Abstract
Trace element and 87Sr/86Sr isotope analyses of fish pectoral fin rays offer non-destructive methods for determining habitat use. In this study, water and fin ray samples were analyzed for Gulf Sturgeon Acipenser oxyrinchus desotoi from the Choctawhatchee River Basin (FL and AL, USA) and compared with reference samples from Atlantic Sturgeon A. o. oxyrinchus held at controlled salinities (0, 10, 33 ppt). Samples were analyzed using inductively coupled plasma mass spectrometry, with a multi-collector for 87Sr/86Sr. In water, Sr, Ba, Mn and Zn differed between freshwater and saline habitats, with increases in Sr and decreases in Ba, Mn and Zn. 87Sr/86Sr decreased upstream to downstream with lowest values in saline habitats. In the reference study, water trace element concentrations and 87Sr/86Sr corresponded to those in pectoral fin rays. 87Sr/86Sr was higher in pectoral fin ray than water, due to influence of diet, which differed with salinity. In wild fish, trace elements in pectoral fin rays indicated freshwater emigration to saline habitats primarily occurred in the second to third growth zone with some heterogeneity in the population (4% <0.3 years, 39% 0.5–1.3 years, 39% 1.5–2.3 years, 17% 2.5–3.3 years). Analyses of 87Sr/86Sr indicated initial locations of Gulf Sturgeon were in the middle river, with few fish in the upper or lower river. Most (74%) juvenile Gulf Sturgeon utilized more than one river region prior to freshwater emigration and 48% moved upstream temporarily based on increased 87Sr/86Sr. After initial freshwater emigration, fish utilized lower-river to saline habitats. Collectively, these studies demonstrate the usefulness of trace element and 87Sr/86Sr analyses in sturgeon pectoral fin rays.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Field-based assessments of habitat use by fishes may be limited by species rarity, difficulty in sampling certain habitats, fish size and cost. Such challenges are particularly exacerbated at early life history stages. Trace element analysis of calcified structures has been used to retrospectively identify movements and habitat use, particularly in anadromous fishes (Elsdon and Gillanders 2003; Nelson et al. 2013). Calcified structures incorporate trace elements from the environment with calcium as fish grow, and chemistry changes can be related to fish age (Campana 1999; Elsdon et al. 2008). This technique has primarily been applied to otoliths, which are relatively inert (Campana 1999), but can also be applied to other calcified structures such as scales and fin rays (Clarke et al. 2007; Allen et al. 2009; Altenritter et al. 2015).
Sturgeons are an imperiled group of long-lived fishes (Birstein 1993), and the use of structures such as fin rays is particularly beneficial due to their non-lethal collection (Collins and Smith 1996; Nguyen et al. 2016). Further, these structures are frequently collected in population monitoring studies for ageing purposes (Nelson et al. 2013) and are frequently easier to age than otoliths (Stevenson and Secor 1999), although accuracy may decrease in fish >14 years of age (Bruch et al. 2009). Early life history stages of most sturgeons are difficult to sample in their natural environments and as a result, they are poorly understood, with particular uncertainty in early freshwater habitat use and timing of seawater entry (Secor et al. 2002). In sturgeons, trace element analyses of pectoral fin rays have been used to determine the timing of seawater entry in Green A. medirostris (Allen et al. 2009), Russian A. gueldenstaedti (Arai et al. 2002), and White A. transmontanus (Veinott et al. 1999) Sturgeons and predict natal origin within freshwater in Shovelnose Scaphirhynchus platorynchus, Pallid S. albus and Lake Sturgeons A. fulvescens (Phelps et al. 2012, 2016). Studies have also shown the usefulness of these techniques in Atlantic Sturgeon A. oxyrinchus oxyrinchus (Stevenson and Secor 1999; Balazik et al. 2012) and using scutes in Shortnose Sturgeon A. brevirostrum (Altenritter et al. 2015). The validation of trace element techniques in sturgeons using reference conditions has been conducted in several studies (Allen et al. 2009; Balazik et al. 2012; Phelps et al. 2012; Altenritter et al. 2015; Carriere et al. 2016), few of which utilized multiple salinities to represent a range of environmental conditions. Further verification is needed, particularly considering the potential physiological regulation of certain trace elements (Sturrock et al. 2015; Loewen et al. 2016).
Another technique, the analysis of strontium (Sr) isotopes, has been used in recent years to aid in identification of natal spawning areas (Hobbs et al. 2007, 2010). Sr is incorporated into bedrock and released by weathering processes. In freshwater drainages it has been measured at constant ratios spanning season and year (Kennedy et al. 2000). The four naturally occurring isotopes of Sr differ in relative abundance: 84Sr (0.56%), 86Sr (9.87%), 87Sr (7.04%) and 88Sr (82.53%) (Capo et al. 1998). 87Sr is formed by the slow radioactive decay of 87Rb, allowing concentrations of 87Sr to be related to age and type of bedrock (Capo et al. 1998). In contrast, concentrations of 86Sr do not change by radioactive decay and are relatively stable in many environments (Capo et al. 1998). The ratio of 87Sr/86Sr is characteristic of freshwater rivers and within river reaches due to differences in bedrock material, whereas oceanic 87Sr/86Sr is relatively homogenous (Capo et al. 1998). Calcified structures in fishes have been shown to relate closely to water concentrations of 87Sr/86Sr (Ingram and Weber 1999; Kennedy et al. 2000), with similar ratios found in otoliths, scales and vertebrae (Kennedy et al. 2000). Studies have primarily examined 87Sr/86Sr in otoliths although a recent study described methodology for reliable analyses in structures such as sturgeon fin rays (Willmes et al. 2016). Analysis of 87Sr/86Sr has particular application to freshwater and low salinity habitat use (Milton and Chenery 2005; Hobbs et al. 2010; Chase et al. 2015; Hodge et al. 2016). Therefore, Sr isotopes offer a means to evaluate use of freshwater river reaches, as long as bedrock and corresponding water signatures are differentiable with river location or distance. This technique has recently been validated in reference groups of sturgeon held under controlled freshwater conditions (Sellheim et al. 2017), and Sr metabolism of sturgeon indicates the pectoral fin ray is a useful structure for assessing Sr due to accumulation and long-term retention (Carriere et al. 2016). However, no studies have documented longer-term validation (> 3 months) of this technique with sturgeons nor evaluated the effects of salinity.
The Gulf Sturgeon (A. o. desotoi) is an imperiled species endemic to the northern Gulf of Mexico (Ross 2001), and has a different genetic stock structure and geographic distribution than a closely-related sub-species, the Atlantic Sturgeon (A. o. oxyrinchus), which occurs along the Atlantic coast (Waldman et al. 2002). Early life history habitat use within freshwater and the timing of emigration to saline habitats are not well known. Few studies have located first year juveniles, with limited evidence indicating freshwater habitat use spans a broad length of a natal river (Sulak and Clugston 1998), similar to predictions from laboratory early dispersal studies (Kynard and Parker 2004). Juveniles are thought to first emigrate from freshwater to saline habitats late in their first year of life (Sulak and Clugston 1998). Habitat use thereafter includes winter use of saline habitats such as estuaries (Fox et al. 2002; Hightower et al. 2002; Sulak et al. 2009) and offshore island regions (Ross et al. 2009). Juveniles and adults return to freshwater habitats where they apparently seek thermal refuge from warm saline habitats during summer months (Hightower et al. 2002). Adults move up-river to spawn in the spring, but there has been limited success in locating actual spawning locations (Fox et al. 2000).
Restoration plans for this species necessitate identification of habitats important to different life history stages, such as for spawning, early life history growth, holding or resting, and foraging. The Choctawhatchee River System in Florida and Alabama, USA, provides an excellent model to evaluate habitat use using trace element and Sr isotopic techniques, due to the relatively simple system consisting of two distinct branches of the main river, a long drainage (> 200 km), and an estuary terminating at the northern Gulf of Mexico. The identification of habitat use at juvenile life history stages and adult spawning regions contributing to recruitment of juveniles would be beneficial to guiding restoration for this species. Therefore, the goal of this project was to determine past habitat use of early-juvenile to adult Gulf sturgeon through trace element and Sr isotopic analyses. Objectives were to quantify trace element concentrations and Sr isotope ratios within the Choctawhatchee River System, validate the use of pectoral fin rays of Gulf Sturgeon for trace element and Sr isotope analysis using reference studies, and analyze wild Gulf Sturgeon pectoral fin rays and develop retrospective habitat use based on these techniques.
