Abstract
Shelter is an environmental feature that provides protection from danger, and its use is an important anti-predator behaviour for juvenile salmonids and a focus of stream restoration programs. However, how shelter availability influences the foraging behaviour of these fishes in the wild is not fully understood. We predicted that juvenile Atlantic salmon would increase their foraging behaviour in a low shelter environment to prioritize food acquisition, and hypothesized this effect would differ between individuals from two populations that are targeted for reintroduction into Lake Ontario given differences in predation experienced by each in the wild. We measured the foraging activity of juvenile Atlantic salmon from the two populations while they were held in pens for 6-day periods in a Lake Ontario tributary that differed in the number of shelters. Over the duration of the study when fish were observed during dawn, midday, and dusk periods, salmon from both populations had odds of foraging that was 77% lower in the low shelter treatments than in the high shelter treatments. These differences in behaviour had no noticeable association with diet or growth rate during the experiment. The two populations tested did not differ in any aspect of foraging or growth. Based on additional observations made at night, the juvenile salmon exhibited a bimodal distribution of foraging, foraging more intensively at midday and at night. Overall, our data show that high shelter availability increases foraging in two populations of Atlantic salmon, suggesting that increasing shelter availability should continue to be a key component of stream restoration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Predation risk is a key factor influencing foraging activity in juvenile fishes (Sih 1980; McNamara and Houston 1987; Lima and Dill 1990). Anti-predator behaviours are ubiquitous and include increasing vigilance (Lima 1987), avoiding areas where predation risk is high (Mikheev et al. 1994), reducing foraging activity during periods when predation risk is high (Breau et al. 2007), and utilizing shelters such as an undercut bank, boulders, coarse woody debris, or any other environmental feature that provides protection from predators, in order to avoid detection by predators (Orpwood et al. 2006; Millidine et al. 2006; Larranaga and Steingrímsson 2015). In particular, shelter use is an important anti-predator behaviour and the use of shelters provides several benefits such as reduced predation risk (Lima and Dill 1990), reduced time spent in vigilance (Lima and Dill 1990; Millidine et al. 2006), and decreased time until resumption of foraging after interruption (Lima and Dill 1990), all factors which could allow for longer foraging bouts. Alternatively, sheltering, like any anti-predator behaviour, can trade-off with foraging and reduce growth rate (Utne et al. 1993; Tupper and Boutilier 1997; Höjesjö et al. 2004).
Obtaining food often involves leaving an area of relative safety and moving into areas with a higher perceived predation risk, resulting in a trade-off that requires optimizing amount of time spent foraging versus the amount of time in a shelter or area of safety (McNamara and Houston 1987; Brown and Kotler 2004). Increases in perceived predation risk may result in unpredictable and variable interruptions in foraging. Because of this, organisms can also change their foraging behaviour to avoid foraging at times of high predation and increase their activity at times of perceived safety (Lima 1986; Houston and McNamara 1993; McNamara et al. 1994, 2005; Sih and McCarthy 2002; MacLeod et al. 2007; Creel et al. 2008; Walters et al. 2017). This “interrupted foraging” response has been previously observed in fishes (Gries et al. 1997; Metcalfe et al. 1998, 1999; Reebs 2002). For example, timing of foraging behaviour can shift from being diurnal to nocturnal or crepuscular (dawn and dusk) in response to high predation risk from diurnal predators (Helfman 1986; Gries et al. 1997; Metcalfe et al. 1998, 1999). Within a species, shelter availability can also influence the diel timing of foraging. For example, during summer in Northern Iceland, juvenile Arctic char (Salvelinus alpinus) forage primarily during twilight hours in high shelter environments to minimize encounter rates with diurnal avian predators but forage during daylight hours in low shelter environments when foraging efficiency is highest (Larranaga and Steingrímsson 2015). Juvenile Atlantic salmon (Salmo salar) in artificial streams display similar behaviour with increased nocturnal activity in the presence of cover compared to the absence of cover (Orpwood et al. 2003). These studies suggest that fishes prioritize growth over survival when shelters are not available by foraging during times of high food availability but higher risk of predation (see also Orpwood et al. 2003; Larranaga and Steingrímsson 2015). The prioritization of growth over survival could be because most predatory fishes are gape-limited and increased individual growth can result in a shorter period of susceptibility to these predators (Persson et al. 1996; Sogard 1997; but see Lima and Dill 1990).
The intensity (frequency and duration) of anti-predator behaviours is often correlated with levels of predation that are experienced by prey populations under natural conditions (Bell 2005). As such, the response to predators may differ among populations as the result of local adaptation to the predation regime (Åbjörnsson et al. 2004). For example, a study of three-spined stickleback (Gasterosteus aculeatus) in California rivers found that fish from the Navarro River, an environment with a high predation rate, remained motionless longer and foraged less following a visual cue of a predator, than those from Putah Creek, an environment with a low predation rate (Bell 2005). Population differences in anti-predator behaviour have also been noted in shelter use. Fathead minnows (Pimephales promelas) from a population allopatric with predatory northern pike (Esox lucius) did not change shelter use when exposed to a visual stimulus of pike (Mathis et al. 1993). However, when minnows from a population sympatric with pike were shown the pike stimulus, they increased shelter use (Mathis et al. 1993). Thus, populations may vary in their intensity of anti-predator behaviours because of local adaptation to the predation risk they encounter in their local environment.
Habitat destruction or degradation is widely regarded as the primary threat to aquatic fauna in North America (Allan and Flecker 1993). Stream restoration programs are widespread throughout much of North America and increasing available shelter and refugia for fish are often a focal point of the restorations (Finstad et al. 2007). Past literature has shown decreased habitat complexity and a lack of shelter impact survival and growth of freshwater organisms (Miller et al. 1989; Nehlsen et al. 1991; Tupper and Boutilier 1997; Suttle et al. 2004) and that increasing habitat complexity and shelter availability increases the survival and growth of fish (e.g. Finstad et al. 2007). With the current trend of increasing shelter availability during stream restoration programs, evaluating how species respond to increases in shelter availability is important.
Juvenile Atlantic salmon (Salmo salar) are visual predators that are primarily active during the day, when they feed on drifting invertebrates (Allen 1941; Keeley and Grant 1997). Because of their reliance on visual hunting, during the day they have a high foraging efficiency (the success rate of capturing prey items for a given foraging strike; Fraser and Metcalfe 1997). Although foraging efficiency is high during the day, so is the risk of predation by diurnal predators (Gotceitas and Godin 1991; Fraser and Metcalfe 1997). In stream environments, the major predators of juvenile Atlantic salmon are diurnal avian predators such as mergansers (Mergus spp.) and kingfishers (Megaceryle spp.) and larger diurnal piscivorous fish such as brook trout (Salvelinus fontinalis; White 1937, 1938; Gotceitas and Godin 1993).
