Abstract
Although chondrichthyans are conspicuously present in shallow waters, many ecological aspects of neritic species in the Humboldt Current System remain unknown. This study provides a first assessment of the diet of seven commercially exploited and understudied sympatric chondrichthyans inhabiting nearshore habitats off the central coast of Peru: four stingrays (Hypanus dipterurus, Myliobatis peruvianus, M. chilensis, and Urotrygon chilensis), a guitarfish (Pseudobatos planiceps), a smooth-hound shark (Mustelus mento), and a chimaera (Callorhinchus callorynchus). A total of 166 stomachs were examined between 2012 and 2015 and prey items were pooled for the total of years for analysis. Although our analysis did not account for inter seasons variability, our results suggest diet partitioning among species, except for the stingrays’ group. A diet based on soft-bottom polychaetes and fish was shared by H. dipterurus, M. peruvianus, and M. chilensis, while soft-bottom polychaetes and crabs were more important in U. chilensis. The smooth-hound shark and guitarfish exhibited a diet dominated by crabs, and the chimaera consumed mainly hard-bottom mollusks. Foraging habitat estimations distinguished two main habitats of association: Benthic, including the stingray U. chilensis, the chimaera, and the smooth-hound shark; and benthic-demersal, including the guitarfish, and the rest of stingrays. A pattern of feeding specialization was observed for H. dipterurus, P. planiceps, and C. callorynchus. Preliminary trophic level estimations based on diet composition placed these species as secondary consumers. Intraspecific dietary variation was assessed for P. planiceps and H. dipterurus as their sampled sizes allowed meaningful comparisons. The diet of P. planiceps varied from small to large sizes but not for H. dipterurus. No differences were detected on diet composition between males and females in either species. Despite the limited temporal resolution, this study provides the first insights of chondrichthyans predatory activity, suggesting diet partitioning among the species of this assemblage in a nearshore habitat of the central coast of Peru. Enhancing the temporal resolution of this type of studies would improve our knowledge on trophic functioning in the Humboldt Current ecosystem.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Understanding the feeding ecology of species provides fundamental information on community dynamics and the functional role species play in the structure and organization of ecosystems (Lindeman 1942). This is particularly relevant for high trophic level predators, which exert a top-down control on communities at lower levels (Heupel et al. 2014). The trophic cascading effect caused by the loss of predators (e.g., decline of large sharks; Myers et al. 2007), however, requires precise information on diet composition and predator-prey interactions to avoid inaccurate generalizations (Grubbs et al. 2016). Spurious correlations when describing complex dynamics may have negative consequences on conservation and management of exploited ecosystems (Grubbs et al. 2016). This is particularly worrying for coastal ecosystems, which congregate a large faunal diversity (Ross 1986), and are increasingly threatened and modified by human activities (Blaber et al. 2000).
Coastal chondrichthyans (sharks, rays, and chimaeras) play a key role in the food web dynamics. Previous studies highlight the importance of rays in promoting diversity and habitat heterogeneity on benthic communities by disturbing the soft bottoms and enhancing nutrients dispersal (VanBlaricom 1982; Thrush et al. 1994). At midwater column, chondrichthyans also regulate the density of demersal and pelagic preys (Ebert and Bizzarro 2007; Navia et al. 2007; Espinoza et al. 2015). Yet, the basic ecology of many such species remains under investigated in many coastal regions, limiting the implementation of ecosystem-based management plans. Around 39% of the coastal and continental chondrichthyans species listed in the IUCN Red List are categorized as Data Deficient, while 43.6% are considered Threatened (Dulvy et al. 2014). Thus, from a conservation point of view, it is crucial to understand the role these species play in coastal ecosystems (Heithaus et al. 2010).
Along the coast of Peru, in the southeast Pacific, fishing pressure threatens a great diversity of chondrichthyans and it is forecasted that this situation may be worsening due to the absence of implemented management measures that regulate their fisheries (Gonzalez-Pestana et al. 2016). At this coastal region, current trophic models are only focused on benthic macroinvertebrates (e.g., Tam et al. 2008), and small teleosts of commercial interest (e.g., Taylor and Wolff 2007; Taylor et al. 2008) without the inclusion of chondricthyans species. This is an important gap of information since the chondrichthyans’ predatory activity might constitute an important part in the complexity of nearshore trophic dynamics (Tobin et al. 2014). Further, sympatric species often segregate their diets, habitat use, and timing of feeding as a mechanism thought to minimize competition (Ross 1986; Bizzarro et al. 2007; Sánchez-Hernández et al. 2011). For example, in the Colombian Pacific, four coastal sympatric species, a smooth-hound shark (Mustelus lunulatus), two guitarfish (Pseudobatos leucorhynchus and Zapteryx xyster), and a ray (Raja velezi) segregate their diet by preying on distinct species of shrimps (Navia et al. 2007). This strategy has been observed at intraspecific levels as well, with factors such as ontogeny and sex being associated with feeding segregation (Bizzarro et al. 2007; Espinoza et al. 2012; López-García et al. 2012). This type of information is currently missing for coastal chondrichthyans in Peru, despite that detailed knowledge of how species diet varies within a species is crucial to understand their feeding ecology.
This study investigates for the first time the diet composition of an assemblage of seven sympatric chondrichthyans reported in Peru (Cornejo et al. 2015) and inhabiting a nearshore habitat at the central coast. The assemblage is composed by four stingrays: Hypanus dipterurus (synonym Dasyatis dipterura; Jordan and Gilbert, 1880), Myliobatis peruvianus (Garman 1913), M. chilensis (Philippi, 1892), and Urotrygon chilensis (Günther, 1872); a guitarfish: Pseudobatos planiceps (synonym Rhinobatos planiceps; Garman, 1880), a smooth-hound shark: Mustelus mento (Cope, 1877); and a chimaera: Callorhinchus callorynchus (Linnaeus, 1758). The objectives were to: (1) describe and compare the diet composition of these seven species, (2) estimate their dietary niche breadth, trophic level and foraging habitat, and (3) examine the influence of sex, size and their interaction term on the diet of H. dipterurus and P. planiceps, the two most frequently recorded species in the study area. The rays’ species in this study are considered as Data Deficient by IUCN Red List, the shark is considered as Near Threatened and the chimaera is of Least Concern. All these species are of commercial importance, are landed year-round, and lack any management measure, except for a minimum catch size established for M. mento.
