Abstract
Orestias is an endemic fish genus of lacustrine and lotic systems distributed on the Andes highland region (Altiplano) of Peru, Bolivia and Chile (9°S to 22°S). Based on morphological characters, taxonomic studies have recognized seven species on the Chilean western southern Altiplano region (17°S - 22°S). The current geographical distribution of Orestias would be associated with historical vicariant events and fluctuations in water levels since the Pleistocene. In this context, this group arises as an interesting model to assess Orestias morphological adaptations in Altiplano systems. Morphological and meristic analyses were performed on ten populations of Orestias in the southern Altiplano. The results showed significant differences among populations. The meristic and morphometric characters were related to physical and chemical properties of their habitat. We considered the systematic validation of the Orestias species and the possible future determination of new species of the populations of O. cf. agassii of Huasco saltpan, Isluga River and Chuviri wetland, which should be tested with trophic, genetic and karyotype analyses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Traditionally, taxonomic studies for the identification of species members have been carried out using morphological and meristic characteristics (Cadrin 2000), where specimens or their populations may present overlaps when characters are invariant. (Swain and Foote 1999; Murta 2000). When fish are exposed to different environmental conditions, they can adjust their morphology and evolve with adaptations that allow them to survive the new conditions (Webb 1984). The environment is a powerful force that models the morphology of organisms during their ontogeny (Costa and Cataudella 2007). Hence, morphology is not only a result of their genetics but also of their environment and behavior (Guill et al. 2003).
Ecosystem dynamism and geographic isolation may enable specimens to experience a wide range of ecological conditions, leading to morphological differences and local adaptations (Schluter 1993, 1996, 2000; Losos et al. 1998; Kocher 2004; Maldonado et al. 2009). For this reason, it is suggested that natural selection may lead species into occupying similar ecological niches with certain morphological and meristic characteristics, in response to equivalent selective pressures (Wainwright 1991, 1996; Hugueny and Pouilly 1999; Cardin and Friedland 1999; Pouilly et al. 2003; Poulet et al. 2005; Merona 2005). The quantification of specific characteristics of a specimen or group of specimens may demonstrate the degree of speciation induced by biotic and abiotic conditions, contributing to the definition of different groups of species (Bailey 1997; Nacua et al. 2010).
To understand the morphological responses of organisms to environmental conditions, patterns of morphological variation have been compared with patterns of variation in ecological characteristics (Norton et al. 1995; Maldonado et al. 2009). These analyses have helped to determine whether there is phenotypic divergence among different species or populations of the same species, and which could have an impact on them, shedding light on the interaction that species have had with their environment since their separation from their dispersal center. A habitat freshwater heterogeneity (lotic and lentic) could potentially create a spatial variation or facilitate morphological plasticity (Franssen 2011). For example, the lotic environments select a body fusiform shape by reducing the resistance of the water stream, obtaining lower energy costs (Webb 1984; Langerhans and Reznick 2010; Foster et al. 2015).
The genus Orestias described in the southern Altiplano (17° to 22° S) inhabits aquatic systems as diverse as high lakes, rivers, saltpans and wetlands (locally called bofedales) of the Andes. Most systematic studies have emphasized the use of meristic and morphological characters for Orestias species characterization. Up to now, species descriptions have mainly considered relationships of body proportions, number and distribution of scales, fin rays and gills characteristics (Arratia 1982; Lauzanne 1982; Parenti 1984a; Costa 1997; Vila et al. 2011, 2013), yet lacking an analysis which would correlate the set of characters with the environmental variations where the species live.
