Abstract
Recent studies have demonstrated that a pyraclostrobin-containing fungicide (Headline® Fungicide—Headline® Fungicide and Headline AMP® Fungicide are registered trademarks of BASF) is toxic to amphibians at environmentally relevant concentrations. However, these studies were performed in a laboratory setting of a worst-case direct exposure in clean media. Interception of spray by the crop canopy and ground cover used by animals for security cover will influence exposure. Thus, risk to amphibians is unclear in an environmentally realistic field environment. We tested exposure and toxicity of Headline AMP® Fungicide to amphibians in multiple agricultural habitat scenarios (e.g., within treated crop vs. grassy areas adjacent to crop) and at two rates during routine aerial application. Specifically, we placed Woodhouse’s toads (Bufo woodhousii) and Blanchard’s cricket frogs (Acris blanchardi) in enclosures located within treated and untreated corn (VT stage, approximate height = 3 m), and in the potential drift area (adjacent to treated corn) during aerial application of Headline AMP Fungicide at either 731 or 1052 ml/ha (70 and 100 % the maximum application rate in corn, respectively). Mean concentrations of pyraclostrobin measured at ground level were ≤19 % of nominal application rate in all areas. Overall, mean mortality of recovered individuals of both species was ≤15 %, and mortality within Headline AMP Fungicide-treated corn (where risk was anticipated to be highest) was <10 %. It is important to understand that application timing, interception by the crop canopy (which varies both within and between crop systems), and timing of amphibian presence in the crop field influences risk of exposure and effects; however, our results demonstrate that amphibians inhabiting VT stage corn during routine aerial application of Headline AMP Fungicide are at low risk for acute mortality, matching existing laboratory results from acute toxicity studies of Headline Fungicide.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mass of fungicides applied per ha of corn, soybean, and wheat crops has at least doubled within the last decade (National Agricultural Statistics Service; http://quickstats.nass.usda.gov/; accessed April 17, 2013). For example, Headline Fungicide (active ingredient or “a.i.” = pyraclostrobin) is used on 90 different crops to control over 50 fungal diseases (http://www.agproducts.basf.us/products/headline-fungicide.html; accessed 14 May 2014), and is widely used in Europe and the Americas (Bross and Mackenroth 2005; Regenstein 2006). Label claims indicate that Headline Fungicide also increases plant health and tolerance to non-fungal stressors (e.g., drought; BASF 2008) and thus yield, which may encourage further use of Headline Fungicide.
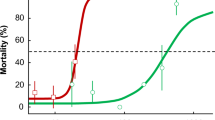
Recent laboratory studies have demonstrated that environmentally relevant concentrations of Headline Fungicide were toxic to amphibians that use wetlands associated with cropland for breeding and development (Tiner 2003; Duellman and Trueb 1986). Belden et al. (2010) and Hooser et al. (2012) reported that both the full formulation of Headline Fungicide, as well as its active ingredient alone (pyraclostrobin), were significantly toxic to larval Bufo cognatus (aquatic phase test) at environmentally relevant concentrations (as low as 5 and 15 μg a.i./L, respectively), as might be expected based on the toxicity of pyraclostrobin to fish (e.g., bluegill sunfish, LC50 = 11.4 μg/L; USEPA 2013). Further, direct application of Headline to terrestrial environments also may pose a risk, as direct application of Headline Fungicide caused about 65 % mortality of juvenile B. cognatus (terrestrial phase test) at the maximum field application rate in North American corn (2.2 µg a.i./cm2; 220 g a.i./ha), but had no effect at 0.1× this rate (Belden et al. 2010). Brühl et al. (2013) also conducted a laboratory study and observed 100 % mortality of juvenile Rana temporaria exposed to Headline Fungicide at the maximum application rate in cereals (220 g a.i./ha), but no effects were observed at 0.1× this rate.
The above studies were performed in controlled laboratory settings under an exposure scenario of direct overspray in clean media, and do not represent risk in an environmentally realistic field environment. Many factors influence exposure and effects of pesticides in natural settings. For example, interception by the crop canopy likely decreases the amount of chemical reaching ground level, affording a degree of protection to animals. However, the amount of spray intercepted by plants and the amount reaching the ground is variable and dictated by factors such as plant species, growth stage, and method of pesticide application (FOCUS 2000; Olesen and Jensen 2013), all of which are influenced or dictated by the pesticide-specific registered use guidelines for specific locations (e.g., states, countries). Indeed, depending on the pesticide, fields may be sprayed when fallow or at times when crop canopy is extensive and interception of spray is predicted to be as much as 90 %. Further, various aspects of an animal’s behavior may influence exposure and effects during routine application of pesticides. These include burying behavior (Seymour 1973), daily activity patterns (Lannoo 2005; Ruibal et al. 1969), and use of microhabitats as refugia (Seebacher and Alford 2002). Thus, it is often unclear whether routine application of pesticides, either by aerial or ground (more typical in Europe) methods, pose a risk to amphibians in or near agriculture. Recent studies have investigated the overlap between estimated pesticide applications and the presence of amphibians in agricultural fields, with emergent predictions of exposure and effects for amphibians under different crop and pesticide use scenarios (Berger et al. 2013; Lenhardt et al. 2015). These predictions range from minimal overlap between amphibians and pesticides to estimates of possible mortality events, even with high pesticide interception rates (Lenhardt et al. 2015). The objective of this study was to investigate the exposure and effects of Headline Fungicide to amphibians during aerial treatment of VT stage corn, the typical corn application in North America. Specifically, we tested exposure and toxicity of Headline AMP Fungicide during treatment of cornfields by placing juvenile Woodhouse’s toads (Bufo woodhousii) and Blanchard’s cricket frogs (Acris blanchardi) in enclosures located within and adjacent to cornfields during application of the fungicide. We predicted low penetration of Headline to the ground in cornfields and thus low exposure and negligible effects on amphibians (Belden et al. 2010; Brühl et al. 2013).
