Abstract
Body shape is a morphological attribute that frequently changes as organisms adapt to environmental fluctuations and optimize the use of available resources. In fish whose distribution includes estuarine and riverine environments, it is common to observe changes in body shape that are related to maneuverability and speed of movement in response to temporal and spatial variation in water flow. Here, through geometric morphometric and linear morphometric analysis, the intraspecific morphological variation of the cichlids Amphilophus trimaculatus, Astatheros macracanthus, and Mayaheros beani was evaluated to determine if there are repeated patterns of variation in body shape associated with estuarine and riverine environments. The three species showed the same trend of morphological variation; in the estuaries, the specimens were generally deeper and robust, with a long head and short caudal peduncle, while river specimens had shallowed and fusiform bodies with a short head and long caudal peduncle. The magnitude of the changes was not the same in the three species, as M. beani showed greater differentiation, and some morphological measures showed changes in opposite directions between the species. These findings indicate that the environment occupied by the species is an important factor in the differentiation of body shape, probably due to water flow, although other factors may determine the magnitude and direction of change in some morphological traits.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Several extrinsic and intrinsic factors affect the morphological expression of living organisms at different levels of organization, as well as at different temporal and spatial scales (Ruehl et al. 2011; Hopper et al. 2017; Scott et al. 2020). From an adaptive point of view, environmental changes can promote the divergence of phenotypic traits and even speciation (Hopper et al. 2017). In aquatic ecosystems, historical and recent environmental changes can cause adaptive divergence in fish in morphological traits associated with habitat use and resource exploitation (Winemiller 1991; Winemiller et al. 1995; Johnson and Belk 2001; Ruehl et al. 2011; Scott et al. 2020). In this sense, when lineages with independent evolutionary histories have been subjected to similar environmental pressures, it is common to observe repeated morphological patterns (Oke et al. 2017).
In fishes, the adaptive importance of repeated morphology has been widely studied at the spatial, temporal, and taxonomic scales (Ruehl et al. 2011). The most frequent and evolutionarily important adaptive changes have been observed in the shape, size, and proportions of the body, due to their functional implications in the swimming performance of organisms during different stages of growth (Costa and Cataudella 2007; Franssen et al. 2013; Sánchez-González and Nicieza 2017; Hernández et al. 2022).
In this sense, the Cichlidae family serves as a reference model for understanding the relationship of historical and recent environmental factors to morphological variation (McMahan et al. 2017; Hernández et al. 2022; Říčan et al. 2023). In the diversification of Neotropical cichlids, changes in body shape have been observed to be recurrent and related to ecological opportunity to inhabit new environments (Říčan et al. 2016; López-Fernández 2021). Repeated morphological patterns observed in several cichlid lineages also reflect different populations’ responses to the same selection pressures (Říčan et al. 2016, 2023; Aguilar-Contreras et al. 2021; López-Fernández 2021).
In several groups of fish, including the Cichlidae family, there exist divergent morphological patterns in body shape that have been linked to swimming efficiency (lotic and lentic) and habitat preference (benthic or pelagic) of organisms under different flowing water conditions (Langerhans and Reznick 2009). In riverine environments where the velocity of water is generally fast and unstable, the body shape of organisms tends to be shallowed and fusiform, while in estuarine environments, where the velocity of water is low or absent, the body shape tends to be deep and robust (Langerhans 2008; Langerhans and Reznick 2009; Franssen et al. 2013; Steele and López-Fernández 2014; Říčan et al. 2016, 2023; Kelley et al. 2017; Aguilar-Contreras et al. 2021). Although flowing water seems to significantly influence the expression of body shape, there are other abiotic and biotic factors that may be important, mainly in species that are distributed in estuarine and riverine environments, such as depth, temperature, turbidity, salinity, dissolved oxygen, habitat structure, and predation (Svanbäck and Eklöv 2002; Eklöv and Svanbäck 2006; Olsson et al. 2007; Langerhans and Reznick 2009; Crampton 2011; Burress et al. 2023).