Atlantic salmon are the focus of reintroduction attempts in Lake Ontario and its tributaries. Atlantic salmon were extirpated from Lake Ontario in the late 1800s (Crawford 2001), and decades of stocking attempts and habitat restoration have yet to re-establish a self-sustaining population (Stewart and Johnson 2014). Currently, the OMNRF stocks juvenile fish from two hatchery populations of Atlantic salmon into Lake Ontario’s tributaries—Sebago Lake and LaHave River—in the hopes of establishing a self-sustaining population (Fig. 1; LOASRP 2019). The Sebago Lake strain was established in the Ontario Fish Culture Program from wild egg collections from the Panther River, Maine (a tributary to Sebago Lake) in 2006 (Fig. 1; OMNR 2011). The LaHave River strain was established from wild egg collections from the LaHave River, Nova Scotia, in 1995 (Fig. 1; OMNR 2011). The source populations of these strains have been reproductively isolated for thousands of years (King et al. 2001) and have different predation regimes in their native environments (Bowlby et al. 2013; Pellerin and Pierce 2015) that may have led to evolved differences in anti-predator behaviours (Houde et al. 2010). Determining if these two populations differ in their anti-predator behaviour and performance in Lake Ontario tributaries is of management concern.
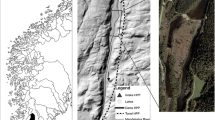
Map of the study site (Duffins Creek) showing locations of the three riffle where the enclosures and drift net were erected in East Duffins Creek at the Greenwood Conservation Area Ajax, ON. Study site is also shown in reference to the Codrington Fish Culture Station (Codrington, ON) where juvenile Atlantic salmon were reared, the Panther River, ME where the Sebago Lake hatchery population naturally occurs, and the LaHave River, NS
Here, we examined if a change in shelter availability changed the rate and the timing of diel foraging in Atlantic salmon from two populations that are targeted for reintroduction into Lake Ontario. Previous literature shows shelter behaviour to be ubiquitous, but the precise means by which salmon use shelter will likely be context and species specific. Prior work on juvenile salmonids suggests that in some contexts, fishes prioritize foraging in low shelter environments (Orpwood et al. 2003; Larranaga and Steingrímsson 2015). We hypothesized that juvenile Atlantic salmon would alter their foraging activity depending on the shelter availability in their environment based on the trade-off between predator avoidance and foraging efficiency. We predicted that, with a lower shelter availability, fish would forage more and prioritize food acquisition. Furthermore, we predicted that in a low shelter environment, fish will forage more heavily during the day prioritizing food acquisition. In a high shelter environment, fish should forage more at night, sheltering during the day and compensating for reduced food availability by foraging more at night. Furthermore, we predicted that if fish foraged more with a decrease in shelter availability, then fish in environments with low shelter availability should have higher growth rates and higher gut content masses than those in environments with high shelter availability. Assuming evolved anti-predator behavioural differences between populations due to their differences in native predation regimes and their persistence in the hatchery setting, we hypothesized that fish from the LaHave and Sebago populations would differ in their foraging activity.
Methods
Study system and juvenile rearing
We used two hatchery strains of Atlantic salmon, the Sebago Lake and LaHave River strains, that are the focus of restoration efforts in the Lake Ontario watershed. Hereafter, the two strains will be referred to as populations. Eggs were fertilized on 2 November 2016 for the Sebago population and 17 November 2016 for the LaHave population and then reared until the fry stage (age 0+; 10 January 2017 for the Sebago population and 13 February 2017 for the LaHave population) at the Ontario Ministry of Natural Resources and Forestry’s (OMNRF) Normandale Fish Culture Station (Normandale, ON). Once the fish reached the fry stage, they were transported to the OMNRF’s Codrington Research Facility (Fig. 1; Codrington, ON). Throughout the experiment, fish were separated by population. Fry was held by population in 73-L white polypropylene tanks (n = 30) until the parr stage (age 8+ months). For the current study, 72 juvenile salmon from each population were haphazardly selected, anesthetized in a bath of tricaine methanesulfonate (MS-222; 15 mg/L) buffered with sodium bicarbonate (15 mg/L), and divided haphazardly into groups of 4. To distinguish individual fish within each group, each fish within a group was then tagged with a subcutaneous injection of fluorescent visual implant elastomer tags (VIE; Northwest Marine Technologies, Shaw Island, WA) on the dorsal surface of the back of one of four colours. After tagging, fish were allowed to recover from the tagging procedure for a minimum of 3 weeks.
Design of field experiment
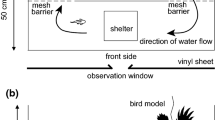
The experiment was conducted in East Duffins Creek, Ontario, Canada, between 1 August and 11 September 2017 (Fig. 1). The experimental setup included 6 enclosures arranged in pairs across three riffle sequences (Figs. 1 and 2a) which is the preferred habitat for juvenile Atlantic salmon (McCrimmon 1954). Each enclosure measured 1.5 m × 1 m × 0.75 m (length × width × height) and was constructed with panels of synthetic nylon net (4 mm stretched mesh size) stapled to 2.54 × 5.08 cm white pine strapping as the frame (Fig. 2). The mesh size was permeable enough to let through most drifting invertebrates and chemical cues, and caused minimal surface disturbance to allow for behavioural observations but small enough to prevent any large predators from entering. The panels were fastened together using 20-cm cable ties and secured to the streambed using 2.44-m steel alloy t-bars. Wires were stretched across the top of the enclosure to deter avian predators. Within each enclosure, the natural substrate was sieved to exclude particles >5 cm. Thus, each enclosure had a thin layer of natural substrate with a mixture of pebbles (<5 cm in diameter), gravel (0.2–1.0 cm), silt, and sand.
A modified Latin squares design was used to test how shelter availability (high vs. low) and population (Sebago Lake vs. LaHave River) affected juvenile foraging behaviour over a 6-day period (Table 1). The two blocking factors were week (n = 6) and riffle (n = 3) and allowed for each treatment and population to be tested in each enclosure. Thus, the number of experimental units was 36. The low shelter treatment had one added boulder placed on top of the substrate (Dolinsek et al. 2007; Bilhete and Grant 2016). The high shelter treatment had five added boulders. The added boulders, which were all ~20 cm in diameter and similar in shape, were collected from other parts of the stream within the vicinity of each riffle and were scrubbed clean with a stiff-bristled brush. At the end of each week, boulders were removed, scrubbed to remove algae and invertebrates, and placed back in the pens.
At the beginning of each week, each enclosure was stocked with four juvenile Atlantic salmon, a density used in previous net pen studies (Larranaga and Steingrímsson 2015; Bilhete and Grant 2016). Prior to stocking, the mass (± 0.1 g) and fork length (± 0.1 mm) of each juvenile salmon were measured. Fish were given 1 day to acclimate to the enclosures (see Larranaga and Steingrímsson 2015; Bilhete and Grant 2016). In the fourth sampling week of the study, from 23 August 2017 to 28 August 2017, a freshet led to a large log crashing into one of the enclosures in riffle 2 that contained fish from the LaHave River population in the high shelter treatment, opening a gap in the enclosure, and allowed all fish contained in the enclosure to escape. As such, this experimental unit (week 4, pen 2, riffle 2) was excluded from all analyses.
Behavioural observations
During each 6-day trial, observations of fish were conducted three times per day within 2-h blocks of time: dawn (from sunrise to 2 h after sunrise), midday (between 11:00 and 15:00), dusk (two 2 prior to sundown to sundown). Sunrise and sunset times were obtained daily from Environment Canada for Pickering, ON (https://weather.gc.ca). One observer stood motionless downstream from the enclosure for 5 min before beginning observations. A fish was considered inactive if it remained motionless in the substrate or under a boulder and active otherwise. A foraging attempt was defined as a movement of more than one body length to capture a potential prey item (Bilhete and Grant 2016) and was distinguished from agonistic behaviours (as described in Keenleyside and Yamamoto 1962) such as chases, charges, and nips in that a foraging attempt was directed at prey and not conspecifics. Foraging attempts are faster than a typical swimming movement and afterwards juvenile salmon typically return to their foraging station. For each active fish found, 10-min focal observations were done to record the number of foraging attempts and the time spent active. The number of fish foraging in the enclosure was also recorded. Observers noted that avian predators (Common mergansers, Mergus merganser; Belted kingfishers, Megaceryle alcyon) were present in the study area, but avian surveys were not conducted.
