Abstract
The density, size and age distribution were investigated for 233 eels, Anguilla japonica, sampled in fresh and brackish water areas of the Kojima Bay-Asahi River system, Okayama, Japan, to evaluate the possible patterns of dispersal of eels that recruit to this area. Migratory histories of 183 eels were categorized into 5 types depending on the Sr and Ca concentrations in their otoliths: (1) brackish water residents (74 fish, 40.4%), which settled in saline water and remained until capture; (2) freshwater residents (46 fish, 25.1%), which settled in freshwater and remained until capture; (3) upstream shifters (3 fish, 1.6%), which settled in saline water and moved upstream into freshwater; (4) downstream shifters (53 fish, 29.0%), which settled in freshwater and moved downstream into saline water; (5) multiple habitat shifters (7 fish, 3.8%), which shifted their habitats between freshwater and saline water more than twice. For eels captured in the brackish water area, fish density decreased with distance in the downstream direction, while the size and age of eels increased. For eels captured in the freshwater area, size and age were greater than those in the upper-most brackish site. These observations suggest that eels in this system initially accumulate in the lower reaches of the river and then disperse in both upstream and downstream directions following their growth.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The Japanese eel, Anguilla japonica, is a catadromous fish species that spawns in waters west of the Mariana Islands, and their larvae migrate to the freshwater and estuarine habitats of East Asia (Tsukamoto 1992, 2006). After metamorphosis into yellow eels, they spend most of their life in continental waters until the onset of sexual maturation (Matsui 1972; Tesch 1977). This anguillid species is an important commercial fish, and recruiting glass eels are intensively captured for use in aquaculture. Recent serious declines of the Japanese eel stock (Tatsukawa 2003; Tsukamoto et al. 2009) require accurate ecological information for effective resource management.
Recent studies based on otolith microchemistry have shown that upstream migration of anguillid eels into freshwaters is facultative, and have confirmed the occurrence of sea eels, or marine residents that have never entered freshwater (Tsukamoto et al. 1998; Tsukamoto and Arai 2001). Thereafter, a considerable number of studies on the variations of life histories of anguillid eels have been done (Jessop et al. 2002; Tzeng et al. 2002, 2003; Arai et al. 2003, 2004; Limburg et al. 2003; Morrison et al. 2003; Kotake et al. 2003, 2004, 2005; Daverat et al. 2005; Daverat and Tomas 2006; Shiao et al. 2006; Lin et al. 2007). These studies found consistent divergences of life history patterns in five species of temperate eels that include river, estuarine and marine residents, and eels that move from one habitat to another once or more during their growth phase.
Yellow eels in freshwater have long been intensively studied about their distribution, abundance, growth, feeding, and other ecological aspects (Tesch 1977; Moriarty 2003). Compared to eels in freshwater habitats, the number of studies on eels in saline habitats (brackish and seawater) is still limited. Most studies on the ecology of eels in saline habitats only described the proportion of migratory types (Arai et al. 2003; Kotake et al. 2003, 2004) and/or only compared the biological aspects of eels (e.g., growth rate) to different habitats such as freshwater (Jessop et al. 2002; Morrison et al. 2003; Tzeng et al. 2003; Arai et al. 2004; Kotake et al. 2005), but little attention has been given to comparisons among eels inhabiting a single saline environment. For a better understanding of the behavior and ecology of eels in brackish and seawater habitats, investigation of eels in a single estuarine system over a small geographic scale is required.