Due to their recent diversification and intricate evolutionary and biogeographic history, understanding the environmental complexity of aquatic ecosystems and its effect on the phenotypic expression of Neotropical cichlids is not a simple task, particularly in the lineages of Northern Middle America, where studies on morphological variation and evolution are still incipient (López-Fernández 2021). In this way, it is necessary to deepen the study of morphodynamics to understand its importance in the processes that have promoted the diversification of one of the most interesting Neotropical cichlid communities. Species with a wide distribution and physiological capacity to occupy different types of environments are ideal for evaluating the adaptive response of morphological traits to the variation of environmental factors; such is the case of the cichlids Amphilophus trimaculatus Günther 1867, Astatheros macracanthus (Günther 1864), and Mayaheros beani (Jordan 1889). These cichlids are found in estuarine and riverine environments on the Pacific slope in Mexico and Guatemala (Miller et al. 2005). Furthermore, Amphilophus trimaculatus and A. macracanthus inhabit the upper part of the Grijalva basin in Mexico (Miller et al. 2005; González-Díaz et al. 2008).
Thus, the objective of this study was to evaluate intraspecific variation in body shape in three distantly related cichlids from Northern Middle America to assess the existence of repeated patterns of variation associated with estuarine and riverine environments. From the morphological patterns reported in other cichlids, it was expected that the body shape of estuarine specimens would be deeper and robust, while in riverine, it would be shallow and fusiform (Říčan et al. 2016; Kelley et al. 2017).
Methods
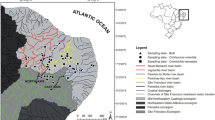
We selected 237 specimens from the Fish Collection of El Colegio de la Frontera Sur (ECOSC) (Supplementary 1): Amphilophus trimaculatus (n = 90; 47 estuarine and 43 riverine environments; standard length (SL) 27.53–213.56 mm); Astatheros macracanthus (n = 54; 33 estuarine and 21 riverine; SL 32.42–147.70 mm); Mayaheros beani (n = 93; 37 estuarine and 56 riverine; SL 39.28–176.46 mm) (Fig. 1).
Morphometric analysis
Each specimen was photographed from the left side using a Sony Alpha A37 digital camera, which was mounted on a tripod to standardize the object distance, and with a ruler placed in each photograph. Body shape was described and compared using geometric morphometric analysis performed with the MorphoJ 1.07a software (Klingenberg 2011). The body shape description was obtained with the configuration of 17 anatomical landmarks (Fig. 2A). Digitization and image processing were performed using tpsDig version 2.31 (Rohlf 2017) and tpsUtil version 1.81 (Rohlf 2018) software.
A landmark configuration used to describe body shape in the geometric morphometric analysis. (1) Anterior end of the upper maxilla, (2) start of the dorsal fin, (3) end of the dorsal fin, (4) upper boundary of the caudal fin, (5) center of the caudal fin, (6) base of the caudal fin, (7) end of the anal fin, (8) origin of the anal fin, (9) origin of the pelvic fin, (10) ventral insertion of the pectoral fin, (11) dorsal insertion of the pectoral fin, (12) most posterior end at the operculum, (13) upper end of the operculum, (14) cleitral fusion, (15) posterior end of the maxilla, (16) anterior extreme of the sphenotic orbit, (17) posterior extreme of the sphenotic orbit. B Measures used in the linear analysis. (a) Anterior end of the upper maxilla to start of the dorsal fin, (b) anterior end of the upper maxilla to most posterior end of the operculum, (c) start and end of the dorsal fin, (d) start of the dorsal fin to ventral insertion of the pelvic fin, (e) end of the dorsal fin to end of the anal fin, (f) upper boundary to base of the caudal fin to base of the caudal fin, (g) end of the anal fin to base of the caudal fin, (h) anterior to posterior extreme of the sphenotic orbit
A generalized Procrustes analysis was performed to eliminate the variation caused by the scale, position, and orientation of the specimens (Zelditch et al. 2004; Adams 2014). Later, the effect of allometry produced by the size differences among specimens was removed using a regression analysis with the Procrustes coordinates and the centroid size. The residual values obtained from the regression were used in the analyses below (Klingenberg 2011).
