Abstract
Cultured organisms undergo genetically-based behavioural changes that may reduce their ability to survive in the wild. This has raised concerns that interbreeding between escaped cultured and wild organisms will generate hybrids exhibiting maladaptive behaviours which may ultimately reduce the fitness of the wild counterpart. We compared anti-predator responses in Atlantic salmon (Salmo salar) from two wild North American populations, the major farmed strain used in regional aquaculture, and their wild-farmed hybrids (F1, F2, and wild backcross). Anti-predator responses of fry (age 0+ parr) were measured under common environmental conditions, using a model of a natural predator (belted kingfisher, Ceryle alcyon). Farmed fry exhibited significantly reduced anti-predator responses relative to fry from both wild populations. The anti-predator responses of wild-farmed hybrid fry were intermediate to those of the parental populations (pure farmed or wild). The magnitude by which wild-farmed hybrids differed in anti-predator responses from pure wild fish also depended on the wild population. These results suggest that: (1) the observed behavioural differences have a genetic basis; (2) wild-farmed hybrids have, on average, reduced anti-predator responses relative to wild fish; and that (3) the effects of wild-farmed interbreeding on anti-predator responses will differ between wild populations. Our study is consistent with the general hypothesis that continual farmed-wild interbreeding may have detrimental effects on the fitness of wild organisms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Raising organisms in artificial environments (e.g. captivity) over several generations is believed to cause genetically-based behavioural changes that deviate from those expressed in the natural environment (Price 1997; Frankham 2008). Such behavioural changes occur when the natural and artificial environments differ in their selective pressures of certain behaviours (e.g. increased tameness towards humans or domestication; Hosey 1997).
Organisms that are cultured for economic benefit are exposed to a variety of selective pressures in aquaculture that differ from those experienced in their natural environment. In fishes, as in many captive-bred populations, there is intentional selection for commercially beneficial traits, such as faster growth rates and delayed maturity, in aquaculture programmes (Gjedrem et al. 1988; Glebe 1998). The aquaculture environment itself may act as an inadvertent selective force, affecting the behaviour of farmed individuals (Gross 1998). Behavioural adaptations to the artificial environment (e.g. changes in aggression) may be beneficial to living in the aquaculture environment, but are more likely to lead to a loss of local adaptation (c.f. Taylor 1991; Garcia de Leaniz et al. 2007) in the natural environment (Fleming et al. 1994; Verspoor 1998; Jonsson and Jonsson 2006).
Farmed Atlantic salmon (Salmo salar) often escape from sea cages and have the potential to enter rivers and interbreed with wild salmon. While the reproductive performance (reflected, for example, by mate and territory acquisition) of farmed salmon can be inferior to that of wild fish (Fleming et al. 1996), there are exceptions (Garant et al. 2003; Weir et al. 2005). Furthermore, numbers of escaped farmed salmon within rivers are sufficiently high that interbreeding between farmed and wild salmon (hereafter, wild-farmed interbreeding) may be widespread. Morris et al. (2008), for example, reported that farmed Atlantic salmon have been detected in 87% of monitored rivers within a 300 km radius of most aquaculture activity in eastern North America.
Differences between farmed salmon and their wild counterparts are often sufficient to raise concerns that wild-farmed interbreeding may have detrimental ecological effects on wild populations (McGinnity et al. 2003; Jonsson and Jonsson 2006). The wild-farmed hybrid offspring may, for example, harbour maladapted traits that were inherited from the farmed parent which may lead to a lower fitness in the natural environment (Fleming et al. 2000; McGinnity et al. 2003; Fraser et al. 2008). This reduction in fitness is thought to have its greatest effect on small or declining wild populations, leading to a decrease in population growth rate and, possibly, local extirpation (Hutchings 1991; Hindar et al. 2006).
One means by which fitness can be reduced is through a reduction of anti-predator responses. Several studies have shown that farmed salmon exhibit reduced anti-predator responses relative to wild fish (Johnsson and Abrahams 1991; Einum and Fleming 1997; Fleming and Einum 1997). The anti-predator response in these studies was measured as the time it took for an early-life history salmon to resume foraging after a simulated predator attack. A faster time to resume foraging can be interpreted as an increased willingness to expose oneself to a predator (Johnsson and Abrahams 1991). The reduced response within groups of farmed salmon may occur for several reasons (Fleming and Einum 1997). First, culture environments experience little natural predation and the anti-predator response may be lost over time. Furthermore, the farmed fish experience considerably higher densities than those experienced in the wild, thus favouring increased aggression and reduced caution. Farmed salmon may also produce more growth hormone relative to wild salmon (e.g. Fleming and Einum 1997; Fleming et al. 2002), potentially increasing the need to forage to a greater degree to meet higher energy demands.
