Abstract
The warm-water species, striped searobin (Prionotus evolans), has colonized Narragansett Bay (Rhode Island, USA) in recent decades. To clarify the high variability of past investigations, the aim of this study was to characterize the diet of striped searobins. Stomach contents from searobins collected throughout Narragansett Bay in 2019 were identified and analyzed. Diet varied ontogenetically, seasonally, and regionally. The ontogenetic changes in diet reflected an expansion of searobin niche breadth: all searobins ate a diet with sand shrimp; however, smaller searobins ate primarily sand shrimp, while larger searobins also consumed crustaceans and finfish. Searobin diet varied by month, but diet composition did not exhibit a singular trend. Regional variation was illustrated by a dominance of mysid shrimp in Upper-Bay searobin samples, while the Mid- and Lower-Bay samples contained larger proportions of other crustacea and sand shrimp. Their flexible diet provides strong evidence that striped searobins are adaptable generalist feeders. Searobin stomachs included nine prey species of economic importance; such predation could become more common as continued increase of habitat use of searobins in Narragansett Bay may result in greater overlap with the occurrence of vulnerable juvenile stages of economically valuable species (e.g., winter flounder). The results of this work contribute to our understanding of the evolving role of the striped searobin in food webs, particularly those in Southern New England (USA).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Generalist feeders consume a wide variety of prey and are often better equipped to adapt to changes in food resources compared with specialized feeders (Jennings and Brander 2010). In warming ecosystems, warm-water generalists may occupy a progressively larger niche as they both consume and compete with the original species of the ecosystem. As environmental change in marine ecosystems may cause variations in prey availability, the role of generalist species in connecting food web nodes in communities will also become increasingly important. This is particularly true in estuaries, which are especially sensitive to environmental change and have been noted to be dominated by generalist species, including crabs, gastropods, and fishes (Miller and Dunn 1980; Dolbeth et al. 2008), both in terms of physiological adaptations and feeding strategy (Elliott and Whitfield 2011).
Narragansett Bay (Rhode Island, USA) is a rapidly warming temperate estuary in the Southern New England region of the Northeast US Continental Shelf Large Marine Ecosystem, an area that has seen significant shifts in temperature and species assemblages in recent decades (Collie et al. 2008; Lucey and Nye 2010). The rising water temperatures have allowed many warm-water summer transient species to colonize the estuary, resulting in a decline of cold-water resident species (Collie et al. 2008). An example of a summer transient species, the striped searobin (Prionotus evolans, family Triglidae) is a benthic omnivore found in coastal temperate waters in the western Atlantic (Collette and Klein-MacPhee 2002). Striped searobins were first recorded in scientific surveys of Narragansett Bay in 1963, though they were not observed regularly until the 1970s. Since their arrival, the species has exploded in abundance, now ranking as the 7th most abundant by biomass in the University of Rhode Island’s Graduate School of Oceanography (GSO) trawl survey during the last decade (Collie et al. 2008; URI GSO Fish Trawl, unpubl. data).
Striped searobin biology is strongly affected by temperature. They spawn in estuaries in summer months and migrate between the spawning grounds and deeper shelf areas with timing mediated by temperature gradients (McBride and Able 1994; McBride et al. 1997; McBride 2002). In Narragansett Bay, the timing of seasonal ingress and egress of striped searobins have each shifted by over a month since the 1960s in response to changing bottom temperatures, which currently range from − 1 to 24 °C and are exhibiting the most rapid warming in winter (Bell et al. 2018; Langan et al. 2021). As the cold edge of a species’ thermal tolerance tracks local climate velocity more closely than the warm extreme (Fredston-Hermann et al. 2020), projected increases in Narragansett Bay temperatures, particularly of winter minimums, will likely expand searobin residence time further and lead to temperatures optimally aligned with the thermal niche of this species (~ 2 to 26 °C; Fulweiler et al. 2015; Bell et al. 2018; Kaschner et al. 2019). Thus, the role of the striped searobin in the food web is likely becoming more influential as climate change continues to shift community structure.