Costa et al. (2008) reported that smaller individuals of Anguilla anguilla in the brackish water zone preferred upper reaches rather than lower reaches, and that eels were more abundant in the middle part of the estuary and decreased both in the upstream and downstream directions. Another study showed that A. anguilla glass eels arriving from the sea and migrating up the estuary accumulated the upper limit of tidal flow and then dispersed as newly transformed yellow eels (Edeline et al. 2007). Based on those two studies, eels might be suggested to settle around the upper limit of tidal flow and then move into both the upper and lower reaches of the river as they grow. For freshwater, this suggestion is strongly supported by a large number of observations that reported size increases and abundance decreases of eels in the upstream direction of river systems (Naismith and Knights 1988, 1993; Haro and Krueger 1991; Barak and Manson 1992; Smogor et al. 1995; Tzeng et al. 1995; Oliveira 1997; Feunteun et al. 2003; Goodwin and Angermeier 2003; Laffaille et al. 2003; Moriarty 2003; Lasne and Laffaille 2008; Yokouchi et al. 2008). In contrast to eels in freshwater, the movement of eels in brackish and seawater is still poorly documented. The objective of this study was to investigate the density and characteristics of the eels in the lower region of the Asahi River and in Kojima Bay to learn about the possible patterns of movement of eels in brackish water using fixed sampling sites distributed over a small geographic scales. Throughout this study our reference to “eels” only concerns species of Anguilla and not other anguilliform fishes.
Materials and methods
Study area
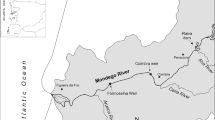
This study was conducted in Kojima Bay and the lower region of the Asahi River, Okayama, Japan (Fig. 1). Kojima Bay is a shallow brackish water area connected to the Seto Inland Sea. Two other considerable freshwater sources are nearby the Asahi River: Lake Kojima and the Hyakken River. The mouth of the Asahi River is free of barriers (falls or dams), while Lake Kojima and the Hyakken River are dammed off to keep the saline water of Kojima Bay away from the freshwater behind the dams. Consequently, aquatic organisms are allowed to pass the mouth of the Asahi River without artificial restraint, while the outlets of Lake Kojima and the Hyakken River are blocked. The Yoshii River enters the lower part of Kojima Bay without artificial restraint same as the Asahi River.
Eels were collected mainly in the brackish water areas in this study. Brackish water and freshwater are divided at the upper limit of tidal influence in the lower Asahi River. The place of this point is determined by a sluice gate 8 km upstream from the river mouth (Fig. 1). From October to May, the gate is open and tidal effects reach about 11 km upstream from the river mouth, while the gate dams tidal flow from June to September. The dividing point of brackish water and freshwater in the Asahi River consequently changes depending on the season, from about 8 km upstream from the river mouth in summer, to about 11 km in other seasons. When the sluice dams the river, a side channel that is about 800 m long connects the upper and lower areas of the sluice gate allowing aquatic animals to move upstream and downstream past this obstacle.
Sampling
Eight sampling sites were in the brackish water area (sites B to I) and one site was in the freshwater area (site A) (Fig. 1). For the brackish water area, sites B and C were located in the lower Asahi River and the rest (sites D to I) were in Kojima Bay. The mean depth and surface water salinity at each sampling time in these sites varied from 0.9 to 3.6 m and from <0.5 to 26.5, respectively (Table 1). Sampling was carried out monthly during 1 year, from September 2007 to August 2008. An exception was the site F, where sampling was done during only 3 months, from September to November 2007. The sluice gate 8 km upstream from the mouth of the Asahi River (upper limit of tidal flow in summer) was arbitrarily determined as the point of reference to represent the distance of sampling sites from the upper limit of tidal flow (Fig. 1).
Eels were collected using refuge traps comprised of 3 cylinders that were open at both ends. In the brackish water area (site B to I), traps were made of gray PVC pipes of 1 m length and 50 mm caliber, and about 30 traps per sampling site were attached to ropes with 5 m intervals. In the freshwater area (site A), bamboo cylinders of 1 m length and various sized calibers (mean ± SD = 47.8 ± 7.9 mm) were used, and 50 traps were arranged with more than 2.5 m intervals. Sampling was undertaken by boat during daytime.
Morphological observations
The total length (TL) of each captured eel was measured to the nearest 1 mm. Sex was determined by visual inspection of gonad morphology. The growth stages of eels (yellow-eel or silver eel stages) were confirmed by the examination of the color of the fish body and pectoral fins according to the silvering index of Okamura et al. (2007).