Methods
Water sample analysis of the Choctawhatchee River System
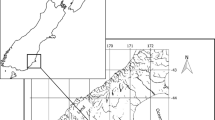
Water samples were collected throughout the Choctawhatchee River System (FL and AL, USA) during July 2015, ranging from the limits of accessibility to sturgeons in the upper reaches to its confluence with the ocean (Fig. 1). The drainage basin has two main river arms, the Choctawhatchee River to the east and the Pea River to the west, meeting near Grenada, AL. After the confluence, the Choctawhatchee River flows approximately 150 km until it empties into a large estuarine region, Choctawhatchee Bay, and then into the Gulf of Mexico near Destin, FL. This river system serves as an excellent model to test the efficacy of trace element techniques for Gulf Sturgeon due to its moderate size, discrete location, and existing information base on Gulf Sturgeon.
Water samples were acidified to pH <2 using 16 N trace element grade nitric acid (Fluka TraceSelect, Sigma Aldrich, St. Louis, MO, USA), filtered (0.45 um, VWR, Radnor, PA, USA) and stored at 4 °C prior to analyses. Water samples were evaluated for trace elements using solution inductively coupled plasma mass spectrometry (ICPMS) to determine the elements with the greatest changes between freshwater, estuarine and oceanic areas. Due to differences in concentration, freshwater samples were diluted 5 times and seawater samples were diluted 10 times for determination of 208Pb, 55Mn, 66Zn, and 137Ba, and 2000 times for 44Ca, 26Mg, and 86Sr, and measured separately. Sample and standard preparation were conducted in a laminar flow hood using ultra high purity nitric acid (Optima grade A467, Fisher Scientific) and Milli-Q deionized water (18.2 MΩ). A Thermofinnigan Element2 Sector Field ICPMS with Cetac SX-100 autosampler was used, with wash time of 60 s, take-up time of 120 s, argon as the sample, cooling, auxiliary gas and 4 runs/10 passes for low resolution elements and 3 runs/10 passes for medium resolution elements.
Water samples were also analyzed for Sr isotopic (87Sr/86Sr) changes using a multi-collector ICPMS following methods by Willmes et al. (2016). For determination of 87Sr/86Sr in water samples, a multi-collector (MC) ICPMS instrument was used (Nu Plasma HR, Nu Instruments Ltd., Wrexham, UK). Samples were introduced with a desolvating nebulizer system (DSN-100), and computer software (Nu Plasma v. 1.4.2011) calculated twice the standard error (SE) from the baseline as the detection limit and also identified outliers. Detectors (sapphire-cased graphite to gold contact faraday cups amplified by 1011 Ohm feedback resistors) were maintained in a pressure and temperature controlled environment and calibrated every 24 h. 87Sr/86Sr data were internally normalized by the measured 86Sr/88Sr ratio relative to the ratio of natural abundances of 86Sr and 88Sr: 0.1194. 85Rb was measured to correct for the potential of small amounts of 87Rb present following Willmes et al. (2016). NIST SRM 987 (Sr carbonate) was analyzed every 6 analyses, and a correction factor was applied to data based on the difference from the established value of 0.710249 (Housh and McMahon 2000). Marine coral was used as an in-house standard.
Fin ray trace element analysis
Gulf Sturgeon fin rays were collected by gill net sets, following Fox et al. (2002), from the Choctawhatchee River, FL on October 13 and 14, 2011 near river km 40, in collaboration with Dr. Stephania Bolden (National Oceanic and Atmospheric Administration) (Fig. 1). Sections (1–2 cm) of the leading pectoral fin ray were collected near the point of articulation using a coping saw from 24 fish ranging from juveniles of approximately 1 m in total length to adults approaching 2 m in length, with juveniles and adults based on size classes identified by Sulak and Clugston (1999).
To better understand relationships between trace element and isotope concentrations in water of varying salinity and pectoral fin rays of wild Gulf Sturgeon, fin ray samples were collected from Atlantic Sturgeon maintained at known salinities. Due to the unavailability of Gulf Sturgeon from restrictions imposed by US endangered species status, Atlantic Sturgeon were used as a reference species. It is important to note that Atlantic and Gulf sturgeon are two sub-species of A. oxyrinchus and so it is reasonable to assume metabolism of Sr isotopes would be the same in both sub-species.
Atlantic Sturgeon were first generation progeny of wild broodstock obtained from the Bears Bluff National Fish Hatchery, Wademalaw Island, SC, in collaboration with the Warm Springs National Fish Hatchery, Warm Springs, GA. Fish were transferred to the Mississippi State University South Farm Aquaculture Facility. Fish were held for approximately one year in fresh water (flow-through well-water) and then transferred to recirculating systems with either fresh water (0 ppt), brackish water (10 ppt) or seawater (33 ppt) for approximately 5 months as described by Allen et al. (2014). Treatment salinities were made using artificial marine salt (Instant Ocean, Blacksburg, VA) and well-water. All treatments were fed the same commercial diet (Soft Moist Diet, Rangen Inc., Buhl, ID, USA) at 1% body weight/day. In order to mark when fish were subjected to different salinity environments, 25 mg oxytetracycline (liquamycin)/kg fish mass was injected into the dorsal musculature following Stevenson and Secor (1999). Fish were injected at the time of increase in salinity and a second time when salinity was returned to freshwater values, with freshwater fish injected at both of the same times. Fish were subsequently transferred to flow-through fresh water for an additional year, euthanized in 200 mg/L tricaine methanesulfonate, and leading pectoral fin rays were collected. Initial mean (± SE) sizes of fish were 436 ± 13 g, 437 ± 4 mm fork length and 513 ± 5 mm total length (n = 43), and final mean sizes of fish were 3245 ± 137 g, 790 ± 10 mm fork length and 905 ± 12 mm total length (n = 34). All procedures were approved by an institutional animal use and care committee (protocol #13–047).
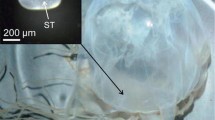
From both wild Gulf Sturgeon and reference Atlantic Sturgeon, leading pectoral fin rays or a 1–2 cm section of the fin ray near the point of articulation were collected using a coping saw, placed in sealed plastic bags on ice, frozen at −20 °C, and then thawed and adhering tissue was cleaned from the surface using forceps and a scalpel. Fin rays were rinsed in deionized water and air dried. Rays were sectioned into 0.5 mm sections using an Isomet low speed saw (Buehler, Lake Bluff, Illinois, USA) with a diamond wafering blade (Allied High Tech Products, Rancho Dominguez, CA, USA). Sections were adhered to 45 × 25 mm glass slides using thermoplastic glue (Crystalbond, Electron Microscopy Services, Hatfield, PA, USA) and polished using 600–3000 grit lapidary film.
Fin ray trace elements (i.e., Ca, Mg, Sr, Ba, Mn, Zn, Pb), were measured using laser ablation (LA) ICPMS. A New Wave Research UP-213 Laser Ablation System with helium carrier gas, and a ThermoFinnigan Element2 Sector Field ICPMS (utilizes magnetic force) with argon as the auxillary, cooling and sample gas were used. For analyses, Iolite 2.2.1 (Paton et al. 2011) was utilized. Trace elements were measured in fin ray growth zones and annuli. Growth zones are thicker layers deposited during the growing season whereas annuli are thinner hypermineralized layers deposited during periods of slow growth (Wright et al. 2002).
Analysis of each sample was preceded by a pre-ablation line transect (40 um wide laser, 0 s laser warm up, speed: 150 um/s, laser output 65%, rep rate 10 Hz) to remove the top layer (3–4 um) of potential contamination along the same transect as the line scan. Line scan ablation parameters consisted of: 30 um wide laser, 30 s laser warm up, speed: 5 um/s (small rays) or 10 um/s (large rays), laser output: 85%, rep rate 20 Hz. During each ablation, one element was analyzed at a time for 0.050 s, divided into ten 0.005 s subsamples, looping through all seven elements (43Ca, 88Sr, 137Ba, 25Mg, 55Mn, 66Zn, 208Pb) repeatedly during each transect. The mean background concentration for each element was removed from total counts and the limit of detection for each element was set at three times the background. Prior to and following each sample, a standard was run (NIST 610). Calcium was set to 27% based on microprobe data for Atlantic Sturgeon (Stevenson and Secor 1999; J.T. Stevenson and D. Secor, Chesapeake Biological Laboratory, University of Maryland, unpublished data).
Fin ray sections were analyzed using line transects from the core to the outer edge along the longest axis (Fig. 2). Polished sections were photographed using a camera (DFC290HD Camera, Leica Microsystems Limited, Wetzlar, Germany) attached to a stereomicroscope (Leica S8AP0) and computer software (Leica Application Suite v. 4.1.0), and laser line transect lengths were determined.