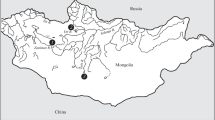
Seven Orestias species have been described for the southern Altiplano (Fig. 1); Orestias piacotensis (Vila 2006), O. laucaensis (Arratia 1982), O. parinacotensis (Arratia 1982), O. chungarensis (Vila and Pinto 1986), O. cf. agassii (Mann, 1954), O. gloriae (Vila et al. 2011) and O. ascotanensis (Parenti, 1984). The validity of the species described for the agassii complex of the southern Altiplano has been questioned by Villwock and Sienknecht (1995, 1996), postulating that they represent ontogenetic stages and that they could also represent geographical variations of the same species. The likely source of discrepancy is that the original descriptions were based on the analyses of a limited number of specimens of the same ontogenetic stage (Villwock and Sienknecht 1995, 1996).
The objective of this study has been to analyze the morphological and meristic differences between the species of Orestias of the southern Altiplano. We hypothesized differences among the populations of Orestias, principally due to historical isolation and particular environmental conditions of each ecosystem of the southern Altiplano. For this, a higher number of individuals have been considered for the morphological and meristic analyses, incorporating adults of different sizes and considering all the species described for this zone, as well as new populations under study.
Material and methods
The study included 258 Orestias specimens collected between 2013 and 2016 (Table 1) in 10 different systems of the southern Altiplano (Fig. 1). The fish were captured using hand nets, as well as electrofishing (SAMUS -725) at deeper places. Fish were fixed with 95% alcohol after being euthanized with Tricaine methanesulfonate (MS222). Posteriorly, at the laboratory, the alcohol was removed and fish were fixed with new 95% alcohol. Finally, the specimens were included to the collection of the Limnology Laboratory of the University of Chile (Table 1).
The species included in this study were O. parinacotensis (Arratia 1982) from Parinacota wetland (18°12’ S; 69°16’ W), O. laucaensis (Arratia 1982) from Lauca River (18°11’ S; 68°16’ W) and Cotacotani lake (18°12’ S; 69°14’ W), O. chungarensis (Vila and Pinto 1986) of Lake Chungará (18°15’ S; 69°07’ W), O. ascotanensis (Parenti 1984a) of Ascotán saltpan (21° S; 68° W), O. piacotensis (Vila 2006) of Piacota lake (18°12’ S; 69°158’ W); O. cf. agassii (Valenciennes, 1846) of Huasco saltpan (20°16’ S; 68°41’ W) O. cf. agassii of Isluga River (19°01’ S; 68°42’ W), O. cf. agassii of Chuviri wetland (18°10’ S; 69°20’ W) and O. gloriae (Vila et al. 2011) of Carcote saltpan (21°17’ S; 68°19’ W). The descriptions of Arratia (1982); Parenti (1984a); Vila and Pinto (1986), Vila (2006) and Vila et al. (2011) were used to determine the species.
For the morphological analyses of the species studied, the following measurements were standardized for each specimen size: eye diameter, head length, pre dorsal length, standard length, total length, pre orbital length, caudal peduncle length, pre anal length, body height, caudal peduncle height, and head height (Fig. 2). For the meristic analyses, the same specimens were used, counting the number of scales of the lateral line, the number of rays of the dorsal, anal, pectoral and caudal fins (Appendix Table 5). Data were analyzed by a MANOVA (Multivariate variance analysis) using all morphometric measurements, grouping specimens by sampled localities, and grouped also by type of environment classifying them into fluvial O. laucaensis, and O. cf. agassii of Isluga; O. cf. agassii from Huasco, O. ascotanensis and O. gloriae in saltpans; O. chungarensis O. piacotensis as lacustrines; O. laucaensis of Cotacotani, O. parinacotensis and O. cf. agassii of Chuviri from wetlands. A Principal Component analysis (PCA) and Linear discriminant analysis (LDA) were performed using Statistica 6.0 (Statsoft) software, with each species as a classifying variable. The discriminant function was evaluated in a classification matrix using the Jacknife option. Subsequently, a Mantel analysis was done to evaluate the correlation between the morphological distances analyzed and the geographic distance between the sampled localities using Mantel 2.0 (Liedloff) software.