Statistical analysis
A principal component analysis (PCA) was performed for each species, including both estuarine and riverine specimens, to determine the pattern of differentiation and distribution in the morphospace. Wireframe graphs of the first two principal components were used to visualize and describe changes in body shape among specimens. Statistical differences in body shape among environments were determined through discriminant function analyses (DFA) with Procrustes and Mahalanobis distances (with 1000 rounds of permutation).
Based on the wireframe graphs, we identified the body sections in which greater variation existed for all species. We obtained eight linear measurements to evaluate whether they were discriminant among the estuarine and riverine groups by species (Fig. 2B). Measurements were obtained from the photographs using the CoordGen8 software (IMP, Sheets 2014), and linear measures were standardized using proportions with respect to the standard length to eliminate variation in specimen size. Later, we conducted a t-test to determine whether significant differences existed according to estuarine and riverine environments. Box plots were elaborated to visualize the variation of measures, which were expressed in proportion to standard length. For statistical analyses and the elaboration of box plots, we used the PAST software, version 4.08 (Hammer et al. 2001).
Results
Amphilophus trimaculatus
In the PCA, the first two components explained 49.9% of the variance (Fig. 3A). Throughout PC1 (34.78%), an overlap of the specimens from both environments was observed; however, specimens from the estuarine environment tended to be located toward the positive axis, while those from the riverine environment trended toward the negative axis. In PC2 (15.12%), there was no morphological separation of the specimens due to the environment. From the wireframe graphs, it was observed that, in the riverine environment, the specimens had shorter heads, longer caudal peduncles, and shallower bodies. In contrast, in the estuarine environment, the specimens had longer heads, shorter caudal peduncles, and deeper bodies. In the discriminant function analysis (DFA) with the Procrustes and Mahalanobis distances, significant differences were found in the body shape of the specimens from both environments (p < 0.001, Table 1). The t-test indicated significant differences in three linear measurements between the specimens using the environment: the anterior edge of the upper jaw to the posterior edge of the operculum (b), the anterior insertion of the dorsal fin to the anterior insertion of the fin pectoral (d), and eye diameter (h) (Fig. 2A; Table 2).
Astatheros macracanthus
The first two components of the PCA explained 42.5% of the variance (Fig. 3B). In the middle axis of PC1 (27.15%), there was an overlap between individuals from both environments; however, there was a tendency for specimens from the riverine environment to be located toward the negative axis and the estuarine individuals toward the positive axis. From the wireframe graphs, it was observed that, in the riverine environment, the specimens had shallowed bodies, shorter heads, and longer caudal peduncles, while estuarine specimens had deeper bodies, longer heads, and shorter caudal peduncles. In PC2 (15.35%), no separation of the specimens by environment was observed. The DFA with the Procrustes and Mahalanobis distances showed that the body shape of both groups demonstrated significant differences (p < 0.001, Table 1). The t-test determined that five linear measurements differed significantly in specimens from each environment. These were the anterior insertion of the dorsal fin to the posterior insertion of the same (c), the anterior insertion of the dorsal fin to the anterior insertion of the pectoral fin (d), the posterior insertion of the dorsal fin to the posterior insertion of the anal fin (e), the posterior insertion of the anal fin to the posterior ventral edge of the caudal peduncle (g), and the diameter of the eye (h) (Fig. 3B; Table 2).