Fin ray 87Sr/86Sr analysis
For fin ray 87Sr/86Sr analysis, a New Wave Research UP213 laser ablation system with the same MC-ICPMS described for Sr isotope water analyses previously was utilized. The carrier gas was helium and makeup gasses were a combination of argon and nitrogen. Analyses consisted of discrete spot ablations, with laser operating parameters of a 30 s warm up, a 12 s delay between each ablation spot, and a 25 s dwell on each spot, at 10 Hz and 65% power. Spot ablations were 55 um-wide, with 10 um in between each spot, with ablation transects conducted from the core to the outer edge along a short axis to avoid organic inclusions (Fig. 2). Internal standards from fin ray sections collected from Green Sturgeon from a saline water source and from White Sturgeon from a freshwater source were run before each slide to check for instrument drift.
To determine relative contributions of diet and water to 87Sr/86Sr in pectoral fin rays, 87Sr/86Sr was measured in pectoral fin rays, commercial fish feed and treatment water. For fin rays, two methods for determining 87Sr/86Sr in treatment regions were compared: laser ablation ICPMS using data from spot ablations within the oxytetracycline marked treatment regions of fin rays (n = fin rays from 6 fish/salinity treatment), and micromilling within the same region of fin rays followed by solution ICPMS. Micromilling utilized a MicroMill Sampling System (1000–3016 model, New Wave, Electro Scientific Industries, Inc., Portland, OR, USA) with a 0.5 mm diameter drill bit (Brasseler USA, Savannah, GA, USA). Fin ray micromilled regions went deeper and sampled a larger area than each discrete ablation spot, due to greater sample volume requirements for analyses. Micromilled fin ray samples (n = 3/salinity) and feed (n = 3) were prepared following Eerkens et al. (2016) and analyzed by solution MC-ICPMS to determine 87Sr/86Sr, similar to water samples. Percent contributions of 87Sr/86Sr in water and diet to 87Sr/86Sr in pectoral fin ray were calculated following Kennedy et al. (2000):
where SrIR = Sr isotope ratio of 87Sr/86Sr; SrIRwater = 87Sr/86Sr in water, SrIRfin ray = 87Sr/86Sr in pectoral fin ray, and SrIRdiet = 87Sr/86Sr in food.
Statistical analysis
Values are reported as mean ± SE. Data for fin ray trace element concentrations, and water and fin ray 87Sr/86Sr from the reference experiment with Atlantic Sturgeon were analyzed using a one-way analysis of variance (ANOVA) with salinity as the independent factor. The relationship between water 87Sr/86Sr and river distance was analyzed with a linear regression. Normality and homogeneity of variance were tested by Shapiro-Wilk and Levene’s tests, respectively. Data transformation (e.g., Log10) was used when necessary to meet ANOVA assumptions. If the overall ANOVA was significant, Tukey’s honestly significant difference (HSD) test was used to compare treatment means. For all analyses, differences were considered significant at P < 0.05 (JMP 12.2.0, SAS Institute Inc., Cary, NC, USA).
Results
Water analyses – Choctawhatchee River System
Both water elemental concentrations (i.e., mg/L or μg/L) and elemental ratios to Ca (e.g., Sr/Ca) were evaluated to determine which had similar patterns to elemental patterns in fin rays for the purpose of life history based movements and habitat (i.e., salinity: freshwater, brackish water [estuarine] or seawater [ocean]) use. In the Choctawhatchee River System, there were distinct differences in trace element concentrations between freshwater, estuarine and ocean regions. Ca, Mg and Sr increased with salinity, Ba and Mn decreased with salinity, and Pb and Zn only changed slightly and were highly variable (Table 1). Ratios of elements relative to Ca had similar relationships (i.e., increasing or decreasing) to water concentrations, albeit large changes in concentration did not necessarily relate to large changes in element/Ca ratios (Table 2). Notably, Sr, which is typically measured in calcified structures, had large (190-fold) concentration increases with salinity (Table 1), but relatively small (2-fold) increases relative to Ca with salinity (Table 2). The greatest differences between freshwater and seawater were seen in decreases in Ba/Ca and Mn/Ca (Table 2). Although Zn/Ca also decreased, concentrations were similar between brackish water and seawater, and Mg/Ca increased with salinity (Table 2).
Similar to water trace element concentrations, Sr isotope ratios (87Sr/86Sr) differed between freshwater and saline habitats (Table 1). Freshwater values were high, whereas brackish water and seawater values were low. Brackish water estuarine samples and seawater ocean samples had similar values (Table 1).
The ratio of 87Sr/86Sr was related to river distance from the estuary, with upriver areas having the highest values and lower river areas, estuary and ocean, having the lowest values (Fig. 3). Both the main stem Choctawhatchee River and tributary streams exhibited this same pattern, although tributaries generally had slightly lower values (Fig. 3). The relationship between 87Sr/86Sr and main stem river distance was y = (5.92 × 10−6)*x + 0.7092, n = 12 locations, p < 0.0001, R2 = 0.94. The relationship between 87Sr/86Sr main stem and tributaries and river distance was y = (4.89 × 10−6)*x + 0.7092, n = 16 locations, p < 0.0001, R2 = 0.83. Importantly for fin ray analyses, both trace element and Sr isotopes differed within the watershed with saline habitats, and in the case of 87Sr/86Sr, the clear relationship with distance in the watershed allowed for prediction of freshwater habitat use.
Water and fin ray analyses – reference systems
Reference conditions were used to establish relationships between water and fin ray trace elements and strontium isotopes for use in prediction of habitat movements in wild fish. In reference conditions for Atlantic Sturgeon, water concentrations of Ca, Mg and Sr increased with salinity and Zn did not change with salinity similar to the Choctawhatchee River System. Although in reference conditions, concentrations of Sr were 3–5 fold higher in freshwater and brackish water (Table 1). Unlike the Choctawhatchee River System, Ba and Mn increased with salinity, and Pb was not detectable (Table 1).
In terms of changes in ratios of elements to Ca, Mg/Ca was at similar levels to the Choctawhatchee River System, increasing with salinity, with an approximately 5-fold increase from freshwater to brackish water and 7-fold increase from freshwater to seawater (Table 2). Notably, Sr/Ca decreased with salinity, with an approximately 3-fold decrease from freshwater to brackish water and seawater, due to higher ratios in freshwater as a result of relatively high Sr and low Ca, unlike the Choctawhatchee River System (Table 2). Although Ba and Mn increased in concentration with salinity (Table 1), their ratios to Ca (Ba/Ca, Mn/Ca) both decreased with salinity, albeit with smaller changes (i.e., Ba: 34–75 fold; Mn: 6–8 fold) compared to the Choctawhatchee River System (i.e., Ba: 20–200 fold; Mn: 13–1300 fold; Table 2). Zn/Ca decreased with salinity, with an approximately 40-fold decrease from freshwater to brackish water and an 80-fold decrease from freshwater to seawater.
In reference fish, fin ray trace element concentrations (i.e., μg /g) had the clearest relationships for Sr and Ba with salinity (Table 3). Sr increased with salinity whereas Ba decreased with salinity, with greater concentrations of Ba in fin rays from fish in freshwater than brackish water and seawater. Fin ray concentrations of Mg, Mn, Pb and Zn did not have a clear increasing or decreasing relationship with salinity (Table 3).
When compared with water trace element concentrations, fin ray concentrations of Sr (i.e., μg/g) (Table 3) showed increases with salinity. This pattern was similar to water concentrations of Sr (i.e., μg/L) which increased (Table 1), but not water concentrations relative to Ca (e.g., Sr/Ca) which decreased (Table 2). In contrast, fin ray concentrations of Ba (Table 3) decreased with salinity. A similar pattern was observed in water Ba/Ca which also decreased with salinity (Table 2), whereas water concentrations of Ba increased with salinity (Table 1).
All water reference 87Sr/86Sr values were lower than those in the Choctawhatchee River System, but notably, the relationship between reference water concentrations of 87Sr/86Sr and salinity were similar, with higher values in freshwater than brackish water and seawater, and very small differences between brackish water and seawater (Table 1). Fin ray 87Sr/86Sr was distinguishable between fish held in freshwater, brackish water and seawater using laser ablation, but only between fish held in freshwater and brackish water or seawater for micromilled and digested samples (Table 4). Ratios of fin ray 87Sr/86Sr from laser ablation were somewhat higher (by 0.00020–0.00044) than water concentrations, although fin ray values from saline habitats were still below water values for freshwater (Table 4). Diet 87Sr/86Sr was determined to be 0.709332 ± 0.000006 by solution MC-ICPMS (n = 3). Contributions of 87Sr/86Sr from water and diet to 87Sr/86Sr in pectoral fin rays were complex and related to salinity. For pectoral fin ray 87Sr/86Sr, the LA-MC-ICPMS method was used to calculate relationships to diet and water due to its use for all other pectoral fin ray samples, and greater precision than solution MC-ICPMS due to more discrete sampling areas and higher replication of subsamples (n = 3–11 ablation spots) within the treatment region of the fin ray. Contribution of 87Sr/86Sr from water was greater than diet, and increased with salinity from 54.1% at 0 ppt to 77.3% at 33 ppt (Table 4), with the equation representing the relationship: y = 53.9962 + 1.4126× −0.0214 × 2, where y = percent contribution to 87Sr/86Sr in pectoral fin rays and x = salinity. Contribution of 87Sr/86Sr from diet decreased with salinity from 45.9% at 0 ppt to 22.7% at 33 ppt (Table 4), with the equation representing the relationship: y = 46.0038–1.4126× +0.0214 × 2.