Supplementary nighttime observations (21:00–23:00) were added to the protocol during weeks 4–6. During the night sampling periods, observations were carried out with infrared cameras because light of shorter wavelengths can influence fish behaviour (Marchesan et al. 2005). Unusually high levels of precipitation throughout the first 3 weeks of the experiment resulted in higher-than-average discharge and high turbidity making nighttime observations difficult with the relatively low resolution of the infrared cameras. Because of the difficulty of observing the fish in the videos, we only recorded time active and the number of fish foraging during the night observation period.
Apart from the freshet that cause the elimination of an experimental unit, all fish used in the study were observed during their time in the enclosures. Water depth ranged from 20 to 40 cm in the net pens during the experiments and was clear enough to observe fish behaviour during each time period during daylight hours. Because of the ability of Atlantic salmon parr to hide in the cobble, a dip net could not be used to remove fish from the pen at the end of each week. To remove fish from the pen, the back panel of the pen was removed, and a weir was fixed to the opening. Using this method, attempts were made to recover all fish, but some escaped in the process. Recaptured salmon were euthanized in a bath containing buffered MS-222 and weighed (± 0.1 g). The digestive tract of each fish was collected and stored in 95% ethanol.
Stomach content analyses
To examine foraging, preserved guts were dissected, and stomach and intestine contents were removed by flushing into a 39-mL Bogorov tray using 80% ethanol. After flushing, items still stuck to the stomach lining were removed using a pointed probe. All stomach contents were then examined under a stereozoom microscope, identified to order using the key on page 8 in Peckarsky et al. (1990) and counted to determine invertebrate abundance in the diet of each individual (Grey 2001). Non-identifiable stomach contents were not counted. All stomach contents, identifiable and non-identifiable, were then filtered from the ethanol solution using a 15-mL Millipore vacuum filter (Fisher Scientific, Ottawa, ON) with a pre-weighed 2.4-cm Whatman glass microfiber filter paper (Fisher Scientific, Ottawa, ON). The gut contents were weighed (± 0.1 mg; wet weight) and then placed in a 60 °C oven (VWR International, Mississauga, ON) for 24 h. The dry gut contents were also weighed (± 0.1 mg).
Physical variables and invertebrate drift
Water depth (± 1 cm) and water temperature (± 0.1 °C) at each riffle were measured every 15 min with HOBO U20L loggers (Onset Inc., Bourne) and HOBO Pendant Temperature/Light 8k loggers (Onset Inc., Bourne). Current velocity (m·s−1) was measured at the start of each 2-h observation block with a Flowtracker (SonTek, San Diego). These physical variables were measured because temperature and current velocity are known to influence the foraging behaviour of Atlantic salmon (Wańkowski and Thorpe 1979; Metcalfe et al. 1998).
To determine invertebrate drift availability at the site, a drift net (30 cm × 40 cm mouth × 80 cm long, 250 μm mesh) with a collection bottle (5 cm × 7.5 cm) was installed 50 m upstream from the first riffle, far enough as to not reduce food availability in the first riffle. Invertebrate drift was defined as the passive downstream transport of invertebrates. The drift net was deployed three times daily at the same time as behavioural observations. The drift net was also deployed during nighttime observation in weeks 4–6. Bottle contents were collected and preserved in 200 mL of 95% ethanol. From each 200 mL sample, two 39 mL subsamples were randomly selected and placed in a 39-mL Bolgorov tray (Wildco, Yulee, FL). Invertebrates present in each subsample were counted under a stereozoom microscope and were identified to order using the key on page 8 found in Peckarsky et al. (1990).
Data analyses
All analyses were conducted in R 3.5.0 (R Core team 2016) using a significance threshold of α = 0.05. For each diel period, the number of foraging attempts (foraging rate) and time active were averaged among individuals in a pen and over the 6 days prior to further analysis to avoid pseudoreplication. Using data for all pens, all weeks, and the three daytime diel periods, the distribution of the number of foraging attempts was determined using the function fitdistr with the package MASS (Ripley 2018). Foraging rate data did not allow for a Gaussian, Poisson, or zero-inflated Poisson model. Thus, in order to analyze the foraging rate, the data were converted to binary data (0 or 1) where if a fish had not committed a foraging strike during a 15-min observation period it would receive a 0 and a 1 if it committed one or more foraging strikes. This variable will henceforth be known as the probability of foraging. The probability of foraging was then analyzed in a fully factorial logistic generalized linear mixed model using function glmer in package lme4 (Bates et al. 2018). This model was tested for the effects of treatment, diel period (dawn, midday, and dusk), and population, and included random intercepts for week (1–6), riffle (1–3), and subject, a number from 1 to 36 which represents each group of 4 fish contained within a pen and observed for 1 week (i.e. the experimental unit). Subject was included to deal with the repeated nature of the activity data across the three diel periods. Akaike Information Criterion (AIC) was used for model selection. Random effects were initially included in models but were removed if they did not explain any of the variance. To compare among diel periods, we used Tukey tests implemented using the glht function in package multcomp (Hothorn et al. 2008). In the final models, the exponentiated coefficients reflect the odds of foraging versus not foraging, and the logit−1-transformed coefficients reflect the probability of foraging.
To evaluate effects of shelter and population among both the daytime and nocturnal observation periods, we used the time fish were active and limited the analysis to the last 3 weeks of the study. The time fish were active was analyzed similarly to the foraging rate. Because the data did not allow for Gaussian, Poisson, or zero-inflated Poisson models, the data were converted to binary and then analyzed using a linear mixed model with the function lmer in the package lme4 (Bates et al. 2018) with the same model structure as the probability of foraging. This variable is henceforth known as the probability of activity.
Fish mass and length were analyzed using a general linear model with the fixed factors treatment and population, and the covariate week. The gut content dry mass was first subjected to a ln(x + 1) transformation and then analyzed using a generalized linear mixed model with a Gaussian distribution with the function lmer in the package lme4 (Bates et al. 2018) to test the effects of treatment, population, and the treatment by population interaction. The ANOVA() function in the package car (Fox et al. 2021) was used to generate analysis-of-variance tables for the models with likelihood-ratio chi-square statistics. Fish length before treatments was also included as a covariate and sampling week, riffle, and subject were included as random effects.
Specific growth rate for each individual fish was calculated using the methods of Ricker (1975) and measured as %·day−1. Specific growth rate values were then ln(x+1) transformed to normalize the data and then compared between treatments and populations using general linear mixed models with treatment, population, the population × treatment interaction, fish length before treatment as a covariate, and random intercepts for week, riffle, and subject. Linear regression analyses were used to test for a relationship between gut content mass and specific growth rate, and time spent active and specific growth rate.