Materials and methods
Study sites
The present study was conducted in the Rainwater Basin of Nebraska (USA) during late July 2013. This region is heavily cultivated with 67 % of total land area planted in corn, soybeans, and wheat (Bishop and Vrtiska 2008), much of which is potentially treated with Headline AMP Fungicide. Ten cornfields were selected for the study from two locations: five in Fillmore County (“southern” fields) and five in Hamilton County (“northern” fields). Average temperature and precipitation in both regions for the month of July were 23 °C and 0.12 cm, respectively. All southern fields were irrigated via center pivot irrigation, and three of five northern fields were irrigated via flood irrigation, with the remaining two irrigated by center pivot irrigation. Three enclosures (see description below) were placed at least 100 m apart in each of three zones: within-spray, potential drift zone, and out-of-spray (spray, drift, and reference, respectively; Fig. 1). In all fields, reference enclosures were at least 100 m from spray. In southern fields, spray and reference zones were at opposite sides of a square 64 ha field, or reference zones were in the corners of the field, as separate sprayed and unsprayed fields were not available. In northern fields, spray and reference zones were in separate sprayed and unsprayed fields. Current drift models (e.g., AgDrift®, Stewart Agricultural Research Services, Macon, MO, USA) predict that spray concentrations from plane applications are reduced to about 20 % of field rate within 15 m of spray (Teske et al. 2002). Thus, drift enclosures for all fields were outside of the cornfields, about 3–15 m from the edge of spray, as dictated by placement constraints at each field. Vegetation in drift enclosures was typically grass of varying height (ranging from 1 cm to 1.5 m high). Concentrations of active ingredient were measured above the canopy and at ground level in all enclosures (see “Exposure Assessment”). As recommended (BASF 2012), corn in all fields was VT (tassel) stage at the time of treatment, which in this study included corn ca. 3 m tall with nearly complete canopy closure.
Schematic representation of experimental fields. Fields contained enclosures at least 100 m apart in one of three zones: within-spray, potential drift zone, and out-of-spray (sprayed corn, drift zone, reference corn). Reference enclosures were at least 100 m from spray in all fields. In southern fields, spray and reference zones were at opposite sides of the field, or reference zones were in the corners of the field. In northern fields, spray and reference zones were in separate sprayed and unsprayed fields. Drift enclosures for all fields were outside of the cornfields about 3–15 m from the edge of spray
Test substance
Previous studies on the toxicity of pyraclostrobin in amphibians used Headline Fungicide (Belden et al. 2010; Hooser et al. 2012; Brühl et al. 2013). Laboratory studies comparing the toxicity of Headline fungicide formulations are in progress; however, Headline Fungicide is being replaced by Headline AMP for use in the US and was scheduled for use on our study sites. Headline AMP Fungicide is an SC (suspension concentrate) formulation and differs from Headline Fungicide (EC-formulation, emulsion concentrate) by removal of naptha (EC: 57.2 % by mass), less pyraclostrobin (13.64 vs. 23.6 % by mass, respectively), and the addition of metconazole (a secondary active ingredient, 5.14 % by mass) and propylene glycol (BASF Corporation 2012). Label use rates of Headline AMP Fungicide in North American corn are between 10 and 14.4 fl. oz./A, which is equivalent to 731 and 1052 ml formulation/ha. These rates correspond to 106–152 g/ha of applied pyraclostrobin, respectively.
Study organisms
Blanchard’s cricket frogs were collected by hand in early/mid July 2013 from ponds in Payne County, Oklahoma (USA). Frogs were housed for about two weeks in our animal facility at Oklahoma State University (OSU) and kept in 37.8 L glass aquaria (≈21 cm L × 28 cm W × 30 cm H) lined with moist soil. Photoperiod was kept at 13:11 light:dark. Frogs were provided water and either 0.4–0.6 cm crickets or flightless Drosophila melanogaster dusted with Miner-All (Sticky Tongue Farms, Sun City, CA, USA) ad libitum. Frogs were transported from OSU to NE and housed upon arrival in plastic containers (≈59 cm L × 43 cm W × 32 cm H) lined with moist soil for no more than five days prior to deployment to fields. Juvenile Woodhouse’s toads were collected by hand from wetlands in Hamilton County, NE 2 days prior to deployment, and housed in plastic containers as described above. All animals of both species were approximately 15–20 mm snout–vent length, similar in size to those used by Belden et al. (2010), and were collected from wetlands not associated with agriculture.