Mayaheros beani
In the PCA, the first two components explained 46.75% of the variance (Fig. 3C). In PC1 (30.59%), there was an overlap of some specimens from both environments; however, many of the riverine specimens were located at the negative axis and the estuarine specimens at the positive axis. In PC2 (16.16%), there was no morphological separation according to environment type. With the wireframe graphs, it was observed that, in the riverine environment, the specimens had shallow bodies, with shorter heads and longer caudal peduncles, while the estuarine specimens had deeper bodies with longer heads and shorter caudal peduncles. The DFA with the Procrustes and Mahalanobis distances showed that the body shape of both groups differed significantly (p < 0.001, Table 1). The t-test indicated that seven linear measurements differed significantly between specimens from each environment: the anterior edge of the upper jaw to the anterior insertion of the dorsal fin (a), the anterior edge of the upper jaw to the posterior edge of the operculum (b), anterior insertion of the dorsal fin to posterior insertion of the dorsal fin (c), anterior insertion of the dorsal fin to anterior insertion of the pectoral fin (d), posterior insertion of the dorsal fin to insertion posterior edge of the anal fin (e), the posterior edge of the caudal peduncle (f), and the posterior insertion of the anal fin to the posterior ventral edge of the caudal peduncle (g) (Fig. 3C; Table 2).
Discussion
Three distance-related cichlids of northern Middle America, A. trimaculatus, A. macracanthus, and M. beani, showed repeated patterns of variation in body shape related to the types of environment they inhabit. In specimens from a riverine environment, the organisms had shallow bodies, with short heads and elongated caudal peduncles. In contrast, the estuarine specimens had deep bodies, long heads, and short caudal peduncles. This pattern of body variation is in agreement with the expected hypothesis and supports what has been documented in other cichlids and groups of fish, which present divergent phenotypes mainly related to the water flow regimes of lotic and lentic environments (Perazzo et al. 2019; Scott et al. 2020; Hernández et al. 2022).
From the functional adaptive standpoint, in riverine environments, the shallow body shape reduces resistance to water flow and optimizes the energy expenditure of organisms to stay in the current. In contrast, in estuarine environments, a robust and deep body shape facilitates faster burst speeds and increased maneuverability (Langerhans and DeWitt 2004; Langerhans 2008; Langerhans and Reznick 2009; Franssen et al. 2013; Scott et al. 2020). For example, in the cichlid Caquetaia kraussii, populations from lentic marsh areas of Colombia have been observed exhibiting a more robust and compact body type, in contrast to those from riverine environments, which have showed a more slender and elongated body type (Hernández et al. 2022). Likewise, in populations of the Midas cichlid (Amphilophus spp.) from the lakes of Nicaragua, the same pattern was observed only in limnetic and benthic environments, respectively (Recknagel et al. 2014).
Changes in head size are associated with the capture and processing of food (Perazzo et al. 2019; Larouche et al. 2022), as well as with the acquirement of dissolved oxygen and/or atmospheric air (Schofield et al. 2009; Gotanda et al. 2012; Hernández et al. 2022). In the cichlids Mayaheros uropthalmus and Pseudocrenilabrus multicolor, it was reported that specimens with larger heads have larger gills, allowing them to obtain oxygen in brackish environments (Schofield et al. 2009; Gotanda et al. 2012). The same pattern of variation has been found in the Poeciliidae family, in which some species subjected to low concentrations of dissolved oxygen in sulfur environments have adapted to this condition by increasing the size of the gills and head (Tobler and Hastings 2011).
Although a repeated pattern of variation has been found to be associated with body height, head size, and caudal peduncle length, the magnitude of the changes in the three species observed here is different in other traits. In the morphospace of M. beani, the separation of the specimens between both environments was greater, and more discriminating linear measurements were also found in this species. Furthermore, in the morphospaces of Astatheros macracanthus and Amphilophus trimaculatus, the separation between specimens from both environments was smaller (Fig. 3), as was the number of discriminating linear measurements (Fig. 4). However, while the environment is a powerful force that shapes the phenotypic expression of these cichlids, it is also important to recognize that genetic, developmental, functional, and phylogenetic factors also contribute to or limit the direction and magnitude of morphological variation in each species (Seilacher 1991).