Food availability was determined by measuring the drift rate of the invertebrates commonly consumed by juvenile Atlantic salmon (McCrimmon 1954). Drift rates were estimated using methods in O’Hop and Wallace (1983). The invertebrate drift rate was measured as number of individuals·m−3·h−1 and drift rates were calculated for all orders. Summary means were produced for each order during each time of day (dawn, dusk, midday, night) over the course of the experiment. Linear regression analysis was used to determine the relationship between activity level and food availability at the scale of time of day for each day throughout the experiment.
Summary means for water temperature and discharge data were produced for each time of day over the course of the experiment. Linear regression analysis was used to determine the relationship between time active and water temperature at the scale of time of day for each day throughout the experiment.
Results
Probability of foraging and activity
The distributions of foraging rate and time active were highly skewed and were dominated by values of 0 strikes·15 min−1 and 0 s, respectively (Figs. 3, 4 and 5; Online Resource 1). In the analysis of probability of foraging, the model with the lowest AIC included treatment and diel period and random intercepts for week. No other model was within ΔAIC of 2 of the selected model. The odds of foraging in the lower shelter treatment was significantly lower—by 77%—than in the high shelter treatment (Table 2). At the baseline diel period of dawn, the probability of foraging was 0.137 in the low shelter treatment and 0.414 in the high shelter treatment. The probability of foraging also depended on time of day, with foraging occurring most frequently at midday (Table 2; Fig. 6). Tukey tests indicated a significant difference in the odds of foraging between midday and dawn (4.9 times higher at midday; z = 2.52, p 0.031), a nearly significant difference between midday and dusk (4.1 times higher at midday; z = 2.27, p = 0.061), and no difference between dusk and dawn (0.2 times higher at dusk; z = 0.300, p = 0.952). The random effect of week explained some of the variation in the model of probability of foraging, as fish were more active in the earlier weeks of the study (variance = 2.61, SD = 1.62; Fig. 4c; Table 2). We repeated these analyses using the probability of activity as a response variable, and all the conclusions held (results not shown).
The foraging rate (strikes·15 min−1) of Atlantic salmon from two populations during 6-day treatments in either high (light grey) or low shelter (dark grey) enclosures. a The foraging rate of fish from the LaHave River and Sebago Lake populations in either the high and low shelter treatments. b The foraging rate of fish in high and low shelter treatments at each diel period. c The foraging rate of fish in high and low shelter treatment over each week. Data shown are the weekly and pen averages. Boxplots show the median, the first and third quartiles, data within the interquartile ranges, and outliers
The time active (s) of Atlantic salmon from two populations from either high (light grey) or low (dark grey) shelter treatments at each diel period during 6-day treatments for the last 3 weeks of observations (22 August–11 September 2017). Boxplots show the median, the first and third quartiles, data within the interquartile range, and outliers. Data shown are the weekly and pen averages
In the analysis of probability of activity during the last 3 weeks of the study, the model with the lowest AIC included diel period and random intercepts for week (Table 3). A model including diel period and treatment and random intercepts for week was within ΔAIC of 2 of the selected model (Table 3). In the top-ranked model, activity differed among the diel periods, but the effect was weaker than in the analysis of probability of foraging (χ2 = 7.4, df = 3, p = 0.061; Fig. 7). The probability of activity was highest at night, and based on Tukey tests, there was a nearly significant difference in the odds of activity between night and dusk (8.1 times higher at night; z = 2.45, p 0.069). The week random effect explained some of the variation in the model (variance = 0.48, SD = 0.69; Table 3). In the second-ranked model with treatment added, the conclusions regarding the effect of diel period were similar (Table 3). However, unlike in the analysis of probability of foraging, there was no significant difference in the odds of activity between the high and low treatment (Table 3).
The modelled effect of diel period on the probability of activity of juvenile Atlantic salmon at the baseline treatment of high shelter in East Duffins Creek during the 6-day observation periods during the last 3 weeks of observations (22 August–11 September 2017). Error bars denote the 95% confidence interval for each estimate
Comparisons of body size
At the start of trials, Sebago Lake fish were longer (mean ± SD; 7.6 ± 0.9 cm) than LaHave River fish (6.6 ± 0.7 cm, t= 8.2, df = 139, p < 0.01). Sebago Lake fish were also heavier (3.9 ± 1.4 g) than LaHave River fish (2.3 ± 0.8 g, t = 9.4, df = 139, p < 0.01). There was no difference in the length (t = 0.76, df = 139, p = 0.45) or mass (t = 0.68, df = 139, p = 0.49) of fish used in high versus low shelter treatments. Fish length (t = 6.42, df = 139, p < 0.01) and mass (t = 5.64, df = 139, p < 0.01) increased with each week.
Gut content mass and specific growth rate
Juvenile salmon successfully foraged for invertebrates while in the pens. There was no difference in the dry mass of gut contents between populations (mean ± SD, Sebago Lake: 12.23 ± 6.86 mg, LaHave River: 9.21 ± 5.79 mg, χ2 = 0.008, df = 1, p = 0.92) and treatments (high shelter: 11.37 ± 7.98 mg, low shelter: 10.35 ± 4.77 mg, χ2 = 0.05, df = 1, p = 0.82) nor was there an interaction between treatment and population (χ2 = 0.52, df = 1, p = 0.47). Fish length was a predictor of gut content mass (χ2 = 54.63, df = 1, p < 0.001) and was retained in the model. Larger fish had heavier gut contents than did smaller fish. The week, riffle, and pen random effects did not account any of the variance and were dropped from the models.
Fish grew while in the pens, but there was no difference in specific growth rate between treatments (mean ± SD; high shelter: 0.660 ± 1.40%·day−1, low shelter: 0.877 ± 1.07%·day−1, χ2 = 0.75, df = 1, p = 0.39) or populations (mean ± SD; Sebago Lake: 0.462 ± 0.797%·day−1, LaHave River: 1.13 ± 1.54%·day−1, χ2 = 0.13, df = 1, p = 0.72). Furthermore, the population by treatment interaction (χ2 = 1.44, df = 1, p = 0.23) was not a predictor of specific growth rate. Fish length was retained as a covariate in the model (χ2 = 11.88, df = 1, p < 0.001) as fish that were large had a smaller specific growth rate. The week random effect explained some of the variance (variance = 0.18, SD = 0.34) but riffle and pen did not and were dropped from the final model. Neither gut content dry mass (t = −0.49, df = 89, p = 0.49, r2 = 0.006) nor time active (t = 1.86, df = 34, p = 0.07) was a predictor of specific growth rate at the individual level.
Invertebrate drift
The drift rates of all orders of invertebrates are found in Online Resource 2. The availability of the three orders of invertebrates consumed the most by juvenile Atlantic salmon over the course of this experiment (Diptera, Ephemeroptera, and Trichoptera) did not appear to differ much between diel periods (Fig. 8a–c). For the total drift rate of all invertebrate orders captured in the drift net, drifting invertebrate abundance did not appear to differ much between diel periods (Fig. 8d). There was no association between the time active and the total invertebrate drift (r2 < 0.01, t = 1.3, df = 96, p = 0.17).