Limited research has examined the extent to which anti-predator responses differ among populations, or among multi-generational wild-farmed hybrid crosses (i.e. F2 or greater generations; Einum and Fleming 1997; Fleming and Einum 1997; Tymchuk et al. 2006). Furthermore, despite extensive evidence that farmed salmon are escaping in eastern North America (Morris et al. 2008), that aquaculture salmon production continues to increase in the region, and that many of the wild populations located near these farms are endangered or otherwise declining (COSEWIC 2006), no studies have examined the anti-predator responses of farmed and wild salmon in the region. Evaluations of the differential effects of multi-generational interbreeding between escaped farm salmon and divergent wild populations are critical for assessing the overall risks posed to wild salmon on species-wide scales (Hutchings and Fraser 2008).
Our study’s objectives were thus to test three hypotheses: (1) that wild salmon had greater anti-predator responses relative to farmed salmon; (2) that two generations of interbreeding between farmed salmon and wild salmon resulted in reduced anti-predator responses in hybrid salmon relative to wild salmon; (3) that the degree of reduction in anti-predator responses of hybrids relative to wild salmon differed between two different river populations.
Materials and methods
Generation of the crosses
Parental fish were obtained from two wild populations from Nova Scotia, Canada: Stewiacke River, in the Inner Bay of Fundy (45° 8′N; 63° 22′W), and Tusket River, from the Southern Upland region (43° 51′N; 65° 58′W). Stewiacke River parents were captured as wild parr and raised in captivity until maturity; Tusket River parents were captured as adults. The farmed strain was originally derived from the Saint John River, New Brunswick (45° 15′N; 66° 3′W), and had experienced four generations in aquaculture at the time our study was initiated in 2001 (Glebe 1998). Wild Stewiacke (STEW), wild Tusket (TUSK), farmed (FARM), and F1 hybrids (F1 STEW-FARM and F1 TUSK-FARM: wild × farmed) crosses were generated in November and December of 2001 at the Aquatron Facility, Dalhousie University (Fig. 1). These crosses were raised under common environmental conditions until maturity to ensure that any behavioural differences observed during the experiment would be attributable to genetic differences and not to environmental differences (Fig. 1).
Additional crosses were generated in November and December 2005, using the 2001 crosses as parents (Fig. 1; Table 1). Prior to generating the 2005 crosses, all adults from the 2001 crosses were individually tagged and fin clipped. DNA was extracted from the fin clips and used to identify the genotypes at five polymorphic microsatellite loci. The genotype information was used for parentage assignments and the assignments were used to avoid inbred matings (i.e. full-sib, half-sib or cousin matings) in the generation of the 2005 crosses. The 2005 crosses permitted the generation of multigenerational hybrids, i.e. F2 hybrids (F2 STEW-FARM and F2 TUSK-FARM: F1 hybrid × F1 hybrid), and wild backcrosses (BC1 STEW-FARM and BC1 TUSK-FARM = wild × F1 hybrid) and thus multigenerational comparisons in anti-predator responses between farmed and both wild populations (Fig. 1). Each consisted of 9–23 chiefly full-sibling families (Table 1), except for TUSK and both F2 hybrids in which each female was crossed to two or three different males. The same parents were used in the generation of multiple crosses. This was necessary to control for female and male representation when comparing trait means between crosses, as parental effects (especially maternal effects) are prevalent in Atlantic salmon (Green 2009).
In May 2005, due to space limitations, all families were pooled within each cross and 500 fish randomly selected from each cross pool were then transferred to one of four or five replicate 100 l tanks for a total of 41 tanks, such that tanks had even densities and even numbers of individuals per family within each cross. Fry were periodically culled at random as they outgrew the space. Fish densities were maintained at equal levels among tanks until the time at which our behavioural comparisons were made.
Predator tank and predator model design
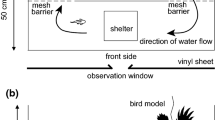
A predator tank was constructed to measure the anti-predator response of salmon fry (age 0+ parr, 113–197 days post yolk absorption; Fig. 2). The predator tank consisted of four testing sections, each measuring 36 cm × 50 cm. Given that fry generally prefer to maintain a position on gravel (Gibson 1993), each section contained a gravel patch (3–10 mm particle size) in addition to a hiding area (i.e. a refuge) in one of the corners constructed out of a piece of ABS pipe that had been cut in half. Fry tend to prefer mean water velocities of 10–40 cm s−1 and depth between 20–40 cm in Nova Scotia and New Brunswick rivers (Morantz et al. 1987). Attempts were made to design the predator tank with these characteristics but, due to logistic constraints, our water velocity and depth were 11 cm s−1 and 11 cm, respectively. A spray bar was positioned at the bottom of the section where it dispensed water directly over the gravel patch and any excess water overflowed an 11 cm wall of opaque acrylic. A cardboard barrier with viewing slits was erected around the tank to minimize any visual disturbance of the fry.
