Abstract
Japanese eel (Anguilla japonica) is a commercially important species; however, its population has declined in recent years. Appropriate conservation management, including habitat protection, is required to reverse this decline. However, their habitat use pattern during the riverine life stage is poorly understood. In this study, we investigated the longitudinal distribution and microhabitat of small-sized (<200 mm total length) and large-sized (≥200 mm total length) Japanese eels observed in 83 and 124 quadrats (1 m × 1 m), respectively, placed at seven stations in the Nikkeshi River in Fukushima Prefecture, Japan. Analysis using generalized linear models revealed that the eel density of both size classes decreased with increasing distance from the river mouth. In addition, the density of small-sized eels, but not large-sized ones, decreased as weir numbers increased. Moreover, analysis using generalized additive models showed that microhabitat uses differed between the size classes. The small-sized eels used near-shore habitats which had low current velocities. Their preferred habitats contained both complex substrates with smaller particle sizes and simple substrates with relatively large particle sizes. In contrast, the large-sized eels used both near-shore habitats with lower current velocities and the center of the river which had high current velocities. They preferred simple riverbed habitats with large particle-sized substrates and no underwater vegetation. These results suggest that there is a size-dependent change in the longitudinal distribution and microhabitat use of Japanese eels during their river life stage. These findings provide valuable information for the conservation and management of Japanese eels in rivers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Japanese eels are catadromous fish that spawn in open seas and grow in continental waters (Tsukamoto et al. 2011). Although Japanese eel is one of the most commercially valuable fishery resources in East Asia, including Japan, the stock has been rapidly decreasing since the 1970s due to habitat degradation (i.e., construction of concrete riverbanks and dams) and overfishing of glass, yellow, and silver eels (Tsukamoto et al. 2009; Itakura et al. 2015; Kaifu et al. 2018; Kaifu 2019). On average, 78.6% of the effective habitats of Japanese eels have been lost in East Asian rivers (Chen et al. 2014). Consequently, this species is currently categorized as endangered in the International Union for Conservation of Nature Red List (Jacoby and Gollock 2014). Therefore, to undertake effective management and conservation measures of wild Japanese eel stocks, it is necessary to understand the ecology of their whole life cycle.
Several ecological aspects of yellow and silver eels inhabiting fresh/brackish waters have been well investigated. For example, based on otolith Sr/Ca ratios, habitats for eels from the elver to silver stages before spawning migration have been reported to extend from coastal to fresh waters (Tsukamoto and Arai 2001; Kaifu et al. 2010; Yokouchi et al. 2012), and their growth rates in coastal/brackish waters are higher than those in freshwater habitats (Yokouchi et al. 2008; Kaifu et al. 2013; Wakiya et al. 2016), as shown in other anguillids (Morrison and Secor 2003; Daverat and Tomás 2006; Walsh et al. 2006; Cairns et al. 2009). Yellow eels use various types of riverbed materials as refuges to avoid predation and strong currents (Aoyama et al. 2005; Tomie et al. 2017; Christoffersen et al. 2018), whereas young eels at the elver and young yellow stages use riverbed materials with smaller particle sizes (Kume et al. 2019). Understanding the habitat uses of target fishes throughout their life history is important to implement appropriate conservation strategies and restore their habitats. Despite extensive studies of the across-life-stage distribution of eels in rivers, including Japanese eels (e.g., Tzeng et al. 1995; White and Nights 1997; Domingos et al. 2006; Lasne and Laffaille 2008; Yokouchi et al. 2008; Kwak et al. 2019), data on the use habitat of micro- and reach-scales by eels in the glass/elver and yellow stages in natural environments are lacking (Laffaille et al. 2003; Johnson and Nack 2013; Kume et al. 2019).
In this context, the aim of the present study was to assess the impact of (i) hydrological and anthropogenic factors and (ii) longitudinal distribution on the riverine habitat use of Japanese eels in a small river, characterized by intensive agricultural use in its catchment area. Moreover, we compared these results between two size classes, small-sized and large-sized eels. In Japan, many small rivers are utilized and modified (i.e. channelized) for paddy field irrigation, leading to degradation of riverine habitats of aquatic organisms. Although such rivers may be potentially used to restore the habitats because they can be easier to apply and monitor than in large rivers, they have not been considered for the conservation efforts of aquatic organisms, including Japanese eels. Thus, knowledge of eel habitat use in these rivers will contribute to the establishment of comprehensive management and conservation strategies for wild eel stocks.