Materials and methods
Study area and sampling
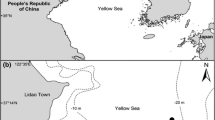
The study area was located on the central coast of Peru (13°15′48.86“ S, 76°18’49.96” W) and encompasses ca. 32 km2 around the marine terminal of PERU LNG (Peru Liquified Natural Gas Co.) (Fig. 1). The seabed around the terminal is dominated by sand and mud with natural scattered rocky patches. Sampling was part of a fish diversity monitoring assessment program, carried out twice per year in October (early Austral spring) and March (late Austral summer) during 2012–2015. The nearshore local conditions are largely influenced by intense year-round coastal upwelling of the Humboldt Current System, with relatively low surface temperatures (14–18 °C), high nutrient levels, and weak seasonality (Taylor et al. 2008).
Map of the study site, showing 26 sampling stations scattered in a nearshore habitat of the central coast of Peru. Black circles (●) correspond to the sampling stations carried out from vessels surveys (at 10 and 20 m depth), and white triangles (∆) show the shoreline sampling stations deployed a long 16 km of the coast around the Marine terminal
Species were collected during surveys carried out from fishery-independent vessels (10 and 20 m depth), and by artisanal fisheries carried out at the shoreline (0–5 m depth) along the study area. Specimens collected from vessels were taken by using surface and bottom multifilament gillnets of 50 to 80 m length (mesh size ca. 7–25 cm) and 3.8 to 12.5 width, and 200 m longlines (100 J-hooks of sizes 4–9 cm). All gears were deployed during daylight for 45 min each and were replicated twice per sampling site. Artisanal fishermen in the shoreline worked during daylight and overnight using a diverse type of gears, including longlines, handlines, and gillnets; all deployed at seven sites throughout 16 km of the shoreline keeping ca. 2 km apart between sites. Information related to gear, and bait type (if used) was obtained through personal communication with artisanal fishermen.
Fish were identified to species level, sexed, weighed, and measured. Total length (TL – tip of the snout to posterior tip of the tail) was obtained for the smooth-hound shark, the chimaera and the guitarfish, while disc width (DW – distance between the wing tips) was obtained for the stingrays. Whole stomachs were removed from each individual and fixed in 10% formalin solution. Prey items were identified in the laboratory to the lowest taxonomic resolution possible; counted and weighted (blotted mass 0.001 g of precision). When the exact number of individuals of a prey item could not be determined due to an advanced digestion degree, we assigned a unit value to avoid overestimation bias. Unity value was applied to a minimal number of prey items, so that digestion rate had not influenced the representation of both, soft and hard prey at prey number level. If bait was found in the stomach content, it was removed from the analysis only if they appeared as recently consumed when compared to the rest of contents.
Data analyses
Diet composition was described at two levels: (1) prey items were described at the lowest taxonomic level of prey identification; and (2) grouped into eight categories considering their taxonomic affinity and the associated substratum type with the goal to identify the chondrichthyans’ most likely foraging habitat: teleost fishes (FISH), hard-bottom mollusks (MOL_hb), soft-bottom mollusks (MOL_sb), epibenthic crabs (CRB), sand burrowing crabs or soft-bottom crabs (CRB_sb), shrimps-like crustaceans (SHR), hard-bottom polychaetes (POL_hb), and soft-bottom polychaetes (POL_sb). Fish prey was grouped in one whole category since the items found corresponded mostly to either unidentified or pelagic fish species. Incidentally ingested matter not considered part of the chodrichthyes’ diet (i.e. plant matter, unidentified inorganic matter, or parasites) were excluded from the analyses.
Cumulative prey curves were constructed using the Clench (1979) equation to evaluate whether the number of stomachs analyzed was large enough to appropriately describe species diet composition. The order in which samples were added to the curve was randomized 999 times to smooth the entry of new species (Ferry and Cailliet 1996). The cumulative number of each prey item and prey group was plotted against the number of stomach examined. Curves that reached an asymptote with a slope (s) at the end of the curve less than 0.1 (e.g. first derivative of the tangent of the slope at the end of the curve) were considered reliable enough as to describe the species diet (Jiménez-Valverde and Hortal 2003).
The Prey-Specific Index of Relative Importance (% PSIRI) (Brown et al. 2012) was calculated to describe the prey contribution in the diet of each species:
This measure combines values of frequency of occurrence (%FO) and prey-specific abundances (%PNi – by counts, %PWi - by wet mass); being the latter a modification of %Ni (mean number) and %Wi (mean weight) defined by Hyslop (1980). PSIRI was proposed as a standardized measure of prey contribution that contrasts with the IRI (Pinkas et al. 1971) because of its additive condition between taxonomic levels. This quality eliminates the bias of the non-additive property of the IRI and facilitates comparisons with other studies (Brown et al. 2012). All calculations were based on the number of non-empty stomachs. Prey diversity and trophic niche breadth of each species was characterized using the Shannon-Wiener diversity index (H′) to the base e (natural logarithm), and the Levin’s standardized Index (B) (Krebs 1999):
where: Hj= H′ of species j, Pi= proportion of prey item i (%Ni /100), n = total number of prey items, Bj = B of species j.
Trophic level (TL) was estimated using the following equation (Cortés 1999):
where: TLj = TL of species j, Pk = proportion of prey group k (%Ni /100), TLk = TL of prey group k obtained from the standardized TL for prey taxa compiled by Ebert and Bizzarro (2007). For the case of FISH, a mean trophic level was calculated from the overall identified teleosts preys using information from FishBase (Froese and Pauly 2016). Chondrichthyes’ association to a foraging habitat was determined by using the index proposed by Bizzarro et al. (2017). For this estimation, prey items were assigned to a general habitat of occurrence, being: 1 = benthic, 2 = demersal, and 3 = pelagic (Bizzarro et al. 2017).