An aerial predator model was used because aerial predators have been shown to induce a greater anti-predator response than in-stream predators (Gotceitas and Godin 1993). A belted kingfisher (Ceryle alcyon) was selected because it is a natural predator of salmon fry in Nova Scotia (Cairns 1998). The dimensions and colours for the belted kingfisher model were acquired from preserved specimens at the Natural History Museum of Nova Scotia. The final model was 30 cm × 37 cm and was constructed out of 20 gauge galvanized wire and papier mâché. The model was painted to resemble a female belted kingfisher, using acrylic paint, and covered in several coats of interior varnish to provide the model limited water resistance.
Measurement and analysis of the anti-predator responses
Between September and November 2006, 20 fry were randomly chosen from pooled family tanks (four to five random fry from each of the four or five replicate pooled family tanks) of each cross (total N = 180) to assess their anti-predator responses. Again, there were several independent families within a cross, and the fry used were a random subsample of the equally pooled families, thus the lack of independence should be minor. Each fry was measured (nearest mm) and weighed (nearest 0.01 g) before being placed into one of the four testing sections of the predator tank. Observations of fry for each cross were distributed evenly among the four testing sections. Each fry was allowed to acclimatize to the tank for 24 h with 10 h of light and no food. Before the fry was ‘attacked’ with the kingfisher, the position of the fry (on the gravel patch, partially inside the refuge, or completely inside the refuge) was noted.
The fry was then ‘attacked’ (i.e. lunging the beak of the model kingfisher towards the fry) until it entered the refuge. The operator of the kingfisher model was hidden behind the cardboard barrier to limit the exposure of the fry to the operator. The number of attacks required before fry entered the refuge was recorded and the timer was started once the fry entered the refuge. Food pellets (N = 2–4) were dropped into the tank every minute where they circulated in the water and were visible to the fry in the refuge.
The refuge time was recorded when the fry completely left the refuge to forage or to return to the gravel patch. That is, the refuge time was calculated as the time from the entry into the refuge to the complete exit of the refuge (c.f. Einum and Fleming 1997; Fleming and Einum 1997). On occasion, fry would only partially emerge from the refuge such that only their heads were visible. This period of partial emergence was termed ‘wait time’. Fry that did not exit the refuge after 20 min had their refuge time recorded as 20 min.
All statistical analyses comparing the crosses for the fry position before the kingfisher attacks, number of kingfisher attacks, refuge time, and wait time were performed in R 2.7.0. (available at http://www.r-project.org/). Statistical significance was set at the α = 0.05 level. Joint-scale analysis (described in Lynch and Walsh 1998) was also used to test the null hypotheses of additive (A) and additive-dominance (AD) inheritance of anti-predator responses between crosses in each wild-farmed comparison. A likelihood ratio test subtracting the A test statistic (χ2) from the AD test statistic was then used to determine which model best fit the data. Epistatic effects could not be estimated using joint-scale analysis because of the limited number of degrees of freedom imposed by the number of crosses.
Results
Fry position in the predator tank prior to the kingfisher attack
Prior to attack by the kingfisher model, fry were either positioned on the gravel patch, partially inside the refuge, or completely inside the refuge. There were no significant differences between crosses for the number of fry positioned completely inside the refuge (Kruskal–Wallis one-way ANOVA, P = 0.434), thus the number of fry positioned completely inside the refuge and the number of fry positioned partially inside the refuge were pooled together. There were significant differences in the proportion of the fry positioned on the gravel patch and the pooled observations (completely/partially inside the refuge) for all crosses except for STEW and BC1 STEW-FARM fry (Fig. 3).
Fry positions in the predator tank prior to the kingfisher model attacks. Open bars are the pooled observations for the positions of completely inside the refuge and partially inside the refuge. Filled bars are the observations of the position on the gravel patch. Percentages reflect the amount of farmed genes in the crosses. *Identify the crosses exhibiting significant differences between the two pooled and gravel patch positions, using two-sided tests for the equality of proportions
Number of kingfisher attacks
All fry eventually entered the refuge after being attacked by the kingfisher (mean number of attacks ± 1SD = 2.89 ± 2.60). There was no relationship between cross and the number of kingfisher attacks required for the fry to enter the refuge (Kruskal–Wallis one-way ANOVA, P = 0.687).
