kg F.W. (Zaccari et al. 2015) and 21–26 mg/kg F.W. (Warman and Havard 1997). In the case of Mo, Ensafi and Khaloo (2005) found 1.48 ng/mL, similar to our values of 1.53–1.80 ng Mo/mL. Among all micronutrients, Mo is often present in plants in lower concentrations (Kirkby and Römheld 2007), agreeing with our results. Fe, Mn, and Cu concentrations were lower than those reported by Warman and Havard (1997), even in the control group. However, this is not surprising or alarming because crop plants only need a small amount of micronutrients. These works show that carrots in our work, even after CYN exposure, were generally within the normal amounts of nutrients detected in this species, explaining that growth and photosynthesis were not compromised by this condition.
It would be of interest to further study the role of these minerals in the plant metabolism. Manganese is part of the structure of some photosynthetic proteins, and Zn is essential for the synthesis of chlorophyll and for metalloenzymes. Copper toxicity could affect plant physiological processes and ultimately plant production (Pinto et al. 2014b), being important as cofactor of antioxidant enzymes (Pinto and Ferreira 2015; Kirkby and Römheld 2007).
The absence of mineral content data in leaves makes the link between the nutrient effect and photosynthesis difficult to establish and rather speculative. Taking into account that most minerals decreased in roots, we could expect a similar effect of CYN in leaves. Since some of these minerals are critical to photosynthesis, the most probable result of such a change in minerals would be the inhibition of photosynthesis. However, this hypothetical situation does not agree with the experimental data on photosynthesis, which leads us to the assumption that, for this particular experiment, mineral, and photosynthesis effects are poorly associated.
Conclusions
In the present study, the effects of ecologically relevant concentrations of CYN (10 and 50 µg/L) were investigated regarding to growth, photosynthetic activity, and mineral content in carrot plants exposed for 30 days to C. ovalisporum extracts contained in the irrigation water. Based on the results, we conclude that exposure to CYN did not have a clear negative effect on the growth of D. carota, and it even caused an increase in the fresh weights of the exposed plants. Furthermore, exposure of carrots to these CYN concentrations did not appear to affect vital biochemical processes such as photosynthesis. Nevertheless, the decrease in mineral content indicate possible alterations in the nutritional value of carrots after exposure to crude extracts of C. ovalisporum containing CYN. This study is the first to evaluate the effects C. ovalisporum extracts on a root vegetable. However, more studies are necessary to understand the effect of this toxin in other environmental scenarios.
References
Azevedo CC, Azevedo J, Osório H, Vasconcelos V, Campos A (2014) Early physiological and biochemical responses of rice seedlings to low concentration of microcystin-LR. Ecotoxicology 23:107–121
Baetz U, Martinoia E (2014) Root exudates: The hidden part of plant defense. Trends Plant Sci 19:90–98
Banker R, Carmeli S, Hadas O, Teltsh B, Porat R, Sukenik A (1997) Identification of cylindrospermopsin in Aphanizomenon ovalisporum (Cyanophyceae) isolated from lake Kinneret, Israel. J Phycol 33:613–616
Bazin E, Mourot A, Humpage AR, Fessard V (2010) Genotoxicity of a freshwater cyanotoxin, cylindrospermopsin, in two human cell lines: Caco-2 and HepaRG. Environ Mol Mutagen 51:251–259
Berry JP, Gibbs PD, Schmale MC, Saker ML (2009) Toxicity of cylindrospermopsin, and other apparent metabolites from Cylindrospermopsis raciborskii and Aphanizomenon ovalisporum, to the zebrafish (Danio rerio) embryo. Toxicon 53:289–299
Beyer D, Surányi G, Vasas G, Roszik J, Erdodi F, M-Hamvas M, Bácsi I, Bátori R, Serfozo Z, Szigeti ZM, Vereb G, Demeter Z, Gonda S, Máthé C (2009) Cylindrospermopsin induces alterations of root histology and microtubule organization in common reed (Phragmites australis) plantlets cultured in vitro. Toxicon 54:440–449
Bogialli S, Bruno M, Curini R, Di Corcia A, Fanali C, Lagana A (2006) Monitoring algal toxins in lake water by liquid chromatography tandem mass spectrometry. Environ Sci Technol 40:2917–2923
Burns J (2008) Toxic cyanobacteria in Florida waters. Adv Exp Med Biol 619:127–137
Capelo A, Santos C, Loureiro S, Pedrosa MA (2012) Phytotoxicity of lead on Lactuca sativa: effects on growth, mineral nutrition, photosynthetic activity and oxidant metabolism. Fresen Environ Bull 21:450–459
Corbel S, Mougin C, Bouaicha N (2014) Cyanobacterial toxins: modes of actions, fate in aquatic and soil ecosystems, phytotoxicity and bioaccumulation in agricultural crops. Chemosphere 96:1–5