A metric multidimensional scaling ordination plot (3D – mMDS) from bootstrapping averages was conducted for a visual examination of the multivariate dispersion of the diet composition of each species (Clarke et al. 2014). Significant differences on diet composition among species were tested using the permutational multivariate analysis of variance (PERMANOVA) (Anderson 2001). PERMANOVA is a semi-parametric test based on a given measure of ecological distance and calculates the significance using permutations. This test is robust enough in cases when data normality and variance homogeneity is not achieved such as in the case of prey groups’ composition. One-way PERMANOVA test was run based on the Bray-Curtis dissimilarity matrix using %W of the prey groups’ contribution to the chondrichthyans’ diet. %W was used for the statistical analyses since this measure best reflects the predator dietary nutritional contribution (Macdonald and Green 1983). Diet composition of all species was compared by pooling the available data sets along the study period (2012–2015) since most species were not recorded in enough numbers per season as to conduct meaningful temporal variation analysis.
Intraspecific significant differences were evaluated only for H. dipterurus and P. planiceps due to their suitable sample size. Two-way PERMANOVA test was conducted to analyze significant effects on diet variability by sex (male or female), ontogeny (immature or mature), and their interaction considering all factors fixed. We classified ontogeny in H. dipterurus in two size classes based on the average size of sexual maturity for males and females individuals of H. dipterurus described by Smith et al. (2007). Due to the absence of prior references for P. planiceps, we used our observations of macroscopic gonadal stages of the guitarfish collected based on a qualitative scale (Snelson et al. 1988; Rojas 2000) to differentiate two size classes. Individuals with a total length less than 100 cm were classified as size class 1 (Sc1, individuals presenting non-developed or reduced gonads), while larger individuals were classified as size class 2 (Sc2, individuals with well-developed gonads or presence of embryos) (Appendix 3). Differences in size distribution of sexes for H. dipterurus and P. planiceps were analyzed with the Kolmogorov-Smirnov test (α = 0.05; Wilcox 2005). Statistical tests were performed using PRIMER v.7 (MDS and PERMANOVA+; Clarke and Gorley 2015), and Statistica v. 7.1 (Clench curves; StatSoft Inc. 2006).
Results
A total of 166 stomach contents of seven chondrichthyes (Hypanus dipterurus, Pseudobatos planiceps, Myliobatis peruvianus, Myliobatis chilensis, Urotrygon chilensis, Mustelus mento, and Callorhinchus callorynchus) were analyzed. All the stomachs analyzed were full, only one empty stomach was found in H. dipteturus (1.82%) during the study period. Specimens analyzed corresponded to early spring season (October) from 2012 to 2014. Only stomach contents of H. dipterurus and P. planiceps’ corresponded to both early spring and late summer seasons throughout the study period; yet, we did not conduct seasonal analysis in diet because of the small sampling sizes collected during the survey seasons. Details on the species’ size intervals and sex ratios are provided in Table 1. When preys were examined to the lowest taxonomic level, cumulative curves were reliable enough only for H. dipterurus, P. planiceps, and M. peruvianus (s ≤ 0.1); but when preys were treated as groups, curves indicated satisfactory sample sizes for all the species, except for U. chilensis (s = 0.26) (Appendix 1). All statistical analyses were performed at the prey group level since cumulative curves depicted sufficient sample sizes; while diet composition was described at both prey items and groups levels.
Diet composition, trophic niche breadth, and trophic level
A total of 44 prey taxa (belonging to 34 families) were identified, comprising decapod crustaceans (40%, 17 prey items: 15 CRB, 2 CRB_sb), mollusks (23%, 10 prey items: 3 MOL_hb, 7 MOL_sb), polychaetes (21%, nine prey items: 2 POL_hb, 7 POL_sb), fish (12%, five prey items), and shrimp-like-crustaceans (4%, two prey items). P. planiceps, H. dipterurus, and C. callorynchus exhibited the lowest values of dietary breadth, whereas high values of prey diversity; contrasting with the rest of species that exhibited broad dietary niches, and varied values of prey diversity (Table 1).
The %PSIRI values for prey groups showed that POL_sb, FISH, MOL_hb, CRB, and CRB_sb were important components in the chondrichthyans diet; while SHR, POL_hb, and MOL_sb were seldom consumed. The diet of the stingrays, H. dipterurus, M. chilensis and M. peruvianus, consisted primarily of POL_sb and FISH. For these species, their consumption of POL_sb was explained by the contribution of the polychaete Abarenicola affinis (%PSIRI > 20). On the other hand, the stingray U. chilensis fed primarily on POL_sb (%PSIRI = 48) and CRB_sb (%PSIRI = 20). However, in contrast with the rest of stingrays, its consumption of POL_sb was explained by polychaetes belonging to the families Onuphidae, Flabelligeridae, and Capitellidae. The guitarfish P. planiceps fed mainly on CRB_sb (%PSIRI = 49) and FISH (%PSIRI = 25); while the smooth-hound shark M. mento fed on CRB and CRB_sb (%PSIRI = 51 and 31, respectively). For both species, the mole crab Emerita analoga made the sole contribution to the category CRB_sb. For M. mento, its diet of CRB was mainly supplied by the crab Platyxanthus orbignyi (%PSIRI = 22) and the hermit crab Pagurus villosus (%PSIRI = 18). The chimaera C. callorynchus, showed a large contribution of MOL_hb in its diet (%PSIRI = 59), with the mussel Semimytilus algosus representing the dominant contributor to this prey category (%PSIRI = 58.6). Even though the consumption of FISH for the whole assemblage was dominated by unidentified teleosts, the small pelagic fish Engraulis ringens was identified as an important prey item for H. dipterurus and M. peruvianus (%PSIRI = 17 and 14, respectively), while Anchoa nassus for M. chilensis (%PSIRI = 14) (Fig. 2, Appendix 2).