Materials and methods
Study site
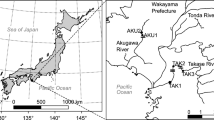
We conducted field surveys in the Nikkeshi River (~11.5 km long, 3.0–25.0 m wide), Fukushima Prefecture, Japan (Fig. 1) on 23–24 April 2018. This river flows into the southern part of the Matsukawa-ura lagoon (6.46 km2), which is connected to the open ocean via a single narrow channel (about 100 m wide) (Fig. 1B). The lower and middle course of this river run through a lowland area that mainly consists of paddy fields. To enable agricultural management, these courses were channelized with concrete, and weirs were built to prevent fine sediment deposition on the riverbeds as well as to maintain a high water level to supply the paddy fields during the agricultural season. Since we sampled at the beginning of the agricultural season, the water levels were relatively high. We randomly chosen seven stations (St. 1–7) in the river (Fig. 1C). All reaches were located entirely within freshwater areas and had concrete banks on both sides. No reared eels were released by fisheries cooperative associations in this river.
Maps (a–c) of the study area. (a) Maps show northern Honshu and Fukushima Prefecture, Japan, (b) Matsukawa-ura lagoon and its tributaries, and (c) the Nikkeshi River. Circles indicate sampling stations. Triangle indicates water gate. Closed and open squares indicate inflatable rubber weir and vertical wall weir, respectively (see Table 2)
Field survey
We collected eels at the seven stations during daytime (Fig. 1). Sampling was performed by three people: one person operated the electrofishing unit (LR-20B, SmithRoot, Vancouver, WA, USA) while the others caught the eels using dip-nets (39 cm × 40 cm opening, 40 cm depth, 1 mm mesh size). We moved upstream, zigzagging across the river to guarantee complete coverage within each station. All stations were sampled once. All sampling procedures of this study were performed in accordance with the “Guidelines for the Use of Fishes in Research” published by the Ichthyological Society of Japan in 2003 (http://www.fish-isj.jp/6nglish/guidelines.html).
We targeted eels encompassing all riverine life stages. In the field, we determined the size class of eels to classify those of small size [<200 mm total length (TL), which corresponds to the elver and yellow stages (Fukuda et al. 2013)] and those of large size [≥200 mm TL, which corresponds to the yellow stage (Okamura et al. 2007)] through visual observation. No glass or silver eels were found during the sampling. The in situ catch points of the collected eels were marked on the riverbed for subsequent measurements using a numbered location marker. The captured eels were then temporarily kept in buckets to avoid repetitive catches. To check the accuracy of our visual measurements, a fraction of the captured eels (Table 1), which were randomly selected, was frozen using dry ice after anesthesia with clove oil (Walsh and Pease 2002) and transported to the laboratory. The TL of small-sized eels was then measured using a digital caliper (to the nearest 0.1 mm) and that of large-sized eels was measured using a scale (to the nearest 1 mm). The TLs of small-sized eels ranged from 52.8 to 127.0 mm (mean = 81.7 mm) and those of large-sized eels ranged from 200 to 657 mm (mean = 351.6 mm) (Fig. 2; Table 1). The remaining eels were released in the same station from where they were caught.