Dai HP, Wei Y, Yang TX, Sa WQ, Wei AZ (2010) Influence of cadmium stress on chlorophyll fluorescence characteristics in Populus 9 canescens. J Food Agric Environ 10:1281–1283
De la Cruz AA, Hiskia A, Kaloudis T, Chernoff N, Hill D, Antoniou MG, He X, Loftin K, O’Shea K, Zhao C, Pelaez M, Han C, Lynch TJ, Dionysiou DD (2013) A review on cylindrospermopsin: the global occurrence, detection, toxicity and degradation of a potent cyanotoxin. Environ Sci Process Impacts 15:1979–2003
El Khalloufi F, Oufdou K, Lahrouni M, El Ghazali I, Saqrane S, Vasconcelos V, Oudra B (2011) Allelopatic effects of cyanobacteria extracts containing microcystins on Medicago sativa-Rhizobia symbiosis. Ecotoxicol Environ Saf 74:431–438
El Khalloufi F, El Ghazali I, Saqrane S, Oufdou K, Vasconcelos V, Oudra B (2012) Phytotoxic effects of a natural bloom extract containing microcystins on Lycopersicon esculentum. Ecotoxicol Environ Saf 79:199–205
Elliott JA (2012) Is the future blue-green? A review of the current model predictions of how climate change could affect pelagic freshwater cyanobacteria. Water Res 46:1364–1371
Ensafi AA, Khaloo SS (2005) Determination of traces molybdenum by catalytic adsorptive stripping voltammetry. Talanta 65:781–788
Enstone DE, Peterson CA, Ma F (2002) Root endodermis and exodermis: structure, function, and responses to the environment. J Plant Growth Regul 21:335–351
Erofeeva EA (2014) Hormesis and paradoxical effects of wheat seedling (Triticum Aestivum L.) parameters upon exposure to different pollutants in a wide range of doses. Dose Response 12:121–135
Freitas M, Azevedo J, Pinto E, Neves J, Campos A, Vasconcelos V (2015) Effects of microcystin-LR, cylindrospermopsin and a microcystin-LR/cylindrospermopsin mixture on growth, oxidative stress and mineral content in lettuce plants (Lactuca sativa L.). Ecotoxicol Environ Saf 116:59–67
Froscio SM, Humpage AR, Wickramasinghe W, Shaw G, Falconer IR (2008) Interaction of the cyanobacterial toxin cylindrospermopsin with the eukaryotic protein synthesis system. Toxicon 5:191–198
Garda T, Riba M, Vasas G, Beyer D, M-Hamvas M, Hajdu G, Tándor I, Máthé C (2015) Cytotoxic effects of cylindrospermopsin in mitotic and non-mitotic Vicia faba cells. Chemosphere 120:145–153
Gutiérrez-Praena D, Jos A, Pichardo S, Moreno IM, Cameán AM (2013) Presence and bioaccumulation of microcystins and cylindrospermopsin in food and the effectiveness of some cooking techniques at decreasing their concentrations: a review. Food Chem Toxicol 53:136–152
Gutiérrez-Praena D, Campos A, Azevedo J, Neves J, Freitas M, Guzmán-Guillén R, Vasconcelos V (2014) Exposure of Lycopersicon Esculentum to microcystin-LR: Effects in the leaf proteome and toxin translocation from water to leaves and fruits. Toxins 6:1837–1854
Guzmán-Guillén R, Prieto AI, González AG, Soria-Díaz ME, Cameán AM (2012) Cylindrospermopsin determination in water by LC–MS/MS: Optimization and validation of the method and application to real samples. Environ Toxicol Chem 31:2233–2238
Guzmán-Guillén R, Prieto AI, Moreno I, Rios V, Vasconcelos VM, Camean AM (2014) Effects of depuration on oxidative biomarkers in Tilapia (Oreochromis niloticus) after subchronic exposure to cyanobacterium producing cylindrospermopsin. Aquat Toxicol 149:40–49
Havens KE (2008) Cyanobacteria blooms: effects on aquatic ecosystems. Adv Exp Med Biol 619:733–747
Hawes MC, Gunawardena U, Miyasaka S, Zhao X (2000) The role of root border cells in plant defense. Trends Plant Sci 5:128–133
Hoeger SJ, Shaw G, Hitzfeld BC, Dietrich DR (2004) Occurrence and elimination of cyanobacterial toxins in two Australian drinking water treatment plants. Toxicon 43:639–649
Humpage A (2008) Toxin types, toxicokinetics and toxicodynamics. In: Hudnel HK (ed) Cyanobacterial harmful algal blooms state of the science and research needs. Springer Press, New York, pp 383–415
Humpage AR, Falconer IR (2003) Oral toxicity of the cyanobacterial toxin cylindrospermopsin in male Swiss albino mice: determination of no observed adverse effect level for deriving a drinking water guideline value. Environ Toxicol 18:94–103
Ibelings BW, Chorus I (2007) Accumulation of cyanobacterial toxins in freshwater “seafood” and its consequences for public health: a review. Environ Pollut 150:177–192
Jia L, Liu Z, Chen W, Ye Y, Yu S, He X (2015) Hormesis effects induced by cadmium on growth and phtotosynthetic performance in a hyperaccumulator Lonicera japonica thunb. J Plant Growth Regul 34:13–21
Kinnear S, Fabbro L, Duivenvoorden L (2008) Variable growth responses of water thyme (Hydrilla verticillata); To whole-cell extracts of Cylindrospermopsis raciborskii. Arch Environ Contam Toxicol 54:187–194
Kinnear S (2010) Cylindrospermopsin: a decade of progress on bioaccumulation research. Mar Drugs 8:542–564
Kirkby EA, Römheld V (2007) Micronutrients in Plant Physiology: Functions, Uptake and Mobility. Proceedings 543. The International Fertilizer Society, York, United Kingdom.