Field exposure and effects
Animals in all zones were housed in open top metal enclosures (Fig. 2) during aerial application of Headline AMP Fungicide. Enclosures were made of 0.2 cm steel and consisted of two halves that fastened together into a 51.8 cm tall cylinder that was 122 cm in diameter (ca. 1 m2). Each enclosure was inserted at least 5 cm into the soil with minimal disturbance to surface litter. In addition, 25 cm wide aluminum flashing (Amerimax Fabricated Products; Bristol, IN, USA) was attached to the top of each enclosure (5 cm overlap) using metal repair tape (3 M Manufacturing Company, St. Paul, MN, USA), yielding an above ground enclosure height of about 66 cm. Final height varied as a function of soil surface irregularities (e.g., furrows). In the event an enclosure could not be effectively inserted into the soil due to dry, packed soil conditions (an issue in some drift locations only), a small amount of soil was used to fill gaps under the edge of the enclosure, with an effort made to minimize disturbing the habitat (approximately 10 % of ground cover within enclosures; Fig. 2). Enclosures were left uncovered to minimize interception of chemical during spray. In order to encompass all possible exposure scenarios within a cornfield (e.g., directly beneath corn where maximum interception is expected vs. middle of two rows), enclosures in crop fields were bisected by a single cornrow and thus spanned an area extending across two furrows. Because corn canopy cover was generally high, canopy cover at ground level in cornfields was estimated using a spherical densiometer (Lemmon 1956) below the lowest corn leaf (approximately 8–10 cm above the ground) for a subset of enclosures (n = 3 enclosures in separate fields).
Animals were transported into fields in 19 L buckets covered with polyester screen to prevent escape. Five individuals (see exception below) of each amphibian species were blindly selected by hand from buckets and placed in each enclosure approximately 18 h prior to spray in southern fields (July 22, 2013 at approximately 1630 h). At least 24 h following treatment of southern fields, effects were assessed and animals were removed from all enclosures (details described below), after which enclosures were removed and re-deployed to northern fields 1 day prior to treatment (July 25, 2013). Animals were placed in enclosures in northern fields as described above approximately 8 h prior to spray (July 26, 2013 at approximately 1000 h). Spray, drift, and reference randomly received animals during deployment. Difference in animal deployment timing between southern and northern fields was due to restricted spray schedules (e.g., early morning vs. evening) and moving enclosures from southern to northern fields. Once established in a given treatment (e.g., within spray), enclosures were used in the same treatment category (treatment, drift, etc.) when moved from southern to northern fields. Due to insufficient numbers of animals, only spray and reference enclosures of three northern fields received Blanchard’s cricket frogs. Despite receiving 0.9 cm rain the night prior to treatment of southern fields (3 days prior to treatment of northern fields), drift and some reference enclosures were located outside irrigated areas of the fields and thus these enclosures were moistened with about 8 L of deionized water immediately before animal deployment.
Methods used to spray all fields were at the discretion of the local commercial applicator responsible for treatments, in concert with the landowner, with the exception of the amount of Headline AMP Fungicide used on northern fields (see below). Southern fields were aerially treated with Headline AMP Fungicide at 731 ml of formulation/ha (approx. 70 % of maximum application rate; equivalent to 10.0 fl. oz./A; BASF Corporation 2012) on July 23, 2013 at approximately 1030 h. This application rate is the most commonly used by local farmers (per local commercial applicators) and is the minimum recommended rate for corn in North America (BASF Corporation 2012). Fungicide mix was applied to fields at about two gallons per acre (ca. 19 L/ha) using CP09 nozzles (The CP® Products Company, Tempe, AZ, USA) while flying at about 210 km/h. Diplomat® crop oil (Rosen’s Inc., Fairmont, MN, USA; 1 pint/ac) was added to the spray mix to reduce drift, consistent with normal operations. Weather conditions at the time of spray in the southern fields were clear and 21 °C, and the reported local wind speed at the time of spray was 9.3 km/h NNW (weather station ME0107, Sutton, NE, USA).
In order to represent a maximum label rate overspray scenario in North American cornfields, northern fields were aerially treated with 1052 ml formulation/ha Headline AMP Fungicide (equivalent to 14.4 fl. oz./A; BASF Corporation 2012) on July 26, 2013 at approximately 1815 h. Spray application was delayed due to elevated wind speeds throughout the day: weather conditions at the time of spray were clear and 23 °C, and the reported local wind speed at the time of spray was 14.8 km/h N (weather station KAUH, Aurora, NE, USA). Diplomat® crop oil (0.24 L) and 54 g of LockDown drift reducer (Aurora Cooperative, Aurora, NE, USA) were added to the spray mix, and spray was applied to fields at the same volume as southern fields using CP09 nozzles with 5-degree nozzle deflection (commonly used to reduce drift) flying about 210 km/h.
Animals were recovered at least 24 h post-spray from both southern and northern field enclosures (on July 24 and 27, 2013, respectively). Animals were placed in polypropylene mouse cages (≈30 cm L × 19 cm W × 13 cm H; two cages per enclosure, one for each species) lined with soil from within their respective enclosure, and covered with polyester screen. Number of alive, dead and missing of each species 24 h post-spray was recorded for each enclosure in the field at the time of recovery. Although most mortality was expected within the first 24 h (Belden et al. 2010), surviving animals were maintained in cages for an additional 24 h after removal from the field (total of 48 h post-spray) to assess for any further mortality. At 48 h post-spray, the number of alive, dead and missing in each mouse cage (some animals escaped from mouse cages) was recorded and the remaining live animals were euthanized in 0.5 % tricaine methanesulfonate (MS-222, Argent Chemical Laboratories, Inc., Redmond, WA, USA). After use for southern fields, cages were cleaned with soap and water and reused for northern fields. All procedures were performed under protocols approved by the OSU Institutional Animal Use and Care Committee (protocol # AS 01-32).