Following eel sampling, river width at three lines within each station and length of each station were measured and used to calculate the area of each station. Three physical environmental variables, including distance from the nearest riverbank (DR: cm), current velocity (CV: cm/s), and substrate composition (%), were measured and used for subsequent analyses. We set 48 eel-present and 35 eel-absent quadrats (1 m × 1 m) of small-sized eels, and 23 eel-present and 101 eel-absent quadrats (1 m × 1 m) of large-sized eels for the abiotic measurements. The absent quadrats were set in three and five reaches for comparisons of small-sized (St. 2–4) and large-sized eels (St. 2–6) at random. DR was measured from the center of each quadrat to the nearest shore using an aluminum ruler (accurate to the nearest 1 cm). Because this river was shallow (mean ± standard deviation = 18.9 ± 10.2 cm depth), the CV at a 60% depth from the water surface was measured three times at the center of the quadrat using an electromagnetic current meter (VE20/VET-200-10PII, KENEK Co., Ltd., Tokyo, Japan); the mean value of the three readings was used for analyses. Substrate composition (%) in each quadrat was recorded by visual observation using four categories: sand/gravel (<4 mm), pebble (4–64 mm), cobble (64–256 mm), and boulder (≥256 mm). We used Simpson’s D (Hunter and Gaston 1988) as an index of substrate complexity (ISC) based on substrate composition in each quadrat. This index ranges from 0 (only one category in substrate composition) and 1 (highly complex substrate composition). We used the dominant substrate (DS) and ISC for quantitative analyses. In addition, the presence/absence (1/0) of underwater vegetation (UWV) in each quadrat was recorded by visual observation.
We also recorded geographical data for each station. Distance from the river mouth (m) of the station was registered using QGIS ver. 2.18.3 software (QGIS development team 2016). Moreover, we counted the number of weirs from the river mouth and measured the heights of weirs using a laser distance meter (DISTOTM D510, Leica Geosystems, Switzerland), as these structures could function as potential barriers against the upstream migration of eels (White and Knights 1997; Ministry of the Environment, Japan 2016; Kume et al. 2019; Kwak et al. 2019). We used the number of weirs and their maximum height above the water surface from the river mouth for analyses. Of the total 15 weirs, 13 were made of concrete and presented a vertical wall, whereas the other two were inflatable rubber weirs that were inflated from April to September for irrigating the paddy fields (Table 2). In Japan, both types of weirs are commonly used in small rivers that flow through paddy areas. The two inflatable rubber weirs have an integrated fish pass so that eels can move upstream. The heights above the water surface of the other weirs ranged from 19 to 154 cm (average 68.3 cm; Table 1). The sediment of upstream of all weirs consisted of natural substrates, while that of downstream was either natural substrates or a concrete floor. Weir surfaces were crusted with periphyton mats (~1 mm thickness).
Statistical analyses
All statistical tests were carried out using R software ver. 3.3.2 (R Core Team 2017).
To assess the difference in each body size class among the sampling stations, we conducted a Kruskal-Wallis test with a post-hoc Steel-Dwass test using the pSDCFlig function in the NSM3 package (Schneider et al. 2018). To explore factors determining the longitudinal distribution of eels in each size class within a river, we used general linear models (GLMs) with a Poisson distribution and a log-link function or a Gaussian distribution and an identity-link function using the glm function. In these models, the number of eels captured was the dependent variable (Table 2), distance from the river mouth, number of weirs, and the maximum height of weirs (except for inflatable rubber weirs with a fish pass) from the river mouth were the predictor variables (Table 3), and the area of each reach was an offset variable (Table 3). Distance from the river mouth had the highest variance inflation factor (VIF), which indicates the existence of remarkably high multicollinearity between this variable and the others (VIF = 50.9 for distance from the river mouth, VIF = 23.6 and 11.4 for the number and maximum height of weirs, respectively; Table 3). However, previous studies revealed that the density of eels decreased with increasing distance from the river mouth (Tzeng et al. 1995; White and Nights 1997; Laffaille et al. 2003; Lasne and Laffaille 2008). For these reasons, we established GLMs with a two-step process.
We firstly tested the effect of distances from the river mouth on eel densities. In these analyses, the number of eels captured was the dependent variable and distance from the river mouth was the predictor variable. Following the establishment of these models, we tested the weir effects (number and maximum height) on eel density. Due to the removal of the distance effect from these models, we used the residuals of the previous GLMs (eel density versus distance from the river mouth) as the dependent variable, and the number of weirs and the maximum height of weirs as the predictor variables. There was no multicollinearity between the number and maximum height of weirs (VIF = 3.3). These VIF values were calculated using the vif function in the car package (Fox and Weisberg 2018).