While diet data for the striped searobin exist, there is little consensus in the literature, and the most comprehensive studies are now decades old. Oviatt and Nixon (1973) reported on searobin diet in Narragansett Bay, but both striped and northern searobins (Prionotus carolinus) were grouped together in the analysis. These two searobin species were found to consume a wide range of prey including shrimp, crab, juvenile finfish, mollusks, and amphipods (Oviatt and Nixon 1973). However, we have since learned these congeners differ in many aspects of their ecology and life history, including growth, thermal niche, and distribution (McBride and Able 1994; Froese and Pauly 2019). Striped searobin diet may have shifted since this original study in response to climate-driven changes in community composition in a warmer Narragansett Bay. In Long Island Sound, NY, striped searobins were found to be a generalist by Richards et al. (1979). Others in the Hudson River estuary, Long Island bays, and across the Northeast US Continental Shelf, however, found that, by weight or volume, striped searobin diet primarily comprised sand and mysid shrimp with little input from other taxa (Steimle et al. 2000; Sagarese et al. 2011) or that their diet was dominated by fishes and crabs (Smith and Link 2010). However, these previous diet studies have highly variable sampling regions, seasonal variation, and small sample sizes. The objective of our study was to quantify the diet of the striped searobin in Narragansett Bay and evaluate how it varies across spatial, seasonal, and ontogenetic scales. We hypothesized that the species has a wide niche breadth and consumes a variety of prey types given the results from previous studies in the region.
Materials and methods
Sample collection and processing
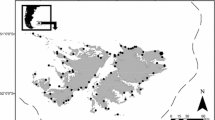
We conducted field sampling from late May to early November 2019. The majority of fish were collected from the GSO bottom trawl survey (Fig. 1), which is conducted weekly at 2 stations in Narragansett Bay (Collie et al. 2008). The Mid-Bay station, Fox Island, is approximately 7 m in depth with mud and shell substrate. The Lower-Bay station, Whale Rock, is just over 20 m in depth with similar substrate. An otter trawl with 5.1-cm (cm) cod end mesh was towed at 3.7 km/h (2 knots) for 30 min, and basic environmental parameters such as temperature (°C) and salinity were recorded. Additional striped searobins were collected from the Rhode Island Department of Environmental Management (DEM) monthly bottom trawl survey, which is conducted monthly at 13 stations in Narragansett Bay. The DEM survey uses an otter trawl fished with a 0.6-cm mesh liner towed at 4.6 km/h (2.5 knots) for 20 min. Searobins retained from the DEM trawl were generally limited to those originating outside of the West Passage area that is sampled by the GSO trawl. Other opportunistic searobin samples included those captured by rod and reel, using artificial bait not included in stomach analysis (number of samples [n] = 3 with identifiable prey), as well as a Mid-Bay experimental midwater trawl (n = 3).
Map of sampling stations in Narragansett Bay where striped searobins (Prionotus evolans) were collected. Samples were collected from the Rhode Island Department of Environmental Management (DEM), University of Rhode Island’s Graduate School of Oceanography (GSO), a midwater trawl survey, or caught via rod and reel (R&R). Most samples came from the GSO Fox Island (FI) and GSO Whale Rock (WR) stations (Table 1)
The processing of each fish consisted of measuring total length (TL, cm), determining sex by visual inspection of the gonads, and weighing (g) the full stomach, empty stomach, and identifying stomach contents aggregated by the lowest discernible taxon with the aid of a dissecting microscope when necessary. Specifically, each prey type was enumerated, weighed, and assigned a level of digestion. About half of the samples were processed on the day of collection; however, approximately 43% of searobins (n = 127) were frozen within a few hours of collection for later dissection.