Otolith analysis
The sagittal otoliths were embedded in epoxy resin and ground to expose the core along the anterior-posterior direction. They were further polished with OP-S liquid and Pt-Pd-coated. A wavelength-dispersive X-ray electron microprobe (JEOL JXA-8900R) was used to measure Sr and Ca concentrations in the otoliths, in a line along the longest axis of each otolith from the core to the edge as described in Tsukamoto and Arai (2001). CaSiO3 and SrTiO3 were used as standards. The accelerating voltage and beam current were 15 kV and 12 nA, respectively. The electron beam was focused on a point 10 μm in diameter, with measurements spaced at 10 μm intervals.
Age determination was done with annual rings on the otolith following the Sr and Ca analysis. The otoliths were repolished to remove the coating, etched with 1% HCl, and thereafter stained with 1% toluidine blue to enhance the otolith rings. The age of specimens was determined by counting the number of the otolith rings, following the method of Nagiec´ and Bahnsawy (1990). We assumed that the otolith rings were deposited annually as discussed by Kotake et al. (2007), and assigned the distinct transition check with about 150 µm radius (elver mark) (Arai et al. 2003) thought to be associated with inshore recruitment to coastal (brackish water) areas as age = 0.
Classification of migratory histories
The migratory histories of eels were categorized depending on the Sr:Ca ratio of their otoliths, according to the criteria of Tsukamoto and Arai (2001): mean Sr:Ca ratio ≥ 6.0 × 10−3 for residing in seawater, 2.5 × 10−3 ≤ Sr:Ca <6.0 × 10−3 in brackish water and Sr:Ca <2.5 × 10−3 in freshwater. Because no full seawater was found in the sampling sites of this study, the criteria was modified as Sr:Ca ratio ≥2.5 × 10−3 for residing in brackish water and Sr:Ca <2.5 × 10−3 in freshwater. We first validated whether this criteria was applicable to eels in this study area, using the Sr:Ca ratio of the otolith edge, which would indicate the salinity of each sampling site. Eels were then categorized into 5 groups (Fig. 2): brackish water residents, which settled in saline water and remained until capture (Type 1); freshwater residents, which settled in freshwater and remained until capture (Type 2); upstream shifters, which changed their habitat only once from saline water to freshwater (Type 3); downstream shifters, which shifted their habitat only once from freshwater to saline water (Type 4); multiple habitat shifters, which shifted their habitat more than twice (Type 5). Eels of which otolith Sr:Ca ratio indicated freshwater residence were categorized into the downstream shifters when they were captured in brackish water, and vice versa.
Sr:Ca variations along line transects from the otolith core to the otolith edge of 6 eels collected in Kojima Bay and the Asahi River, with different migratory types. Filled circles and open circles represent the position of the elver marks and otolith rings, respectively. Type 1: brackish water resident; Type 2: freshwater resident; Type 3: upstream shifter; Type 4: downstream shifter; Type 5a and b: multiple habitat shifters
Data analysis
Density indices were calculated as catch per 30 traps. For spatial distribution analysis, we separated eels captured in the brackish water areas (sites B to I) from those in the freshwater area (site A), to analyze the detailed distribution of eels in brackish water. For the brackish water area, the correlation between the distance from the upper limit of tidal flow (the point of reference) and density, size and age of the eels captured were tested using liner regression analysis. For eels in freshwater (site A), the same characteristics were compared with those in the upper-most brackish sampling site (site B). The catch data from January to March 2008 were excluded from the density analysis because no eels were caught at any site in this period.
Results
Size, age and sex
A total of 233 eels were collected and confirmed as yellow eels, while no silver eels were found during the survey. The total length of 218 eels was measured while 15 eels were released because of surpassing the limit of monthly allowable catch permitted for our research. A total of 187 eels were successfully examined for age determination.
The total length of eels caught in the freshwater area (site A) ranged from 347 to 674 mm with a mean of 492.4 mm, those in the lower river area (sites B and C) ranged from 204 to 698 mm with a mean of 417.3 mm, and those in Kojima Bay (sites D to I) ranged from 338 to 756 mm with a mean of 556.0 mm (Table 2, Fig. 3). Eels caught in Kojima Bay were larger in size than those in the freshwater and the lower river area, respectively, and eels caught in the freshwater area were larger in size than those in the lower river area (p < 0.001, student t-test with Bonferroni correction).