Exposure assessment
A preliminary study was performed to determine the stability and extraction efficiency of pyraclostrobin on samplers intended for use in the field. Six cellulose chromatography papers (Whatman® Grade 3 MM, Maidstone, Kent, UK) were attached to bamboo poles and treated with the same concentration of Headline AMP Fungicide via micropipette. Three samplers were immediately placed in vials and extracted, while three were left outside during a clear day for 3 h. Mean (±SE) recovery immediately after treatment was 98 % (±4 %) and after 3 h recovery was 84 % (±4 %).
The same type of chromatography paper used in preliminary studies was also used to quantify aerially applied fungicide deposition above the vegetation canopy and at ground level. Two papers (total exposed surface area = 133 cm2) were attached to bamboo poles secured to cornstalks or stuck in the ground at opposite sides of each enclosure (drift enclosures), positioning the paper just above the canopy (>3 m above ground in corn). Ground level papers were cut into circles (surface area = 37.39 cm2), placed in canning jar bands (Ball®, TMs Ball Corporation, Broomfield, CO, USA), and placed in a threaded-side down position, slightly embedded into the ground during spray, resulting in an exposed chromatography paper surface area of 23.76 cm2. This approach kept the papers from touching the soil (approximately 1 cm above the soil surface) and prevented animals from using them as refugia. Three ground samplers were placed within each enclosure in all treatments, and spray enclosures were paired with an additional eight ground samplers approximately 3 m outside the enclosure, four directly under corn (“in row”) and four between corn rows (“out of row”) to represent varying exposure scenarios within directly treated cornfield. Ground samplers outside spray enclosures were spaced 1 m apart and extended away from the enclosure parallel to the row. All samplers were collected within 3 h post-spray, followed by extraction with 25 ml of ultra resi-analyzed ethyl acetate (J.T. Baker®, Avantor Performance Materials, Center Valley, PA, USA), and stored at −30 °C until analysis. Internal standards including deuterated chrysene and deuterated perylene (AccuStandard, New Haven, CT, USA) were added directly to each sample. Above-canopy samplers from the treatment group were not concentrated prior to analysis, while all above-canopy drift and reference samples were concentrated 5× and all ground level samples were concentrated 10×. Samples were analyzed for pyraclostrobin using a gas chromatograph/mass spectrometer (GC/MS) (Agilent 5975c, Santa Clara, CA, USA). The GC inlet temperature was 270 °C and the oven program started at 110 °C and ramped up over 16.25 min to 295 °C. Method detection limits were calculated to be 0.003 µg a.i./cm2. Since it required 1–3 h to harvest samplers and place solvent in the vials, the concentration of all samples was corrected based on 84 % recovery of active ingredient.
Statistical analyses
Unless otherwise noted, an alpha of 0.05 was used to determine significance in all tests. Each field represents the experimental unit; thus, pyraclostrobin concentrations were averaged for spray, drift, and reference areas of each field, then normalized to percent of nominal application prior to all analyses. A two-way analysis of variance (ANOVA) on data ranked across all treatments was used to investigate the main effects and interaction of filter location (above or below the canopy) and treatment (spray, drift, and reference) on concentrations of pyraclostrobin. When the interaction was significant, concentrations above and below the canopy were compared in each individual treatment using Wilcoxon rank sum tests, and the main effect of treatment was tested separately for above and below samples using a Kruskal–Wallis one-way ANOVA. Multiple comparisons between treatments were examined with Wilcoxon rank sum tests with a Bonferroni adjusted alpha of 0.0167 (Zar 2009) when the main effect of treatment was significant. To determine if enclosures had an effect on concentrations of pyraclostrobin at ground level, mean concentrations in and out of spray enclosures were compared using a Wilcoxon rank sum test. Pyraclostrobin concentrations in row and out of row were compared using a paired t test. Wilcoxon rank sum tests were used to determine differences in mortality between treatment areas for each species. All statistical procedures were performed using the software SAS v9.3 (SAS Institute, Cary, NC, USA).
Results and discussion
Above canopy pesticide deposition
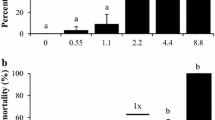
Concentrations of pyraclostrobin measured above the canopy in all treatments were variable, but differed (p < 0.001) and followed a distinct pattern of decreasing concentration from treated to reference zones (Fig. 3). For southern and northern fields, mean concentrations of pyraclostrobin above the canopy in Headline AMP Fungicide-treated corn were 0.99 and 1.30 µg a.i./cm2, respectively, corresponding to approximately 94 and 86 % of nominal application rate (1.06 and 1.52 µg a.i./cm2, respectively). Paper samplers above the canopy did not always maintain perfectly perpendicular when dampened from irrigation or because of movement of the corn stalk used for support, which may have reduced surface area exposed to spray. Further, short-term changes in wind speed/direction, pressure, and temperature not reflected in local weather data may have influenced the pattern of spray deposits above the canopy and resulted in lower recoveries (Salyani and Cromwell 1992).