Analyses
For most analyses, prey species were assigned to broad taxonomic groups to reflect similarity of functional trophic roles and to reduce variation in the data. An expanded diet with more specific prey categorization can be found in Supplemental Table 1. Descriptive statistics of diet are reported with 3 metrics: percent weight (%W), percent count (%N), and frequency of occurrence (%FO). It is important to note, however, that prey counts were not able to be made on all searobin samples (i.e., if the prey were extremely well digested). Therefore, %N is only included in Supplemental Table 1. Searobins were clustered by tow and size category for the weight and count metrics as described by Buckel et al. (1999) and Latour et al. (2008). Size categories were determined a priori based on age group in order to evaluate possible ontogenetic differences in diet. Size bins of small (TL < 18.5 cm; age 0–1), medium (18.5 cm ≤ TL < 27.4 cm; age 2–4), and large ( TL ≥ 27.5 cm; age 5 +) searobins were created using size-at-age information for New England striped searobins (McBride 2002). Month and Bay region factors for analysis were also chosen a priori. The frequency of occurrence was calculated as the percent of non-empty searobin stomachs that contained a particular prey taxon. Searobins with empty stomachs (n = 10) or searobins with stomachs consisting of only unknown prey or plant and inorganic material (n = 4) were removed from statistical analyses. Analyses were completed in R version 3.6.0 (R Core Team 2019).
Multivariate analyses similar to the methods of Davis et al. (2020) were used to analyze the compositional diet data of collected striped searobin. The prey weight proportion data, of identified prey types only, were first arcsine-square root transformed to reduce the impact of highly abundant prey categories. Significant differences (alpha level of 0.05) in the diet were identified with a permutational multivariate analysis of variance (PERMANOVA) on the Bray–Curtis dissimilarity matrix of the transformed data using the package “vegan” in R (Oksanen et al. 2019). Here, the modified PERMANOVA test statistic proposed by Anderson et al. (2017) was used to accommodate heterogeneous group-wise dispersions. The effects of month of capture, region of capture, length, and sex on dietary composition were tested as covariates. Significant covariates were identified with a backward selection procedure, whereby variables were retained if their removal resulted in an increase of at least 0.01 in the residual coefficient of multiple determination (R2). Because their order in the PERMANOVA formula can impact estimated significance, the covariates were also permuted during the selection process to ensure consistent results.
Niche breadth was used to compare intraspecific differences in resource use among searobin categories. This metric can be used to examine if a predator is acting as a “generalist” or “specialist” (Colwell and Futuyma 1971). Niche breadth was calculated with Levin’s standardized niche breadth Bi (Eq. 1.),
where pij is the clustered proportion of total prey weight for cluster i that was made up of prey j, and Ni is the number of prey groups eaten by predators in cluster i (Levins 1968). The resulting Bi varies between 0 and 1, where 0 represents a specialist predator in which all the diet is made up of a single prey category and a value of 1 represents a full generalist for which every prey is an equally important component of the diet. We calculated niche breadth for the striped searobin using the clustered prey categorization schemes described for the PERMANOVA.
The impact of the striped searobin on commercially important species was examined by identifying prey types as commercially important if the species is caught and sold by commercial vessels in New England waters (Atlantic Coastal Cooperative Statistics Program 2019). Categories such as “unknown flatfish” were not classified as commercially important as there are both commercially important and unimportant flounder species.
Results
Diet and diet variability
Striped searobins (n = 303) were collected from 46 unique sampling events (i.e., tows) from 14 locations in Narragansett Bay (Fig. 1; Table 1). There were 71 clusters (i.e., a sample of searobins of a given size category caught in a given tow). Ten searobins had empty stomachs, and another four had no identifiable prey. Due to sampling difficulties, some searobin samples had missing information such as TL (n = 3) or were of undetermined sex (n = 18). The collected searobins ranged from 10.3 to 44 cm TL, and consequently, the majority were assigned to the large size bin (n = 232), followed in abundance by medium (n = 46), and small (n = 12). There were 136, 128, and 25 searobins with stomachs containing identifiable prey collected from the Lower-, Mid-, and Upper-Bay regions, respectively. The most searobins were collected in July (n = 121), and the fewest searobins with prey were caught in August (n = 9). We collected 134 female and 139 male searobins, with the rest classified as unknown or immature. Bottom water temperature during searobin sampling events ranged from 10 to 24 °C.
Diet was variable among individual searobins. Many stomachs contained only a small number of different prey groups; on average, only 2.2 groups of the 8 identified broad prey categories were found. Despite these variations, several patterns emerged from formal analyses. The PERMANOVA indicated that the diet of the sampled searobins varied by TL, region, and month (Table 2). Sampled females reached larger sizes than males (Wilcoxon Rank Sum test, P < 0.001, Supplemental Fig. 1) and had more stomach contents-per-centimeter TL by weight (Wilcoxon Rank Sum test, P < 0.001).