Morphometric measurements and meristic used in this study. 1 eye diameter. 2 head length. 3 pre dorsal length. 4 standard length. 5 total length. 6 dorsal fin rays. 7 preorbital length. 8 body height. 9 lateral line scales. 10 caudal peduncle height. 11 pectoral fin rays. 12 caudal peduncle length. 13 anal fin rays. 14 caudal fin rays. 15 preanal length. 16 head height
For the analyses of geometric morphology, images taken on the left side of the 258 specimens were used, with a high-resolution digital camera (Canon SX 530, 16 Mega Pixel, with 1x optical zoom). Eleven landmarks were established and located on each individual (Fig. 3) using the TPSDig2 program (Rohlf 1990; Rohlf and Slice 1990). To eliminate the external variation of the shape, a General Procrust analysis (GPA) was performed, where the coordinates of the specimens were aligned (moved, rotated, and scaled to fit each other) using the Generalized least-squares Procrustes superimposition (GLS). This method adjusts one configuration over another by minimizing the sum of the squares of the distances among landmarks (Rohlf 1990; Rohlf and Slice 1990). The Relative Warp results were then obtained as the main components of the variation between specimens in the multivariate space. The Relative Warp data were used for the consensus configuration of each locality, and the Euclidean distance ordering analysis was performed using the UPGMA algorithm (Sneath and Sokal 1973). A correlation analysis was performed between the first three Relative Warp components and the chemical and physical water variables of the freshwater systems analyzed (Appendix Table 6). Finally, a MANOVA analysis and a paired analysis were done using the software Morpheus (Slice 2000).
Landmarks used in this study. 1 Tip of snout. 2 Middle point of eyes. 3 insertion of the operculum on the ventral profile. 4 beginning of operculum. 5 superior insertion of the pectoral fin. 6 inferior insertion of the pectoral fin. 7 anterior insertion of the anal fin. 8 posterior insertion of the anal fin. 9 inferior insertion of the caudal fin. 10 superior insertion of the caudal fin. 11 anterior insertion of the dorsal fin
Results
The examined characters in meristic analyses overlap, and they do not differ among the different sizes of each species studied. These results are similar to the obtained by Parenti (1984a). The Principal Component analysis indicated that the first two axes account for 84.96% of the variance. On the first axis, the pre orbital length and head length accounts for most of the variance, while on the second axis corresponds to the eye diameter, the dorsal and caudal fin. This analysis showed unclear patterns, such as that O. ascotanensis is grouped in negative values in the morphometric space for both axes, while, for O. chungarensis, it did not display a clear differentiation among the rest of the species in the morphometric space (Fig. 4). The MANOVA revealed significant differences between localities for the characters analyzed (Wilks’ Lambda = 0.0026 F = 12.32, df = 135, p < 0.01). There are also significant differences for each of the characters measured in the ANOVA carried out among the analyzed localities (p < 0.01). Besides, differences among species were observed in Tukey’s a posteriori test (Table 2).
The morphometric measurements showed differences among some species groups. Orestias ascotanensis showed higher values in the predorsal length and the caudal peduncle length. O. laucaensis of the Lauca River and Cotacotani wetland showed the higher values for head length and O. chungarensis and O. gloriae presented higher values in the head height compared to the rest of the species. On the other hand, the pre orbital distance showed the lowest values in O. ascotanensis, O. chungarensis and O. gloriae.
The exploration of MANOVA about the differentiation of meristic characters and morphometric measurements in different types of environments, showed significant differences (Wilks’ Lambda = 0.39378, F = 7.6, df = 30, p < 0.01). The ANOVA also determined significant differences (p < 0.05) as did Tukey’s a posteriori test for all characters except for the pre anal length, eye diameter and the number of rays on the pectoral and caudal fin, among the different type of environments.