Dietary composition of seven species of chondrichthyans determined with the percentage prey-specific index of relative importance (%PSIRI) of each prey group: CRB (crabs), CRB_sb (soft-bottom crabs), FISH, MOL_hb (hard-bottom mollusks), MOL_sb (soft-bottom mollusk), POL_hb (hard-bottom polychaetes), POL_sb (soft-bottom polychaetes), and SHR (shrimp). See also detailed %PSIRI information in Appendix 2
Regarding the habitat of prey occurrence, two main foraging habitats were estimated: Benthic (FH mean = 1.2 ± 0.1 SD), comprising U. chilensis, M. mento, and C. callorynchus; and Benthic-demersal (FH mean = 1.6 ± 0.2 SD), including P. planiceps, and the rest of the stingrays (Table 1). The mean estimated trophic level for the whole assemblage was 3.67 ± 0.20 (SD) and ranged from 3.33 to 3.96. The highest trophic level was estimated for species preying on a greater amount of FISH (%PSIRI > 25), such as M. peruvianus, H. dipterurus, M. chilensis and P. planiceps. Species feeding mainly on invertebrates (i.e. U. chilensis, M. mento, and C. callorynchus) exhibited lower trophic levels (Table 1).
A visual examination of the metric MDS of bootstrap averages of samples belonging to each elasmobranch species suggest that overall the multivariate dispersion did not overlap (Fig. 3). PERMANOVA analysis detected significant differences in prey composition among chondrichthyan species (Pseudo-F(6, 165) = 10.35, p < 0.05). Within the stingrays group, pair-wise comparisons detected significant differences only between H. dipterurus and U. chilensis (t = 2.34, p < 0.05). The guitarfish P. planiceps and the smooth-hound shark M. mento showed also significant differences in their diets (t = 1.80, p < 0.05); as well as the chimaera C. callorynchus with the rest of the assemblage (p < 0.05).
3D mMDS ordination plot from bootstrapped averages (color symbols) of diet composition of each chondrichthyans species calculated from a Bray-Curtis similarity matrix (based on the average %W of the prey groups consumed by each species). Black symbols represent averages (av) of the repeated bootstrap averages per each species
Intra-specific diet composition of Hypanus dipterurus and Pseudobatos planiceps
Cumulative curves by sex and size class for H. dipterurus and P. planiceps indicated reliable sample sizes (s ≤ 0.1) at prey groups level (Appendix 1). Size distribution per sex for both species showed no significant differences (K-S test, p > 0.05). No significant differences were detected on diet composition at prey group level for the factor sex in H. dipterurus (Pseudo-F(1, 49) = 1.98, p > 0.05) and in P. planiceps (Pseudo-F(1, 45) = 0.12, p > 0.05). Similarly, no significant differences were found on the diet composition between size classes in H. dipterurus (Pseudo-F(1, 49) = 1.32, p > 0.05); however, P. planiceps showed significant differences between size classes (Pseudo-F(1, 45) = 8.14, p < 0.05). FISH consumption increased in importance in the diet of larger individuals, while CRB_sb were dominant in smaller individuals (Table 2). No significant differences were found between the interaction of size class and sex for H. dipterurus (Pseudo-F(1,49) = 0.54, p > 0.05), neither for P. planiceps (Pseudo-F(1,45) = 0.71, p > 0.05).
Discussion
This study describes the diet composition of seven sympatric chondrichthyans in coastal waters of the central coast of Peru. Our findings provide preliminary evidence of feeding partitioning among sympatric species (a smooth-hound shark, five rays, and a chimaera), as well as an important predatory activity in benthic and pelagic habitats. Our results offer novel information and a baseline for reference for a much more detailed trophic understanding of the neritic coastal ecosystem off central Peru.
Cumulative prey curves were considered reliable for three of the seven species when preys were analyzed at the lowest taxonomic level, and for the total of species studied when preys were grouped (with exception of U. chilensis). While increasing the sampling effort reduces the accumulation of rare-prey species, we believe that the prey resolution provided in this study is sufficient as to provide a preliminary assessment of the diet composition and feeding differences of the chondrichthyans’ assemblage.