Refuge time and wait time
The shortest mean refuge time and second lowest variance was observed for the farmed fry (Fig. 4). Conversely, the longest mean refuge times, coupled with high variances, were observed for the wild fry in both populations. The hybrid crosses were intermediate in refuge time and associated variance to those of the parental populations (pure farmed or wild). There were significant differences in the median and mean refuge times between the crosses (Table 2). The ranks from shortest to longest mean refuge time for the Stewiacke-farmed crosses were: FARM < F2 STEW-FARM < BC1 STEW-FARM < F1 STEW-FARM < STEW. The ranks from shortest to longest mean refuge time for the Tusket-farmed crosses were: FARM < F1 TUSK-FARM < F2 TUSK-FARM < BC1 TUSK-FARM < TUSK. Excluding the farmed data, there was also a marginally significant population × cross interaction in refuge time (Factorial ANOVA, P = 0.092). In addition, there were no statistically significant differences among the coefficients of variation (CVs) for the Stewiacke-farmed crosses (Homogeneity of CV test, Zar 1999, P = 0.269) and Tusket-farmed crosses (P = 0.342).
Boxplots of refuge time for the various fry crosses. Box area is proportional to the dispersion in the data. The bold line in the box represents the median and the star represents the mean. Dots represent outliers that were included in analyses. The upper and lower horizontal line connected to the box by dashed lines represent the largest and smallest observation, respectively, that was not an outlier. Percentages represent the amount of farmed genes in the crosses
In both populations, there was a trend for a reduced mean refuge time in hybrid crosses relative to the wild crosses as the amount of farmed genes in the hybrid crosses increased. Accordingly, joint-scale analysis revealed that the refuge time anti-predator responses in both wild-farmed comparisons fit an additive inheritance model (Stewiacke-farmed: \( \chi_{\text{A}}^{2} = 0.455 \), df = 3, P = 0.929; Tusket-farmed: \( \chi_{\text{A}}^{2} = 0.258 \), df = 3, P = 0.968). Inclusion of dominance inheritance did not significantly increase the fit in either comparison (Stewiacke-farmed: \( \chi_{\text{A}}^{2} - \chi_{\text{AD}}^{2} = 0.004 \), df = 1, P = 0.951; Tusket-farmed: \( \chi_{\text{A}}^{2} - \chi_{\text{AD}}^{2} = 0.210 \), df = 1, P = 0.647).
The number of fry that were observed with a wait time was not significantly different among the crosses (Kruskal–Wallis one-way ANOVA, P = 0.434). Fry that did not have an observation of wait time were excluded from analyses comparing the wait times among crosses (remaining N = 120). There were significant differences in the median and mean wait times between the Stewiacke-farmed crosses and Tusket-farmed crosses (Table 2). However, after excluding the farmed data, there was no significant population × cross interaction (Factorial ANOVA, P = 0.551). Furthermore, there were no statistically significant differences among the CVs between the Stewiacke-farmed crosses (Homogeneity of CV test, P = 0.349) and Tusket-farmed crosses (P = 0.842).
The relationship between wait time and cross was similar to that between refuge time and cross, i.e. an increase in the amount of farmed genes in a cross was associated with shorter mean wait time in the hybrid crosses relative to wild fish for both wild populations (Fig. 5). Accordingly, joint-scale analysis revealed that the wait time anti-predator responses in both wild-farmed comparisons fit an additive inheritance model (Stewiacke-farmed: \( \chi_{\text{A}}^{2} = 0.152 \), df = 3, P = 0.985; Tusket-farmed: \( \chi_{\text{A}}^{2} = 0.135 \), df =3, P = 0.987). Inclusion of dominance inheritance did not significantly increase the fit in either comparison (Stewiacke-farmed: \( \chi_{\text{A}}^{2} - \chi_{\text{AD}}^{2} = 0.039 \), df = 1, P = 0.843; Tusket-farmed: \( \chi_{\text{A}}^{2} - \chi_{\text{AD}}^{2} = 0.0003 \), df = 1, P = 0.985).