The age of eels caught in the freshwater area (site A) ranged from 3 to 14 years with a mean of 7.6 years, those in the lower river area (sites B and C) ranged from 2 to 11 years with a mean of 4.5 years, and those in Kojima Bay (sites D to I) ranged from 3 to 12 years with a mean of 6.4 years (Table 2, Fig. 3). Eels caught in the freshwater area were older than those in the lower river area and Kojima bay, respectively, and eels caught in Kojima Bay were older than those in the lower river area (p < 0.05, student t-test with Bonferroni correction).
Female eels were predominant in freshwater (site A), the lower river (sites B and C) and Kojima Bay (sites D to I) (98.3%, 69.1% and 99.0%, respectively, Table 2). The ratio of male eels of the lower river was higher than those of the freshwater area and Kojima Bay, respectively, and the ratio of male eels of Kojima Bay was higher than that of the freshwater area (p < 0.001, x 2-test with Bonferroni correction). 26.5% of the eels captured at the lower river were too small to determine their sex.
Proportion of migratory types
A total of 183 eels were successfully examined for otolith Sr:Ca analysis. Average Sr:Ca ratio within 50 μm from the otolith edge of eels sampled in freshwater area and brackish water area were 1.8 ± 0.4 and 5.8 ± 1.2, respectively (mean ± SD). For 98.0% of eels sampled in freshwater, average Sr:Ca ratio of the otolith edge was lower than 2.5 × 10−3. For 97.7% of eels sampled in brackish water, average Sr:Ca ratio of the otolith edge was higher than 2.5 × 10−3. These results confirmed that the criteria used in Tsukamoto and Arai (2001) was applicable to the eels in this study area.
In the freshwater area (Site A), the freshwater residents were predominant (90.2%) and the habitat shifters (upstream shifters and multiple habitat shifters) were rare (5.9% and 3.9%, respectively) (Table 2). In contrast, there were a considerable number of the downstream shifters in the brackish water area (40.2%, sites B to I combined), even though the marine residents were predominant (56.1%, sites B to I combined). The multiple habitat shifters were also scarce in the brackish water area (3.8%, sites B to I combined). The ratio of the downstream shifters in Kojima Bay (57.1%, sites D to I) was higher than those in the lower Asahi River (16.4%, sites B and C) (p < 0.001, x 2-test).
Spatial distribution
Brackish water area
The density of yellow eels decreased significantly with distance away from the upper limit of tidal flow (the point of reference) in the Asahi River for the overall sample examined (p < 0.05) and for the brackish water residents (p < 0.001) in the liner regression analysis (Fig. 4). This tendency was not found for the downstream shifters (p = 0.286). Eels of the multiple habitat shifters were rare (n = 5) and they were scattered in the brackish water area (Table 2).
The total length of eels increased significantly with distance away from the upper limit of tidal flow of the Asahi River for the overall sample (p < 0.001), brackish water residents (p < 0.001) and the downstream shifters (p < 0.05) (Fig. 4). The smallest eel captured in Kojima Bay (sites D to I) was 338 mm, while 22 eels smaller than this size were captured in the Asahi River (sites B and C).
The age of yellow eels increased significantly with distance away from the upper limit of tidal flow in the Asahi River for the overall sample (p < 0.001) and the brackish water residents (p < 0.001), but not for the downstream shifters (p = 0.185) (Fig. 4). The youngest eel captured in Kojima Bay (sites D to I) was 3 years old, while in the lower Asahi River (sites B and C), the youngest was 2 years old. The total length and age of the multiple habitat shifters was not compared among sites because of the small sample size (n = 5).
Freshwater area
Both the total length and age of eels from the freshwater area were significantly greater than those from the adjacent sampling site (site B) (p < 0.05, student t-test) (Table 2). The density index of freshwater site (mean ± SD = 1.36 ± 4.25) was not different from that of the site B (mean ± SD = 2.69 ± 6.05) (p = 0.236, student t-test).