Diet composition and trophic level of coastal chondrichthyans
Although analyses were conducted pooling all sampling years, distinct diet compositions were observed among the studied species, except within the stingrays group. The dietary overlap among the stingrays may be explained by their comparable habitat of association (coastal benthic-demersal habitats) and feeding behavior. H. dipterurus showed a diet based mainly on pelagic fish and soft-bottoms polychaetes, and secondarily on hard-bottom mollusks. This contrasts with other studies in the North Pacific that reported a diet composed by decapods (Bizzarro 2005) and stomatopods (Navarro-González et al. 2012). The geographic variation in prey composition of H. dipterurus suggests a wide trophic adaptability, feature required to cope with the changing availability of prey of the environment. In the central coast of Peru, polychaetes constitute the most abundant infaunal colonizer of the soft bottoms (Tarazona et al. 2003); hence, this taxon might be considered the most important food item for the benthic feeders at this geographic region. For the eagle rays (M. peruvianus and M. chilensis), a comparable diet based on polychaetes and fish was reported in immature individuals (<60 cm DW) from the northern coast of Peru (Torres Mora 1978; Castañeda 1994). In the present study, only small individuals were captured (M. peruvianus < 75 cm DW, M. chilensis < 54.6 cm DW), coinciding their diets with those reported for the northern populations. Diet studies on members of the family Urotrygonidae conducted across all seasons have documented a broad consumption of crustaceans and secondarily polychaetes (Flores-Ortega et al. 2011; Navia et al. 2011); similar diet composition to what was found for U. chilensis in our study area, although herein polychaetes had greater importance in this species diet. The predatory activity of Urotrygonidae species on crustaceans and polychaetes seems to be a common feature regardless latitude and seasonality. Interestingly, even though a diet based on soft-bottom polychaetes was common in all stingrays; polychaetes species consumed by U. chilensis contrasted with the rest of stingrays. U chilensis diet was based mainly on polychaetes of the families Onuphidae, Flabelligeridae, and Capitellidae; while for the rest of stingrays their diet was based entirely on the polychaete Abarenicolla affinis. This pattern of predation on different prey items corresponding to a same taxon has been reported in other coexisting batoids (Navia et al. 2007; Navarro-González et al. 2012), and might be interpreted as a strategy to reduce prey overlapping with other stingrays consuming similar prey resources. The smooth-hound shark M. mento and the guitarfish P. planiceps consumed mainly crabs and fish, although in variable proportions. M. mento preyed predominantly on crustaceans (e.g., both epibenthic and infaunal crabs), while P. planiceps preyed mainly on soft-bottom crabs and fish. The presence of crustaceans on the diet of M. mento and P. planiceps may be attributed to their mouth morphology. Heemstra (1997) described M. mento dentition (i.e. broadly rounded hemispherical-teeth with no discernible cusps) as a special structure adapted for gridding hard-bodied prey, similar to the dentition pattern on the genus Pseudobatos (De la Rosa-Meza 2010). This broad consumption of crustaceans has also been reported in other species of both genera Pseudobatus and Mustelus (Polo-Silva and Grijalba-Bendeck 2008; De la Rosa-Meza et al. 2013; Amariles et al. 2017). Diet composition of the chimaera C. callorynchus was the most dissimilar among the studied species and it was dominated by the hard-bottom mollusk Semimytilus algosus. This prey group was also preferred by individuals from other geographic locations. For example, at the continental shelf of Patagonia, Argentina, this species was reported preying mainly on the bivalves Zygochlamys patagonica and Pitar rostratus (Di Giacomo and Perier 1996); however, in the central-south coast of Chile, their diet was dominated by both brachyuran crabs and bivalve mollusks (Pedraza and Cubillos 2009).
Overall, the entire assemblage was ranked in the secondary order of consumers (TP = 3.7±0.2 mean) due to their predatory activity over benthic and pelagic organisms. Equivalent results have been reported for stingrays and smooth-hound sharks (Dasyatidae = 3.62, Urotrygonidae = 3.52, and Myliobatinae = 3.37, Triakidae = 3.8, Jacobsen and Bennett 2013) and for the chimaera C. callorynchus in other coastal regions (3.4, Froese and Pauly 2016). High values of prey diversity and feeding specialization were found for H. dipterurus, P. planiceps, and C. callorynchus, coinciding with previous reports for these species (Di Giacomo and Perier 1996; Navia et al. 2007; Navarro-González et al. 2012). Contrastingly, the rest of the assemblage (U. chilensis, M. peruvianus, M. chilensis, and M. mento) showed a broad trophic niche (>0.6 Levin index); yet, the few prey items recorded on these species’ diets may reflect few prey available, thus providing early insights of trophic specialization. A pattern consisting on a high diet specialization, despite a large prey spectrum seems to be a feature for Urotrygon spp. and Mustelus spp. in other geographic areas (Navia et al. 2007; Navarro-González et al. 2012; Amariles et al. 2017); likewise, M. chilensis and M. peruvianus suggest moderate levels of feeding specialization in the northern coast of Peru (Torres Mora 1978; Castañeda 1994). We contemplate the fact that the small sample sizes may hide the diet variability precluding us reaching broader conclusion with the present data.
Dietary characteristics can also serve as an indicator of foraging habitat (Bizzarro et al. 2017; Kemper et al. 2017). In the study area, the species U. chilensis, M. mento, and C. callorynchus showed a strong association to the benthic habitat, with a preference for soft bottoms in U. chilensis, hard bottoms in C. callorynchus, and both types of sediments in M. mento. On the other hand, H. dipterurus, M. peruvianus, M. chilensis, and P. planiceps show a benthic-demersal habitat association due to their predatory activity over both pelagic and benthic prey, preferring all soft bottoms. Since most of these fishes disturb the benthos when searching for preys, their feeding activity would influence the structure of the sea floor communities as observed in similar species (VanBlaricom 1982; Thrush et al. 1994). However, the characteristics of this ecological role should be addressed in future studies.
Intraspecific dietary comparisons in Hypanus dipterurus and Pseudobatos planiceps
The stingray (H. dipterurus) and the guitarfish (P. planiceps) exhibited similar prey consumption between sexes. A lack of sex-biased diet has also been observed in other species of the same genus such as Hypanus longus (synonym Dasyatis longa, López-García et al. 2012) and Pseudobatos spp. (Polo-Silva and Grijalba-Bendeck 2008; De la Rosa-Meza et al. 2013). This suggests a non-sex segregate predatory role within the food web of the studied nearshore habitat. Ontogeny, however, did affect the diet, but only for P. planiceps. For this species, the soft-bottom crab Emerita analoga was more important in the diet of smaller individuals, while fishes were more abundant in the larger ones. A characteristic shift from crustaceans to fish prey items has also been observed for other guitarfish species across the Pacific coast (e.g., Valenzuela-Quiñonez 2009; Blanco-Parra et al. 2012; Espinoza et al. 2015). A dietary switch from small to larger sizes may be attributed to larger gape sizes (Kolmann et al. 2015), higher metabolic requirements (Carlson et al. 2004), and the greater mobility in the water column showed by large individuals (Kemper et al. 2017).
A diet dominated by small pelagic fishes, particularly Peruvian anchovy (Engraulis ringens) makes H. dipterurus vulnerable to interactions with the Peruvian anchovy fisheries. This fact generates concern since bycatch represents a significant cause of mortality on chondrichthyans globally (Dulvy et al. 2014). Further, a vast intrusion of the Peruvian anchovy within the nearshore zones has been reported during warm periods (i.e. summers and El Niño events) because of the characteristic upwelling and temperature shifts of the Humboldt current system (Ochoa et al. 2010). This typical fluctuation in the Peruvian anchovy spatial distribution may change their availability in the nearshore zone, and in turn, the feeding dynamics of H. dipterurus. Yet, how the environmental and prey dynamics may alter the feeding interactions of this stingray is a topic that deserves further research.