Fin ray analyses – Gulf Sturgeon – trace elements
Gulf Sturgeon fin ray trace element concentrations were evaluated as a group and by timing of freshwater emigration and saline entry. As a group, mean values were determined for each element and each age for all 24 fish, based on mean values for laser ablation line transect portions in growth zones or annuli (Fig. 4). In fin rays, saline entry was indicated by a rapid increase in Sr, which increases with salinity, and rapid decreases in Ba, Mn and to a lesser degree Zn, which all decrease with salinity (Tables 1 and 2). Mg and Pb were not very useful for identifying saline habitat use based on high variability (Figs. 4 and 5) and lack of differences in water between salinities (Tables 1 and 2).
Mean (± standard error) trace element concentrations of a strontium, b barium, c manganese, d zinc, e magnesium and f lead in Gulf Sturgeon (Acipenser oxyrinchus desotoi) fin ray growth zones for fish presumably entering saline water in growth zone (GZ) 2 (~1.5 yrs. old; n = 9 fish), GZ 3 ~2.5 yrs. old; n = 9 fish) or GZ 4 (~3.5 yrs. old; n = 4 fish)
When all fin rays were analyzed together as a group, Sr was the only element analyzed that clearly increased with age, reaching a plateau by 3.3 years of age. Pb increased, but was highly variable at older ages. Ba, Mn and Zn all decreased with age and reached plateaus between 2.3–4.3 years of age. From 4.3–7.3 years of age, there was relatively little change in trace element concentrations. Differences in fin ray chemistry were evident between growth zones and annuli (Fig. 4). Sr decreased in annuli compared to growth zones, and Zn decreased in growth zones compared to annuli (Fig. 4). Mn was slightly decreased in growth zones, and no clear differences were observed between growth zones and annuli in Ba, Mg and Pb.
When fin rays were analyzed by timing of freshwater emigration and saline entry, there were four patterns: saline entry within growth zone 1 (n = 1), 2 (n = 9), 3 (n = 9) or 4 (n = 4). Thus, 4% of fish emigrate within growth zone 1 (~0.3 years old), 39% emigrate in growth zone 2 (~0.5–1.3 years old), 39% emigrate in growth zone 3 (~1.5–2.3 years old) and 17% emigrate in growth zone 4 (~2.5–3.3 years old). The laser ablation for one fish missed the first growth zone, so this fish was not included in life history type analyses.
Fin ray analyses – Gulf Sturgeon – Predicted freshwater habitat use based on 87Sr/86Sr
Retrospective habitat use in freshwater was predicted based on 87Sr/86Sr in the Choctawhatchee River System (Fig. 6). Assumptions were that water concentrations of 87Sr/86Sr correspond directly to fin ray concentrations, which has been shown for scales and vertebrae in Atlantic salmon (Kennedy et al. 2000). Increases in fin ray 87Sr/86Sr were considered to be related to up-river movements into areas where water 87Sr/86Sr was higher, and similarly, decreases in fin ray 87Sr/86Sr were considered to be down-river movements.
Three methods were used to assess the natal signature of Gulf Sturgeon, the ablation spot nearest the core, the first three ablation spots nearest the core or the first freshwater area utilized based on a cluster of 87Sr/86Sr values from sequential ablation spots. Habitat use was partitioned into regions of the Choctawhatchee River system (Fig. 6) for greater understanding of population patterns. Results for all three techniques were similar, with 1 fish (4%) in upper-river habitats, 0–1 fish (0–4%) in upper-middle river habitats, 8–9 fish (35–39%) in middle river habitats, 12–14 fish (52–61%) in middle-lower river habitats and 0–1 fish (0–4%) in lower river habitats (Table 5).
Gulf Sturgeon moved within the Choctawhatchee River System, based on separate clustering of 87Sr/86Sr values from ablation spots. A total of 17 fish (74%) had more than one area of freshwater utilized, with nine fish (39%) moving to the middle river region and eight fish (35%) moving to the middle-lower river region (Table 5). In addition, 11 fish (48%) moved upriver for at least a brief period of time based on higher 87Sr/86Sr values compared to values from the ablation spot closest to the core. The highest values of 87Sr/86Sr from an ablation spot for each fish indicated that fish were located upstream as far as the upper river (one fish; 4%), the upper-middle river (one fish; 4%), the middle river (17 fish; 74%) or the middle-lower river (four fish; 17%) at some point in their life history (Table 5).
Down-river movements of Gulf Sturgeon, based on 87Sr/86Sr values, indicated that 11 fish (46%) moved to the estuary-ocean and 13 fish (54%) moved to the lower river region at some point in their life history based on the lowest 87Sr/86Sr ablation spot values for each fish (Table 5). The overall “saline” signature as determined by a cluster of low 87Sr/86Sr values from ablation spots following freshwater residence, indicated 23 fish (96%) spent most of their time in the lower river and one fish (4%) spent most of its time in the estuary/ocean.
Discussion
The use of trace elements and Sr isotopes for reconstructing movements of sturgeons has been conducted in relatively few species. Further, use of these techniques in other fishes in structures beyond otoliths is not common. This study establishes the use of these techniques in Gulf Sturgeon pectoral fin rays, evaluating water composition, fin ray incorporation, and predicted habitat use of wild Gulf Sturgeon based on water and pectoral fin ray composition of trace elements and 87Sr/86Sr, and comparison with reference fish held in controlled conditions.
Water analyses – Choctawhatchee River System
Trace elements that are divalent cations substitute for Ca during mineral deposition into calcified structures (Blumenthal 1990), and the rate at which they are exchanged is generally considered to be proportional primarily to their concentration in water and their relative concentration to Ca (Walther and Thorrold 2006). Evaluation of groups of trace elements including both increasing and decreasing elements in relation to salinity can be helpful for better partitioning of past habitat use. In the Choctawhatchee River System, water concentrations of Sr, Mg, Ba, Mn and Pb appeared the most promising for comparison with concentrations in Gulf Sturgeon pectoral fin rays, due to increases in Sr and Mg with salinity and decreases in Ba, Mn and Pb. Relationships of these elements to Ca were somewhat different than their total concentration, with Sr/Ca showing small increases relative to salinity, although Ba/Ca and Mn/Ca had large decreases in relationship to salinity.
Water ratios of 87Sr/86Sr also showed differences between freshwater and saline habitats, but were not as useful for distinguishing within saline habitats due to small differences between estuarine and oceanic salinities. However, relationships between water 87Sr/86Sr and river distance showed a steady decrease from headwaters of the drainage basin to the ocean, suggesting the possibility of broadly identifying natal location and freshwater habitat use.
Water and fin ray analyses – Reference systems
In reference conditions for Atlantic Sturgeon, water concentrations of Sr increased with salinity similar to the Choctawhatchee River system, however, Sr/Ca decreased with salinity. Unlike the Choctawhatchee River System, water concentrations of Ba and Mn increased with salinity, but their ratios to Ca decreased with salinity, albeit Mn was at a much smaller level of decrease. Therefore, concentrations were not directly transferrable to the Choctawhatchee River System, but relationships could still be used to verify whether trace elements were deposited relative to water concentrations. Water 87Sr/86Sr was lower than in fin rays but similar in relationship to salinity between reference conditions and the Choctawhatchee River System.
In pectoral fin rays of reference fish, Sr increased and Ba decreased relative to salinity (Table 3), and were the most useful trace elements for evaluating occupancy of different salinities. The other elements analyzed (Mg, Mn, Pb and Zn) did not have a clear relationship with salinity. Interestingly, although Mg increased in water samples with salinity, there was no relationship with salinity in the fin ray. Notably, Mg was the only element analyzed that occurred at greater concentrations in the water than Ca. The processes of substitution for Ca are likely complex and differ based on the relative concentration of the element. Further, the regulation of certain trace elements such as Mg and Zn are also important for biological processes in the body and are physiologically regulated, complicating environmental relationships to deposition in calcified structures (Loewen et al. 2016). Similar to this reference study, in Green Sturgeon, pectoral fin ray Sr/Ca increased with salinity and Ba/Ca decreased with salinity, and were useful for delineating habitat use (Allen et al. 2009).