Because the PERMANOVA indicated that striped searobin diet varied by TL, the average diet was reported in the three different size classes (Table 3). The diet table with expanded prey categories is included in Supplemental Table 1. When plotted in niche breadth coordinate space, it is clear the diet of striped searobins of all sizes was non-specialized (Fig. 2). Small and medium searobins had a dominance of sand and grass shrimp (Crangon spp. and Palaemonetes spp., respectively), and these shrimps remained important prey for large searobins. Costello (1990) describes points falling under the “feeding strategy” line representing a heterogeneous diet, and all other prey types, except sand shrimp, fell into the quadrants of rare prey and generalized feeding. The ontogenetic diet shift was also illustrated by the expansion of the niche breadth of searobins by size class. Using the prey categories reported in Table 3, small searobins had the narrowest niche (BSm = 0.05), and this increased in the larger size classes (BMed = 0.14 and BLg = 0.57).
Visualization of striped searobin (Prionotus evolans) diet in the style suggested by Costello (1990). The upper left quadrant represents the specialization of prey, the upper right is dominant prey, the bottom right is a generalized diet, and the bottom left shows rarer prey items
Though the PERMANOVA showed that diet varied significantly by month, the composition of searobin diet through time did not exhibit a singular trend (Fig. 3). Early in the sampling season, sand shrimp (45–64%) and other crustaceans such as mantis shrimp (Squilla empusa, 24–31%) dominated the stomach weight. In July, amphipods increased substantially to 27.7%W of the diet, and mysid shrimp increased to 18.5%W of the diet. In early fall, there was a much higher proportion of crabs and fishes in their stomachs (crab: 27.2–50.7%W, fish: 5.9–33.3%W).
Diet also varied significantly by region within Narragansett Bay. Approximately half of the total prey weight came from the Lower-Bay stations, where prey groups were most evenly represented (Fig. 4). The smallest fraction of total prey weight came from the Upper-Bay stations (10.8%W), where diets were primarily composed of mysid shrimp. The Mid-Bay stations had large proportions of other crustaceans and sand shrimp. Fish were represented in similar proportions (8.9–12.6%W) in all regions.
Commercial fisheries impacts
Throughout the sampling period, nine distinct prey species of commercial importance were found in the searobin stomach samples (Table 4). The commercially important fish prey specimens were primarily in post-larval or juvenile life stages. By weight, October had the largest amount of commercially important prey, though prey species showed varied temporal patterns. In total, 7% of sampled searobins had consumed prey of commercial importance, and these commercially important prey species made up 4% of the clustered prey weight (7% total). When examining only fish prey, 50% of the prey weight was unidentifiable to genus or species level. However, commercially important species made up 80% of the total identifiable fish prey by weight and 76% by count.
Discussion
Narragansett Bay striped searobins exhibited highly variable diets, consuming a wide array of taxa. The large amount of individual variation indicates a flexible diet whereby searobins consume nearly any species of appropriate size that they encounter, supporting the idea that this species is a generalist feeder as suggested by some previous research (Richards et al. 1979). While multiple searobin species had been qualitatively reported to consume shrimp, crab, fish, mollusks, and amphipods (Oviatt and Nixon 1973), this study confirms that all of these groups are consumed by the striped searobin specifically. Squid predation was not documented in this earlier research and may represent a diet expansion such that the searobins have taken advantage of increasing squid abundance and residence time in the Bay in recent decades (Collie et al. 2008).
Our results clearly showed ontogenetic expansion in the searobin diet. In general, smaller searobins depended more on small shrimp and other small benthic taxa, while larger searobins consumed a greater variety of prey items including fish, crab, and other crustaceans. The clear relationship between diet and TL and between TL and sex likely resulted in the lack of significant association between diet and sex. Ontogenetic diet expansion in striped searobins mimics the patterns seen in many fish species, such as summer flounder, bluefish (Pomatomus saltatrix), Atlantic cod (Gadus morhua), and goosefish (Lophius americanus), whereby larger individuals consume more fish and larger crustaceans (Armstrong et al. 1996; Buckel et al. 1999; Link and Garrison 2002; Latour et al. 2008). Large searobins still consumed shrimps and benthos, but a wider assortment of larger prey species were also found. From this diet expansion, we can conclude that as searobins grow, so does their trophic connectivity within the food web.