Mean (±SE) pyraclostrobin concentrations (µg/cm2) measured above the canopy in southern (1.06 μg a.i./cm2, corresponding to 731 ml formulation/ha, 70 % maximum label rate in North American corn) and northern (1.52 μg a.i./cm2, corresponding to 1052 ml formulation/ha, maximum label rate in North American corn) fields for treated corn (Spray), potential drift zone (Drift), and untreated corn (Reference). Concentrations represent the average of five replicate fields. Treatments from each replicate field contained three separate enclosures at which concentrations were measured, which were then averaged to yield a mean concentration for each treatment prior to calculating means. Letter groupings denote treatment areas that did not differ significantly (p > 0.05) based on overall mean percent of nominal application rate for each treatment area
Mean concentrations of pyraclostrobin above the canopy in drift enclosures (ca. 3–15 m from treated crops) were 0.21 and 0.45 µg/cm2 for southern and northern fields, respectively, corresponding to 20 and 29 % of nominal application rate, and differed from concentrations above the canopy in the spray area (p = 0.007). Drift concentrations above the canopy in the present study are similar to those predicted by current spray drift models (e.g., AgDrift®), which predicts approximately 20 % of nominal application 10 m downwind from the edge of treatment (Teske et al. 2002). It is important to note that the purpose of studying spray drift was not to quantify spray drift but rather to give insight on potential exposure of animals outside of cornfields in a region that is likely to have spray drift.
Mean concentrations of pyraclostrobin above the canopy in reference enclosures were 0.12 and 0.03 µg/cm2 for southern and northern fields, respectively, corresponding to 11 and 2 % of nominal application rate. Mean pyraclostrobin concentrations measured above the canopy did not differ between reference and drift enclosures (p = 0.271, Fig. 3). Due to limitations in available fields, reference enclosures in southern fields were located within the same fields as spray enclosures, but outside the spray zone. Thus, reference enclosures in southern fields could have been exposed to drift during treatment. In contrast, reference enclosures in northern fields were in separate, completely untreated fields from spray and drift enclosures.
Pesticide penetration through canopy
Pyraclostrobin penetration to ground level was reduced in all treatment areas (Fig. 4). Mean concentrations at ground level in spray enclosures (expected to be highest among all zones) in southern and northern fields were 0.17 and 0.14 µg a.i./cm2, respectively (approximately 16 and 9 % of nominal application rate or 17 and 11 % of concentrations measured above canopy), and differed from concentrations above the canopy (p < 0.001). Interception was expected as the corn canopy was dense above the spray enclosures, with a mean (±SE) canopy cover of 96 % (±0.23 %) as measured at ground level.
Mean (±SE) pyraclostrobin concentrations (µg/cm2) measured at ground level in southern (1.06 μg a.i./cm2, corresponding to 731 ml formulation/ha, 70 % maximum label rate in North American corn) and northern (1.52 μg a.i./cm2, corresponding to 1052 ml formulation/ha, maximum label rate in North American corn) fields for treated corn (Spray), potential drift zone (Drift), and untreated corn (Reference). Concentrations represent the average of five replicate fields. Treatments from each replicate field contained three separate enclosures at which concentrations were measured, which were then averaged to yield a mean concentration for each treatment prior to calculating means. Letter groupings denote treatment areas that did not differ significantly (p > 0.05) based on overall mean percent of nominal application rate for each treatment area
Enclosures could have influenced spray deposition, given their diameter and height. However, concentrations of pyraclostrobin within enclosures in corn did not differ from samples taken adjacent to those enclosures in the corn (p = 0.14), indicating no measurable influence on exposure. Pyraclostrobin measured outside spray enclosures at ground level in the corn row were 8 % of nominal compared to 12 % for those measured out of corn rows, but these differences were not significant (p = 0.133; Table 1). Variability in pyraclostrobin concentrations measured at ground level was high between sampler locations with some samplers receiving a high level of exposure (90th percentile concentrations in and out of corn rows, and within enclosures were 19, 27, and 31 % of the nominal maximum application rate in corn, respectively; Table 1). Indeed, maximum concentrations in and out of corn rows, and within enclosures were 0.53, 1.39, and 0.68 μg a.i./cm2, and all three exposure scenarios contained samplers that were below detection. In contrast, variability during preliminary lab experiments assessing stability of pyraclostrobin on paper samplers was low, and thus, our field results suggest a patchy exposure environment during routine overspray of cornfields. Consequently, a subset of a resident population of organisms could be exposed to a higher concentration as compared to the mean value. For instance, the 90th percentile in spray enclosures typically is 2–3× higher than mean values. Accordingly, some of the amphibians were likely exposed to 2–3× higher concentrations than the average (i.e., 10 % of animals were exposed to rates of at least 0.45 and 0.31 µg a.i./cm2 in southern and northern fields, respectively).