Discriminatory linear measures expressed in percent of standard length for estuarine specimens (E) and riverine specimens (R). Statistically different species marked with an asterisk. Graphs with mean and standard deviation. The description of the linear measures is in Fig. 2B
The behavior of the linear measurements between the analyzed species from both environments had some differences. For example, in M. beani, the caudal peduncle was deeper in specimens from estuarine environments and shallower in riverine specimens. In A. macracanthus, the length from the anterior edge of the mouth to the origin of the dorsal fin in riverine specimens was longer than in estuarine specimens. Finally, in A. trimaculatus, the diameter of the eye was greater in riverine specimens than in estuarine ones, indicating that variation in body shape can vary within these species. While it is true that repeated patterns of variation in body shape are common in fish, and even expected to some extent, in some morphological traits, the direction and magnitude of changes are not predictable and may be the result of local selection pressures or intrinsic factors to the species. It is known that, when several species face a common environmental gradient, their divergence patterns can exhibit some shared and some unique traits (Langerhans and Dewitt 2004).
Conclusions
The study of morphological variation in three distantly related cichlids from Northern Middle America showed a repeated pattern in body shape associated with environment type. Specimens from riverine environments had shallower bodies, shorter heads, and elongated caudal peduncles, while those from estuarine environments exhibited deeper bodies, longer heads, and shorter caudal peduncles. Despite these convergences in the pattern of variation, the magnitude of the morphological changes was not the same across these species, with M. beani specimens showing the greatest differentiation. Furthermore, some morphological traits of the three species even showed changes in opposite directions. Thus, the environment seems to notably influence the phenotypic expression of body shape in the three species; however, other intrinsic factors of the species also seem to influence the magnitude and direction of changes in other morphological traits.
Data availability
All the data used in this study are presented in its text and figures.
References
Adams DC (2014) A generalized K statistic for estimating phylogenetic signal from shape and other high-dimensional multivariate data. Syst Biol 63(5):685–697. https://doi.org/10.1093/sysbio/syu030
Aguilar-Contreras Y, González-Díaz AA, Mejía O, Rodiles-Hernández R (2021) Morphometric variation of Middle-American cichlids: Theraps– Paraneetroplus clade (Actinopterygii: Cichliformes: Cichlidae). Acta Ichthyol Piscat 51(4):403–412. https://doi.org/10.3897/aiep.51.69363
Burress ED, Piálek L, Casciotta J, Almirón A, Říčan O (2023) Rapid parallel morphological and mechanical diversification of south American pike cichlids (Crenicichla). Syst Biol 72(1):120–133. https://doi.org/10.1093/sysbio/syac018
Costa C, Cataudella S (2007) Relationship between shape and trophic ecology of selected species of sparids of the Caprolace coastal lagoon (Central Tyrrhenian sea). Environ Biol Fish 78:115–123. https://doi.org/10.1007/s10641-006-9081-9
Crampton WGR (2011) An ecological perspective on diversity and distributions. In: Albert JS, Reis RE (eds) Historical biogeography of neotropical freshwater fishes. University of California Press, Berkeley, USA, pp 165–189
Eklöv P, Svanbäck R (2006) Predation risk influences adaptive morphological variation in fish populations. Am Nat 167(3):440–452. https://doi.org/10.1086/499544
Franssen NR, Harris J, Clark SR, Schaefer JF, Stewart LK (2013) Shared and unique morphological responses of stream fishes to anthropogenic habitat alteration. Proc R Soc B 280:20122715. https://doi.org/10.1098/rspb.2012.2715