Chondrichthyans were caught as part of a seasonal monitoring program of fish diversity within the influence area of PERU LNG marine terminal; hence our sample sizes were limited to the number of individuals caught during each survey. Since seasonal variability could not be carefully examined, we recommend caution when interpreting these results in term of temporal variation. The findings of this study show a first description of the diet of these co-existing species for the central coast of Peru. However, further studies are recommended to improve the resolution of these chondrichthyans’ diet since biological and environmental variables are observed to influence the species feeding behavior. Since these chondrichthyans are considered an important regional fishery resource, a better understanding of their feeding interactions and ecological role can have a positive impact towards their conservation and management in the coast of Peru.
References
Amariles DF, Navia AF, Giraldo A (2017) Food resource partitioning of the Mustelus lunulatus and Mustelus henlei (Elasmobranchii: Carcharhiniformes). Environ Biol Fish 100:717–732. https://doi.org/10.1007/s10641-017-0598-x
Anderson MJ (2001) A new method for non-parametric multivariate analysis of variance. Austral Ecol 26:32–46. https://doi.org/10.1111/j.1442-9993.2001.01070.pp.x
Bizzarro JJ (2005) Fishery biology and feeding ecology of rays in Bahía Almejas, Mexico. M.S. Thesis. California State University, Moss Landing Marine Lab, San Francisco
Bizzarro JJ, Robinson HJ, Rinewalt CS, Ebert DA (2007) Comparative feeding ecology of four sympatric skate species off Central California, USA. Environ Biol Fish 80:197–220. https://doi.org/10.1007/s10641-007-9241-6
Bizzarro JJ, Yoklavich MM, Wakefield WW (2017) Diet composition and foraging ecology of U.S. Pacific coast groundfishes with applications for fisheries management. Environ Biol Fish 100:375–393. https://doi.org/10.1007/s10641-016-0529-2
Blaber SJM, Cyrus DP, Albaret JJ, Ching CV, Day JW, Elliott M, Fonseca MS, Hoss DE, Orensanz J, Potter IC, Silvert W (2000) Effects of fishing on the structure and functioning of estuarine and nearshore ecosystems. ICES J Mar Sci 57:590–602. https://doi.org/10.1006/jmsc.2000.0723
Blanco-Parra MP, Galván-Magaña F, Márquez-Farías JF, Niño-Torres CA (2012) Feeding ecology and trophic level of the banded guitarfish, Zapteryx exasperata, inferred from stable isotopes and stomach contents analysis. Environ Biol Fish 95:65–77. https://doi.org/10.1007/s10641-011-9862-7
Brown SC, Bizzarro JJ, Cailliet GM, Ebert DA (2012) Breaking with tradition: redefining measures for diet description with a case study of the Aleutian skate Bathyraja aleutica (Gilbert 1896). Environ Biol Fish 95:3–20. https://doi.org/10.1007/s10641-011-9959-z
Carlson JK, Goldman KJ, Lowe CG (2004) Metabolism, energetic demand, and endothermy. In: Carrier JC, Musick JA, Heithaus MR (eds) Biology of sharks and their relatives, 2nd edn. CRC Press, Boca Raton, pp 269–286
Castañeda J (1994) La pesquería artesanal y biología pesquería de especies de importancia económica en caleta San José, Lambayeque-Perú (febrero 1991-septiembre 1992). Tesis de Biología. Universidad Nacional Mayor de San Marcos, pp 23–36
Clarke KR, Gorley RN (2015) PRIMER v7: User Manual/Tutorial. PRIMER-E, Plymouth, UK
Clarke KR, Gorley RN, Sommerfield PJ, Warwick RM (2014) Change in marine communities: an approach to statistical analysis and interpretation, 3rd edn. Primer-E, Plymouth
Clench HK (1979) How to make regional lists of butterflies: some thoughts. J Lepid Soc 33:216–231
Cornejo R, Vélez-Zuazo X, González-Pestana A, Kouri JC, Mucientes G (2015) An updated checklist of Chondrichthyes from the Southeast Pacific off Peru. Check list 11:1809. https://doi.org/10.15560/11.6.1809
Cortés E (1999) Standardized diet compositions and trophic levels of sharks. ICES J Mar Sci 56:707–717. https://doi.org/10.1006/jmsc.1999.0489
De la Rosa-Meza K (2010) Ecomorfología mandibular y dietas de batoideos en el Golfo de California. Ph.D. Thesis. Centro de Investigaciones Científicas y de Educación Superior de Ensenada. BC México, pp 1–308
De la Rosa-Meza K, Sosa-Nishizaki O, de la Cueva-Salcedo H (2013) Feeding habits of the speckled guitarfish Rhinobatos glaucostigma (Elasmobranchii, Batoidea) in the southeastern gulf of California. Cienc Mar 39:277–290. https://doi.org/10.7773/cm.v39i3.2229
Di Giacomo EE, Perier MR (1996) Feeding habits of cockfish, Callorhinchus callorhynchus (Holocephali: Callorhynchidae), in Patagonian waters (Argentina). Mar Freshw Res 47:801–808. https://doi.org/10.1071/MF9960801
Dulvy NK, Fowler SL, Musick JA, Cavanagh RD, Kyne PM, Harrison LR, Carlson JK, Davidson LNK, Fordham SV, Francis MP, Pollock CM, Simpfendorfer CA, Burgess GH, Carpenter KE, Compagno LJV, Ebert DA, Gibson C, Heupel MR, Livingstone SR, Sanciangco JC, Stevens JD, Valenti S, White WT (2014) Extinction risk and conservation of the world’s sharks and rays. eLife 3:e00590. https://doi.org/10.7554/eLife.00590