Concentrations of pyraclostrobin at ground level in drift (grass/weeds) and reference enclosures (corn), as a percentage of above-canopy concentrations, tended to be higher than for ground level in treated enclosures (Figs. 3, 4). However, these data are variable and concentrations below the canopy in drift enclosures were not significantly different from those measured above (p = 0.677). Mean concentrations of pyraclostrobin measured at ground level in drift enclosures were 0.20 and 0.13 µg a.i./cm2 in southern and northern field drift enclosures (19 and 8 % of nominal application rate, respectively) as compared to 0.21 and 0.45 µg/cm2 for above canopy. Mean concentrations at ground level in the drift enclosures were similar to concentrations found in sprayed enclosures (Fig. 4). Thus, an organism’s exposure may be similar between areas of direct spray and adjacent to direct spray, particularly if interception by the canopy is low in the drift zone, which may frequently be the case. Similarly, in the southern fields, some reference enclosures were in corners of sprayed fields and in corn that was not irrigated and the canopy much less developed. Thus, drift may have occurred in these reference plots, and we observed high penetration rates as concentrations of pyraclostrobin did not differ above and below the canopy (p = 0.421). The higher penetration rates in reference and drift enclosures may provide insight into fields with less developed canopy during potential spraying times such as corn and soybeans in early development. In contrast, reference enclosures in northern fields were located in separate, well-irrigated fields with healthy corn. Concentrations measured at ground level in these enclosures were near zero (mean ± SE = 0.00073 ± 0.0015 μg a.i./cm2).
Observed effects in amphibians
Recovery (i.e., percentage of initially deployed collected at each time point) of Woodhouse’s toads was high (>90 %; Table 2). Mortality was ≤4 % in the first 24 h, and ≤12 % thereafter, among all fields and did not differ between treatments (p = 0.366 and 0.200 in the first 24 h and thereafter, respectively; Table 2). Of 16 total mortalities observed in southern fields (from a total of 223 recovered toads), only three occurred within 24 h post-spray, and occurred in two spray enclosures within a single field (of three total spray enclosures; 4 % mortality of recovered animals). Pyraclostrobin concentrations at ground level in these enclosures correspond to the 39th and 70th percentiles of treated enclosures in southern fields (0.07 and 0.19 μg/cm2, respectively), and no mortality was observed in the third spray enclosure, which had the highest pyraclostrobin concentration at ground level (0.29 μg/cm2, corresponding to the 74th percentile). The remaining mortalities were from drift and reference enclosures, and occurred between 24 and 48 h post-spray. Despite wetting prior to animal deployment, soil from drift and reference enclosures was drier than in irrigated cornfields. Although holding times in enclosures were less than 48 h (approximately 24 h) and drift enclosures had good vegetative coverage, vegetative coverage within non-irrigated cornfield (reference enclosures) consisted of dry, dead corn, and animals of both species were frequently observed hiding under this material, as well as burying under the roots of corn stalks. Anurans on dry substrates may lose more than 20 % of their body mass daily from evaporative water loss (McClanahan and Baldwin 1969); thus, animals maintained on dry soil from drift and reference areas may have dehydrated despite the addition of water to enclosures and mouse cages. Further, mortality of animals due to chemical exposure is expected to occur quickly, less than 24 h post-exposure (Belden et al. 2010), suggesting that 48 h mortalities were likely not compound related. Mortality of toads was similar in northern fields (≤3 % in the first 24 h, and ≤12 % thereafter; Table 2), with no mortality in the treatment group and most mortalities occurring in drift enclosures.
Recovery of Blanchard’s cricket frogs was lower than for Woodhouse toads, particularly in southern fields (mean = 52 % at 24 h and 86 % thereafter; Table 3). Standard toxicity testing protocols for risk assessment instruct that missing test organisms be scored as a mortality (http://oehha.ca.gov/ecotox/pdf/SoilTox120208.pdf; accessed 20 February 2014); however, these tests are commonly performed in controlled lab settings from which escape is unlikely. Cricket frogs were frequently observed on corn plants and likely used corn plants and stalks of grass (drift enclosures) as platforms to escape enclosures, despite the height added by the aluminum flashing. Covering enclosures with screen would have prevented or minimized escape, but also may have interfered with spray penetration to ground level, thus reducing environmental realism. As described above, Belden et al. (2010) reported >90 % of observed mortality of juvenile anurans occurred within 24 h of exposure to Headline Fungicide, and dead individuals would have been readily observable in a 1 m2 enclosure during the time frame (24 h) of this study. Thus, we felt justified in classifying animals as missing if we could not find them in the enclosures. Some cricket frogs also escaped during the second 24 h period, as we did not anticipate how difficult it would be to secure cricket frogs in our temporary cages.
Mortality of cricket frogs was generally low in both southern and northern fields (≤8 and 2 % at 24 h, respectively, and ≤25 and 3 %, thereafter, respectively; Table 3) and overall did not differ between treatments (p = 0.768 and 0.531 at 24 h and thereafter, respectively; Table 3). Mortality in spray enclosures never exceeded 7 %. Similar to Woodhouse’s toads, mortality of cricket frogs in southern fields was primarily from drift and reference enclosures, and likely related to desiccation issues as previously described (Table 3). In northern fields, cricket frogs were not deployed to drift enclosures and mortalities of cricket frogs were low in both spray and reference enclosures (Table 3). Both spray and reference enclosures in northern fields were in irrigated cornfields, and soil on which animals were maintained was moist throughout the study.