The LDA, showed significant differences among species (Wilks’ Lambda: 0.00265, F = 12.32, df = 135, p < 0.01), with 100% correct classification for O. chungarensis of Lake Chungará and O. ascotanensis of the Ascotán saltpan, and 90% correct classification for O. gloriae of the Carcote saltpan, O. cf. agassii from the Isluga River and the Huasco saltpan and O. laucaensis from the Lauca River. The species O. cf. agassii of the Chuviri wetland, O. parinacotensis of the Parinacota wetland and O. laucaensis from the Cotacotani wetland had values above 80%, and only O. piacotensis from the Piacota lake resulted in lower correct classification (68%) (Table 3). The Mantel analysis showed a non-significant correlation between geographic distance and morphological differentiation (r = 0.42, p = 0.089).
The results of the geometric morphometric analysis indicated differences among groups for localities. The MANOVA indicated significant differences among localities (p = 0.001). The ANOVA results on the data grouped by species indicated that there are significant differences among the groups (p < 0.01). The a posteriori t test also showed significant differences among species (p < 0.001) for all cases, except O. piacotensis with O. laucaensis from Cotacotani (Table 4).
The analysis of morphometric differences can also be approached graphically using the Thin Plate Splines (TPS), which allows observing the degree of deformation of the morphometric conformation of one species concerning another (Bookstein 1991; Toro et al. 2010). As for the Relative Warps, the first two axes accounted for 22.73% and 20.76% of the variance of the shape data, respectively. The landmarks that most contributed to the interspecific differences were the middle point eye, insertion of the operculum on the ventral profile, tip of snout, the superior insertion of the pectoral and inferior insertion of the pectoral fin (Fig. 5).
The analysis of multiple correlations incorporating the chemical composition of the studied systems showed a significant correlation of the first axis to sulfates (p < 0.05), calcium (p < 0.05) and pH (p < 0.05). The UPGMA diagram showed morphological differences between two groups, with a cluster of individuals from Ascotán, Carcote and Parinacota (chlorate systems) and a second cluster from Piacota – Lauca and Isluga – Cotacotani and Huasco (sulfate systems). These results were related to the environmental characteristics of the watersheds (Fig. 6).
Discussion
As it has been extensively reported and discussed, Orestias is a specious genus distributed from northern Peru to southern Chile (Parenti 1984a: Lüssen et al. 2003; Maldonado et al. 2009; Esquer Garrigos 2013; Vila et al. 2011; Arratia et al. 2017). The analyses of meristic and morphometric characteristics showed a high degree of overlap of the ranges among species, making it difficult to describe species using external morphology and meristics. However, new approaches such as both univariate and multivariate analysis, allow differentiating species with the total character set. The morphometric analyses have a discriminatory power for the different species of Orestias, demonstrating that each species presents a combination of values of the analyzed characters that allows to discriminate them correctly from each other, according to what was reported in the original descriptions of Arratia (1982), Parenti (1984a), Vila and Pinto (1986), Vila (2006) and Vila et al. (2011). Orestias piacotensis is the only species not to be classified correctly in the discriminant analysis. This can be explained by geographical proximity to other species, and the lack of correlation between morphometric differentiation and geographical distances when performing the Mantel test. Nevertheless, specific karyotypes study allows confirming the species validity (Vila et al. 2010).
According to Parenti (1984a) Orestias genus is divided into four monophyletic complexes species including those of the Chilean Altiplano in the agassii complex. This is one of the complexes with higher morphological diversity and has been adapted to a wider variety of habitat characteristics (Lauzanne 1982; Parenti 1984a, b; Maldonado et al. 2009; Vila et al. 2013; Esquer Garrigos 2013). The studies carried out considering the types of fluvial, lacustrine and wetlands environments fish showed significant differences in both multivariate and univariate analyses. The morphological measurements and geometric morphometry analyses showed possible adaptations to these different habitats. There are various research dealing with changes in the body shape of fish in lotic and lentic habitats. (Robinson and Wilson 1994; Taylor et al. 1997; Hendry et al. 2000; Pakkasmaa and Piironen 2000; Brinsmead and Fox 2002; Gaston and Lauer 2015). Values obtained in some measurements, for example, the predorsal length, were significantly higher in O. ascotanensis and O. laucaensis, which live in streams and rivers, in contrast to O. cf. agassii, which lives in wetlands and saltpans.