Drifting a Diptera (larvae, pupae, and adults), b Ephemeroptera (larvae, pupae), c Trichoptera (larvae), and d total invertebrate drift catch during each diel period and day in East Duffins Creek, ON from 1 August to 11 September 2017. Boxplots show the median, first and third quartiles, interquartile range, and outliers according to the 1.5 IQR. Data presented are for the last 3 weeks of sampling. Night sampling for invertebrates began in week 3 (11 August 2017)
Environmental factors
The environmental factors varied throughout the course of the experiment (Fig. 7). Average water temperature across all days was 16.6 °C (± SD = 2.2; range= 10.7–22.9 °C). Average water temperature was the lowest during dawn (5:00–9:00, 14.9 ± 1.8 °C) but was similar during midday (9:00–18:00, 17.3 ± 2.2 °C), dusk (18:00–21:30, 17.6 ± 2.1 °C), and night (21:30–5:00, 17.0 ± 2.1 °C; Fig. 9a). The average stream discharge across all days was 0.72 m3·s−1 (± 0.15; range= 0.53–1.3 m3·s−1; Fig. 9b). There was an association between time active and water temperature (r2 = 0.08, t = 3.86, df = 96, p = 0.001) at the level of time of day for each day throughout the experiment.
Discussion
Across the entire study, shelter availability had a significant influence on the probability of foraging of juvenile Atlantic salmon in field enclosures, but only when analyzing the daytime observations. During midday and crepuscular periods, fish in the high shelter treatment were more likely to be foraging than those in the low shelter treatment. In general, fish were more likely to forage at midday than at dawn and dusk. In the final 3 weeks of the experiment, we were able to determine that fish are also quite active at night. However, there was no effect of shelter availability on the probability of activity in the last 3 weeks, perhaps because fish were generally less active in the latter half of the study. Overall, these results do not support our predictions that fish will forage more during the day prioritizing food acquisition when in an environment with low shelter availability. These results disagree with those found in previous studies that observed an increase in daytime foraging rate of Arctic char and Atlantic salmon in low shelter treatments (Orpwood et al. 2003; Larranaga and Steingrímsson 2015). Despite this, our results support the role shelter availability plays in mediating daily foraging activity.
A reduction in foraging activity has been proposed to reduce the risk of detection by predators when in a high predation risk environment (Maiorana 1976; Fraser and Huntingford 1986; Lima and Dill 1990). The Atlantic salmon in our low shelter environments may have had a reduced probability of foraging to reduce their probability of detection in an environment with a high-perceived predation rate. Indeed, reductions in foraging have been observed in several taxa in response to an increase in perceived predation risk (Metcalfe et al. 1987; Dalton and Flecker 2014; Zanette et al. 2014). The juvenile Atlantic salmon in both treatment groups may also have avoided foraging during dawn due to higher perceived predation risk, because piscivorous birds such as double crested cormorants (Phalacrocorax auritus; Anderson et al. 2004), bald eagles (Haliaeetus leucocophalus; Watson et al. 1991), and mergansers (Mergus spp.; Sjöberg 1985) are known to forage at the greatest intensity at dawn, and belted kingfishers (Megaceryle alcyon) were observed foraging at dawn, at times within 50 m of pens at all three riffles, during this study (Therrien, C. pers. obs.). Shelter can provide a reduction in the perceived risk of predation resulting in greater risk-taking by organisms (Lima and Dill 1990)—in our case foraging during the day. Furthermore, large spawning adult Chinook salmon (Oncorhynchus tshawytscha) began to run considerably throughout the last 2 weeks of the experiment, and it is possible that juvenile Atlantic perceived them as predators. Future research should pair avian surveys with fish observations to better understand fish-predator interactions.
Animals often exhibit modal patterns in foraging, foraging intensely at certain times while decreasing activity at other times. Here, foraging was most intense during midday and night. Thus, fish in our study showed a bimodal distribution of activity. A bimodal distribution of foraging activity is common in fish (Clark and Levy 1988; Fraser et al. 1995; Fraser and Metcalfe 1997) and is thought to result from maximizing foraging efficiency while decreasing predation risk (Fraser et al. 1993, 1995; Fraser and Metcalfe 1997). The first mode typically corresponds to the midday period, the diel period with the highest light intensity. Indeed, Atlantic salmon have the highest foraging efficiency during this period and preferentially forage during this time due to the ease of locating and intercepting drifting prey items (Clark and Levy 1988; Fraser and Metcalfe 1997). The other mode in the bimodal distribution corresponded to the night diel period. Despite having the lowest foraging efficiency at night, nocturnal foraging by Atlantic salmon has been documented and is thought to be the time when Atlantic salmon experience the lowest predation risk (Fraser et al. 1993, 1995; Fraser and Metcalfe 1997). The raw data also hints at less intense bimodal foraging under low shelter availability, but we could not detect any statistical evidence that the effect of shelter treatment depends on the diel period.
Temperature may also play a role in explaining diel foraging patterns. For example, low rates of foraging at dawn could have been driven by water temperatures, which were always the lowest during the dawn period. Moreover, we found that fish were less active in cooler water temperatures. Previous work shows that Atlantic salmon at low late summer/early autumn temperatures feed nocturnally (Fraser et al. 1993, 1995) and decrease their food consumption with decreasing temperature (Fraser et al. 1993; Nicieza and Metcalfe 1997; Fraser and Metcalfe 1997; Railsback and Rose 1999; Gillooly et al. 2001). Changes in temperature also may explain the trend of decreased foraging activity over the course of the experiment, as water temperature decreased as the experiment progressed. Moreover, food availability does not appear to be driving this trend towards nighttime foraging as invertebrate drift did not appear to differ between diel periods and there was no relationship between the rate of drifting invertebrates and the activity level of juvenile Atlantic salmon. Temperature and not food availability thus appeared to play a large role in mediating diel activity patterns of juvenile Atlantic salmon in this study.
We found no difference in the gut content mass or specific growth rate between treatments, despite fish in the high shelter treatments having a higher probability of foraging than those in the low shelter treatment. Previous studies in a number of taxa found a depression of foraging in environments with a high-perceived predation risk that led to differences in growth and food consumption (Metcalfe et al. 1987, Thaler et al. 2012, 2013, Dalton and Flecker 2014, Zanette et al. 2014). The lack of observable differences in gut content mass may be the result of compensatory feeding of fish during the night. Night is the longest time period, ranging from dusk until dawn, and averaged 9 h over the course of our experiment. Furthermore, fish generally showed high activity during the nighttime observations. Previous studies have documented extensive nocturnal foraging in juvenile Atlantic salmon and have proposed it as a mechanism to reduce predation risk (e.g. Fraser et al. 1993). The Atlantic salmon in our low shelter treatment could have been taking advantage of lower predation risk at night to perform compensatory feeding. Additionally, some studies have shown differences in growth rate between individuals in environments differing in predation risk may not arise until late in life in the form of changes in body composition, development, and physiology (Steiner and Buskirk 2009; Thaler et al. 2012). Finally, the 6-day treatment length may have been too short to detect differences in growth rate. Other studies exposing fish to treatments with different levels of shelters for similar lengths of time (Larranaga and Steingrímsson 2015; 9 days, Bilhete and Grant 2016; 7 days) also failed to find differences in specific growth rates between treatments.