González-Díaz AA, Quiñones M, Rodiles-Hernández R, Velásquez-Martínez J (2008) Fishes of La Venta River in Chiapas, Mexico. Zootaxa 1685(1):47–54. https://doi.org/10.11646/zootaxa.1685.1.3
Gotanda KM, Reardon EE, Murphy SMC, Chapman LJ (2012) Critical swim speed and fast-start response in the African cichlid Pseudocrenilabrus multicolor victoriae: convergent performance in divergent oxygen regimes. Can J Zool 90(5):545–554. https://doi.org/10.1139/z2012-019
Hammer Ø, Harper DAT, Ryan PD (2001) PAST: paleontological statistics software package for education data analysis. Paleontologia Electronica 4(1):e4
Hernández J, Villalobos-Leiva A, Bermúdez A, Ahumada-C D, Suazo MJ, Correa M, Díaz A, Benítez HA (2022) Ecomorphology and morphological disparity of Caquetaia kraussii (Perciformes: Cichlidae) in Colombia. Animals 12(23):3438. https://doi.org/10.3390/ani12233438
Hopper GW, Morehouse RL, Tobler M (2017) Body shape variation in two species of darters (Etheostoma, Percidae) and its relation to the environment. Ecol Freshw Fish 26:4–18. https://doi.org/10.1111/eff.12245
Johnson JB, Belk MC (2001) Predation environment predicts divergent life-history phenotypes among populations of the livebearing fish Brachyrhaphis rhabdophora. Oecologia 126:142–149. https://doi.org/10.1007/s004420000504
Kelley JL, Davies PM, Collin SP, Grierson PF (2017) Morphological plasticity in a native freshwater fish from semiarid Australia in response to variable water flows. Ecol Evol 7:6595–6605. https://doi.org/10.1002/ece3.3167
Klingenberg CP (2011) MorphoJ: an integrated software package for geometric morphometrics. Mol Ecol Resour 11:353–357. https://doi.org/10.1111/j.1755-0998.2010.02924.x
Langerhans RB (2008) Predictability of phenotypic differentiation across flow regimes in fishes. Integr Comp Biol 48(6):750–768. https://doi.org/10.1093/icb/icn092
Langerhans RB, DeWitt TJ (2004) Shared and unique features of evolutionary diversification. Am Nat 164(3):335–349. https://doi.org/10.1086/422857
Langerhans RB, Reznick D (2009) Ecology and evolution of swimming performance in fishes: predicting evolution with biomechanics. In: Domenici P, Kapoor BG (eds) Fish locomotion: an etho-ecological perspective. CRC, USA, pp 200–248
Larouche O, Gartner S, Westneat M, Evans K (2022) Mosaic evolution of the skull in labrid fishes involves differences in both tempo and mode of morphological change. Syst Biol 72(2):419–432. https://doi.org/10.1093/sysbio/syac061
López-Fernández H (2021) Neotropical riverine cichlids: adaptive radiation and macroevolution at continental scales. In: Abate MA, Noakes DLG (eds) The behavior, ecology, and evolution of cichlid fishes. Springer Dordrecht, Corvallis, USA, pp 135–173. https://doi.org/10.1007/978-94-024-2080-7_5
McMahan CD, Kutz J, Murray C, Chakrabarty P, Geheber AD, Elías D (2017) Objectively measuring subjectively described traits: geographic variation in body shape and caudal coloration pattern within Vieja Melanurus (Teleostei: Cichlidae). Rev Biol Trop 65(2):623–631. https://doi.org/10.15517/rbt.v65i2.25500
Miller RR, Minckley WL, Norris S (2005) Freshwater fishes of Mexico. University Chicago, Chicago
Oke KB, Rolshausen G, LeBlond C, Hendry AP (2017) How parallel is parallel evolution? A comparative analysis in fishes. Am Nat 190(1):1–16. https://doi.org/10.1086/691989
Olsson J, Svanbäck R, Eklöv P (2007) Effects of resource level and habitat type on behavioral and morphological plasticity in eurasian perch. Oecologia 152:48–56. https://doi.org/10.1007/s00442-006-0588-8
Perazzo GX, Corrêa F, Salzburger W, Gava A (2019) Morphological differences between an artificial lentic and adjacent lotic environments in a characid species. Rev Fish Biol Fisher 29:935–949. https://doi.org/10.1007/s11160-019-09582-y
Recknagel H, Elmer KR, Meyer A (2014) Crater Lake habitat predicts morphological diversity in adaptative radiations of cichlid fishes. Evolution 68:2145–2155. https://doi.org/10.1111/evo.12412