It has been postulated that Orestias speciation should be the result of habitat fragmentation of one or more ancestral populations during the Pleistocene (Northcote 2000; Placzek et al. 2006). Populations would have coexisted in the big paleo lakes the southern Altiplano regions, since speciation data obtained are previous to the last paleolakes and not necessarily the species interplayed keeping strong philopatry preventing their interbreeding (Fraser et al. 2004). On the other hand, the freshwater systems could have been influenced by the Pleistocene dry period, with changes in the water quality, specifically, in the salinity content. Recently researches show the importance of the vicariant events in the Altiplano region, postulated that the allopatric morphological variation would be the result of adaptation to the different freshwater systems characteristics (Vila et al. 2011, 2013).
The morphological diversification of the genus was probably also caused by the differences structure and composition of the aquatic biota (Northcote 2000; Márquez-García et al. 2009). Despite the scarce information related to the behavior and diet of the Orestias populations in the southern Altiplano, studies have described the Orestias as generalist predators of zooplankton and benthic macro invertebrates (Riveros et al. 2012; Guerrero et al. 2015). Although there are no significant differences of Orestias diets, the proportion and location of prey differ among species. On the other hand, studies about the composition and structure of the aquatic biota of the river, wetlands and lake systems of the southern Altiplano, present differences in the composition and structure of their aquatic biodiversity (Márquez-García et al. 2009).
The differences observed in the position of the mouth (snout tip) among habitats may reflect differences in the diet. Mid-water feeding fish have terminal mouths, benthic fish have sub terminal mouths, and surface fish have superior mouths (Keast and Webb 1966; Winemiller 1992; Lauzanne 1982; Moyle and Cech 2000; Northcote 2000; Maldonado et al. 2009). Many authors have suggested that Orestias differentiate in feeding from small plankton to larger prey, such as insects and mollusks. Accordingly, their morphology would have evolved according to its feeding habits (Lauzanne 1982; Pinto and Vila 1987; Parker and Kornfield 1995; Maldonado et al. 2009; Riveros et al. 2012; Guerrero et al. 2015). A complete morphological work done by Arratia et al. (2017) has described a new genus and species of killifish at Chancacolla river basin of the southern Altiplano, reconfirming the importance of the morphological adaptations of these specious fish through million years of evolution.
In conclusion, significant differences among species and populations were found, which have not yet been described in relation to their complete meristic and morphometric characters, in disagreement with the reports of some authors, who have claimed that the Orestias species would be geographical variations of the same species (Villwock and Sienknecht 1995, 1996; Lüssen et al. 2003). The systematic validation of the genus Orestias and the possible determination of new species for the populations in southern Altiplano systems is considered.