Different populations of animals may exhibit differences in anti-predator behaviours as a result of local adaptations to the predation regime they experience (Åbjörnsson et al. 2004). Despite the potential for different predation regimes in their native environments (Bowlby et al. 2013; Pellerin and Pierce 2015), there was no difference in the probability of foraging, the probability of activity, both during the day and overall, total gut mass, or specific growth rate between the fish originally from Sebago Lake and LaHave River populations. These results do not support the hypothesis that the two populations of Atlantic salmon differ in their foraging activity as a result of evolved differences in predator avoidance behaviour, but are consistent with the results of a previous study comparing anti-predator behaviour in these two strains in a captive setting (Lau 2016). Lau (2016) speculated that the lack of observable differences in foraging behaviour between the two strains was a result of their captive breeding history. The LaHave River and Sebago Lake populations of Atlantic salmon have spent 8 and 3 generations, respectively, in captivity (OMNR 2011) which could have relaxed selection leading to the loss of any genetic difference in anti-predator behaviour. Álvarez and Nicieza (2003) found brown trout (Salmo trutta) after two generations in a captive breeding program were insensitive to predation risk and differed in their diel pattern of activity compared to wild conspecifics. Houde et al. (2010) found juvenile Atlantic salmon response to an avian predator cue decreased as hatchery ancestry increased and de Mestral and Herbinger (2013) found second-generation captive bred Atlantic salmon displayed more risk-taking behaviour than first-generation when in the presence of an avian predator model. Comparatively, Lau (2016) found that progeny of a LaHave River salmon crossed with a Mersey River salmon, a strain with no history of captivity, exhibited more intense anti-predator behaviours than did fish from the LaHave River alone. The multiple generations that both populations have spent in the hatchery could have eliminated any differences in anti-predator behaviours these two populations may display in the wild and contribute to the lack of differences in foraging behaviour observed. Although the relaxed selection hypothesis is intriguing, it may be that Sebago Lake and LaHave River populations simply do not have evolved differences in juvenile anti-predator behaviour.
Increasing available shelter and refugia for fish is often a focal point of restoration programs (Finstad et al. 2007). The Lake Ontario Atlantic Salmon Restoration Project is no different and features shelter restoration as one of the project’s focuses (Lake Ontario Atlantic Salmon Restoration Program 2019). In our study, increased shelter availability increased the foraging intensity and activity level, which we interpret as beneficial for growth of Atlantic salmon. Past literature has shown that increasing habitat complexity and shelter availability increases the survival and growth of fish, particularly Atlantic salmon (Finstad et al. 2007) and McCrimmon (1954) determined a lack of shelter to be one of the factors hindering the restoration success of planted Atlantic salmon in Duffins Creek. As such, increasing shelter availability should continue to be a key component of stream restorations and managers should continue to improve available habitat for stream fishes.
Availability of data and material
Raw data and r script are included as supplementary information.
References
Åbjörnsson K, Hansson L-A, Brönmark C (2004) Responses of prey from habitats with different predator regimes: local adaptation and heritability. Ecology 85:1859–1866. https://doi.org/10.1890/03-0074
Allan JD, Flecker AS (1993) Biodiversity conservation in running waters. BioSci 43:32–43. https://doi.org/10.2307/1312104
Allen KR (1941) Studies on the biology of the early stages of the salmon (Salmo salar). J Anim Ecol 10:47–76. https://doi.org/10.2307/1342
Álvarez D, Nicieza AG (2003) Predator avoidance behaviour in wild and hatchery-reared brown trout: the role of experience and domestication. J Fish Biol 63:1565–1577. https://doi.org/10.1111/j.1095-8649.2003.00267.x
Anderson CD, Roby DD, Collis K (2004) Foraging patterns of male and female double-crested Cormorants nesting in the Columbia River estuary. Can J Zool 82:541–554. https://doi.org/10.1139/z04-019
Bates D, Maechler M, Bolker B (2018) Linear mixed-effects models using “Eigen” and S4. https://github.com/lme4/lme4. Accessed 25 Aug 2019
Bell AM (2005) Behavioural differences between individuals and two populations of stickleback (Gasterosteus aculeatus). J Evol Biol 18:464–473. https://doi.org/10.1111/j.1420-9101.2004.00817.x
Bilhete C, Grant JWA (2016) Short-term costs and benefits of habitat complexity for a territorial fish. Ethology 122:151–157. https://doi.org/10.1111/eth.12456
Bowlby HD, Gibson AJF, Levy A (2013) Recovery potential assessment for southern upland Atlantic salmon: status, past and present abundance, life history and trends. DFO Can. Sci. Advis. Sec. Doc. 2013/005
Breau C, Weir LK, Grant JW (2007) Individual variability in activity patterns of juvenile Atlantic salmon (Salmo salar) in Catamaran Brook, New Brunswick. Can J Fish Aquat Sci 64:486–494. https://doi.org/10.1139/f07-026
Brown JS, Kotler BP (2004) Hazardous duty pay and the foraging cost of predation. Ecol Lett 7:999–1014. https://doi.org/10.1111/j.1461-0248.2004.00661.x
Clark CW, Levy DA (1988) Diel vertical migrations by juvenile sockeye salmon and the antipredation window. Am Nat 131:271–290
R Core team (2016) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria http://www.r-project.org/. Accessed 1 Jan 2016
Crawford SS (2001) Salmonine introductions to the Laurentian Great Lakes: an historical review and evaluation of ecological effects. Can Spec Pub Fish Aqua Sci 132:205
Creel S, Winnie JA, Christianson D, Liley S (2008) Time and space in general models of antipredator response: tests with wolves and elk. Anim Behav 76:1139–1146. https://doi.org/10.1016/j.anbehav.2008.07.006
Dalton CM, Flecker AS (2014) Metabolic stoichiometry and the ecology of fear in Trinidadian guppies: consequences for life histories and stream ecosystems. Oecologia 176:691–701. https://doi.org/10.1007/s00442-014-3084-6
de Mestral GL, Herbinger MC (2013) Reduction in antipredator response detected between first and second generations of endangered juvenile Atlantic salmon Salmo salar in a captive breeding and rearing programme. J Fish Biol 83:1268–1286. https://doi.org/10.1111/jfb.12221
Dolinsek IJ, Grant JWA, Biron PM (2007) The effect of habitat heterogeneity on the population density of juvenile Atlantic salmon Salmo salar L. J Fish Biol 70:206–214. https://doi.org/10.1111/j.1095-8649.2006.01296.x
Finstad AG, Einum S, Forseth T, Ugedal O (2007) Shelter availability affects behaviour, size-dependent and mean growth of juvenile Atlantic salmon. Freshw Biol 52:1710–1718. https://doi.org/10.1111/j.1365-2427.2007.01799.x