To examine the microhabitat use of eels in each size class in the river, we used general additive models (GAMs) with a binominal distribution and a logit link function, using the gam function in the mgcv package (Wood 2018). We used the presence/absence of eel data (1/0) as the binary response variable, and the physical environmental data (DR, CV, DS, ISC, and presence/absence of UWV; Table 4) as predictor variables. Three predictor variables (DR, CV, and ISC) were fitted by smoothing splines. Efficiency of the smoothing parameters was estimated by minimizing the unbiased risk estimator.
For model selection of the GLMs and GAMs, we used the dredge function in the MuMIn package (Bartoń 2017). The best model was selected using Akaike’s information criterion (AIC), which stipulates that the best model for any candidate set applied to a given data set is that with the lowest AIC value. Following Burnham and Anderson (2002), models with ΔAIC <2 were assumed to be reasonable alternatives to the best model, and thus were retained.
Results
We collected 48 small-sized (mean = 81.7 mm TL; range = 52.8–127.0 mm TL) and 23 large-sized eels (mean = 351.6 mm TL; range = 200–657 mm TL; Fig. 2; Table 1). The body sizes of small-sized eels in St. 1 were significantly smaller than those in St. 2 and St. 3 (Steel-Dwass test, w = 4.877, p = 0.003 for St. 1 vs. St. 2; w = 5.554, p = 0.001 for St. 1 vs. St. 3; w = 0.402–2.090, p = 0.451–0.992 for other pairs). In contrast, the TLs of large-sized eels were not significantly different among the stations (Kruskal-Wallis test, χ2 = 6.016, p = 0.305).
We evaluated the distribution of Japanese eels within the river using GLMs. In this study system, the densities of both size classes decreased with increasing distance from the river mouth (Table 5). In contrast, weir effects on eel distribution differed between size classes (Table 6). The density of small-sized eels decreased as the number of weirs increased, while the density of large-size eels remained unchanged in relation to weir number.
We used GAMs to examine the microhabitat uses of eels within the river, three models with ΔAIC <2 were selected in both small-sized and large-sized eels (Table 7). In this study system, different physical environmental factors affected eel density depending on their body size (Table 7). For small-sized eels, two physical factors (DR and CV) were significantly important (Table 7). Small-sized eels preferentially used near-shore habitats (<60 cm DR; Fig. 3e) with low CV (<20 cm/s; Fig. 3c). There were no relationships between other physical factors (ISC and UWV) and eel density. There were two GAM model peaks relating to the complex substrate: one peak had an ISC of approximately 0.1 with a larger particle size (high abundance of cobble stones), and the other had an ISC of approximately 0.6 with a smaller particle size (high abundance of pebble stones) (Fig. 3a and d). The peak’s occurrence probabilities were slightly lower in habitats with UWV compared with those with no vegetation (Fig. 3b).
Results of general additive models (full model) on the probability of small-sized eels (<200 mm in total length) occurrence in different microhabitats, including (a) dominant substrate (DS), (b) underwater vegetation (UWV), (c) current velocity (CV), (d) index of substrate complexity (ISC), and (e) distance from the nearest riverbank (DR). Circles indicate all quadrats. Lines with shaded areas (or upper and lower brackets) indicate estimated curves (or lines) with 95% confidence bands. Tick marks on x-axes show the location of observations along the range of continuous explanatory variables
In contrast, the density of large-sized eels was significantly related to four physical factors (DR, CV, DS, and UWV). Moreover, there were weak effects of ISC on large-sized eel occurrence. There were two peaks of DR and CV in the GAM models (Fig. 4e) indicating a positive relationship between large-sized eel density and the following: near-shore habitats (20–40 cm DR) with a low CV (<15 cm/s) and the centers of the river (70–110 cm DR) with a relatively high CV (approximately 55 cm/s). The riverbeds of theses habitats were mainly composed of large substrates (cobble stones and boulders, Fig. 4a), in a relatively simple structure (<0.3 ISC, Fig. 4d). The density of large-sized eels was not dependent on the presence of UWV in their habitats (Fig. 4b).