References
Arratia G (1982). Peces del Altiplano de Chile. In: Veloso A, Bustos E (eds) El hombre y los ecosistemas de montaña MAB-6. El ambiente natural y las poblaciones humanas de Los Andes del Norte Grande de Chile, Volumen I. La vegetación y los vertebrados inferiores de los pisos altitudinales entre Arica y El Lago Chungará. ROSTLAC, UNESCO, Montevideo, Uruguay, pp 93–133
Arratia G, Vila I, Lam N, Guerrero CJ, Quezada-Romegialli C (2017) Morphological and taxonomic descriptions of a new genus and species of killifishes (Teleostei: Cyprinodontiformes) from the high Andes of northern Chile. PLoS One 12(8):e0181989
Bailey KM (1997) Structural dynamics and ecology of flatfish populations. J Sea Res 37:269–280
Bookstein FL (1991) Morphometric tools for landmark data: geometry and biology. Cambridge University Press, Cambridge
Brinsmead J, Fox MG (2002) Morphological variation between lake- and streamdwelling rock bass and pumpkinseed populations. J Fish Biol 61:1619–1638
Cadrin SX (2000) Advances in morphometric analysis of fish stock structure. Rev Fish Biol Fish 10:91–112
Cardin SH, Friedland KD (1999) The utility of image processing techniques for morphometric analysis and stock identification. Fish Res 43:129–139
Costa WJEM (1997) Phylogeny and classification of the Cyprinodontidae revisited (Teleostei: Cyprinodontiformes): are Andean and Anatolian killifishes sister taxa? J Comp Biol 2(1):1–17
Costa C, Cataudella S (2007) Relationship between shape and trophic ecology of selected species of Sparids of the Caprolace coastal lagoon (Central Tyrrhenian Sea). Environ Biol Fish 78:115–123
Esquer Garrigos YS (2013) Multi-scale evolutionary analysis of a high altitude freshwater species flock: diversification of the agassizii complex (Orestias, Cyprinodontidae, Teleostei) across the Andean Altiplano. Doctoral thesis. Museum National D’Histoire Naturelle
Foster K, Bower L, Piller K (2015) Getting in shape: habitat-based morphological divergence for two sympatric fishes. Biol J Linn Soc 114 (1):152-162
Franssen NR (2011) Anthropogenic habitat alteration induces rapid morphological divergence in a native stream fish. Evol Appl 4:791–804
Fraser DJ, Lippe C, Bernatchez L (2004) Consequences of unequal population size, asymmetric gene flow and sex-biased dispersal on population structure in brook charr. Mol Ecol 13:67–80
Gaston KA, Lauer TE (2015) Morphometric variation in in lentic and lotic systems. J Fish Biol 86(1):317-332
Guerrero CJ, Méndez MA, Vila I (2015) Caracterización trófica de Orestias (Teleostei: Cyprinodontidae) en el Parque Nacional Lauca. Gayana (Concepción) 79(1):18–25
Guill JM, Heins DC, Hood CS (2003) The effect of phylogeny on interspecific body shape variation in darters (Pisces: Percidae). Syst Biol 52(4):488–500
Hendry AP, Wenburg JK, Bentzen P, Volk EC, Quinn TP (2000) Rapid evolution of reproductive isolation in the wild: evidence from introduced Salmon. Science 290(5491):516–518
Hugueny B, Pouilly M (1999) Morphological correlates of diet in an assemblage of west African freshwater fishes. J Fish Biol 54(6):1310–1325
Keast A, Webb D (1966) Mouth and body form relative to feeding ecology in the fish fauna of a small Lake, lake Opinicon, Ontario. J Fish Res Board Can 23:1845–1874
Kocher TD (2004) Adaptive evolution and explosive speciation: the cichlid fish model. Nat Rev Genet 5:288–298
Langerhans RB, Reznick DN (2010) Ecology and evolution of swimming performance in fishes: predicting evolution with biomechanics. In: Domenici P, Kapoor BG, editors. Fish locomotion: An eco-ethological perspective. Enfield, NH: Science (pp 200–248)
Lauzanne L (1982) Les Orestias (Pisces, Cyprinodontidae) du Petit lac Titicaca. Rev Hydrobiol Trop 15:39–70
Losos JB, Jackman TR, Larson A, de Queiroz K, Rodriguez-Schettino L (1998) Contingency and determinism in replicated adaptive radiations of island lizards. Science 279(5359):2115–2118
Lüssen A, Falk TM, Villwock W (2003) Phylogenetic patterns in populations of Chilean species of the genus Orestias (Teleostei: Cyprinodontidae): results of mitochondrial DNA analysis. Mol Phylogenet Evol 29(1):151–160