Ebert DA, Bizzarro JJ (2007) Standardized diet compositions and trophic levels of skates (Chondrichthyes: Rajiformes: Rajoidei). Environ Biol Fish 80:221–237. https://doi.org/10.1007/s10641-007-9227-4
Espinoza M, Clarke TM, Villalobos-Rojas F, Wehrtmann IS (2012) Ontogenetic dietary shifts and feeding ecology of the rasptail skate Raja velezi and the brown smoothhound shark Mustelus henlei along the Pacific coast of Costa Rica, Central America. J Fish Biol 81:1578–1595. https://doi.org/10.1111/j.1095-8649.2012.03410.x
Espinoza M, Munroe SEM, Clarke TM, Fisk AT, Wehrtmann IS (2015) Feeding ecology of common demersal elasmobranch species in the Pacific coast of Costa Rica inferred from stable isotope and stomach content analyses. J Exp Mar Biol Ecol 470:12–25. https://doi.org/10.1016/j.jembe.2015.04.021
Ferry LA, Cailliet GM (1996) Sample size sufficiency and data analysis: are we characterizing and comparing diet properly? In: MacKinlay D, Shearer K (eds) Feeding ecology and nutrition in fish: proceedings of the symposium on the feeding ecology and nutrition in fish, international congress on the biology of fish, San Francisco, CA, 14–18 July 1996, pp 71–80
Flores-Ortega JR, Godínez-Domínguez E, González-Sansón G, Rojo-Vázquez JA, Corgos A, Morales-Jáuregui MY (2011) Feeding habits of three round stingrays (Rajiformes: Urotrygonidae) in the central Mexican Pacific. Cienc Mar 37:279–292. https://doi.org/10.7773/cm.v37i3.1871
Froese R, Pauly D (2016) FishBase. World Wide Web electronic publication. www.fishbase.org. Accessed 4 January 2018
Gonzalez-Pestana A, Kouri JC, Velez-Zuazo X (2016) Shark fisheries in the Southeast Pacific: a 61-year analysis from Peru. F1000Res 3:164. https://doi.org/10.12688/f1000research.4412.2
Grubbs RD, Carlson JK, Romine JG, Curtis TH, McElroy WD, McCandless CT, Cotton CF, Musick JA (2016) Critical assessment and ramifications of a purported marine trophic cascade. Sci Rep 6:20970. https://doi.org/10.1038/srep20970
Heemstra PC (1997) A review of the smooth-hound sharks (genus Mustelus, family Triakidae) of the western Atlantic Ocean, with descriptions of two new species and a new subspecies. Bull Mar Sci 60:894–928
Heithaus MR, Frid A, Vaudo JJ, Worm B, Wirsing AJ (2010) Unraveling the ecological importance of elasmobranchs. In: Carrier JC MJ, Heithaus MR (ed) Sharks and their relatives II. CRC Press: Boca Raton, pp 611–637
Heupel MR, Knip DM, Simpfendorfer CA, Dulvy NK (2014) Sizing up the ecological role of sharks as predators. Mar Ecol Prog Ser 495:291–298
Hyslop EJ (1980) Stomach contents analysis—a review of methods and their application. J Fish Biol 17:411–429. https://doi.org/10.1111/j.1095-8649.1980.tb02775.x
Jacobsen IP, Bennett MB (2013) A comparative analysis of feeding and trophic level ecology in stingrays (Rajiformes; Myliobatoidei) and electric rays (Rajiformes: Torpedinoidei). PLoS One 8:e71348. https://doi.org/10.1371/journal.pone.0071348
Jiménez-Valverde A, Hortal J (2003) Las curvas de acumulacion de especies y la necesidad de evaluar los inventarios biologicos. Revista Ibérica de Aracnología 8:151–161
Kemper JM, Bizzarro JJ, Ebert DA (2017) Dietary variability in two common Alaskan skates (Bathyraja interrupta and Raja rhina). Mar Biol 164:52. https://doi.org/10.1007/s00227-017-3078-0
Kolmann MA, Huber DR, Motta PJ, Grubbs RD (2015) Feeding biomechanics of the cownose ray, Rhinoptera bonasus, over ontogeny. J Anat 227:341–351. https://doi.org/10.1111/joa.12342
Krebs CJ (1999) Ecological methodology, 2nd edn. Harper and row publishers, New York, p 654
Lindeman RL (1942) The trophic-dynamic aspect of ecology. Ecology 23:399–417. https://doi.org/10.2307/1930126
López-García J, Navia AF, Mejía-Falla PA, Rubio EA (2012) Feeding habits and trophic ecology of Dasyatis longa (Elasmobranchii: Myliobatiformes): sexual, temporal and ontogenetic effects. J Fish Biol 80:1563–1579. https://doi.org/10.1111/j.1095-8649.2012.03239.x
Macdonald JS, Green RH (1983) Redundancy of variables used to describe importance of prey species in fish diets. Can J Fish Aquat Sci 40:635–637. https://doi.org/10.1139/f83-083
Myers RA, Baum JK, Shepherd TD, Powers SP, Peterson CH (2007) Cascading effects of the loss of apex predatory sharks from a coastal ocean. Science 315:1846–1850. https://doi.org/10.1126/science.1138657
Navarro-González J, Bohórquez-Herrera J, Navia A, Cruz-Escalona V (2012) Composición trófica de batoideos en la plataforma continental frente a Nayarit y Sinaloa, México. Cienc Mar 38:347–362
Navia AF, Mejía-Falla PA, Giraldo A (2007) Feeding ecology of elasmobranch fishes in coastal waters of the Colombian eastern tropical Pacific. BMC Ecol 7:8–8. https://doi.org/10.1186/1472-6785-7-8
Navia AF, Torres A, Mejía-Falla PA, Giraldo A (2011) Sexual, ontogenetic, temporal and spatial effects on the diet of Urotrygon rogersi (Elasmobranchii:Myliobatiformes). J Fish Biol 78:1213–1224. https://doi.org/10.1111/j.1095-8649.2011.02931.x
Ochoa N, Taylor MH, Purca S, Ramos E (2010) Intra- and interannual variability of nearshore phytoplankton biovolume and community changes in the northern Humboldt current system. J Plankton Res 32:843–855. https://doi.org/10.1093/plankt/fbq022