Discussion
Migratory types
In this study and previous ones, the freshwater regions of rivers are typically dominated by freshwater residents, and estuarine regions are a mixture of residents and habitat shifters. For the freshwater area in the Asahi River (site A), the freshwater residents were predominant, while upstream shifters and multiple habitat shifters were rare (Table 2). These results were in agreement with the previous studies on 3 species of the genus Anguilla in which all or most eels caught in freshwater habitats were freshwater residents (A. japonica: Tsukamoto and Arai 2001; Tzeng et al. 2002, A. rostrata: Morrison et al. 2003, A. anguilla: Daverat and Tomas 2006). In the lower Asahi River (sites B and C) and Kojima Bay (sites D to I), a considerable number of eels of habitat shifters (downstream shifters and multiple habitat shifters) were found and most of them were downstream shifters that had initially entered freshwater (Table 2). Similar results were reported for A. japonica (Tzeng et al. 2002), A. rostrata (Morrison et al. 2003) and A. anguilla (Daverat and Tomas 2006). The ratio of downstream shifters was significantly higher in the lower river (sites B and C) than in bay (sites D to I) (Table 2). A possible reason of this tendency is discussed below.
In the Gironde River water system in France, it was suggested that a part of the freshwater population of A. anguilla moves downstream to the estuary or coastal zone after achieving a certain size (300 mm) (Daverat and Tomas 2006). This study found similar results for A. japonica with the minimum size of downstream shifters caught in the brackish water areas being 330 mm (Fig. 4).
Comparison of eels in different habitats
The total length of eels caught in Kojima Bay (sites D to I) was larger than those in the freshwater area (site A), while the age of eels in the bay was younger than those in the freshwater area (Table 2, Fig. 3). This indicates faster growth of eels in the bay than those in the freshwater areas. This is consistent with the previous studies that showed higher growth rate of eels in brackish water area than those in freshwater area for 3 species of the genus Anguilla: A. japonica (Kotake et al. 2005), A. rostrata (Morrison et al. 2003) and A. anguilla (Daverat and Tomas 2006). Both the size and age of eels caught in the lower river area (sites B and C) were smallest in the three areas: freshwater area (site A), lower river area and the bay (sites D to I).
Densities of anguillid eels have been suggested to effect their sex ratio, with greater densities leading higher proportion of males (DeLeo and Gatto 1996; Holmgren and Mosegaard 1996; Krueger and Oliveira 1999). The ratio of male eels was the highest at the lower Asahi River (sites B and C) (Table 2) where the density was higher (Fig. 4), even though the ratio was low (4.4%). This higher ratio of male eels could be related not only to the density of eels, but also to the size and age of eels. Helfman et al. (1987) suggested that male A. rostrata leave continental habitats to the spawning area at a smaller size and age than females. In this study, the size and age were smallest at the lower river area where the ratio of males was the highest (Table 2, Fig. 3). For the other areas, the absence of smaller eels could be the reason for the lower ratios of male eels.
Movement of eels
The density, size and age of yellow eels in the present study were related to the distance from the upper limit of tidal flow in the Asahi River. A considerable number of eels smaller and younger than any of the eels from Kojima Bay (sites D to I) were captured in the lower Asahi River (sites B and C) (Fig. 4), despite the use of completely identical sampling gear in the brackish water area. Moreover, the size and age of eels increased with distance from the upper limit of tidal flow in the Asahi River both for the overall sample examined and the brackish water residents. These results indicate that eels in Kojima Bay had probably moved out from the estuary of the Asahi River (above site B) back into the bay, even though it is possible that a part of the eels in the bay had moved from areas other than the Asahi River, such as the Yoshii River.
Some biological characteristics of eels caught in the freshwater area were compared to those in another sampling site of the same system. Both the age and size of eels captured in the freshwater area (site A) were significantly greater than the upper-most brackish site (site B) (Table 2). Although there were slight differences in the sampling gear used at sites A and B, these results are in agreement with previous studies suggesting that eels in upper reaches would have moved upstream from lower reaches following their growth (Barak and Manson 1992; Yokouchi et al. 2008). Consequently, eels in this study area would be suggested to initially accumulate just after recruitment in the area between the site A and B (first settlement) and then a part of the eels gradually move in both upstream and downstream directions following their growth. The first settlement point of glass eels was considered to be equivalent to the upper limit of tidal flow by Edeline et al. (2007). According to this supposition, the fact that the upper limit of tidal flow in the Asahi River is between the site A and B is consistent with the observation that eels in this system disperse outward from this area. However, this study only examined eels more than 2 years of age so glass eel accumulation in this area was not validated.