Two techniques, micromilling of fin ray samples followed by digestion and solution MC-ICPMS analysis or laser ablation MC-ICPMS from fin ray cross sections, were used to determine whether fin ray 87Sr/86Sr was directly comparable to water 87Sr/86Sr in reference fish. Laser ablation MC-ICPMS had greater precision in sampling and was used for quantification of the contribution of water and diet to fin ray 87Sr/86Sr. Fin ray 87Sr/86Sr was higher than in water, presumably due to the influence of diet. Water contribution was greater than diet to fin ray 87Sr/86Sr, although the contribution was not a static rate, with water contribution increasing with increasing salinity (Table 4). Sellheim et al. (2017) recently found incorporation of White Sturgeon fin ray 87Sr/86Sr is more closely related to water than diet with a lower diet contribution (8%) than the present study, although the study was of a shorter duration (three months) and conducted only in fresh water. Importantly, 87Sr/86Sr in natural diets has been shown to relate directly or very closely to 87Sr/86Sr in the surrounding river water (Ingram and Weber 1999; Kennedy et al. 2000) in contrast to the commercial diet used for reference fish in this study. Therefore, reference fish were useful for establishing diet is also an important influence on 87Sr/86Sr in pectoral fin rays, and the relative influence is complex, changing with salinity. However, in wild fish, diet presumably would originate from the nearby environment and would reflect water 87Sr/86Sr.
In reference fish, annuli were present immediately prior to oxytetracycline marks from injections administered in mid-September. This indicates annuli are deposited in late summer to early fall (i.e., August–September), which also corresponds with timing of annuli deposition in wild Gulf Sturgeon (USFWS 1995). One growth zone and one annulus are deposited every year in Choctawhatchee River Gulf Sturgeon (Baremore and Rosati 2014) and Atlantic Sturgeon (Stevenson and Secor 1999).
Fin ray analyses – Gulf Sturgeon – Trace elements
Increases in Gulf Sturgeon fin ray Sr and decreases in Ba, Mn and to a lesser degree Zn were useful for delineating freshwater emigration and saline entry, whereas Mg and Pb were not very useful for identifying habitat use. In wild fish, Mn was a very good marker of freshwater or saline habitat use, contrasting inversely with Sr. However, in reference fish, Mn was not a clear marker of saline transition. Presumably, the much larger Mn concentration gradient between freshwater and seawater in the Choctawhatchee River System contributed to the distinct changes in Mn in the fin rays.
Trace element analyses revealed heterogeneity within the wild population of Gulf Sturgeon in the Choctawhatchee River System, with 4% of fish emigrating from freshwater to saline water within growth zone 1 (~0.3 years old), 39% emigrating in growth zone 2 (~0.5–1.3 years old), 39% emigrating in growth zone 3 (~1.5–2.3 years old) and 17% emigrating in growth zone 4 (~2.5–3.3 years old). Relative stasis in Sr, Ba, Mn and Zn from 4.3–7.3 years of age indicated prolonged habitat use of estuary and oceanic waters during this time period.
Laser ablation ICPMS typically occurs in a transect from the core or origin of a calcified structure to the outside edge where new deposition is occurring. Important to handling of the data are potential differences in mineral composition between growth zones, deposited when fish are growing, and annuli, deposited when fish are growing very slowly or not at all (Stevenson and Secor 1999). For Gulf Sturgeon, notable differences existed in concentrations of Sr, Zn and Mn between growth zones and annuli. These differences were beneficial for identifying age, but indicate combining data from both areas may complicate interpretation of life history patterns.
Fin ray analyses – Gulf Sturgeon – 87Sr/86Sr
Retrospective habitat use in freshwater, from 87Sr/86Sr, was based on the assumption that water concentrations of 87Sr/86Sr correspond directly to concentrations in calcified structures such as fin rays, previously verified in scales and vertebrae of Atlantic salmon (Kennedy et al. 2000). Diet has also been shown to affect fin ray 87Sr/86Sr (Kennedy et al. 2000), similar to the reference fish in this experiment. However, diet sources within a river system could be expected to relate directly to water 87Sr/86Sr based on very close or direct relationships between 87Sr/86Sr in water and calcified structures in fish (Ingram and Weber 1999; Kennedy et al. 2000).
In this study, pectoral fin ray 87Sr/86Sr data indicated natal habitats were primarily in middle (35–39%) or middle-lower (52–61%) river habitats, with few fish (0–4%) in upper or lower portions of the drainage (Table 5; Fig. 6). Most (74%) juveniles moved to more than one freshwater region prior to emigration to saline waters, generally within the middle to middle-lower region of the river system. Interestingly, almost half of the fish (48%) moved upriver at some point in their early life history, albeit rarely beyond the middle river region. Field studies have found wide dispersal of age-0 juveniles including locations upriver of known spawning grounds (Sulak and Clugston 1998). Up- and down-river movements have also been shown using 87Sr/86Sr in juvenile life stages of other freshwater species (Chase et al. 2015).
In contrast to trace element data, 87Sr/86Sr data on downriver movements indicated many fish moved to lower river habitats, but the transition to saline water was not as clear. Data for 87Sr/86Sr showed decreases at similar ages to the trace element data, but in outer regions of the fin ray cross section the resolution wasn’t as clear because a short axis of the fin ray was used making determination of freshwater emigration more problematic based on thin outer growth zones and annuli. When interpreted with the trace element data, it would appear all of these fish were utilizing saline and lower river habitats after the first few years.
Comparison with field studies
Comparison of these data with field studies on the timing of life history movements of Gulf Sturgeon is important for assessing accuracy and providing greater clarity due to difficulty of sampling of early life history stages of sturgeon. Adult Gulf Sturgeon enter the Choctawhatchee River in the spring, from late March to summer, with spawning fish moving to upriver regions as far as rkm 180 in the Pea and Choctawhatchee Rivers (Fox et al. 2000). Spawning occurs from mid-April to early May above rkm 100, based on limited egg collection from both rivers from approximately rkm 110 to 130, near the confluence of the Choctawhatchee and Pea Rivers (Fox et al. 2000). During summer months, post-spawn and most non-spawning fish occupy the lower river at discrete locations between river km 40–50, below the confluence of the Choctawhatchee River and Holmes Creek, although immature fish occupy a broader area from rkm 0–60 (Hightower et al. 2002). Thus, data from this study are supported by the locations of known spawning and river occupancy of juveniles and adults.
Gulf sturgeon appear to utilize freshwater as a thermal refuge from high summer estuarine and ocean temperatures (Hightower et al. 2002). Adult Gulf Sturgeon are not known to forage during this period (Sulak and Clugston 1999) and may lose body-weight (Sulak and Randall 2002). Fish emigrate to estuarine and oceanic habitats in the fall (September–November) with decreasing temperature, barometric pressure changes (Grammer et al. 2015), decreased day length and increased river discharge (Heise et al. 2005). Foraging and growth occur primarily over a 4–6 month period from November to April in saline habitats (Sulak and Randall 2002). In the Choctawhatchee River System, winter and spring habitat use by Gulf Sturgeon is characterized by foraging in Choctawhatchee Bay in salinities ranging from 6 to 18 ppt by the majority (57%) of males, although a number of males (43%) and most females (66%) have been documented to move into oceanic habitats in the Gulf of Mexico (Fox et al. 2002). Adults from the Pascagoula and Pearl Rivers have been documented in shallow regions between barrier islands (Ross et al. 2009). Winter habitat use by juveniles occurs in estuaries and moderately saline (0.5–18 ppt) habitats near river mouths, with occasional movements into marine habitats during extreme cold events (Sulak et al. 2009; Peterson et al. 2016). Data from this study also indicate juveniles occupy saline habitats early in their life history. Juvenile Gulf and Atlantic Sturgeons have relatively high salinity tolerance from an early age compared to many sturgeon species in part due to rapid growth conferring large body sizes (Altinok et al. 1998; Allen and Cech Jr 2007; Allen et al. 2014).
Young-of-year juveniles (age-0) have been particularly difficult to locate. In the Choctawhatchee River System, age-0 fish have not been documented. In the Suwannee River, age-0 (described as <350 mm TL) and age-1+ (up to 449 mm TL) Gulf Sturgeon have been found from rkm 12–237.5 (Sulak and Clugston 1998). Gut contents indicate age-0 juveniles do eat in freshwater, with diet consisting of insects and oligochaetes (Mason and Clugston 1993). Laboratory studies also indicate embryos have very rapid embryo-larval development, remain hidden, and larvae-juveniles have a 1-step downstream migration, with dispersal patterns resulting in a broad distribution (Kynard and Parker 2004). In the Suwannee River, age-0 fish have been predicted to move downriver to the river mouth region in late January to mid-February after 9–10 months in freshwater (Sulak and Clugston 1998), although fall spawning has been suggested for up to 20% of the population (Randall and Sulak 2012). The wide use of the river and the presence of fish over age-1 in far upriver locations (Sulak and Clugston 1998) indicate the potential for heterogeneity in juvenile Gulf Sturgeon habitat use, similar to the finding of a range of ages initiating downriver movements to saline habitats in this study.