The spatiotemporal variability in the searobin diet supports the idea that this omnivore may be able to adapt to new regions, seasons, and climatic conditions. Diet varied by both Bay region and month, though there were no discernable unidirectional trends in the prey composition. Due to the overall limited sample size of searobins from the Upper-Bay, a greater proportion of the samples were caught via rod and reel. Future studies with more samples from non-bottom trawl gears could further examine the effect that gear type has on striped searobin diet. Spatial patterns in diet data for searobins have been found before, such as in Long Island Sound, where searobin diets changed with substrate type and depth (Richards et al. 1979). Spatiotemporal variability also provides an explanation of why past studies reported such dissimilar diets; these patterns could reflect differences in prey availability. Striped searobins have been shown to feed opportunistically in laboratory studies where searobin diet reflected the initial relative abundance of the prey types offered (Manderson et al. 1999). While we believe the diet reflects the underlying prey field, additional data are needed on the distributions of prey species and benthos in Narragansett Bay to confirm this and conduct further analyses.
Narragansett Bay is an important nursery habitat for many fish species (Keller et al. 1999), and striped searobins could be major predators for the juvenile and larval stages of commercially important species. We found 9 distinct species of commercial importance in searobin stomachs, which may be an underestimate due to the exclusion of unknown categories (i.e., cancer crab or unidentified fish), and the difficulty of identifying partially digested juvenile fish. Striped searobins have been known to prey on larval winter flounder, scup, and black sea bass in experimental settings (Manderson et al. 1999; Scharf et al. 2006), and species of commercial importance have been found in stomach contents previously (Richards et al. 1979). Striped searobins have expanded their traditional residence time in Narragansett Bay beyond the summer months (Langan et al. 2021), providing additional prey opportunities when overlapping with young of the year fishes. As waters have warmed in recent years, striped searobins are regularly present in the trawl surveys in the Bay from May to November, overlapping with the peak of fish larvae and therefore settling juveniles (Keller et al. 1999). Where prey species previously had temporal refuge, the extended residence of striped searobins in Narragansett Bay in early summer and late fall has created the opportunity to overlap with and possibly predate the early life stages of species such as Atlantic cod and summer flounder (Keller et al. 1999). If residence time continues to expand into spring and winter, life stages of additional commercially important prey species such as Atlantic menhaden (Brevoortia tyrannus) and tautog (Tautoga onitis) may become vulnerable (Keller et al. 1999). Given that spatiotemporal overlap controls the intensity of predation interactions (Durant et al. 2007), continued expansion of the geographic range and residence time of the striped searobin could impact these nursery habitats.
The striped searobin is an example of a species primed for success in the face of climate change. Striped searobin abundance in Narragansett Bay has increased by threefold since the 1990s (GSO Trawl data), and they are now one of the most abundant species in the estuary (Collie et al. 2008). This species has a wide thermal tolerance and can utilize both estuarine and offshore habitats (McBride and Able 1994; McBride et al. 1997). The striped searobin follows the pattern of poleward expansion due to climate change seen in many species (Pinsky et al. 2013) and is increasingly observed in more northern waters (Northeast Fisheries Science Center Ecosystem Dynamics and Assessment Branch 2020). Generalist invaders are typically “winners” that outcompete their specialized-feeding native counterparts (Olden et al. 2004; Layman and Allgeier 2012). In Narragansett Bay, examples of specialist feeders include bluefish (Pomatomus saltatrix), weakfish (Cynoscion regalis), and to a lesser extent striped bass (Morone saxatilis), which serve as the basis for important fisheries (Smith and Link 2010). Dominance by fast-growing generalist feeders in ecosystems is likely to only grow with changing climatic and environmental conditions (Brustolin et al. 2019). This dominance may be particularly true for the striped searobin, for which top-down control is likely to be limited by the lack of an active fishery and their absence from the diet of regional piscivores (Bowman et al. 2000; Innes-Gold et al. 2020).