Results of general additive models (full model) on the probability of large-sized eel (≥200 mm in total length) occurrence in different microhabitats. Figures show the results of (a) dominant substrate (DS), (b) underwater vegetation (UWV), (c) current velocity (CV), (d) index of substrate complexity (ISC), (e) distance from the nearest riverbank (DR) with a left panel inset in focus view. Circles indicate all quadrats. Lines with shaded areas (or upper and lower brackets) indicate estimated curves (or lines) with 95% confidence bands. Tick marks on x-axes show the location of observations along the range of continuous explanatory variables
Discussion
The present study provides important data on the longitudinal distribution and microhabitat use of Japanese eels of two different size classes during their riverine life stages. As shown in previous studies, we found that the density of both small-sized and large-sized eels decreased with increasing distance from the river mouth (Tzeng et al. 1995; White and Nights 1997; Laffaille et al. 2003; Lasne and Laffaille 2008; Yokouchi et al. 2008). We also found a negative relationship between weirs and the density of small-sized eels but not large-sized eels. It is well known that weirs can prevent the upstream migration of anguillid eels, contributing to the loss of longitudinal connectivity within a watershed (White and Knights 1997; Feunteun 2002; Ministry of the Environment, Japan 2016; Kume et al. 2019; Kwak et al. 2019). Our results support those of our previous study which found that the density of small-sized eels decreased as the number of weirs increased (Kume et al. 2019). However, no relationship was found between small-sized eels and maximum weir heights (Kume et al. 2019). Weirs higher than 40 cm have been reported to hinder the upstream migration of Japanese eel elvers (Ministry of the Environment, Japan 2016). It is likely that the occurrence of periphyton mats on the weir surface, which was observed in this study, may help young eels (especially glass eels and elvers) to climb the weirs in our study system (Solomon and Beach 2004). In contrast, we found that the density of large-sized eels was not significantly related to either the number or the maximum height of weirs. Kwak et al. (2019) reported that dams shorter than 3.0 m in height did not prevent the upstream migration of yellow American eels (A. rostrata). In addition, yellow eels tend to show a small home range in a river (Laffaille et al. 2005; Itakura et al. 2018). Therefore, we suggest that eels can spread their distribution within a river when they are at the glass and elver stages and then they will stay there until the onset of maturation (i.e. the silver eel stage) when they move out to sea. Alternatively, large-sized eels may repeatedly move within a river until the onset of maturation. However, further research is needed to elucidate eel movement in rivers in relationship to eel growth stage and size. In addition, the climbing abilities of the two size classes we studied remain unclear. Thus, there is a need for further studies on the relationship between eel body size and weirs, particularly studies that can exclude any effects from proximity to the river mouth.
Moreover, analysis of the microhabitat uses of small-sized and large-sized eels suggested that different physical factors affected eel occurrence depending on body size. The relationship between distance from the nearest riverbank and eel density was strong for small-sized eels but weak for large-sized eels. Our findings suggest that small-sized eels use the near-shore habitat preferentially, while large-sized eels inhabit a much wider area from the near-shore to the center of a river. These results coincide with those of a previous study on European eel (A. anguilla) (Laffaille et al. 2003). Our results also revealed the strong influence of CV on the habitat uses of both small-sized and large-sized eels. The density of small-sized eels increased in habitats with a CV of <20 cm/s. Previous studies demonstrated that small-sized eels preferred habitats with a slower water flow (Barbin and Krueger 1994; Linton et al. 2007). For example, American eels with a small body size (mean 56 mmTL) could not maintain their positions in water columns with a CV exceeding 35–40 cm/s (Barbin and Krueger 1994). In our study, however, large-sized eel density peaked twice, when the CV was approximately 15 and 55 cm/s. Previous studies showed that yellow European eels (mean 444 mmTL), corresponding to the large size class in our study, could swim at 30–55 cm/s under experimental conditions (Quintella et al. 2010). Therefore, the CV is likely to affect how well anguillid eels maintain their positions in the river based on the limitation of their swimming ability.
The riverbed structure is also an important factor for eel habitats because eels settle and hide in various types of riverbed materials during different riverine life stages (Dou and Tsukamoto 2003; Aoyama et al. 2005; Tomie et al. 2013, 2017; Christoffersen et al. 2018). In our study site, the small-sized eels were observed in very complex substrates, composed of small riverbed materials (i.e. pebbles) which created spatially heterogenous habitats (Kume et al. 2019). They were also observed in relatively simple substrates, containing large particle sizes (i.e. cobble). Small gaps can be found between these large particles which may provide opportunities for small-sized eels to hide. By contrast, the large-sized eels used a relatively simple riverbed with large particle sizes (i.e., boulder). Such riverbed materials create larger gaps in which large-sized eels can hide. Therefore, small-sized eels can settle and hide in a wider range of substrate types, while large-sized eels are likely to require larger gaps between larger particles in which to settle and hide.