Říčan O, Pangrácová A, Rodriguez Haro CE, Říčanová Š (2023) Repeated ecomorphological divergence in Bujurquina (Teleostei: Cichlidae) body shape. J Vertebr Biol 72(23004):1–20. https://doi.org/10.25225/jvb.23004Rohlf FJ (2017) TpsDig, Thin Plate Spline Digitise, version 2.31. Department of Ecology and Evolution, University of New York Stony Brook
Říčan O, Piálek L, Dragová K, Novák J (2016) Diversity and evolution of the middle American cichlid fishes (Teleostei: Cichlidae) with revised classification. Vertebr Zool 66:1–102. https://doi.org/10.3897/vz.66.e31534
Rohlf FJ (2018) TpsUtil, thin plate spline utility, version 1.81. Department of Ecology and Evolution, University of New York Stony Brook
Ruehl CB, Shervette V, Dewitt TJ (2011) Replicated shape variation between simple and complex habitats in two estuarine fishes. Biol J Linn Soc 103(1):147–158. https://doi.org/10.1111/j.1095-8312.2011.01626.x
Sánchez-González JR, Nicieza AG (2017) Phenotypic convergence of artificially reared and wild trout is mediated by shape plasticity. Ecol Evol 7(15):5922–5929. https://doi.org/10.1002/ece3.3156
Schofield PJ, Loftus WF, Fontaine JA (2009) Salinity effects on behavioural response to hypoxia in the non-native mayan cichlid Cichlasoma urophthalmus from Florida Everglades wetlands. J Fish Biol 74:1245–1258. https://doi.org/10.1111/j.1095-8649.2009.02192.x
Scott S, Rojas P, Vila I (2020) Meristic and morphological differentiation of Orestias species (Teleostei; Cyprinodontiformes) from the southern Altiplano. Environ Biol Fish 103:939–951. https://doi.org/10.1007/s10641-020-00995-4
Seilacher A (1991) Self-organizing mechanisms in morphogenesis and evolution. In: Schmidt-Kittler N, Vogel K (eds) Constructional morphology and evolution. Springer, Berlin, Heidelberg, pp 251–271. https://doi.org/10.1007/978-3-642-76156-0_17
Sheets HD (2014) Integrated Morphometrics Package 8: 2014. [IMP]
Steele S, López-Fernández H (2014) Body size diversity and frequency distributions of neotropical cichlid fishes (cichliformes: Cichlidae: Cichlinae). PLoS ONE 9e106336. https://doi.org/10.1371/journal.pone.0106336
Svanbäck R, Eklöv P (2002) Effects of habitat and food resources on morphology and ontogenetic growth trajectories in perch. Oecologia 131(1):61–70. https://doi.org/10.1007/s00442-001-0861-9
Tobler M, Hastings L (2011) Convergent patterns of body shape differentiation in four different clades of poeciliid fishes inhabiting sulfide springs. Evol Biol 38(4):412–421. https://doi.org/10.1007/s11692-011-9129-4
Winemiller KO (1991) Ecomorphological diversification in lowland freshwater fish assemblages from five biotic regions. Ecol Monogr 61:343–365. https://doi.org/10.2307/2937046
Winemiller KO, Kelso-Winemiller LC, Brenkert AL (1995) Ecomorphological diversification and convergence in fluvial cichlid fishes. Environ Biol Fish 44:235–261. https://doi.org/10.1007/BF00005919
Zelditch ML, Swiderski D, Sheets D, Fink W (2004) Geometric morphometrics for biologist: a primer. Elsevier Academy
Acknowledgements
AAGD and MSB thank SNI-CONAHCYT, and MSB thanks CONACHYT for the postdoctoral grant and SNI-CONACHYT. We thank anonymous reviewers for their helpful comments on the manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Alfonso A. González-Díaz and Miriam Soria-Barreto. The first draft of the manuscript was written by Alfonso A. González-Díaz and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
Care was taken in the handling and use of the captured fish in accordance with SEMARNAT’s laws, guidelines, and policies. Sampling was authorized under the fishing permit number PPF/DGOPA249/14 from CONAPESCA.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
González-Díaz, A.A., Soria-Barreto, M. & Martínez-Cárdenas, L. Repeated patterns in the body shape of distantly related estuarine and riverine cichlids from Northern Middle America. Environ Biol Fish 107, 335–345 (2024). https://doi.org/10.1007/s10641-024-01534-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10641-024-01534-1