Maldonado E, Hubert N, Sagnes P, De Merona B (2009) Morphology-diet relationships in four killifishes (Teleostei, Cyprinodontidae, Orestias) from Lake Titicaca. J Fish Biol 74(3):502–520
Márquez-García M, Vila I, Hinojosa LF, Méndez MA, Carvajal JL, Sabando MC (2009) Distribution and seasonal fluctuations in the aquatic biodiversity of the southern Altiplano. Limnol Ecol Manag Inland Waters 39(4):314–318
Merona B (2005) Alteration of fish diversity downstream from PetitSaut dam in French Guiana. Implication of ecological strategies of fish species. Hydrobiologia 551:33–47
Moyle P, Cech J (2000) Fishes: an introduction to ichthyology, fourth edn. Prentice-Hall, Upper Saddle River
Murta AG (2000) Morphological variation of horse mackerel (Trachurus trachurus) in the Iberian and north African Atlantic: implications for stockidentification. ICES J Mar Sci 57:1240–1248
Nacua SS, Dorado EL, Torres MAJ, Demayo CG (2010) Body shape variation between two populations of the white goby, Glossogobius giuris (Hamilton and Buchanan). Res J Fish Hydrobiol 5(1):44–51
Northcote TG (2000) Ecological interactions among an orestiid (Pisces Cyprinodontidae) species flock in the littoral zone of Lake Titicaca. Advances in ecological research, vol 31 book series. Adv Ecol Res 3:399–420
Norton SF, Luczkovich JJ, Motta PJ (1995) The role of ecomorphological studies in the comparative biology of fishes. Environ Biol Fish 44(1–3):287–304
Pakkasmaa S, Piironen J (2000) Water velocity shapes juvenile salmonids. Evol Ecol 14:721–730
Parenti LR (1984a) A taxonomic revision of the Andean killifish genus Orestias (Cyprinodontiformes, Cyprinodontidaae). Bull Am Mus Nat Hist 178:107–214
Parenti LR (1984b) Biogeography of the Andean killifish genus Orestias with comments on the species flock concept. In: Echelle AA, Kornfield I (eds) Evolution of fish species flocks. University of Maine at Orono Press, Orono
Parker A, Kornfield I (1995) Molecular Perspective on Evolution and Zoogeography of Cyprinodontid Killifishes (Teleostei; Atherinomorpha). Copeia 1995 (1):8
Pinto M, Vila I (1987) Relaciones tróficas y caracteres morfofucionales de Orestiaslaucaensis Arratia 1982 (PISCES: CYPRINODONTIDAE). An Mus Hist Nat Valpso 18:77–84
Placzek C, Quade J, Patchett PJ (2006) Geochronology and stratigraphy of late Pleistocene lake cycles on the southern Bolivian Altiplano: implications for causes of tropical climate change. Geol Soc Am Bull 118:515–532
Pouilly M, Lino F, Bretenoux JG, Rosales C (2003) Dietary-morphological relationships in a fish assemblage of the Bolivian Amazonian floodplain. J Fish Biol 62(5):1137–1158
Poulet N, Reyjol Y, Collier H, Lek S (2005) Does fish scale morphology allow the identification of population leuciscus burdigalensis in river Viaur (SW France). Aquat Sci 67:122–127
Riveros J, Vila I, Mendez MA (2012) Trophic niche of Orestias agassii (Cuvier and Valenciennes, 1846) in the streams system of salar de Huasco (20 degrees 05’S, 68 degrees 15’W). Gayana 76(2):79–91
Robinson BW, Wilson DS (1994) Character release and displacement in fishes: a neglected literature. Am Nat 144:596–627
Rohlf FJ (1990) Rotacional fit (Procrustes) methods. In: Rohlf FJ, Bookstein FL (eds) Proceedings of the Michigan Morphometrics workshop. Special publication N°2. An Arbor: Univ. of Michigan Museum of Zoology, pp 227-236
Rohlf FJ, Slice DE (1990) Extensions of the Procrustes method for the optimal superimposition of landmarks. Syst Zool 39:40–59
Schluter D (1993) Adaptive radiation in sticklebacks - size, shape, and habitat use efficiency. Ecology 74(3):699–709
Schluter D (1996) Ecological causes of adaptive radiation. Am Nat 148: S40-S64. Supplement: Suppl. S
Schluter D (2000) Ecological character displacement in adaptive radiation. Am Nat 156: S4-S16. Supplement: Suppl. S
Slice D (2000) Morpheus et al: Software for morphometric research. Department of Ecology and Evolution, State University of New York, Stony Brook, NY.