Previous studies revealed that glass eels and elvers preferred to use habitats with UWV (Laffaille et al. 2003; Johnson and Nack 2013). However, small-sized eels, including the elver and yellow eel stages, showed no tendency to use habitats with the presence of UWV in the present study. Combining our results with those from previous studies suggests that UWV requirement differs between glass eels, elvers, and yellow eels. Thus, further studies on the UWV requirements of small-sized eels at each riverine life stage are needed. However, we also found that large-sized eels avoid habitats with UWV. Previous studies found that large-sized eels hid in UWV in relatively large rivers (Laffaille et al. 2003; Johnson and Nack 2013). Thus, both size classes of eels are likely to use UWV as their habitats.
Overall, the present study revealed size-dependent changes in the distribution and microhabitat use of Japanese eels during riverine life stages. Firstly, weirs acted as barriers to prevent eel upstream migration, and the density of small-sized eels decreased as the number of the weirs increased. The small-sized eels used near-shore habitats with low current velocities as well as relatively complex substrates with smaller particle sizes. The large-sized eels, however, used open habitats, ranging from the near-shore to the center of the river, with low current velocities and simple substrates composed of large particles. These findings provide useful information for the conservation and management of Japanese eels in rivers flowing through paddy fields, which have not yet been used as habitats for the conservation of aquatic organisms, including Japanese eels. Since Japanese eels may require a variety of habitat types depending on river sizes and riverine life stage, the generality and applicability of the obtained results should be validated in several rivers in the future to promote the comprehensive management and conservation of wild eel stocks as well as to implement effective river restoration.
References
Aoyama J, Shinoda A, Sasai S, Miller MJ, Tsukamoto K (2005) First observations of the burrows of Anguilla japonica. J Fish Biol 67:1534–1543
Barbin GP, Krueger WH (1994) Behaviour and swimming performance of elvers of the American eel, Anguitta rostrata, in an experimental flume. J Fish Biol 45:111–121
Bartoń K (2017) Multi-Model Inference. https://cran.r-project.org/web/packages/MuMIn/MuMIn.pdf. Accessed January 30, 2019
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information-theoretic approach. Springer, New York
Cairns DK, Secor DA, Morrison WE, Hallett JA (2009) Salinity-linked growth in anguillid eels and the paradox of temperate-zone catadromy. J Fish Biol 74:2094–2114
Chen J-Z, Huang S-L, Han Y-S (2014) Impact of long-term habitat loss on the Japanese eel Anguilla japonica. Estuar Coast Shelf Sci 151:361–369
Christoffersen M, Svendsen JC, Kuhn JA, Nielsen A, Martjanova A, Støttrup JG (2018) Benthic habitat selection in juvenile European eel Anguilla anguilla: implications for coastal habitat management and restoration. J Fish Biol 93:996–999
Daverat F, Tomás J (2006) Tactics and demographic attributes in the European eel Anguilla anguilla in the Gironde watershed, SW France. Mar Ecol Prog Ser 307:247–257
Domingos I, Costa JL, Costa MJ (2006) Factors determining length distribution and abundance of the European eel, Anguilla anguilla, in the river Mondego (Portugal). Freshw Biol 51:2265–2281
Dou S-Z, Tsukamoto K (2003) Observations on the nocturnal activity and feeding behavior of Anguilla japonica glass eels under laboratory conditions. Environ Biol Fish 67:389–395
Feunteun E (2002) Management and restoration of European eel population (Anguilla anguilla): an impossible bargain. Ecol Engineer 18:575–591