The decrease of eel density downstream from the upper limit of tidal flow in the Asahi River for both the overall sample and the brackish water residents (Fig. 4) suggests dispersal. Some authors have suggested that yellow eel movements in rivers mainly result from this kind of movements (Smogor et al. 1995; Feunteun et al. 2003; Ibboston et al. 2003; Edeline et al. 2007). Because high density would lead to stronger competition for food and space, the movements of eels in the brackish water area could be explained as density-dependent dispersal resulting in reduced competition. Density-dependent dispersal might explain the greater ratio of the downstream shifters in Kojima Bay (sites D to I) than in the lower Asahi River (sites B and C) where there are high densities of eels (Table 2). In their downstream movement from freshwater to brackish water, passing through the high-density area of the Asahi River would result in a high proportion of downstream shifters in Kojima Bay.
The habitat use pattern observed in this system could be typical for other species of the genus Anguilla, because similar tendencies were reported in A. anguilla. In the Gironde River, France, and the coastal area around the river mouth, the smallest sized yellow-phase European eels were captured in the estuary (Daverat and Tomas 2006). In Portugal, it was reported that smaller yellow-phase European eels preferred the upper brackish water zone, rather than lower reaches, and eels were more abundant in the middle of the estuary and decreased both in the upstream and downstream directions (Costa et al. 2008).
This study showed that yellow eels appear to have initially accumulated in the upper estuary (between site A and B) and then dispersed both in upstream and downstream directions. The estuary of the lower Asahi River, and probably also the lower Yoshii River, therefore could be important habitats for the initial settlement and the early juvenile stage of eels that recruit into the Kojima Bay and its inlet rivers. For the purpose of eel stock conservation, these areas should be carefully managed to avoid over-fishing, pollution and obstacles inhibiting eel movement into and away from these areas.
References
Arai T, Kotake A, Lokman PM, Miller MJ, Tsukamoto K (2004) Evidence of different habitat use by New Zealand freshwater eels Anguilla australis and A. dieffenbachii, as revealed by otolith microchemistry. Mar Ecol Prog Ser 266:213–225
Arai T, Kotake A, Ohji M, Miller MJ, Tsukamoto K, Miyazaki N (2003) Occurrence of sea eels of Anguilla japonica along the Sanriku coast of Japan. Ichthyol Res 50:78–81
Barak NAE, Manson CF (1992) Population density, growth and diet of eels, Anguilla anguilla L., in two rivers in eastern England. Aquaculture and Fisheries Management 23:59–70
Costa JL, Domingos I, Assis CA, Almeida PR, Moreira F, Feunteun E, Costa MJ (2008) Comparative ecology of the European eel, Anguilla anguilla (L., 1758), in a large Iberian river. Environ Biol Fish 81:421–434
Daverat F, Tomas J (2006) Tactics and demographic attributes in the European eel Anguilla anguilla in the Gironde watershed, SW France. Mar Ecol Prog Ser 307:247–257
Daverat F, Tomas J, Lahaye M, Palmer M, Elie P (2005) Tracking continental habitat shifts of eels using otolith Sr/Ca ratios: validation and application to the coastal, estuarine and riverine eels of the Gironde–Garonne–Dordogne watershed. Mar Freshw Res 56:619–627
DeLeo GA, Gatto M (1996) Trends in vital rates of the European eel: evidence for density dependence? Ecological Applications 6:1281–1294