Fox J, Weisberg S, Adler D (2021) Package “car.” Version 3.0-11. https://cran.r-project.org/web/packages/car/index.html. Accessed 5 Nov 2020
Fraser D, Huntingford FA (1986) Feeding and avoiding predation hazard: the behavioral response of the prey. Ethology 73:56–68. https://doi.org/10.1111/j.1439-0310.1986.tb00999.x
Fraser NHC, Metcalfe NB (1997) The costs of becoming nocturnal: feeding efficiency in relation to light intensity in juvenile Atlantic salmon. Funct Ecol 11:385–391. https://doi.org/10.1046/j.1365-2435.1997.00098.x
Fraser NHC, Metcalfe NB, Thorpe JE (1993) Temperature-dependent switch between diurnal and nocturnal foraging in salmon. Proc R Soc Lond B 252:135–139. https://doi.org/10.1098/rspb.1993.0057
Fraser NHC, Metcalfe NB, Heggenes J, Thorpe JE (1995) Low summer temperatures cause juvenile Atlantic salmon to become nocturnal. Can J Zool 73:446–451. https://doi.org/10.1139/z95-051
Gillooly JF, Brown JH, West GB et al (2001) Effects of size and temperature on metabolic rate. Science 293:2248–2251. https://doi.org/10.1126/science.1061967
Gotceitas V, Godin J-GJ (1993) Effects of aerial and in-stream threat of predation on foraging by juvenile Atlantic salmon (Salmo salar). In: Gibson RJ, Cutting RE (eds) Production of juvenile Atlantic salmon, Salmo salar, in natural waters. pp 35–41
Gotceitas V, Godin J-GJ (1991) Foraging under the risk of predation in juvenile Atlantic salmon (Salmo salar L.): effects of social status and hunger. Behav Ecol Sociobiol 29:255–261. https://doi.org/10.1007/BF00163982
Grey J (2001) Ontogeny and dietary specialization in brown trout (Salmo trutta L.) from Loch Ness, Scotland, examined using stable isotopes of carbon and nitrogen. Ecol Freshw Fish 10:168–176. https://doi.org/10.1034/j.1600-0633.2001.100306.x
Gries G, Whalen KG, Juanes F, Parrish DL (1997) Nocturnal activity of juvenile Atlantic salmon (Salmo salar) in late summer: evidence of diel activity partitioning. Can J Fish Aquat Sci 54:1408–1413. https://doi.org/10.1139/f97-046
Helfman GS (1986) Behavioural responses of prey fishes during predator-prey interactions. In: Felder ME, Lander GV (eds) Predator-prey relationships: perspectives and approaches from the study of lower vertebrates. University of Chicago Press, Chicago, pp 135–156
Höjesjö J, Johnsson J, Bohlin T (2004) Habitat complexity reduces the growth of aggressive and dominant brown trout (Salmo trutta) relative to subordinates. Behav Ecol Sociobiol 56:286–289. https://doi.org/10.1007/s00265-004-0784-7
Hothorn T, Bretz F, Westfall P (2008) Simultaneous inference in general parametric models. Biom J 50:346–363
Houde ALS, Fraser DJ, Hutchings JA (2010) Reduced anti-predator responses in multi-generational hybrids of farmed and wild Atlantic salmon (Salmo salar L.). Conserv Genet 11:785–794. https://doi.org/10.1007/s10592-009-9892-2
Houston AI, McNamara JM (1993) A theoretical investigation of the fat reserves and mortality levels of small birds in winter. Ornis Scand 24:205–219. https://doi.org/10.2307/3676736
Keeley ER, Grant J (1997) Allometry of diet selectivity in juvenile Atlantic salmon (Salmo salar). Can J Fish Aquat Sci 54:1894–1902. https://doi.org/10.1139/f97-096
Keenleyside MHA, Yamamoto FT (1962) Territorial behaviour of juvenile Atlantic salmon (Salmo salar L.). Behaviour 19:139–169
King TL, Kalinowski ST, Schill WB et al (2001) Population structure of Atlantic salmon (Salmo salar L.): a range-wide perspective from microsatellite DNA variation. Mol Ecol 10:807–821. https://doi.org/10.1046/j.1365-294X.2001.01231.x
Larranaga N, Steingrímsson SÓ (2015) Shelter availability alters diel activity and space use in a stream fish. Behav Ecol 26:578–586. https://doi.org/10.1093/beheco/aru234
Lau M (2016) Innate and learned predator recognition in four strains of captive-bred Atlantic salmon, Salmo salar. Dissertation, University of Western Ontario
Lima SL (1986) Predation risk and unpredictable feeding conditions: determinants of body mass in birds. Ecol 67:377–385. https://doi.org/10.2307/1938580
Lima SL (1987) Vigilance while feeding and its relation to the risk of predation. J Theor Biol 124:303–316
Lima SL, Dill LM (1990) Behavioral decisions made under the risk of predation: a review and prospectus. Can J Zool 68:619–640. https://doi.org/10.1139/z90-092
[LOASRP] Lake Ontario Atlantic Salmon Restoration Program (2019) Bring back the salmon - 2019 newsletter. http://www.bringbackthesalmon.ca/wpcontent/uploads/2019/07/Newsletter-summer-2019.pdf. Accessed 13 Nov 2020
MacLeod R, Lind J, Clark J, Cresswell W (2007) Mass regulation in response to predation risk can indicate population declines. Ecol Lett 10:945–955. https://doi.org/10.1111/j.1461-0248.2007.01088.x
Maiorana VC (1976) Predation, submergent behavior, and tropical diversity. Evol Theory 1:157–177
Marchesan M, Spoto M, Verginella L, Ferrero EA (2005) Behavioural effects of artificial light on fish species of commercial interest. Fish Res 73:171–185. https://doi.org/10.1016/j.fishres.2004.12.009
Mathis A, Chivers DP, Smith RJF (1993) Population differences in responses of fathead minnows (Pimephales promelas) to visual and chemical stimuli from predators. Ethology 93:31–40. https://doi.org/10.1111/j.1439-0310.1993.tb00976.x
McCrimmon HR (1954) Stream studies on planted Atlantic salmon. J Fish Res Board Can 11:362–403
McNamara JM, Houston A (1987) Starvation and predation as factors limiting population-size. Ecology 68:1515–1519
McNamara JM, Houston AI, Lima SL (1994) Foraging routines of small birds in winter: a theoretical investigation. J Avian Biol 25:287–302. https://doi.org/10.2307/3677276
McNamara JM, Barta Z, Houston AI, Race P (2005) A theoretical investigation of the effect of predators on foraging behaviour and energy reserves. Proc R Soc London B 272:929–934. https://doi.org/10.1098/rspb.2004.3037
Metcalfe NB, Huntingford FA, Thorpe JE (1987) The influence of predation risk on the feeding motivation and foraging strategy of juvenile Atlantic salmon. Anim Behav 35:901–911. https://doi.org/10.1016/S0003-3472(87)80125-2
Metcalfe NB, Fraser NHC, Burns MD (1998) State–dependent shifts between nocturnal and diurnal activity in salmon. Proc R Soc London B 265:1503–1507. https://doi.org/10.1098/rspb.1998.0464
Metcalfe NB, Fraser NHC, Burns MD (1999) Food availability and the nocturnal vs. diurnal foraging trade-off in juvenile salmon. J Anim Ecol 68:371–381. https://doi.org/10.1046/j.1365-2656.1999.00289.x
Mikheev VN, Metcalfe NB, Huntingford FA, Thorpe JE (1994) Size-related differences in behaviour and spatial distribution of juvenile Atlantic salmon in a novel environment. J Fish Biol 45:379–386. https://doi.org/10.1111/j.1095-8649.1994.tb01320.x
Miller RR, Williams JD, Williams JE (1989) Extinctions of north American fishes during the past Century. Fisheries 14:22–38. https://doi.org/10.1577/1548
Millidine KJ, Armstrong JD, Metcalfe NB (2006) Presence of shelter reduces maintenance metabolism of juvenile salmon. Funct Ecol 20:839–845. https://doi.org/10.1111/j.1365-2435.2006.01166.x
Nehlsen W, Williams JE, Lichatowich JA (1991) Pacific salmon at the crossroads: stocks at risk from California, Oregon, Idaho, and Washington. Fisheries 16:4–21