The striped searobin is not the only generalist species to capitalize on a warming Narragansett Bay. Little skate (Leucoraja erinacea), black sea bass, and scup also consume a wide variety of prey species with none dominating their diets, and all are warm-water species increasing in abundance in Narragansett Bay (Collie et al. 2008; Szczepanski 2013; Malek et al. 2016). As species expand in the new environmental conditions, striped searobins could represent a competitor species to these taxa of greater commercial importance. Striped searobins prey on most other major functional groups in Narragansett Bay, are present in every area of the Bay, and remain for a large part of the year, which could give them a competitive advantage. These widespread interspecies interactions will certainly lead to feedbacks on the energy flow and productivity of the Narragansett Bay ecosystem.
Understanding the diet and diet variability is the first step in fully measuring the impact of the striped searobin on the food webs of newly colonized ecosystems. This species rapidly established itself in a new region, yet it has not been the focus of much study in comparison to other species with expanding ranges. The lack of economic importance of this species means it has been largely overlooked in the literature despite exhibiting substantial predation on and competition with better-studied species. Knowledge gaps regarding in situ prey fields available to striped searobins prevent us from completing a more quantitative report on the impact of the striped searobin on the Narragansett Bay food web. Additionally, close to half our samples were frozen, which could have increased the amount of unidentifiable prey due to tissue degradation during freezing and thawing. Finally, a greater understanding of striped searobin consumption rates and the factors that control that rate would improve estimates of total predation on the other groups of the system. Despite these gaps, the diet composition data presented in this study are useful for food web modeling studies (e.g., Innes-Gold et al. 2020) where trophic connections are represented quantitatively. In conclusion, the combination of their increased presence and abundance, wide thermal niche, and generalist feeding strategy positions the striped searobin for continued success in Narragansett Bay and further poleward expansion into other estuaries.
Data availability
The data that support the findings of this study are available from the corresponding author, AH, upon reasonable request.
References
Anderson MJ, Walsh DCI, Robert Clarke K et al (2017) Some solutions to the multivariate Behrens-Fisher problem for dissimilarity-based analyses. Aust N Z J Stat 59:57–79. https://doi.org/10.1111/anzs.12176
Armstrong MP, Musick JA, Colvocoresses JA (1996) Food and ontogenetic shifts in feeding of the goosefish, Lophius americanus. J Northwest Atl Fish Sci 18:99–89. https://doi.org/10.2960/j.v18.a7
Atlantic Coastal Cooperative Statistics Program (2019) ACCSP public data warehouse: non-confidential commercial landings. https://www.accsp.org (Accessed Jul 2020)
Bell RJ, Wood A, Hare J et al (2018) Rebuilding in the face of climate change. Can J Fish Aquat Sci 75:1405–1414. https://doi.org/10.1139/cjfas-2017-0085
Bowman RE, Stillwell CE, Michaels WL, Grosslein MD (2000) Food of northwest Atlantic fishes and two common species of squid. US Dep Commer NOAA Tech Memo NMFS-NE 155. https://doi.org/10.5962/bhl.title.4024
Brustolin MC, Nagelkerken I, Moitinho Ferreira C et al (2019) Future ocean climate homogenizes communities across habitats through diversity loss and rise of generalist species. Glob Chang Biol 25:3539–3548. https://doi.org/10.1111/gcb.14745
Buckel JA, Fogarty MJ, Conover DO (1999) Foraging habits of bluefish, Pomatomus saltatrix, on the U.S. East Coast Continental Shelf. Fish Bull 97:758–775
Collette BB, Klein-MacPhee G (2002) Fishes of the Gulf of Maine, 3rd edn. Smithsonian Institution, Washington, DC