Kittler K, Schreiner M, Krumbein A, Manzei S, Koch M, Rohn S, Maul R (2012) Uptake of the cyanobacterial toxin cylindrospermopsin in Brassica vegetables. Food Chem 133:875–879
Kosma DK, Rice A, Pollard M (2015) Analysis of aliphatic waxes associated with root periderm or exodermis from eleven plant species. Phytochemistry 117:351–362
Lahrouni M, Oufdou K, El Khalloufi F, Baz M, Lafuente A, Dary M, Pajuelo E, Oudra B (2013) Physiological and biochemical defense reactions of Vicia faba L.-Rhizobium symbiosis face to chronic exposure to cyanobacterial bloom extract containing microcystins. Environ Sci Pollut Res 20:5405–5415
Li TQ, Tao Q, Di ZZ, Lu F, Yang XE (2014) Effect of elevated CO2 concentration on photosynthetic characteristics of hyperaccumulator Sedum alfredii under cadmium stress. J Integr Plant Biol 57:653–660
M-Hamvas M, Máthé C, Vasas G, Jámbrik K, Papp M, Beyer D, Mészáros I, Borbély G (2010) Cylindrospermopsin and microcystin-LR alter the growth, development and peroxidase enzyme activity of white mustard (Sinapis alba L.) seedlings, a comparative analysis. Acta Biol Hung 61:35–48
Machado JFF (2014) Implicações do Uso de Água Contaminada com Microcistina na Qualidade de Daucus Carota (Cenoura). Dissertação de Mestrado. Universidade do Porto, Portugal
Madhana Sekhar K, Rachapudi VS, Mudalkar S, Reddy AR (2014) Persistent stimulation of photosynthesis in short rotation coppice mulberry under elevated CO2 atmosphere. J Photochem Photobiol B: Biol 137:21–30
Maxwell K, Johnson N (2000) Cholorophyll fluorescence – A practical guide. J Exp Bot 51:659–668
Metcalf JS, Barakate A, Codd GA (2004) Inhibition of plant protein synthesis by the cyanobacterial hepatotoxin, cylindrospermopsin. FEMS Microbiol Lett 235:125–129
Mitrovic SM, Allis O, Furey A, James KJ (2005) Bioaccumulation and harmful effects of microcystin-LR in the aquatic plants Lemna minor and Wolffia arrhiza and the filamentous alga Chladophorafracta. Ecotoxicol Environ Saf 61:345–352
Moreira C, Azevedo J, Antunes A, Vasconcelos V (2012) Cylindrospermopsin: occurrence, methods of detection and toxicology. J Appl Microbiol 114:605–620
Nicolle C, Simon G, Rock E, Amouroux P, Rémésy C (2004) Genetic variability influences carotenoid, vitamin, phenolic, and mineral content in white, yellow, purple, orange and dark orange carrot cultivars. J Am Soc Hortic Sci 129:523–529
Pearson L, Mihali T, Moffitt M, Kellmann R, Neilan B (2010) On the chemistry, toxicology and genetics of the cyanobacterial toxins, microcystin, nodularin, saxitoxin and cylindrospermopsin. Mar Drugs 8:1650–1680
Pflugmacher S, Jung K, Lundvall L, Neumann S, Peuthert A (2006) Effects of cyanobacterial toxins and cyanobacterial cell-free crude extract on germination of alfalfa (Medicago sativa) and induction of oxidative stress. Environ Toxicol Chem 25:2381–2387
Pinto E, Almeida AA, Aguiar AA, Ferreira IM (2014a) Changes in macrominerals, trace elements and pigments content during lettuce (Lactuca sativa L.) growth: influence of soil composition. Food Chem 152:603–611
Pinto E, Aguiar AA, Ferreira IM (2014b) Influence of soil chemistry and plant physiology in the phytoremediation of Cu, Mn, and Zn. Crit Rev Plant Sci 33:351–373
Pinto E, Ferreira IMPLVO (2015) Cation transporters/channels in plants: tools for nutrient biofortification. J Plant Physiol 179:64–82
Prieto A, Campos A, Cameán A, Vasconcelos V (2011) Effects on growth and oxidative stress status of rice plants (Oryza sativa) exposed to two extracts of toxin-producing cyanobacteria (Aphanizomenon ovalisporum and Microcystis aeruginosa). Ecotoxicol Environ Safe 74:1973–1980