Considerations for application of data to retrospective habitat use
Potential limitations of data for determining retrospective habitat use need to be considered, which include limitations of variability in water trace element and 87Sr/86Sr, limitations based on ecology of Gulf Sturgeon, limitations of fin ray formation, and limitations of the sampling technique for assessing 87Sr/86Sr. Application of water 87Sr/86Sr may be limited by variability within the drainage basin and by variability between seasons. Within the Choctawhatchee River main stem, water 87Sr/86Sr had a clear relationship with river distance, with high values farther inland and low values near the coast. Within the drainage basin, 87Sr/86Sr in tributaries followed this pattern, albeit with often slightly higher or lower values than the main stem river. Variability in water 87Sr/86Sr within seasons and between years may also be present, although Sr signatures are based on underlying bedrock weathering (Capo et al. 1998), presumably contributing to a fairly stable water concentration over time. Stability in water 87Sr/86Sr has been shown across seasons and years in other systems (Kennedy et al. 2000).
Gulf sturgeon spawning and early life history use may pose certain limitations on application of fin ray 87Sr/86Sr data for retrospective habitat use. Gulf Sturgeon, like most other sturgeons, swim upriver to spawn, potentially beyond natal rearing habitats. For many sturgeons, larvae may have one or two periods of “swim-up” where they swim vertically upwards into the water current and are carried downstream to nursery habitats rich in food resources. Laboratory experiments on larval and juvenile Gulf and Atlantic Sturgeon have shown a variable length 1-step migration with short and long-distance migrants suggesting a broad river distribution of fish (Kynard and Horgan 2002; Kynard and Parker 2004).
The fin ray itself also has potential limitations in application of 87Sr/86Sr data that need to be considered. While otoliths are calcium carbonate, supplied by endolymph, known to form by daily accretion and remain relatively inert from subsequent remodeling (Pannella 1971; Campana 1999), these assumptions do not necessarily hold true for fin rays. In sturgeons, fin rays are lepidotrichia covered by dermal bone (Findeis 1997), which is typically composed of a collagen matrix with mineralized calcium phosphate (hydroxyapatite, Ca10(PO4)6(OH)2) and supplied by systemic vasculature (Dacke 1979). The potential for remodeling is not well understood. The overall function of otoliths and fin rays is also different. Whereas otoliths function for orientation, fin rays function as structural support for fins, as a part of the skeletal system. Similarly, the rate of accretion of the hydroxyapatite matrix of fin rays may be more closely related to growth rate than that of otoliths. The rate of fin ray accretion during rapid juvenile growth has been estimated at 6 μm/d with a duration of 22 d needed to retain a water chemical signature, when fish were held at 18 °C in fresh water (Sellheim et al. 2017). However, fin rays may incorporate water chemistry over much smaller time scales (Phelps et al. 2012), and detection ability may relate in part to method of analysis. In this study, for both reference Atlantic Sturgeon and wild Gulf Sturgeon, the widest bands were associated with early growth zones, with growth zones farther from the core becoming progressively thinner. Growth rates are typically fastest in juvenile life history stages, which would correspond to the widest growth zones found near the fin ray core. The potential for remodeling may be greater in larger, older individuals, although this has not been demonstrated. Further, mobilization of systemic calcium by reproductive female fishes is often associated with osteoclasts (Persson et al. 1998), which are present in regions of bone but not in otoliths. Therefore, juvenile fish may provide the least concern for interpretation of chemical signature information on early life history habitat use when utilizing fin rays. However, several studies, including the present reference study, indicate chemical composition of fin rays is retained for long periods of time (Allen et al. 2009; Carriere et al. 2016), possibly because bone remodeling is not extensive in many fishes compared to mammals (Meunier 2002).
Unlike otoliths, detection of a maternal signature in fin rays is likely to be difficult. In sturgeons, distinguishable precursors to fin rays begin to form in the pectoral fin by 14 d postfertilization or 4 d prior to exogenous feeding (Davis et al. 2004). Interestingly, studies on larval sturgeons have demonstrated Sr/Ca ratios reflect environmental concentrations from hatching onwards (Phelps et al. 2012). The location of collection of the fin ray is also important, with fin ray sections closer to the point of articulation providing better information from early life history (Wright et al. 2002), due to growth in width and length with time. In this study, fin ray sections analyzed were close to the point of articulation. Analyses of trace elements used the long axis of the fin ray which provided better resolution of growth zones and annuli between years, but included more organic materials and fin ray inclusions within the fin ray. The short axis was used for 87Sr/86Sr analyses, but had less resolution in growth zones beyond the first few years due to thinner bands of growth zones and annuli. Ablation spots were useful for providing discrete information for a particular location with little signal carryover, whereas line transects were beneficial for obtaining continuous data.
Conclusion
In reference Atlantic Sturgeon held in freshwater, estuarine or oceanic salinities, concentrations of trace elements and 87Sr/86Sr were relative to water concentrations indicating fin rays can be used for determination of past movements. In Gulf Sturgeon from the Choctawhatchee River System, Sr, Ba, Mn and to a lesser degree Zn were useful trace elements for reconstructing life history, particularly movements between freshwater and saline water.
Initial freshwater emigration of Gulf Sturgeon to saline waters occurred from age 0.3–3.3 years based on decreases in Ba and Mn and increases in Sr, with 4% of fish emigrating at age 0.3 years (growth zone 1), 39% at age 0.5–1.3 years (growth zone 2), 39% at age 1.5–2.3 years (growth zone 3) and 17% at age 2.5–3.3 years (growth zone 4). Prior to movements to saline water, Gulf Sturgeon were using habitats throughout most of the length of the river, with the exception of extreme upper river habitats based on 87Sr/86Sr. Initial locations of Gulf Sturgeon which may reflect natal habitat, were generally in the middle and middle-lower river, with few fish in the upper or lower river. The majority (74%) of Gulf Sturgeon utilized more than one river region prior to initial emigration based on 87Sr/86Sr.
Information from field studies on early life history stages is very limited for most sturgeons, including Gulf Sturgeon. Trace element and 87Sr/86Sr generally corroborated with the limited results from field studies, providing a clearer picture of heterogeneity in habitat use within the population and more detailed information on freshwater habitat use and initial entry to saline habitats at early life history stages. Future studies would benefit from an evaluation of seasonal and annual variability in trace element and Sr isotope water concentrations and analysis of fin rays from telemetered fish in the Choctawhatchee River System.