Fox J, Weisberg S (2018) Companion to Applied Regression. https://cran.r-project.org/web/packages/car/car.pdf. Accessed February 24, 2019
Fukuda N, Miller MJ, Aoyama J, Shinoda A, Tsukamoto K (2013) Evaluation of the pigmentation stages and body proportions from the glass eel to yellow eel in Anguilla japonica. Fish Sci 79:425–438
Hunter PR, Gaston MA (1988) Numerical index of the discriminatory ability of typing systems: an application of Simpson’s index of diversity. J Clin Microbiol 26:2465–2466
Itakura H, Kitagawa T, Miller MJ, Kimura S (2015) Declines in catches of Japanese eels in rivers and lakes across Japan: have river and lake modifications reduced fishery catches? Lands Ecol Engineer 11:147–160
Itakura H, Miyake Y, Kitagawa T, Kimura S (2018) Site fidelity, diel and seasonal activities of yellow-phase Japanese eels (Anguilla japonica) in a freshwater habitat as inferred from acoustic telemetry. Ecol Freshw Fish 27:737–751
Jacoby DMP, Gollock, MJ (2014) Anguilla japonica. The IUCN Red List of Threatened Species 2014:e.T166184A1117791
Johnson JH, Nack CC (2013) Habitat use of American eel (Anguilla rostrata) in a tributary of the Hudson River, New York. J Appl Ichthyol 29:1073–1079
Kaifu K (2019) Challenges in assessments of Japanese eel stock. Mar Policy 102:1–4
Kaifu K, Tamura M, Aoyama J, Tsukamoto K (2010) Dispersal of yellow phase Japanese eels Anguilla japonica after recruitment in the Kojima Bay-Asahi River system, Japan. Environ Biol Fish 88:273–282
Kaifu K, Miller MJ, Yada T, Aoyama J, Washitani I, Tsukamoto K (2013) Growth differences of Japanese eels Anguilla japonica between fresh and brackish water habitats in relation to annual food consumption in the Kojima Bay-Asahi River system, Japan. Ecol Freshw Fish 22:127–136
Kaifu K, Yokouchi K, Higuchi T, Itakura H, Shirai K (2018) Depletion of naturally recruited wild Japanese eels in Okayama, Japan, revealed by otolith stable isotope ratios and abundance indices. Fish Sci 84:757–763
Kume M, Terashima Y, Wada T, Yamashita Y (2019) Longitudinal distribution and microhabitat use of young Japanese eel Anguilla japonica in a small river flowing through paddy areas. J Appl Ichthyol 35:876–883
Kwak TJ, Engman AC, Lilyestrom CG (2019) Ecology and conservation of the American eel in the Caribbean region. Fish Manag Ecol 26:42–52
Laffaille P, Feunteun E, Baisez A, Robinet T, Acou A, Legault A, Lek S (2003) Spatial organisation of European eel (Anguilla anguilla L.) in a small catchment. Ecol Freshw Fish 12:254–264
Laffaille P, Acou A, Guillouët J (2005) The yellow European eel (Anguilla anguilla L.) may adopt a sedentary lifestyle in inland freshwaters. Ecol Freshw 14:191–196
Lasne E, Laffaille P (2008) Analysis of distribution patterns of yellow European eels in the Loire catchment using logistic models based on presence–absence of different size-classes. Ecol Freshw Fish 17:30–37
Linton ED, Jónsson B, Noakes DLG (2007) Effects of water temperature on the swimming and climbing behavior of glass eels, Anguilla spp. Environ Biol Fish 78:189–192
Ministry of the Environment, Japan (2016) Report of the project on conservation measures of Japanese eels 2015. Ministry of the Environment, Japan, Tokyo, Japan (in Japanese)
Morrison WE, Secor DH (2003) Demographic attributes of yellow-phase American eels (Anguilla rostrata) in the Hudson River estuary. Can J Fish Aquat Sci 60:1487–1501
Okamura A, Yamada Y, Yokouchi K, Horie N, Mikawa N, Utoh T, Tanaka S, Tsukamoto K (2007) A silvering index for the Japanese eel Anguilla japonica. Environ Biol Fish 80:77–89
QGIS Development Team (2016) QGIS geographic information system. Open Source Geospatial Foundation Project. http://www.qgis.org. Accessed 30 April 2019