Quesada A, Moreno E, Carrasco D, Paniagua T, Wörmer L, De Hoyos C, Sukenik A (2006) Toxicity of Aphanizomenon ovalisporum (Cyanobacteria) in a Spanish water reservoir. Eur J Phycol 41:39–45
Quiblier C, Susanna W, Isidora ES, Mark H, Aurélie V, Jean-François H (2013) A review of current knowledge on toxic benthic freshwater cyanobacteria - Ecology, toxin production and risk management. Water Res 47:5464–5479
Rücker J, Stüken A, Nixdorf B, Fastner J, Chorus I, Wiedne C (2007) Concentrations of particulate and disolved cylindrospermopsin in 21 Aphanizomenon-dominated temperate lakes. Toxicon 50:800–809
Saker ML, Eaglesham GK (1999) The accumulation of cylindrospermopsin from the cyanobacterium Cylindrospermopsis raciborskii in tissues of the Redclaw crayfish Cherax quadricarinatus. Toxicon 37:1065–1077
Saker ML, Metcalf JS, Codd GA, Vasconcelos VM (2004) Accumulation and depuration of the cyanobacterial toxin cylindrospermopsin in the freshwater mussel Anodonta cygnea. Toxicon 43:185–194
Saqrane S, Ouahid Y, El Ghazali I, Oudra B, Bouarab L, del Campo FF (2009) Physiological changes in Triticum durum, Zea mays, Pisum sativum and Lens esculenta cultivars, caused by irrigation with water contaminated with microcystins: a laboratory experimental approach. Toxicon 53:786–796
Scherz H, Kirchhoff E (2006) Trace elements in foods: zinc contents of raw foods—A comparison of data originating from different geographical regions of the world. Critical Review. J Food Compos Anal 19:420–433
Shaw GR, Seawright AA, Moore MR, Lam PKS (2009) Cylindrospermopsin, a cyanobacterial alkaloid: evaluation of its toxicologic activity. Ther Drug Monit 22:89–92
Van Apeldoorn ME, Van Egmond HP, Speijers GJA, Bakker GJI (2007) Toxins of cyanobacteria. Mol Nutr Food Res 51:7–60
Vasas G, Gáspár A, Surányi G, Batta G, Gyémánt G, M-Hamvas M, Máthé C, Grigorszky I, Molnaár E, Borbeély G (2002) Capillary electrophoretic assay and purification of cylindrospermopsin, a cyanobacterial toxin from Aphanizomenon ovalisporum, by plant test (blue-green sinapis test). Anal Biochem 302:95–103
Warman PR, Havard KA (1997) Yield, vitamin and mineral contents of organically and conventionally grown carrots and cabbage. Agr Ecosyst Environ 61:155–162
White PJ, Brown PH (2010) Plant nutrition for sustainable development and global health. Ann Bot 105:1073–1080
Zaccari F, Cabrera MC, Ramos A, Saadoun A (2015) In vitro bioaccessibility of β-carotene, Ca, Mg and Zn in landrace carrots (Daucus carota, L.). Food Chem 166:365–371
Žegura B, Gajski G, Štraser A, Garaj-Vrhovac V (2011) Cylindrospermopsin induced DNA damage and alteration in the expression of genes involved in the response to DNA damage, apoptosis and oxidative stress. Toxicon 58:471–479
Funding
This work was partially funded by Fundação para a Ciência e Tecnologia (FCT), under the framework of the project UID/Multi/04423/2013, by Ministerio de Ciencia e Innovación of Spain under the project AGL2009-10026, and by Junta de Andalucía under the project P09-AGR-4672. Remedios Guzmán’s work is supported by the Spanish Ministerio de Educación fellowship. Alexandre Campos work is supported by a post-doctoral grant (SFRH/BPD/103683/2014) from FCT.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Guzmán-Guillén, R., Campos, A., Machado, J. et al. Effects of Chrysosporum (Aphanizomenon) ovalisporum extracts containing cylindrospermopsin on growth, photosynthetic capacity, and mineral content of carrots (Daucus carota). Ecotoxicology 26, 22–31 (2017). https://doi.org/10.1007/s10646-016-1737-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-016-1737-4