Boxplots of wait time for the various fry crosses. The symbols are the same as those described in the caption for Fig. 4
There was no relationship between the number of kingfisher attacks and refuge time or wait time for any of the crosses (Table 3). That is, a higher number of kingfisher attacks was not associated with a heightened anti-predator response (i.e. an increase in either refuge or wait time). In addition, the difference between refuge time and wait time did not differ significantly between crosses (Kruskal–Wallis one-way ANOVA, P = 0.464). That is, certain crosses were not waiting significantly longer at the edge of the refuge before completely emerging in comparison to other crosses. None of the variables measured (fry position, refuge time, wait time, and number of kingfisher attacks) was correlated with fry length or mass (Table 4). There were also no tank effects on any of the variables measured (Table 4).
Discussion
Differences in anti-predator responses between wild and farmed fish
Farmed fry emerged from their refuges earlier than those from both wild populations. The same trend was observed for wait time, thus suggesting a genetic link between wait time and refuge time. If the refuge time of the wild fry reflects an adaptive response, then the reduction in refuge time, as observed for the hybrids, may translate to a reduction in hybrid fitness. The behavioural differences between farmed and wild salmon were most likely genetically-based because the fish had been raised in a common environment and there was no correlation between any of the behaviours and length or mass. However, the reduced refuge time response in farmed fry could be related to the different ancestry of the fry and not necessarily to artificial or inadvertent selection. That is, our study did not test directly for the effects of ancestry versus the effects of domestication because wild Saint John River salmon were not tested. Alternatively, some studies have suggested that fish that produce more growth hormone (and have a faster growth rate as a consequence) have a more reduced anti-predator response because they have a higher need to forage and may be more willing to take risks (e.g. Johnsson and Björnsson 1994; Johnsson et al. 1996; Fleming and Einum 1997). Nevertheless, differences in refuge time between farmed and wild fry in the present study could not be explained by differences in growth rate. Notably, wild Tusket fry and parr grew marginally faster than farmed fry and parr (Lawlor 2003; D. J. Fraser and J. A. Hutchings, unpublished data), yet the former had longer refuge times. In addition, the wild populations did not differ in their anti-predator responses in terms of refuge time, wait time, and coefficient of variation suggesting that, at least at the geographic scale examined in this study, they exhibited similar anti-predator responses. Collectively, these results suggested that selection may have contributed to a reduction of anti-predator responses in the aquaculture environment.
Conserved aspects of the anti-predator responses
Salmon fry from different crosses did not differ significantly in the number of kingfisher attacks it took for them to retreat to the refuge nor in the number of observations of wait times. Furthermore, the difference between wait time and refuge time did not differ among crosses. That is, wild fry did not spend a longer period of time at the edge of the refuge before emerging than the other crosses. These results suggest that some wild elements of the anti-predator response, such as the initial reaction time to the predator and the time taken to observe one’s surroundings before leaving a refuge, have been retained in farmed and hybrid salmon, despite the potential for artificial or inadvertent selection in captivity to select for more ‘risk taking’ behaviours. Interestingly, using the same methodology, Einum and Fleming (1997) and Fleming and Einum (1997) also documented a lack of differentiation in the time it took population crosses to enter a refuge (wild, farmed, and F1 hybrids; Imsa and Lone River wild populations from Norway).
Effects of wild-farmed hybridization on anti-predator responses
In both wild-farmed comparisons, hybridization generated F1 and F2 hybrids that exhibited intermediate (i.e. additive) refuge and wait times relative to parental populations. These results were consistent with those reported from the two other studies that have compared salmonid refuge times between wild-farmed hybrids and their parental populations (Einum and Fleming 1997; Tymchuk et al. 2006). In addition, the coefficients of variation as a standardized measure of dispersion did not differ among the crosses. Thus, despite changes to the mean anti-predator response in hybrids, we found no evidence that hybridization increased or decreased the dispersion around the means. Variation may have increased if there was increased non-additive genetic variation in the crosses between populations without fixed allelic differences or if there was the recombination of genotypes in the crosses; variation may have decreased if the crosses had been between populations with fixed allelic differences (Edmands 1999).
We did, however, find some evidence, albeit limited, that the magnitude of the effects of wild-farmed interbreeding on anti-predator responses differed between wild populations for refuge time. That is, of the two wild-farmed comparisons, the differences in average refuge times were greatest between Stewiacke versus Stewiacke-farmed hybrids. Although our data suggested a strong additive component to the inheritance of anti-predator responses, the failure to reject an additive model could also be attributed to the large variance in the data. In addition, epistasis could not be tested for directly due to the low number of crosses which limited the number of degrees of freedom.