Nicieza AG, Metcalfe NB (1997) Growth compensation in juvenile Atlantic salmon: responses to depressed temperature and food availability. Ecology 78:2385–2400
O’Hop J, Wallace JB (1983) Invertebrate drift, discharge, and sediment relations in a southern Appalachian headwater stream. Hydrob 98:71–84. https://doi.org/10.1007/BF00019252
[OMNR] Ontario Ministry of Natural Resources (2011) Stocks catalogue. Fish and Wildlife Branch, Ontario Ministry of Natural Resources, Peterborough, Ontario
Orpwood JE, Griffiths SW, Armstrong JD (2003) Effects of body size on sympatric shelter use in over-wintering juvenile salmonids. J Fish Biol 63:166–173. https://doi.org/10.1111/j.1095-8649.2003.00206.x
Orpwood JE, Griffiths SW, Armstrong JD (2006) Effects of food availability on temporal activity patterns and growth of Atlantic salmon. J Anim Ecol 75:677–685. https://doi.org/10.1111/j.1365-2656.2006.01088.x
Peckarsky BL, Fraissinet PR, Penton MA, Conklin DJ Jr (1990) Freshwater macroinvertebrates of northeastern North America. Cornell University Press
Pellerin J, Pierce Jr. UD (2015) The Crooked River: characteristics, history, and fisheries management. Maine Department of Inland Fisheries and Wildlife
Persson L, Andersson J, Wahlstrom E, Eklov P (1996) Size-specific interactions in lake systems: predator gape limitation and prey growth rate and mortality. Ecology 77:900–911. https://doi.org/10.2307/2265510
Railsback SF, Rose KA (1999) Bioenergetics modeling of stream trout growth: temperature and food consumption effects. Trans Am Fish Soc 128:241–256
Reebs SG (2002) Plasticity of diel and circadian activity rhythms in fishes. Rev Fish Biol Fish 12:349–371. https://doi.org/10.1023/A:1025371804611
Ricker WE (1975) Computation and interpretation of biological statistics of fish populations. Bull Fish Res Board Can 191. http://www.dfo-mpo.gc.ca/Library/1485.pdf
Ripley B (2018) Support functions and datasets for Venables and Ripley’s MASS. http://www.stats.ox.ac.uk/pub/MASS4. Accessed 25 Aug 2019
Sih A (1980) Optimal behavior: can foragers balance two conflicting demands. Science 210:1041–1043
Sih A, McCarthy TM (2002) Prey responses to pulses of risk and safety: testing the risk allocation hypothesis. Anim Behav 63:437–443. https://doi.org/10.1006/anbe.2001.1921
Sjöberg K (1985) Foraging activity patterns in the goosander (Mergus merganser) and the red-breasted merganser (M. serrator) in relation to patterns of activity in their major prey species. Oecologia 67:35–39. https://doi.org/10.1007/BF00378448
Sogard SM (1997) Size-selective mortality in the juvenile stage of teleost fishes: a review. Bull Mar Sci 60:1129–1157
Steiner UK, Buskirk JV (2009) Predator-induced changes in metabolism cannot explain the growth/predation risk tradeoff. PLOS ONE 4:e6160
Stewart TJ, Johnson T (2014) Lake effects on survival of Lake Ontario Atlantic salmon. Ontario Ministry of Natural Resources and Forestry, Alliston, Ontario
Suttle KB, Power ME, Levine JM, McNeely C (2004) How fine sediment in riverbeds impairs growth and survival of juvenile salmonids. Ecol App 14:969–974. https://doi.org/10.1890/03-5190
Thaler JS, McArt SH, Kaplan I (2012) Compensatory mechanisms for ameliorating the fundamental trade-off between predator avoidance and foraging. PNAS 109:12075–12080. https://doi.org/10.1073/pnas.1208070109
Thaler JS, Contreras H, Davidowitz G (2013) Effects of predation risk and plant resistance on Manduca sexta caterpillar feeding behaviour and physiology. Ecol Entomol 39:210–216. https://doi.org/10.1111/een.12086
Tupper M, Boutilier RG (1997) Effects of habitat on settlement, growth, predation risk and survival of a temperate reef fish. Mar Ecol Prog Ser 151:225–236
Utne ACW, Aksnes DL, Giske J (1993) Food, predation risk and shelter: an experimental study on the distribution of adult two-spotted goby Gobiusculus flavescens (Fabricius). J Exp Mar Biol Ecol 166:203–216. https://doi.org/10.1016/0022-0981(93)90219-E
Walters BT, Cheng TNN, Doyle J et al (2017) Too important to tamper with: predation risk affects body mass and escape behaviour but not escape ability. Funct Ecol 31:1405–1417. https://doi.org/10.1111/1365-2435.12851
Wańkowski JWJ, Thorpe JE (1979) Spatial distribution and feeding in Atlantic salmon, Salmo salar L. juveniles. J Fish Biol 14:239–247. https://doi.org/10.1111/j.1095-8649.1979.tb03515.x
Watson JW, Garrett MG, Anthony RG (1991) Foraging ecology of bald eagles in the Columbia River Estuary. J Wild Manage 55:492–499. https://doi.org/10.2307/3808981
White HC (1937) Local feeding of kingfishers and mergansers. J Biol Board Can 3:323–338. https://doi.org/10.1139/f37-016
White HC (1938) The feeding of kingfishers: food of nestlings and effect of water height. J Fish Res Bd Can 4:48–52. https://doi.org/10.1139/f38-004
Zanette LY, Clinchy M, Suraci JP (2014) Diagnosing predation risk effects on demography: can measuring physiology provide the means? Oecologia 176:637–651. https://doi.org/10.1007/s00442-014-3057-9
Acknowledgements
Support for use of study site was provided by the Toronto Region Conservation Authority under authority of a Permission to Enter permit (CFN 77069.10). The movement of Atlantic salmon into Duffins Creek was approved by OMNRF Aquatic Research and Monitoring Section under License to Collect Fish for Scientific Research Purposes (#1087209). Special thanks to Simon Bonner and Liana Zanette for their advice on the experiment design, Jake Ruegg and Bill Sloan for their help with fish husbandry, and Nigel Davies for help with data collection.
Funding
This study was funded by NSERC Discovery Grants to YM and BN, an NSERC Strategic Partnership Grant for Projects to BN, and a Canada Foundation for Innovation – Leaders Opportunity fund to YM. This research was also supported by the Ontario government (Queen Elizabeth II Graduate Scholarship to CT). Support for the rearing and housing of juvenile Atlantic salmon was provided by the Ontario Ministry of Natural Resources and Forestry (OMNRF) Fish Culture Section as well as the Codrington and Normandale Fish Culture Stations.
Author information
Authors and Affiliations
Contributions
C.T., Y.M., and B.N. all helped conceive the original idea and methodology. C.T. collected the data, conducted data processing, and drafted the manuscript. C.T. and Y.M. contributed to data analysis. All authors discussed the results and contributed to the final manuscript. Supervision was provided by Y.M. and B.N.
Corresponding author
Ethics declarations
Ethics approval
This study was approved by the Animal Ethics Committee of the University of Western Ontario (Protocol Number: 2010–214) and the OMNRF Aquatic Research and Monitoring Section Animal Care Committee (AUP ACC #151).
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Therrien, C.A., Morbey, Y.E. & Neff, B.D. Hide or seek: presence of stream shelter influences the foraging behaviour of juvenile Atlantic salmon (Salmo salar, Linnaeus 1758). Environ Biol Fish 105, 1–18 (2022). https://doi.org/10.1007/s10641-021-01176-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10641-021-01176-7