Collie JS, Wood AD, Jeffries HP (2008) Long-term shifts in the species composition of a coastal fish community. Can J Fish Aquat Sci 65:1352–1365. https://doi.org/10.1139/F08-048
Colwell RK, Futuyma DJ (1971) On the measurement of niche breadth and overlap. Ecology 52:567–576. https://doi.org/10.2307/1934144
Costello MJ (1990) Predator feeding strategy and prey importance : a new graphical analysis. J Fish Biol 36:261–263
Davis M, Chamberlin J, Gardner J et al (2020) Variable prey consumption leads to distinct regional differences in Chinook salmon growth during the early marine critical period. Mar Ecol Prog Ser 640:147–169. https://doi.org/10.3354/meps13279
Dolbeth M, Martinho F, Leito R et al (2008) Feeding patterns of the dominant benthic and demersal fish community in a temperate estuary. J Fish Biol 72:2500–2517. https://doi.org/10.1111/j.1095-8649.2008.01856.x
Durant JM, Hjermann DØ, Ottersen G, Stenseth NC (2007) Climate and the match or mismatch between predator requirements and resource availability. Clim Res 33:271–283
Elliott M, Whitfield AK (2011) Challenging paradigms in estuarine ecology and management. Estuar Coast Shelf Sci 94:306–314. https://doi.org/10.1016/j.ecss.2011.06.016
Fredston-Hermann A, Selden R, Pinsky M et al (2020) Cold range edges of marine fishes track climate change better than warm edges. Glob Chang Biol 26:2908–2922. https://doi.org/10.1111/gcb.15035
Froese F, Pauly D (2019) FishBase. http://fishbase.org/. Accessed Jun 2020
Fulweiler RW, Oczkowski AJ, Miller KM et al (2015) Whole truths vs. half truths - and a search for clarity in long-term water temperature records. Estuar Coast Shelf Sci 157:A1–A6. https://doi.org/10.1016/j.ecss.2015.01.021
Innes-Gold A, Heinichen M, Gorospe K et al (2020) Modeling 25 years of food web changes in Narragansett Bay (USA) as a tool for ecosystem-based management. Mar Ecol Prog Ser 654:17–33. https://doi.org/10.3354/meps13505
Jennings S, Brander K (2010) Predicting the effects of climate change on marine communities and the consequences for fisheries. J Mar Syst 79:418–426. https://doi.org/10.1016/j.jmarsys.2008.12.016
Kaschner K, Kesner-Reyes K, Garilao C et al (2019) Aquamaps: predicted range maps for aquatic species. www.aquamaps.org (Accessed Jun 2020)
Keller AA, Klein-MacPhee G, Burns JSO (1999) Abundance and distribution of ichthyoplankton in Narragansett Bay, Rhode Island, 1989–1990. Estuaries 22:149–163
Langan J, Puggioni G, Oviatt C et al (2021) Climate alters the migration phenology of coastal marine species. Mar Ecol Prog Ser 660:1–18. https://doi.org/10.3354/meps13612
Latour RJ, Gartland J, Bonzek CF, Johnson RMA (2008) The trophic dynamics of summer flounder (Paralichthys dentatus) in Chesapeake Bay. Fish Bull 106:47–57
Layman CA, Allgeier JE (2012) Characterizing trophic ecology of generalist consumers: a case study of the invasive lionfish in The Bahamas. Mar Ecol Prog Ser 448:131–141. https://doi.org/10.3354/meps09511
Levins R (1968) Evolution in changing environments: some theoretical explorations, No. 2. Princeton University Press, Princeton
Link JS, Garrison LP (2002) Trophic ecology of Atlantic cod Gadus morhua on the Northeast US Continental Shelf. Mar Ecol Prog Ser 227:109–123. https://doi.org/10.3354/meps227109
Lucey SM, Nye JA (2010) Shifting species assemblages in the Northeast US Continental Shelf Large Marine Ecosystem. Mar Ecol Prog Ser 415:23–33. https://doi.org/10.3354/meps08743
Malek AJ, Collie JS, Taylor DL (2016) Trophic structure of a coastal fish community determined with diet and stable isotope analyses. J Fish Biol 89:1513–1536. https://doi.org/10.1111/jfb.13059
Manderson JP, Phelan BA, Bejda AJ et al (1999) Predation by striped searobin (Prionotus evolans, Triglidae) on young- of-the-year winter flounder (Pseudopleuronectes americanus, Walbaum): examining prey size selection and prey choice using field observations and laboratory experiments. J Exp Mar Bio Ecol 242:211–231. https://doi.org/10.1016/S0022-0981(99)00105-7
McBride RS (2002) Spawning, growth, and overwintering size of searobins (Triglidae: Prionotus carolinus and P. evolans). Fish Bull 100:641–647