There were also some other nuances in the effects of hybridization on fry behaviour between the wild populations. Notably, wild Stewiacke and BC1 Stewiacke fry did not exhibit a strong preference for remaining on the gravel patch during the acclimatization period relative to wild Tusket fry or other crosses. This was somewhat unexpected given that fry have been reported to prefer to maintain a position on gravel (Gibson 1993). Although it is interesting to speculate that the differences in preference for holding on gravel patches might reflect adaptive differentiation between Tusket and Stewiacke juvenile environments (c.f. Taylor 1991; Garcia de Leaniz et al. 2007), we are unaware of any major differences in their habitats that might account for the differences observed here.
Possible consequences to wild fish populations
Wild-farmed hybrids exhibited reduced anti-predator responses relative to wild fry. As a consequence, hybrid fry may be more at risk of predation in the wild, contributing to a reduction in mean fitness in the wild population, especially if the rate at which wild-farmed hybridization occurs is considerable. Fleming et al. (2000) estimated a 70% early survival of F1 farmed-wild hybrid Atlantic salmon relative to wild salmon in a native river. The authors attributed reduced anti-predator responses as one possible reason for the lower survival of hybrids (c.f. Einum and Fleming 1997; Fleming and Einum 1997). Conversely, McGinnity et al. (2004) found that, over several generations of juvenile hatchery-rearing, hatchery salmon from the same genetic origin as wild salmon did not exhibit differential adult survival relative to wild fish. This was despite genetic changes in the hatchery fish in growth, age of smolting, and other life history aspects. In addition, the hatchery salmon program in McGinnity et al. (2004) did not intentionally select for economically beneficial traits, such as increase growth rate and delayed maturity, as in farmed salmon programs. Nevertheless, the hatchery-reared salmon had been exposed to natural predators as post-juveniles each generation, where as farmed fishes spend their entire life-cycle in captivity and are not exposed to predators. Furthermore, farmed salmon are not exposed to natural selection via the exposure to predators before they reproduce, and this could be an explanation for the reduced anti-predator responses observed in our farmed fish that were raised in captivity for several generations.
A loss of hybrid fry through predation could affect wild populations indirectly through a reduction in overall population size (Einum and Fleming 1997). It could also directly affect wild populations if the increased catchability of hybrid fry by predators attracts more predators into the area. Furthermore, with a trend of less variation in the behavioural template than wild fish, hybrids would be expected to have, on average, a reduced capacity to adapt to the changes in the environment relative to wild fry (Freeman and Herron 2004).
With an increasing number of generations of artificial selection in the aquaculture environment, it is also expected that farmed fish will become more genetically and behaviourally distinct from wild populations (Gross 1998; Verspoor 1998; Hutchings and Fraser 2008). Given that the farmed Saint John River strain is now in its sixth generation of aquaculture (Quinton et al. 2005), but was only in its fourth generation of aquaculture in this study, it is expected that the hybridization between escaped farmed salmon currently used in aquaculture and wild salmon in Eastern Canada may result in an even greater reduction of anti-predator responses and, thus, fitness in wild-farmed hybrids.
Our results contribute to the growing number of studies that have documented genetically-based behavioural differences in farmed and wild-farmed hybrids relative to wild fish. The present study is distinct, however, in illustrating the importance of examining multiple wild populations for risk assessment. The effects of multi-generational wild-farmed hybridization varied somewhat for different wild populations. Greater reduction of anti-predator responses in wild-farmed hybrids relative to the wild state for some wild populations may pose a greater risk to the persistence and recovery of these populations relative to wild populations with lesser degrees of reduction in such responses. Perhaps more importantly, the effects of hybridization on anti-predator responses were consistently in a direction that is likely to be disadvantageous in the natural environment for both wild populations.