R Core Team (2017) R: a language and environment for statistical computing. Foundation for Statistical Computing, Vienna. http://www.r-project.org/. Accessed January 30, 2019
Schneider G, Chicken E, Becvarik R (2018) Functions and datasets to accompany Hollander, Wolfe, and chicken - Nonparametric statistical methods, Third edition. https://cran.r-project.org/web/packages/NSM3/NSM3.pdf. Accessed February 7, 2019
Solomon DJ, Beach MH (2004) Fish pass design for eel and elver (Anguilla anguilla). RandD technical report W2-070/TR. Environment agency, Bristol, UK
Tomie JPN, Cairns DK, Courtenay SC (2013) How American eels Anguilla rostrata construct and respire in burrows. Aquat Biol 19:287–296
Tomie JPN, Cairns DK, Hobbs RS, Desjardins M, Fletcher GL, Courtenay SC (2017) American eels (Anguilla rostrata) substrate selection for daytime refuge and winter thermal sanctuary. Mar Freshw Res 68:95–105
Tsukamoto K, Arai T (2001) Facultative catadromy of the eel Anguilla japonica between freshwater and seawater habitats. Mar Ecol Prog Ser 220:265–276
Tsukamoto K, Aoyama J, Miller MJ (2009) Present status of the Japanese eel: resources and recent research. Am Fish Soc Symp 58:21–35
Tsukamoto K, Chow S, Otake T, Kurogi H, Mochioka N, Miller MJ, Aoyama J, Kimura S, Watanabe S, Yoshinaga T, Shinoda A, Kuroki M, Oya M, Watanabe T, Hata K, Ijiri S, Kazeto Y, Nomura K, Tanaka H (2011) Oceanic spawning ecology of freshwater eels in the western North Pacific. Nat Commun 2:179
Tzeng W-N, Cheng P-W, Lin F-Y (1995) Relative abundance, sex ratio and population structure of the Japanese eel Anguilla japonica in the Tanshui River system of northern Taiwan. J Fish Biol 46:183–201
Wakiya R, Kaifu K, Mochioka N (2016) Growth conditions after recruitment determine residence-emigration tactics of female Japanese eels Anguilla japonica. Fish Sci 82:729–736
Walsh CT, Pease BC (2002) The use of clove oil as an anaesthetic for the longfinned eel, Anguilla reinhardtii (Steindachner). Aquaculture Res 33:627–635
Walsh CT, Pease BC, Hoyle SD, Booth DJ (2006) Variability in growth of longfinned eels among coastal catchments of South-Eastern Australia. J Fish Biol 68:1693–1706
White EM, Knights E (1997) Dynamics of upstream migration of the European eel, Anguilla anguilla (L.), in the rivers Severn and Avon, England, with special reference to the effects of man-made barriers. Fish Manag Ecol 4:311–324
Wood S (2018) Mixed GAM computation vehicle with automatic smoothness estimation. https://cran.r-project.org/web/packages/mgcv/mgcv.pdf. Accessed January 30, 2019
Yokouchi K, Aoyama J, Oka HP, Tsukamoto K (2008) Variation in the demographic characteristics of yellow-phase Japanese eels in different habitats of the Hamana Lake system, Japan. Ecol Freshw Fish 17:639–652
Yokouchi K, Fukuda N, Miller MJ, Aoyama J, Daverat F, Tsukamoto K (2012) Influences of early habitat use on the migratory plasticity and demography of Japanese eels in Central Japan. Estuar Coast Shelf Sci 107:132–140
Acknowledgements
We thank the staff at Soma Branch of Fukushima Prefectural Fisheries Experimental Station, Shigeho Ijiri, Moemi Horiuchi, Tomoya Hori, Takuji Noda, Hideto Nakanishi, and Daichi Tyushi, for supporting the field work.
Funding
This work was supported by the River Fund of the River Foundation (2018–5211-002) to M.K., JSPS KAKENHI (16H02563) to Y.Y., and the Link Again Program of the Nippon Foundation - Kyoto University Joint Project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kume, M., Terashima, Y., Kawai, F. et al. Size-dependent changes in habitat use of Japanese eel Anguilla japonica during the river life stage. Environ Biol Fish 103, 269–281 (2020). https://doi.org/10.1007/s10641-020-00957-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10641-020-00957-w