McBride RS, Able KW (1994) Reproductive seasonality, distribution, and abundance of Prionotus carolinus and P. evolans (Pisces: Triglidae) in the New York Bight. Estuar Coast Shelf Sci 38:173–188
McBride RS, O’Gorman JB, Able KW (1997) Interspecific comparisons of searobin movements, size structure, and abundance in the temperate western North Atlantic. Fish Bull 96:303–314
Miller JM, Dunn ML (1980) Feeding strategies and patterns of movement in juvenile estuarine fishes. In: Kennedy VS (ed) Estuarine Perspectives, pp 437–448. Academic Press, Inc. New York
Northeast Fisheries Science Center Ecosystem Dynamics and Assessment Branch (2020) Fisheries habitat in the Northeast U.S. Shelf ecosystem: striped searobin. https://www.fisheries.noaa.gov/new-england-mid-atlantic/ecosystems/fisheries-habitat-northeast-us-shelf-ecosystem#striped-searobin
Oksanen FJ, Blanchet G, Friendly M et al (2019) _vegan: Community Ecology Package_. R package version 2.6-4, <https://cran.r-project.org/package=vegan>
Olden JD, Poff NLR, Douglas MR et al (2004) Ecological and evolutionary consequences of biotic homogenization. Trends Ecol Evol 19:18–24. https://doi.org/10.1016/j.tree.2003.09.010
Oviatt CA, Nixon SW (1973) The demersal fish of Narragansett Bay: an analysis of community structure, distribution and abundance. Estuar Coast Mar Sci 1:361–378. https://doi.org/10.1016/0302-3524(73)90026-1
Pinsky ML, Worm B, Fogarty MJ et al (2013) Marine taxa track local climate velocities. Science 341:1239–1242. https://doi.org/10.1126/science.1239352
R Core Team (2019) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/
Richards SW, Mann JM, Walker JA (1979) Comparison of spawning seasons, age, growth rates, and food of two sympatric species of searobins, Prionotus carolinus and Prionotus evolans, from Long Island Sound. Estuaries 2:255–268. https://doi.org/10.2307/1351572
Sagarese SR, Cerrato RM, Frisk MG (2011) Diet composition and feeding habits of common fishes in Long Island bays, New York. Northeast Nat 18:291–314
Scharf FS, Manderson JP, Fabrizio MC (2006) The effects of seafloor habitat complexity on survival of juvenile fishes: species-specific interactions with structural refuge. J Exp Mar Bio Ecol 335:167–176. https://doi.org/10.1016/j.jembe.2006.03.018
Smith BE, Link JSJ (2010) The trophic dynamics of 50 finfish and 2 squid species on the Northeast US Continental Shelf. US Dep Commer NOAA Tech Memo NMFS-NE 216. Northeast Fisheries Science Center, Woods Hole
Steimle FW, Pikanowski RA, McMillan DG et al (2000) Demersal fish and American lobster diets in the lower Hudson - Raritan Estuary. US Dep Commer NOAA Tech Memo NMFS-NE 161. Northeast Fisheries Science Center, Woods Hole
Szczepanski JA (2013) Feeding ecology of skates and rays in Delaware and Narragansett Bays. PhD dissertation, University of Rhode Island, Kingston, RI. ProQuest Dissertations Publishing, 3558628
Acknowledgements
This material is based upon work supported in part by the National Science Foundation under EPSCoR Cooperative Agreement #OIA-1655221. We thank N. Santos and C. McManus for assisting us in gathering samples and the associated station data. We also thank Captain S. Barber for the operation of the GSO fish trawl. This is a contribution of the Rhode Island Marine Fisheries Institute.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
The Graduate School of Oceanography Fish Trawl is funded by the University of Rhode Island and the Rhode Island Department of Environmental Management, and the collection protocol is approved by the URI’s Institutional Animal Care and Use Committee Board as #AN1920-024.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Heinichen, M., Innes-Gold, A., Langan, J.A. et al. Describing the diet of a generalist feeder: the striped searobin (Prionotus evolans) from Narragansett Bay, Rhode Island (USA). Environ Biol Fish 106, 697–706 (2023). https://doi.org/10.1007/s10641-023-01410-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10641-023-01410-4