Edeline E, Beaulaton L, Barh RL, Elie P (2007) Dispersal in metamorphosing juvenile eel Anguilla anguilla. Mar Ecol Prog Ser 344:213–218
Feunteun E, Laffaille P, Robinet T, Briand C, Baisez A, Oliver JM, Acou A (2003) A review of upstream migration and movements in inland waters by anguillid eels: toward a general theory. In: Aida K, Tsukamoto K, Yamauchi K (eds) Eel biology. Springer-Verlag, Tokyo, pp 191–213
Goodwin KR, Angermeier PL (2003) Demographic characteristics of American eel in Potomac River drainage, Virginia. Trans Am Fish Soc 132:524–535
Haro AJ, Krueger WH (1991) Pigmentation, otolith rings, and upstream migration of juvenile American eel (Anguilla rostrata) in a coastal Rhode Island stream. Can J Zool 69:812–814
Helfman GS, Facey DE, Hales LS Jr, Bozeman EL Jr (1987) Reproductive ecology of the American eel. Am Fish Soc Symp 1:42–56
Holmgren K, Mosegaard H (1996) Implications of individual growth status on the future sex of the European eel. J Fish Biol 49:910–925
Ibboston A, Smith J, Scarlett P, Aprahamian MW (2003) Colonisation of freshwater habitats by the European eel Anguilla anguilla. Freshw Biol 47:1696–1706
Jessop BM, Shiao J-C, Iizuka Y, Tzeng W-N (2002) Migratory behaviour and habitat use by American eels Anguilla rostrata as revealed by otolith microchemistry. Mar Ecol Prog Ser 233:217–229
Kotake A, Arai T, Ohji M, Yamane S, Miyazaki N, Tsukamoto K (2004) Application of otolith microchemistry to estimate the migratory history of Japanese eel Anguilla japonica on the Sanrilu coast of Japan. J Appl Ichthyol 20:150–153
Kotake A, Arai T, Okamura A, Yamada Y, Utoh T, Oka HP, Miller MJ, Tsukamoto K (2007) Ecological aspects of the Japanese eel, Anguilla japonica, collected from coastal areas of Japan. Zool Sci 24:1213–1221
Kotake A, Arai T, Ozawa T, Nojima S, Miller MJ, Tsukamoto K (2003) Variation in migratory history of Japanese eels, Anguilla japonica, collected in coastal waters of the Amakusa islands, Japan, inferred from otolith Sr/Ca ratios. Mar Biol 142:849–854
Kotake A, Okamura A, Yamada Y, Utoh T, Arai T, Miller MJ, Oka H, Tsukamoto K (2005) Seasonal variation in the migratory history of the Japanese eel Anguilla japonica in Mikawa Bay, Japan. Mar Ecol Prog Ser 293:213–225
Krueger WH, Oliveira K (1999) Evidence for environmental sex determination in the American eel, Anguilla rostrata. Environ Biol Fish 55:381–389
Laffaille P, Feunteun E, Baisez A, Robinet T, Acou A, Legault A, Lek S (2003) Spatial organization of European eel (Anguilla anguilla L.) in a small catchment. Ecol Freshw Fish 12:254–264
Lasne E, Laffaille P (2008) Analysis of distribution patterns of yellow European eels in the Loire catchment using logistic models based on presence-absence of different size-classes. Ecol Freshw Fish 17:30–37
Limburg KE, Svedang H, Elfman M, Kristiansson P (2003) Do stocked freshwater eels migrate? Evidence from the Baltic suggests ‘yes’. In: Dixon DA (ed) Biology, management and protection of catadromous eels. Symposium 33. American Fisheries Society, Bethesda, pp 275–284
Lin YJ, Luzys L, Shiao JC, Iizuka Y, Tzeng WN (2007) Growth differences between naturally recruited and stocked European eel Anguilla anguilla from different habitats in Lithuania. J Fish Biol 71:1773–1787
Matsui I (1972) Eel biology (in Japanese). Kouseisha Kouseikaku, Tokyo
Moriarty C (2003) The yellow eel. In: Aida K, Tsukamoto K, Yamauchi K (eds) Eel Biology. Springer-Verlag, Tokyo, pp 89–105
Morrison WE, Secor DH, Piccoli PM (2003) Estuarine habitat use by Hudson River American eels as determined by otolith strontium:calcium ratios. In: Dixon DA (ed) Biology, management and protection of catadromous eels. Symposium 33. American Fisheries Society, Bethesda, pp 87–100