Previous laboratory studies by Belden et al. (2010) and Brühl et al. (2013) reported significant mortality of juvenile anurans (70 and 100 % mortality of B. cognatus and R. temporaria, respectively) following direct overspray of Headline Fungicide at the maximum application rate in corn (2.2 μg a.i./cm2). However, in both studies there was no significant mortality at 0.1× this rate (0.22 μg a.i./cm2), a level just greater than the maximum mean concentration of pyraclostrobin measured at ground level in the present study (0.20 μg a.i./cm2; Fig. 4). Consequently, it is not surprising that mortality of both species was not correlated with pesticide exposure and was low in all zones in our test system, which was modeled after common application methods in North America. Note however that exposure may differ in other crop systems or with different formulations, application methods, timing, and crop cover. For example, Priaxor® (BASF 2013a) is a pyraclostrobin-based formulation recommended for corn at a V5–V18 stage of development (BASF 2013b), which will have lower spray interception than VT corn (see interception estimates in FOCUS 2000). Note however that increased penetration to the ground will be offset by application of less active ingredient, as the recommended application rate for Priaxor on corn is 4–8 oz/ac compared to 10–14.4 for Headline AMP (292–584 vs. 731–1052 ml/ha, respectively).
Although the full extent of cropland use by amphibians is unknown, many species of amphibians breed in wetlands embedded in cropland (Gray et al. 2004) and return to breed in their natal ponds (Pechmann et al. 2001; Breden 1987). Dispersal distance of emerging metamorphs into the surrounding upland may be limited by jump distance (Semlitsch 2008), vulnerability to desiccation (particularly high in juveniles, and may vary by species; Semlitsch 2008), and structural barriers (e.g., roads; Vos et al. 2007). In addition, irrigated crop may be the best or most attractive habitat surrounding natal wetlands. Thus, amphibians that breed in wetlands associated with agriculture may occupy cropland.
Corn was sprayed by plane at VT stage in this study, as is commonly practiced for most Headline Fungicide applications in North American cornfields (BASF Corporation 2012), and corresponding mortality risk appears low under these conditions (tassel stage). Concentrations of pyraclostrobin at ground level within and adjacent to treated cornfield were reduced by interception by corn or grass canopy. Canopy cover measured in cornfields in the present study was high (> 95 %), typical of canopy for VT stage corn (Quinn and Laflen 1983). However, crop fields (including corn) and adjacent drift zones are not uniform; fields also contain areas of poor or no growth with less canopy cover (e.g., turn rows, dry conditions) that may be treated purposefully or inadvertently. Indeed, pyraclostrobin concentrations at ground level were variable with ten percent of enclosures surpassing 26 % penetration. Nonetheless, mortality of amphibians in all areas was low to non-existent, a result consistent with previous laboratory toxicity data (Belden et al. 2010). Lastly, Headline Fungicide is labeled for multiple types of crops and crop stages and exposure potential will vary among these correspondingly different conditions (e.g., canopy coverage, row spacing; Daughtry et al. 1992). Amphibians, especially in the presence of embedded wetlands, will likely be found in a variety of different crop types, including those approved for treatment with Headline AMP Fungicide (e.g., soybeans; BASF Corporation 2012; Attademo et al. 2005). Thus, amphibian exposure to the fungicide during aerial application may be possible, depending on the crop type and their location within the field. Additional work is necessary to investigate exposure and effects of Headline Fungicide during aerial application to other crops with differing canopy cover.
References
Attademo AM, Peltzer PM, Lajmanovich RC (2005) Amphibians occurring in soybean and implications for biological control in Argentina. Agr Ecosyst Environ 106(4):389–394. doi:10.1016/j.agee.2004.08.012
BASF Corporation (2008) Headline® Fungicide supplemental label. NVA 2008-04- 088-0327. Research Triangle Park, NC. http://agproducts.basf.us/products/label-and-msds/headline-fungicide-supp-label-4.pdf
BASF Corporation (2012) Headline AMP® Fungicide label. NVA 2012-04-343-0153. Research Triangle Park, NC. http://agproducts.basf.us/products/label-and-msds/headline-amp-fungicide-label.pdf
BASF Corporation (2013a) Priaxor® Fungicide label. NVA 2012-04-372-0228. Research Triangle Park, NC. http://agproducts.basf.us/products/label-and-msds/priaxor-fungicide-label.pdf
BASF Corporation (2013b) Corn fungicide solution guide. APN#13-02-013. http://www.agproducts.basf.us/products/research-library/sequential-corn-technical-information-bulletin.pdf
Belden J, McMurry S, Smith L, Reilley P (2010) Acute toxicity of fungicide formulations to amphibians at environmentally relevant concentrations. Environ Toxicol Chem 29:2477–2480. doi:10.1002/etc.297
Berger G, Graef F, Pfeffer H (2013) Glyphosate applications on arable fields considerably coincide with migrating amphibians. Sci Rep 3:2622