Pedraza M, Cubillos LA (2009) Hábitos alimentarios y dieta del pejegallo (Callorhinchus callorhynchus Linneaus, 1958) en la zona centro-sur de Chile (33°S a 41°S). In: XXIX Congreso de Ciencias del Mar, Concepción, 2009. Instituto de Investigación Pesquera, Concepción, p 203
Pinkas L, Oliphant M, Iverson L (1971) Food habits of albacore bluefin tuna and bonito in California waters. University of California. https://escholarship.org/uc/item/7t5868rd. Accessed 6 Jan 2018
Polo-Silva C, Grijalba-Bendeck M (2008) Espectro trófico de la raya guitarra Rhinobatos percellens (Walbaum, 1792)(Elasmobranchii: Rhinobatidae) en Santa Marta, Caribe Colombia. Mem Fund La Salle Cien Nat 169:21–33
Rojas P (2000) Contribución al conocimiento biológico de Mustelus lunulatus un recurso pesquero potencial en el Pacífico Colombiano. Tesis de Biología. Universidad del Valle, Cali, pp 1–59
Ross ST (1986) Resource partitioning in fish assemblages: a review of field studies. Copeia 1986:352–388. https://doi.org/10.2307/1444996
Sánchez-Hernández J, Vieira-Lanero R, Servia MJ, Cobo F (2011) Feeding habits of four sympatric fish species in the Iberian Peninsula: keys to understanding coexistence using prey traits. Hydrobiologia 667:119–132. https://doi.org/10.1007/s10750-011-0643-2
Smith WD, Cailliet GM, Melendez EM (2007) Maturity and growth characteristics of a commercially exploited stingray, Dasyatis dipterura. Mar Freshw Res 58:54–66. https://doi.org/10.1071/MF06083
Snelson FF, Williams-Hooper SE, Schmid TH (1988) Reproduction and ecology of the Atlantic stingray, Dasyatis sabina, in Florida coastal lagoons. Copeia 1988:729–739. https://doi.org/10.2307/1445395
StatSoft Inc (2006) Electronic statistics textbook. Statsoft, Tulsa
Tam J, Taylor MH, Blaskovic V, Espinoza P, Michael Ballón R, Díaz E, Wosnitza-Mendo C, Argüelles J, Purca S, Ayón P, Quipuzcoa L, Gutiérrez D, Goya E, Ochoa N, Wolff M (2008) Trophic modeling of the northern Humboldt current ecosystem, part I: comparing trophic linkages under La Niña and El Niño conditions. Prog Oceanogr 79:352–365. https://doi.org/10.1016/j.pocean.2008.10.007
Tarazona J, Gutiérrez D, Paredes C, Indacochea A (2003) Overview and challenges of marine biodiversity research in Peru. Gayana (Concepción) 67:206–231. https://doi.org/10.4067/S0717-65382003000200009
Taylor MH, Wolff M (2007) Trophic modeling of eastern boundary current systems: a review and prospectus for solving the" Peruvian puzzle". Rev Peru Biol 14:87–100
Taylor MH, Wolff M, Mendo J, Yamashiro C (2008) Changes in trophic flow structure of Independence Bay (Peru) over an ENSO cycle. Prog Oceanogr 79:336–351
Thrush SF, Pridmore RD, Hewitt JE, Cummings VJ (1994) The importance of predators on a sandflat: interplay between seasonal changes in prey densities and predator effects. Mar Ecol Prog Ser 107:211–222 http://www.jstor.org/stable/24842678
Tobin AJ, Mapleston A, Harry AV, Espinoza M (2014) Big fish in shallow water; use of an intertidal surf-zone habitat by large-bodied teleosts and elasmobranchs in tropical northern Australia. Environ Biol Fish 97:821–838. https://doi.org/10.1007/s10641-013-0182-y
Torres Mora AO (1978) Biología y pesquería de Myliobatis chilensis y M. peruvianus en la caleta San José, Junio 1976 a Marzo 1977. Tesis de Biología. Universidad Nacional Pedro Ruiz Gallo, pp 1–71
Valenzuela-Quiñonez F (2009) Hábitos alimenticios del pez guitarra Rhinobatos productus en el alto Golfo de California. M.S. Thesis. Centro de Investigaciones Biológicas del Noroeste (CIBNOR), La Paz, B.C.S. México, pp 1-82
VanBlaricom GR (1982) Experimental analyses of structural regulation in a marine sand community exposed to oceanic swell. Ecol Monogr 52:283–305. https://doi.org/10.2307/2937332
Wilcox R (2005) Kolmogorov–Smirnov test. In: encyclopedia of biostatistics, vol 4. John Wiley and Sons, ltd. https://doi.org/10.1002/0470011815.b2a15064
Acknowledgements
We thank PERU LNG and the Smithsonian Conservation Biology Institute for funding this study. We also thank the artisanal fishermen of Chincha and the staff of The Environment Management S.A.C. for their data collection, sample analyses and logistic support during the study, in particular to R. Canales, F. Castagnino and F. Menéndez. We extend our gratitude to Chris Harrod, Alfonso Alonso, and John Ramirez for their constructive comments that helped improve the manuscript. Five anonymous reviewers provided helpful comments on an early version of this manuscript. This publication is contribution No. 44 of the Biodiversity Monitoring and Assessment Program, implemented by the Center for Conservation and Sustainability of the Smithsonian Conservation Biology Institute.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(DOCX 505 kb)
Rights and permissions
About this article
Cite this article
Silva-Garay, L., Pacheco, A.S. & Vélez-Zuazo, X. First assessment of the diet composition and trophic level of an assemblage of poorly known chondrichthyans off the central coast of Peru. Environ Biol Fish 101, 1525–1536 (2018). https://doi.org/10.1007/s10641-018-0797-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10641-018-0797-0