Sneath PH, Sokal RR (1973) Numerical taxonomy San Francisco, Freeman: Stony Brook, NY
Swain DP, Foote CJ (1999) Stocks and chameleons: the use of phenotypic variation in stock identification. Fish Res 43:1123–1128
Taylor EW, Egginton S, Taylor SE, Butler PJ (1997) Factors which limit swimming performance at different temperatures. In: Wood CM, McDonald DG (eds) Global warming: implications for freshwater and marine fish. Cambridge University Press, pp 105–133
Toro IM, Manríquez SG, Suazo GI (2010) Morfometría geométrica y el estudio de las formas biológicas: de la morfología descriptiva a la morfología cuantitativa. Int J Morphol 28:977–990
Vila I (2006) A new species of killifish (Teleostei; Cyprinodontiformes) from the southern Altiplano, Chile. Copeia 3:471–476
Vila I, Pinto M (1986) A new species of killifish (Pisces, Cyprinodontidae) from the Chilean Altiplano. Rev Hydrobiol Trop 19:233–239
Vila I, Scott S, Lam N, Iturra P, Mendez MA (2010) Karyological and morphological analysis of divergence among species of the killifish genus Orestias (Teleostei: Cyprinodontidae) from the southern Altiplano. Origin and phylogenetic interrelation ships of Teleosts, pp 471–480
Vila I, Scott S, Mendez MA, Valenzuela F, Iturra P, Poulin E (2011) Orestias gloriae, a new species of cyprinodontid fish from saltpan spring of the southern high Andes (Teleostei: Cyprinodontidae). Ichthyol Explor Freshw 22(4):345–353
Vila I, Morales P, Scott S, Poulin E, Véliz D, Harrod C, Méndez MA (2013) Phylogenetic and phylogeographic analysis of the genus Orestias (Teleostei: Cyprinodontidae) in the southern Chilean Altiplano: the relevance of ancient and recent divergence processes in speciation. J Fish Biol 82(3):927–943
Villwock W, Sienknecht U (1995) Intraspezifische variabilitét im genus Orestias Valenciennes 1839 (Teleostei: Cyprinodontidae) and zum problem der artidentitét. Mitt Zoological Museum of the University of Hamburg 92:381–398
Villwock W, Sienknecht U (1996) Contribución al conocimiento e historia de los peces chilenos. Los Cyprinodóntidos del género Orestias Val. 1839 (Teleostei: Cyprinodontidae) del altiplano chileno. Medio Ambiente 13:119–126
Wainwright PC (1991) Ecomorphology: experimental functional anatomy for ecological problems. Am Zool 31:680–693
Wainwright PC (1996) Ecological explanation through functional morphology: the feeding biology of sunfishes. Ecology 77:1336–1343
Webb D (1984) Form and function in fish swimming. Sci Am 251:58–68
Winemiller KO (1992) Ecological divergence and convergence in freshwater fishes. Natl Geogr Res 8:308–327
Acknowledgments
Comisión Nacional Científica y Tecnológica Project 1140543.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All procedures involving animals were performed in accordance with the standards of the Universidad de Chile Bioethics Committee and under authorization of Subsecretaría de Pesca, Chile, exempted resolution #1103.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
Rights and permissions
About this article
Cite this article
Scott, S., Rojas, P. & Vila, I. Meristic and morphological differentiation of Orestias species (Teleostei; Cyprinodontiformes) from the southern Altiplano. Environ Biol Fish 103, 939–951 (2020). https://doi.org/10.1007/s10641-020-00995-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10641-020-00995-4