References
Allen PJ, Cech JJ Jr (2007) Age/size effects on juvenile green sturgeon, Acipenser medirostris, oxygen consumption, growth, and osmoregulation in saline environments. Environ Biol Fish 79(3–4):211–229. https://doi.org/10.1007/s10641-006-9049-9
Allen PJ, Hobbs JA, Cech JJ Jr, Van Eenennaam JP, Doroshov SI (2009) Using trace elements in pectoral fin rays to assess life history movements in sturgeon: estimating age at initial seawater entry in Klamath River green sturgeon. Trans Am Fish Soc 138(2):240–250. https://doi.org/10.1577/T08-061.1
Allen PJ, Allen PJ, Mitchell ZA, DeVries RJ, Aboagye DL, Ciaramella MA, Ramee SW, Stewart HA, Shartau RB (2014) Salinity effects on Atlantic sturgeon (Acipenser oxyrinchus oxyrinchus Mitchill, 1815) growth and osmoregulation. J Appl Ichthyol 30(6):1229–1236. https://doi.org/10.1111/jai.12542
Altenritter ME, Kinnison MT, Zydlewski GB, Secor DH, Zydlewski JD (2015) Assessing dorsal scute microchemistry for reconstruction of shortnose sturgeon life histories. Environ Biol Fish 98(12):2321–2335. https://doi.org/10.1007/s10641-015-0438-9
Altinok I, Galli SM, Chapman FA (1998) Ionic and osmotic regulation capabilities of juvenile Gulf of Mexico sturgeon, Acipenser oxyrinchus desotoi. Comp Biochem Physiol A 120(4):609–616. https://doi.org/10.1016/S1095-6433(98)10073-9
Arai T, Levin AV, Boltunov AN, Miyazaki N (2002) Migratory history of the Russian sturgeon Acipenser gueldenstaedti in the Caspian Sea, as revealed by pectoral fin spine Sr:ca ratios. Mar Biol 141(2):315–319
Balazik MT, McIninch SP, Garman GC, Fine ML, Smith CB (2012) Using energy dispersive x-ray fluorescence microchemistry to infer migratory life history of Atlantic sturgeon. Environ Biol Fish 95(2):191–194. https://doi.org/10.1007/s10641-012-9979-3
Baremore IE, Rosati JD (2014) A validated, minimally deleterious method for aging sturgeon. Fish Bull 112(4):274–282. https://doi.org/10.7755/FB.112.4.4
Birstein VJ (1993) Sturgeons and paddlefishes: threatened fishes in need of conservation. Conserv Biol 7(4):773–787. https://doi.org/10.1046/j.1523-1739.1993.740773.x
Blumenthal NC (1990) The in vitro and in vivo uptake of trace elements by hydroxyapatite. In: Priest ND, Van de Vyver FL (eds) Trace metals and fluoride in bones and teeth. CRC Press, Boca Raton
Bruch RM, Campana SE, Davis-Foust SL, Hansen MJ, Janssen J (2009) Lake sturgeon age validation using bomb radiocarbon and known-age fish. Trans Am Fish Soc 138(2):361–372. https://doi.org/10.1577/T08-098.1
Campana SE (1999) Chemistry and composition of fish otoliths: pathways, mechanisms and applications. Mar Ecol Prog Ser 188:263–297. https://doi.org/10.3354/meps188263
Capo RC, Stewart BW, Chadwick OA (1998) Strontium isotopes as tracers of ecosystem processes: theory and methods. Geoderma 82(1-3):197–225. https://doi.org/10.1016/S0016-7061(97)00102-X
Carriere B, Gillis D, Halden N, Anderson G (2016) Strontium metabolism in the juvenile Lake sturgeon, Acipenser fulvescens (Rafinesque, 1817), and further evaluation of the isotope as a marking tool for stock discrimination. J Appl Ichthyol 32(2):258–266. https://doi.org/10.1111/jai.13019
Chase NM, Caldwell CA, Carleton SA, Gould WR, Hobbs JA (2015) Movement patterns and dispersal potential of Pecos bluntnose shiner (Notropis simus pecosensis) revealed using otolith microchemistry. Can J Fish Aquat Sci 72(10):1575–1583. https://doi.org/10.1139/cjfas-2014-0574
Clarke AD, Telmer KH, Shrimpton JM (2007) Elemental analysis of otoliths, fin rays and scales: a comparison of bony structures to provide population and life-history information for the Arctic grayling (Thymallus arcticus). Ecol Freshw Fish 16(3):354–361. https://doi.org/10.1111/j.1600-0633.2007.00232.x
Collins MR, Smith TIJ (1996) Sturgeon fin ray removal is nondeleterious. North Am J Fish Manag 16(4):939–941. https://doi.org/10.1577/1548-8675(1996)016<0939:SFRRIN>2.3.CO;2
Dacke CG (1979) Calcium regulation in sub-mammalian vertebrates. Academic Press, London
Davis MC, Shubin NH, Force A (2004) Pectoral fin and girdle development in the basal Actinopterygians Polydon spathula and Acipenser transmontanus. J Morphol 262(2):608–628. https://doi.org/10.1002/jmor.10264
Eerkens JW, Carlson T, Malhi RS, Blake J, Bartelink EJ, Barfod GH, Estes A, Garibay R, Glessner J, Greenwald AM, Lentz K, Li H, Marshall CK (2016) Isotopic and genetic analyses of a mass grave in central California: implications for precontact hunter-gatherer warfare. Am J Phys Anthropol 159(1):116–125. https://doi.org/10.1002/ajpa.22843
Elsdon TS, Gillanders BM (2003) Reconstructing migratory patterns of fish based on environmental influences on otolith chemistry. Rev Fish Biol Fish 13:219–235
Elsdon TS, Wells BK, Campana SE, Gillanders BM, Jones CM, Limburg KE, Secor DH, Thorrold SR, Walther BD (2008) Otolith chemistry to describe movements and life-history parameters of fishes: hypotheses, assumptions, limitations and inferences. In: Gibson RN, Atkinson RJA, Gordon JDM (eds) Oceanography and marine biology: an annual review, vol 46. CRC Press, Boca Raton, pp 297–330. https://doi.org/10.1201/9781420065756.ch7
Findeis EK (1997) Osteology and phylogenetic interrelationships of sturgeons (Acipenseridae). Environ Biol Fish 48(1/2/3/4):73–126. https://doi.org/10.1023/A:1007372832213
Fox DA, Hightower JE, Parauka FM (2000) Gulf sturgeon spawning migration and habitat in the Choctawhatchee River system, Alabama-Florida. Trans Am Fish Soc 129(3):811–826. https://doi.org/10.1577/1548-8659(2000)129<0811:GSSMAH>2.3.CO;2
Fox DA, Hightower JE, Parauka FM (2002) Estuarine and nearshore marine habitat use by gulf sturgeon from the Choctawhatchee River system, Florida. In: Van Winkle W, Anders PJ, Secor DH, Dixon DA (eds) Biology, management, and protection of north American sturgeon. American Fisheries Society, Symposium 28, Bethesda, p 111–126
Grammer PO, Mickle PF, Peterson MS, Havrylkoff J-M, Slack WT, Leaf RT (2015) Activity patterns of gulf sturgeon (Acipenser oxyrinchus desotoi) in the staging area of the Pascagoula River during fall outmigration. Ecol Freshw Fish 24(4):553–561. https://doi.org/10.1111/eff.12168
Heise RJ, Slack WT, Ross ST, Dugo MA (2005) Gulf sturgeon summer habitat use and fall migration in the Pascagoula River, Mississippi, USA. J Appl Ichthyol 21(6):461–468. https://doi.org/10.1111/j.1439-0426.2005.00701.x
Hightower JE, Zehfuss KP, Fox DA, Parauka FM (2002) Summer habitat use by gulf sturgeon in the Choctawhatchee River, Florida. J Appl Ichthyol 18(4-6):595–600. https://doi.org/10.1046/j.1439-0426.2002.00402.x
Hobbs JA, Bennett WA, Burton J, Gras M (2007) Classification of larval and adult Delta smelt to nursery areas by use of trace elemental fingerprinting. Trans Am Fish Soc 136(2):518–527. https://doi.org/10.1577/T06-087.1
Hobbs JA, Lewis LS, Ikemiyagi N, Sommer T, Baxter RD (2010) The use of otolith strontium isotopes (87Sr/86Sr) to identify nursery habitat for a threatened estuarine fish. Environ Biol Fish 89(3-4):557–569. https://doi.org/10.1007/s10641-010-9672-3
Hodge BW, Wilzbach MA, Duffy WG, Quinones RM, Hobbs JA (2016) Life history diversity in Klamath River steelhead. Trans Am Fish Soc 145(2):227–238. https://doi.org/10.1080/00028487.2015.1111257
Housh T, McMahon TP (2000) Ancient isotopic characteristics of Neogene potassic magmatism in western new Guinea (Irian Jaya, Indonesia). Lithos 50(1-3):217–239. https://doi.org/10.1016/S0024-4937(99)00043-2
Ingram BL, Weber PK (1999) Salmon origin in California's Sacramento-San Joaquin river system as determined by otolith stronitum isotopic composition. Geology 27(9):851–854. https://doi.org/10.1130/0091-7613(1999)027<0851:SOICSS>2.3.CO;2
Kennedy BP, Blum JD, Folt CL, Nislow KH (2000) Using natural strontium isotopic signatures as fish markers: methodology and application. Can J Fish Aquat Sci 57(11):2280–2292. https://doi.org/10.1139/f00-206
Kynard B, Horgan M (2002) Ontogenetic behavior and migration of Atlantic sturgeon, Acipenser oxyrinchus oxyrinchus, and shortnose sturgeon, A. brevirostrum, with notes on social behavior. Environ Biol Fish 63(2):137–150. https://doi.org/10.1023/A:1014270129729
Kynard B, Parker E (2004) Ontogenetic behavior and migration of Gulf of Mexico sturgeon, Acipenser oxyrinchus desotoi, with notes on body color and development. Environ Biol Fish 70(1):43–55. https://doi.org/10.1023/B:EBFI.0000022855.96143.95