References
Cairns DK (1998) Diet of cormorants, mergansers, and kingfishers in northeastern North America. Can Tech Rep Fish Aquat Sci No 225
Committee on the Status of Endangered Wildlife in Canada (COSEWIC) (2006) COSEWIC assessment and update status report on the Atlantic salmon Salmo salar (Inner Bay of Fundy populations) in Canada. Available via www.sararegistry.gc.ca. Accessed 19 January 2009
Edmands S (1999) Heterosis and outbreeding depression in interpopulation crosses spanning a wide range of divergence. Evol Int J Org Evol 53:1757–1768. doi:10.2307/2640438
Einum S, Fleming IA (1997) Genetic divergence and interactions in the wild among native, farmed and hybrid Atlantic salmon. J Fish Biol 50:634–651. doi:10.1111/j.1095-8649.1997.tb01955.x
Fleming IA, Einum S (1997) Experimental tests of genetic divergence of farmed from wild Atlantic salmon due to domestication. ICES J Mar Sci 54:1051–1063
Fleming IA, Jonsson B, Gross MR (1994) Phenotypic divergence of sea-ranched, farmed, and wild salmon. Can J Fish Aquat Sci 51:2808–2824. doi:10.1139/f94-280
Fleming IA, Jonsson B, Gross MR et al (1996) An experimental study of the reproductive behaviour and success of farmed and wild Atlantic salmon (Salmo salar). J Appl Ecol 33:893–905. doi:10.2307/2404960
Fleming IA, Hindar K, Mjølnerød IB et al (2000) Lifetime success and interactions of farm salmon invading a native population. Proc R Soc Lond B Biol Sci 267:1517–1523. doi:10.1098/rspb.2000.1173
Fleming IA, Agustsson T, Finstad B et al (2002) Effects of domestication on growth physiology and endocrinology of Atlantic salmon (Salmo salar). Can J Fish Aquat Sci 59:1323–1330. doi:10.1139/f02-082
Frankham R (2008) Genetic adaptation to captivity in species conservation programs. Mol Ecol 17:325–333. doi:10.1111/j.1365-294X.2007.03399.x
Fraser DJ, Cook AM, Eddington JD et al (2008) Mixed evidence for reduced local adaptation in wild salmon resulting from interbreeding with escaped farmed salmon: complexities in hybrid fitness. Evol Appl 1:501–512. doi:10.1111/j.1752-4571.2008.00037.x
Freeman S, Herron JC (2004) Evolutionary Analysis, 3rd edn. Pearson Prentice Hall, Toronto
Garant D, Fleming IA, Einum S et al (2003) Alternative male life-history tactics as potential vehicles for speeding introgression of farm salmon traits into wild populations. Ecol Lett 6:541–549. doi:10.1046/j.1461-0248.2003.00462.x
Garcia de Leaniz CG, Fleming IA, Einum S et al (2007) A critical review of adaptive genetic variation in Atlantic salmon: implications for conservation. Biol Rev Camb Philos Soc 82:173–211. doi:10.1111/j.1469-185X.2006.00004.x
Gibson RJ (1993) The Atlantic salmon in fresh water: spawning, rearing and production. Rev Fish Biol Fish 3:39–73. doi:10.1007/BF00043297
Gjedrem T, Gjerde B, Refstie T (1988) A review of quantitative genetic research in salmonids at AKVAFORSK. In: Weir BS, Eisen EJ, Goodman MM et al. (eds) Proceedings of the Second International Conference on Quantitative Genetics, Sinauer Associates Inc, Sunderland
Glebe BD (1998) Atlantic salmon broodstock development programs. Can Stock Assess Secret Res Doc 98/157
Gotceitas V, Godin J-GJ (1993) Effects of aerial and in-stream threat of predation on foraging by juvenile Atlantic salmon (Salmo salar). In: Gibson RJ, Cutting RE (eds) Production of juvenile Atlantic salmon, Salmo salar, in natural waters, Can Spec Pub Fish Aquat Sci vol 118, pp. 35–41
Green BS (2009) Maternal effects in fish populations. Adv Mar Biol 54:1–105. doi:10.1016/S0065-2881(08)00001-1
Gross MR (1998) One species with two biologies: Atlantic salmon (Salmo salar) in the wild and in aquaculture. Can J Fish Aquat Sci 55:131–144. doi:10.1139/cjfas-55-S1-131
Hindar K, Fleming IA, McGinnity P et al (2006) Genetic and ecological effects of salmon farming on wild salmon: modelling from experimental results. ICES J Mar Sci 63:1234–1247. doi:10.1016/j.icesjms.2006.04.025
Hosey GR (1997) Behavioural research in zoos: academic perspectives. Appl Anim Behav Sci 51:199–207. doi:10.1016/S0168-1591(96)01104-5
Hutchings JA (1991) The threat of extinction to native populations experiencing spawning intrusions by cultured Atlantic salmon. Aquaculture 98:119–132. doi:10.1016/0044-8486(91)90377-J
Hutchings JA, Fraser DJ (2008) The nature of fisheries- and farming-induced evolution. Mol Ecol 17:294–313. doi:10.1111/j.1365-294X.2007.03485.x