Bishop AA, Vrtiska M (2008) Effects of the wetlands reserve program on waterfowl carrying capacity in the rainwater basin region of south-central Nebraska. US Fish and Wildlife Service, Grand Island
Breden F (1987) The effect of post-metamorphic dispersal on the population genetic structure of Fowler’s toad, Bufo woodhousei fowleri. Copeia 1987(2):386–395. doi:10.2307/1445775
Bross M, Mackenroth C (2005) Summary of residue data and MRL proposals supporting the use of BAS 500 F containing formulations in multiple fruit and vegetable crops. BASF 2005/1023124; In: Pyraclostrobin (210). http://www.fao.org/fileadmin/templates/agphome/documents/Pests_Pesticides/JMPR/Evaluation06/Pyraclostrobin06.pdf. Accessed 29 April 2014
Brühl CA, Schmidt T, Pieper S, Alscher A (2013) Terrestrial pesticide exposure in amphibians: an underestimated cause of global decline? Sci Rep 3:1135. doi:10.1038/srep01135
Daughtry C, Gallo K, Goward S, Prince S, Kustas W (1992) Spectral estimates of absorbed radiation and phytomass production in corn and soybean canopies. Remote Sens Environ 39(2):141–152. doi:10.1016/0034-4257(92)90132-4
Duellman WE, Trueb L (1986) Biology of the amphibians. John Hopkins University Press, Baltimore
FOCUS (2000) “FOCUS groundwater scenarios in the EU review of active substances” Report of the FOCUS Groundwater Scenarios Workgroup, EC document reference Sanco/321/2000 rev.2, 202 pp
Gray MJ, Smith LM, Brenes R (2004) Effects of agricultural cultivation on demographics of southern high plains amphibians. Conserv Biol 18(5):1368–1377. doi:10.1111/j.1523-1739.2004.00089.x
Hooser EA, Belden JB, Smith LM, McMurry ST (2012) Acute toxicity of three strobilurin fungicide formulations and their active ingredients to tadpoles. Ecotoxicology 21(5):1458–1464. doi:10.1007/s10646-012-0899-y
Lannoo MJ (2005) Amphibian declines: the conservation status of United States species. University of California Press, Oakland
Lemmon PE (1956) A spherical densiometer for estimating forest overstory density. Forest Sci 2(4):314–320
Lenhardt PP, Brühl CA, Berger G (2015) Temporal coincidence of amphibian migration and pesticide applications on arable fields in spring. Basic Appl Ecol 16(1):54–63
McClanahan L Jr, Baldwin R (1969) Rate of water uptake through the integument of the desert toad, Bufo punctatus. Comp Biochem Physiol 28(1):381–389. doi:10.1016/0010-406X(69)91351-6
Olesen MH, Jensen PK (2013) Collection and evaluation of relevant information on crop interception. EFSA Supporting Publication 2013: EN-438
Pechmann JK, Estes R, Scott D, Gibbons JW (2001) Amphibian colonization and use of ponds created for trial mitigation of wetland loss. Wetlands 21(1):93–111
Quinn N, Laflen J (1983) Characteristics of raindrop throughfall under corn canopy. Trans ASAE 26(5):1445–1450
Regenstein H (2006) Pyraclostrobin, dossier for the second residue JMPR evaluation. BASF: 2006/1009448. In: Pyraclostrobin (210). http://www.fao.org/fileadmin/templates/agphome/documents/Pests_Pesticides/JMPR/Evaluation06/Pyraclostrobin06.pdf. Accessed 29 April 2014
Ruibal R, Tevis L Jr, Roig V (1969) The terrestrial ecology of the spadefoot toad Scaphiopus hammondii. Copeia 1969:571–584. doi:10.2307/1441937
Salyani M, Cromwell R (1992) Spray drift from ground and aerial applications. Tran ASAE 35(4):1113–1120
Seebacher F, Alford RA (2002) Shelter microhabitats determine body temperature and dehydration rates of a terrestrial amphibian (Bufo marinus). J Herpetol 36(1):69–75
Semlitsch RD (2008) Differentiating migration and dispersal processes for pond-breeding amphibians. J Wildlife Manage 72(1):260–267. doi:10.2193/2007-082
Seymour RS (1973) Physiological correlates of forced activity and burrowing in the spadefoot toad, Scaphiopus hammondii. Copeia 1973:103–115. doi:10.2307/1442364
Teske ME, Bird SL, Esterly DM, Curbishley TB, Ray SL, Perry SG (2002) AgDrift®: a model for estimating near-field spray drift from aerial applications. Environ Toxicol Chem 21(3):659–671. doi:10.1002/etc.5620210327
Tiner R (2003) Geographically isolated wetlands of the United States. Wetlands 23(3):494–516
US Environmental Protection Agency (2013) ECOTOX user guide: ECOTOXicology Database System. Version 4.0. [cited 21 March 2015]. Available at: http://cfpub.epa.gov/ecotox/
Vos CC, Goedhart PW, Lammertsma DR, Spitzen-Van der Sluijs AM (2007) Matrix permeability of agricultural landscapes: an analysis of movements of the common frog (Rana temporaria). Herpetol J 17(3):174–182
Zar J (2009) Biostatistical Analysis, 5th. Prentice Hall, Upper Saddle River
Acknowledgments
Funding for this research was provided by BASF Corporation. We thank T. Raskevitz and D. Daniel for help collecting animals and assistance during data collection. Animals were collected under Oklahoma Department of Wildlife Conservation permit #5825 and Nebraska Game and Parks Commission permit #344.
Conflict of interest
The authors state they have no conflict of interest. The work was performed under contract with the BASF Corporation.
Ethical Approval
All experiments complied with the current laws of the United States.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cusaac, J.P.W., Mimbs, W.H., Belden, J.B. et al. Terrestrial exposure and effects of Headline AMP® Fungicide on amphibians. Ecotoxicology 24, 1341–1351 (2015). https://doi.org/10.1007/s10646-015-1509-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-015-1509-6