Loewen TN, Carriere B, Reist JD, Halden NM, Anderson WG (2016) Linking physiology and biomineralization processes to ecological inferences on the life history of fishes. Comp Biochem Physiol A 202:123–140. https://doi.org/10.1016/j.cbpa.2016.06.017
Mason WT, Clugston JP (1993) Foods of the Gulf sturgeon in the Suwannee River, Florida. Trans Am Fish Soc 122(3):378–385. https://doi.org/10.1577/1548-8659(1993)122<0378:FOTGSI>2.3.CO;2
Meunier FJ (2002) Types of calcified structures: skeleton. In: Panfili J, de Pontual H, Troadec H, Wright PJ (eds) Manual of fish sclerochronology. Ifremer-IRD, Brest, France, pp 65–88
Milton DA, Chenery SR (2005) Movement patterns of barramundi Lates calcarifer, inferred from 87Sr/86Sr ratios in otoliths, indicate non-participation in spawning. Mar Ecol Prog Ser 301:279–291. https://doi.org/10.3354/meps301279
Nelson TC, Doukakis P, Lindley ST, Schreier AD, Hightower JE, Hidlebrand LR, Whitlock RE, Webb MAH (2013) Research tools to investigate movements, migrations, and life history of sturgeons (Acipenseridae), with an emphasis on marine-oriented populations. PLoS One 8(8):e71552. https://doi.org/10.1371/journal.pone.0071552
Nguyen PL, Jackson ZJ, Peterson DL (2016) Comparison of fin ray sampling methods on white sturgeon Acipenser transmontanus growth and swimming performance. J Fish Biol 88(2):655–667. https://doi.org/10.1111/jfb.12866
Pannella G (1971) Fish otoliths: daily growth layers and periodical patterns. Science 173(4002):1124–1127. https://doi.org/10.1126/science.173.4002.1124
Paton C, Hellstron J, Paul B, Woodhead J, Hergt J (2011) Iolite: freeware for the visualisation and processing of mass spectrometric data. J Anal Atom Spectrom 26(12):2508–2518. https://doi.org/10.1039/c1ja10172b
Persson P, Sundell K, Bjornsson BT, Lundqvist H (1998) Calcium metabolism and osmoregulation during sexual maturation of river running Atlantic salmon. J Fish Biol 52(2):334–349. https://doi.org/10.1111/j.1095-8649.1998.tb00801.x
Peterson MS, Havrylkoff J-M, Grammer PO, Mickle PF, Slack WT (2016) Consistent spatiotemporal estuarine habitat use during emigraton or immigration of a western population of gulf sturgeon. Trans Am Fish Soc 145(1):27–43. https://doi.org/10.1080/00028487.2015.1091382
Phelps QE, Whitledge GW, Tripp SJ, Smith KT, Garvey JE, Herzog DP, Ostendorf DE, Ridings JW, Crites JW, Hrabik RA, Doyle WJ, Hill TD (2012) Identifying river of origin for age-0 Scaphirhynchus sturgeons in the Missouri and Mississippi rivers using fin ray microchemistry. Can J Fish Aquat Sci 69(5):930–941. https://doi.org/10.1139/f2012-038
Phelps QE, Hupfeld RN, Whitledge GW (2016) Lake sturgeon Acipenser fulvescens and shovelnose sturgeon Scaphirhynchus platorynchus environmental life history revealed using pectoral fin-ray microchemistry: implications for interjurisdictional conservation through fishery closure zones. J Fish Biol 90(2):626–639. https://doi.org/10.1111/jfb.13242
Randall MT, Sulak KJ (2012) Evidence of autumn spawning in Suwannee River gulf sturgeon, Acipenser oxyrinchus desotoi (Vladykov, 1955). J Appl Ichthyol 28(4):489–495. https://doi.org/10.1111/j.1439-0426.2012.01960.x
Ross ST (2001) The inland fishes of Mississippi. University Press of Mississippi, Jackson
Ross ST, Slack WT, Heise RJ, Dugo MA, Rogillio H, Bowen BR, Mickle P, Heard RW (2009) Estuarine and coastal habitat use of gulf sturgeon (Acipenser oxyrinchus desotoi) in the north-central Gulf of Mexico. Estuar Coasts 32(2):360–374. https://doi.org/10.1007/s12237-008-9122-z
Secor DH, Anders PJ, Van Winkle W, Dixon DA (2002) Can we study sturgeons to extinction? What we do and don't know about the conservation of north American sturgeons. In: Van Winkle W, Anders PJ, Secor DH, Dixon DA (eds) Biology, management, and protection of north American sturgeons. American Fisheries Society, Bethesda, p 3–9
Sellheim K, Willmes M, Hobbs JA, Glessner JJG, Jackson ZJ, Merz JE (2017) Validating fin ray microchemistry as a tool to reconstruct the migratory history of white sturgeon Acipenser transmontanus. Trans Am Fish Soc 146(5):844–857. https://doi.org/10.1080/00028487.2017.1320305
Stevenson JT, Secor DH (1999) Age determination and growth of Hudson River Atlantic sturgeon, Acipenser oxyrinchus. Fish Bull 97:153–166
Sturrock AM, Hunter E, Milton JA, EIMF, Johnson RC, Waring CP, Trueman CN (2015) Quantifying physiological influences on otolith microchemistry. Methods Ecol Evol 6(7):806–816. https://doi.org/10.1111/2041-210X.12381
Sulak KJ, Clugston JP (1998) Early life history stages of gulf sturgeon in the Suwannee River, Florida. Trans Am Fish Soc 127(5):758–771. https://doi.org/10.1577/1548-8659(1998)127<0758:ELHSOG>2.0.CO;2
Sulak KJ, Clugston JP (1999) Recent advances in life history of Gulf of Mexico sturgeon, Acipenser oxyrinchus desotoi, in the Suwannee River, Florida, USA: a synopsis. J Appl Ichthyol 15(4–5):116–128. https://doi.org/10.1111/j.1439-0426.1999.tb00220.x
Sulak KJ, Randall M (2002) Understanding sturgeon life history: enigmas, myths, and insights from scientific studies. J Appl Ichthyol 18(4-6):519–528. https://doi.org/10.1046/j.1439-0426.2002.00413.x
Sulak KJ, Randall MT, Edwards RE, Summers TM, Luke KE, Smith WT, Norem AD, Harden WM, Lukens RH, Parauka F, Bolden S, Lehnert R (2009) Defining winter trophic habitat of juvenile gulf sturgeon in the Suwanee and Apalachicola rivermouth estuaries, acoustic telemetry investigations. J Appl Ichthyol 25(5):505–515. https://doi.org/10.1111/j.1439-0426.2009.01333.x
U.S. Fish and Wildlife Service and Gulf States Marine Fisheries Commission (1995) Gulf sturgeon recovery plan. Fish and Wildlife, Atlanta, p 170
Veinott G, Northcote T, Rosenau M, Evans RD (1999) Concentrations of strontium in the pectoral fin rays of the white sturgeon (Acipenser transmontanus) by laser ablation sampling - inductively coupled plasma - mass spectrometry as an indicator of marine migrations. Can J Fish Aquat Sci 56(11):1981–1990. https://doi.org/10.1139/f99-120
Waldman JR, Grunwald C, Stabile J, Wirgin I (2002) Impacts of life history and biogeography on the genetic stock structure of Atlantic sturgeon Acipenser oxyrinchus oxyrinchus, gulf sturgeon A. oxyrinchus desotoi, and shortnose sturgeon A. brevirostrum. J Appl Ichthyol 18(4-6):509–518. https://doi.org/10.1046/j.1439-0426.2002.00361.x
Walther BD, Thorrold SR (2006) Water, not food, contributes the majority of strontium and barium deposited in the otoliths of a marine fish. Mar Ecol Prog Ser 311:125–130. https://doi.org/10.3354/meps311125
Willmes M, Glessner JJG, Carleton SA, Gerrity PC, Hobbs JA (2016) 87Sr/86Sr isotope ratio analysis by laser ablation MS-ICP-MS in scales, spines, and fin rays as a non-lethal alternative to otoliths for reconstructing fish life history. Can J Fish Aquat Sci doi 73(12):1852–1860. https://doi.org/10.1139/cjfas-2016-0103
Wright PJ, Panfili J, Morales-Nin B, Geffen AJ (2002) Types of calcified structures. In: Panfili J, Pontual H, Troadec H, Wright PJ (eds) Manual of fish sclerochronology. Ifremer-IRD coedition, Brest, France, pp 30–88
Acknowledgments
We thank the National Marine Fisheries Service and Northern Gulf Institute for funding, grant#: NA11OAR4320199; Kent Ware and James Henne, US Fish and Wildlife Service Bears Bluff National Fish Hatchery, Wadmalaw Island, SC, USA for providing juvenile Atlantic Sturgeon; Zachary Mitchell, Heather Stewart, Daniel Aboagye, Shane Ramee, Michael Ciaramella, Charlie Culpepper and Mack Fondren and staff at the Mississippi State University South Farm Aquaculture Facility for assistance with fish care and sampling; Joseph Mitchell for expertise on micromilling; Panseok Yang at the University of Manitoba and Justin Glessner at the University of California Davis for assistance with ICPMS analyses, the Mississippi Agricultural and Forestry Experiment Station (MAFES) and the US Department of Agriculture (USDA) Agricultural Research Service and the USDA National Institute of Food and Agriculture, State project#: 1005154 for facility support; Austin Omer for assistance with figures; and two anonymous reviewers for helpful comments in the review of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Allen, P.J., DeVries, R.J., Fox, D.A. et al. Trace element and strontium isotopic analysis of Gulf Sturgeon fin rays to assess habitat use. Environ Biol Fish 101, 469–488 (2018). https://doi.org/10.1007/s10641-018-0713-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10641-018-0713-7