Johnsson JI, Abrahams MV (1991) Interbreeding with domestic strains increases foraging under threat of predation in juvenile steelhead trout (Oncorhynchus mykiss): an experimental study. Can J Fish Aquat Sci 48:243–247. doi:10.1139/f91-033
Johnsson JI, Björnsson BT (1994) Growth hormone increases growth rate, appetite and dominance in juvenile rainbow trout, Oncorhynchus mykiss. Anim Behav 48:177–186. doi:10.1006/anbe.1994.1224
Johnsson JI, Petersson E, Jönsson E et al (1996) Domestication and growth hormone alter antipredator behaviour and growth patterns in juvenile brown trout, Salmo trutta. Can J Fish Aquat Sci 53:1546–1554. doi:10.1139/cjfas-53-7-1546
Jonsson B, Jonsson N (2006) Culture Atlantic salmon in nature: a review of their ecology and interactions with wild fish. ICES J Mar Sci 63:1162–1181. doi:10.1016/j.icesjms.2006.03.004
Lawlor J (2003) Genetic differences in fitness-related traits among populations of wild and farmed Atlantic salmon, Salmo salar. MSc thesis, Dalhousie University, Halifax
Lynch M, Walsh JB (1998) Genetics and analysis of quantitative traits. Sinauer Associates, Inc., Sunderland
McGinnity P, Prödohl P, Fergusin A et al (2003) Fitness reduction and potential extinction of wild populations of Atlantic salmon, Salmo salar, as a result of interactions with escaped farm salmon. Proc R Soc Lond B Biol Sci 270:2443–2450. doi:10.1098/rspb.2003.2520
McGinnity P, Prödohl P, Ó Maoiélidigh N et al (2004) Differential lifetime success and performance of native and non-native Atlantic salmon examined under communal natural conditions. J Fish Biol 65(Suppl. A):173–187. doi:10.1111/j.0022-1112.2004.00557.x
Morantz DL, Sweeney RK, Shirvell CS et al (1987) Selection of microhabitat in summer by juvenile Atlantic salmon (Salmo salar). Can J Fish Aquat Sci 44:120–129. doi:10.1139/f87-015
Morris MRJ, Fraser DJ, Heggelin AJ et al (2008) Prevalence and recurrence of escaped farmed Atlantic salmon (Salmo salar) in eastern North American rivers. Can J Fish Aquat Sci 65:2807–2826. doi:10.1139/F08-181
Price EO (1997) Behavioural genetics and the process of animal domestication. In: Grandin T (ed) Genetics and the behaviour of domestic animals. Academic Press, San Diego, pp 31–65
Quinton CD, McMillan I, Glebe BD (2005) Development of an Atlantic salmon (Salmo salar) genetic improvement program: genetic parameters of harvest body weight and carcass quality traits estimated with animal models. Aquaculture 247(Spec Issues 1–4):211–217 Genetics in Aquaculture VIII
Taylor EB (1991) A review of local adaptation in Salmonidae, with particular reference to Pacific and Atlantic salmon. Aquaculture 98:185–207. doi:10.1016/0044-8486(91)90383-I
Tymchuk WE, Biagi C, Withler R et al (2006) Growth and behavioral consequences of introgression of a domesticated aquaculture genotype into a native strain of coho salmon. Trans Am Fish Soc 135:442–455. doi:10.1577/T05-181.1
Verspoor E (1998) Genetic impacts on wild Atlantic salmon (Salmo salar L) stocks from escaped farm conspecifics: an assessment of risk. Can Stock Assess Secret Res Doc 98/156
Weir LK, Hutchings JA, Fleming IA et al (2005) Spawning behaviour and success of mature male Atlantic salmon (Salmo salar) parr of farmed and wild origin. Can J Fish Aquat Sci 62:1153–1160. doi:10.1139/f05-032
Zar JH (1999) Biostatistical analysis, 4th edn. Prentice-Hall, New Jersey
Acknowledgments
The work was supported by the Natural Sciences and Engineering Research Council (Canada) through an undergraduate student research award to ASH, a post-doctoral fellowship to DJF, and a Strategic Grant to JAH. ASH was also supported by an Atlantic Salmon Federation Olin Fellowship. We thank at Dalhousie University J. Eddington (Aquatron facility), M. Merrimen, R. Myers, K. Tae, L. Weir, and P. Debes. We also thank P. Amiro (Department of Fisheries and Oceans Nova Scotia Region), J.-G. Godin (Carleton University, Ottawa), A. Hebda (Natural History Museum of Nova Scotia), A. D-S. Houde (Saint Mary’s University, Halifax), the constructive comments of two anonymous reviewers, and E. Anderson. Petrie the belted kingfisher.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Houde, A.L.S., Fraser, D.J. & Hutchings, J.A. Reduced anti-predator responses in multi-generational hybrids of farmed and wild Atlantic salmon (Salmo salar L.). Conserv Genet 11, 785–794 (2010). https://doi.org/10.1007/s10592-009-9892-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10592-009-9892-2