Nagiec´ M, Bahnsawy MH (1990) Age and growth of female eels, Anguilla anguilla L., in a Polish lake, Jeziorak Lake, Mazurian Lake District, Poland. Aquacult Fish Manage 21:459–470
Naismith IA, Knights B (1988) Migrations of elvers and juvenile European eels, Anguilla anguilla L, in the River Thames. J Fish Biol 33:161–175
Naismith IA, Knights B (1993) The distribution, density and growth of the European eel, Anguilla anguilla, in the freshwater catchment of the River Thames. J Fish Biol 42:217–226
Okamura A, Yamada Y, Yokouchi K, Horie N, Mikawa N, Utoh T, Tanaka S, Tsukamoto K (2007) A silvering index for the Japanese eel Anguilla japonica. Environ Biol Fish 80:77–89
Oliveira K (1997) Movements and growth rates of yellowphase American eels in the Annaquatucket River, Rhode Island. Trans Am Fish Soc 126:638–646
Shiao JC, Lozys L, Iizuka Y, Tzeng WN (2006) Migratory patterns and contribution of stocking to the population of European eel in Lithuanian waters as indicated by otolith Sr:Ca ratios. J Fish Biol 69:749–769
Smogor RA, Angermeier PL, Gaylord CK (1995) Distribution abundance of American eels in Virginia Streams: tests of null models across spatial scales. Trans Am Fish Soc 124:789–803
Tatsukawa K (2003) Eel resources in East Asia. In: Aida K, Tsukamoto K, Yamauchi K (eds) Eel biology. Springer-Verlag, Tokyo, pp 293–298
Tesch FW (1977) The Eel biology and management of anguillid eels. Chapman and Hall, London
Tsukamoto K (1992) Discovery of the spawning area for Japanese eel. Nature 356:789–791
Tsukamoto K (2006) Spawning of eels near a seamount. Nature 439:929
Tsukamoto K, Arai T (2001) Facultative catadromy of the eel Anguilla japonica between freshwater and seawater habitats. Mar Ecol Prog Ser 220:265–276
Tsukamoto K, Aoyama J, Miller MJ (2009) Status of the Japanese eel: Resources and Recent Research. In: Casselman JM, Cairns DK (eds) Eels at the edge: science, status, and conservation concerns. American Fisheries Society, Symposium 58 Bethesda, Maryland, pp 21–35
Tsukamoto K, Nakai I, Tesch WV (1998) Do all freshwater eels migrate? Nature 396:635–636
Tzeng WN, Cheng PW, Lin FY (1995) Relative abundance, sex ratio and population structure of the Japanese eel Anguilla japonica in the Tanshui River system of northern Taiwan. J Fish Biol 46:183–201
Tzeng WN, Iizuka Y, Shiao JC, Yamada Y, Oka HP (2003) Identification and growth rates comparison of divergent migratory contingents of Japanese eel (Anguilla japonica). Aquaculture 216:77–86
Tzeng WN, Shiao JC, Iizuka Y (2002) Use of otolith Sr:Ca ratios to study the riverine migratory behaviors of Japanese eel Anguilla japonica. Mar Ecol Prog Ser 245:213–221
Yokouchi K, Aoyama J, Oka HP, Tsukamoto K (2008) Variation in the demographic characteristics of yellow-phase Japanese eels in different habitats of the Hamana Lake system, Japan. Ecol Freshw Fish 17:639–652
Acknowledgements
We thank Shimizu-Suisan and Momotaro-Suisan for voluntary helping our eel sampling, and the Fisheries Cooperatives of Kojima Bay and the Fisheries Division of Okayama Prefecture for cooperation. Critical comments from MJ Miller and K Yokouchi were highly appreciated. This work was partly supported by the Sasakawa Scientific Research Grant from The Japan Science Society.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kaifu, K., Tamura, M., Aoyama, J. et al. Dispersal of yellow phase Japanese eels Anguilla japonica after recruitment in the Kojima Bay-Asahi River system, Japan. Environ Biol Fish 88, 273–282 (2010). https://doi.org/10.1007/s10641-010-9640-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10641-010-9640-y