Abstract
Patients with extensive-stage small-cell lung cancer (ES-SCLC) have high relapse rates and poor prognosis. Anlotinib monotherapy has shown promising efficacy for patients with ES-SCLC and has a non-overlapping toxicity profile with chemotherapy. Therefore, the study aims to assess the efficacy and safety of the addition of anlotinib to platinum-chemotherapy as first-line therapy for patients with ES-SCLC. ES-SCLC patients without systemic chemotherapy and immunotherapy were recruited. Eligible patients received anlotinib (12 mg/day, on day 1–14) of a 21-day cycle, with concomitant etoposide (100 mg/m2, on day 1–3) plus cisplatin (75 mg/m2, on day 1) or carboplatin (AUC = 4–5, on day 1) for 4–6 cycles, followed by indefinite anlotinib maintenance therapy. The primary endpoint was progression-free survival (PFS). Secondary endpoints included objective response rate (ORR), disease control rate (DCR), overall survival (OS). Between Jan 15, 2019 and Dec 31, 2020, 25 patients were enrolled. At the data cut-off time (November 3, 2021), the median follow-up was 14.3 months. Median PFS was 10.3 months (95% CI: 6.0–14.5) and median OS was 17.1 months (95% CI: 11.1–19.3). The ORR and DCR were 90% and 100%, respectively. The most common grade 3 or worse treatment-related adverse events were neutropenia (50%), leukopenia (35%), thrombocytopenia (25%), fatigue (10%), nausea (10%), hyponatremia (10%), anemia (10%). One patient discontinued treatment due to treatment-related adverse events. No treatment-related death occurred. Anlotinib plus platinum–chemotherapy as first-line therapy for ES-SCLC has anti-tumor activity, and showed acceptable tolerability. These results provide a basis for future randomized controlled trials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Small cell lung cancer (SCLC) is a poorly differentiated neuroendocrine neoplasm and accounts for about 15% of all lung cancers, which is characterized by high relapse rates and proliferative rates [1,2,3]. At the time of diagnosis, about two-thirds of all cases of SCLC present with extensive-stage (ES) disease (ES-SCLC) [4]. Usually, overall survival is limited to approximately 8–13 months in ES-SCLC, and the 5-year survival rate is disappointingly low with only < 2% [5].
Until recently, platinum (cisplatin or carboplatin) and etoposide doublet chemotherapy has remained the standard regimen for first-line therapy in patients with ES-SCLC and may provide modest improvements in survival [6,7,8,9]. However, despite initial high response rates, median overall survival (mOS) is limited to approximately 10 months, and the median progression-free survival (mPFS) is only 3 months [9,10,11]. Numerous efforts have been made in more than two decades to improve the therapeutic regimens of ES-SCLC, such as adding PD-L1 inhibitors atezolizumab to carboplatin and etoposide for ES-SCLC resulted in an improvement in mOS 12.3 months and mPFS 5.2 months [12]. Additionally, the CASPIAN trial also demonstrated that durvalumab plus platinum–etoposide for treatment-naive patients with ES-SCLC has significant improvement in overall survival, with median mOS 13.0 months [4]. Nevertheless, the addition of PD-1/PD-L1 inhibitors to chemotherapy does not reproduce the major improvements of survival benefit observed in clinical trials in non-small cell lung cancer and other malignant diseases. Thus, any therapeutic regimen likely to improve the first-line treatments for ES-SCLC would be a valuable asset and worthy of further exploration.
Anlotinib hydrochloride, is a newly developed oral small molecule inhibitor of multiple receptor tyrosine kinases, which blocks tumor angiogenesis and proliferation by inhibiting the vascular endothelial growth factor (VEGF) receptors (1/2/3) and other major tyrosine kinase receptors, such as FGFR1-4, PDGFR a/b, c-Kit, and FLT3 [13]. It has been approved as a third-line treatment for locally advanced or metastatic non-small cell lung cancer (NSCLC) since 2018 [14]. A variety of clinical trials have been demonstrated the clinical benefits of anlotinib in various tumors, such as locally advanced or metastatic medullary thyroid cancer [15], metastatic renal cell carcinoma [16], chemotherapy-refractory metastatic esophageal squamous cell carcinoma [17]. Meanwhile, preclinical studies also revealed the potential anti-tumor activity of anlotinib either alone or combined with chemotherapy in human xenograft models of multiple cancer types [18,19,20]. Furthermore, previously multicenter phase II clinical trials suggested that anlotinib or combined with etoposide capsules as third-line or further-line treatment for ES-SCLC can significantly prolong the PFS and OS, and has an acceptable safety profile [21, 22]. The latest real-world exploratory study also demonstrated that anlotinib monotherapy showed encouraging effectiveness and acceptable safety profile for patients with ES-SCLC who failed the previous chemotherapy treatment [23]. Encouraged by its promising beneficial outcomes for tumors, we speculated that anlotinib combined with platinum/cisplatin or platinum /carboplatin may act as an effective first-line treatment regimen to improve the ES-SCLC patients’ outcomes.
Therefore, we conducted this phase II clinical study to assess the efficacy and safety of anlotinib plus etoposide and cisplatin/carboplatin in treatment-naive patients with ES-SCLC.
Materials and methods
Study design
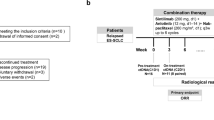
This was a single-center, single-arm phase II study (ChiCTR2000035043) of the anlotinib plus etoposide and cisplatin/carboplatin as first-line therapy for Chinese patients with ES-SCLC. This study was conducted in Cancer Hospital of Zhengzhou and approved by the ethics committee of the Third People's Hospital of Zhengzhou city (Ethical approval number: SY20190004), and conducted in accordance with Good Clinical Practice guidelines and the Declaration of Helsinki. Written informed consent was obtained from all patients before enrollment.
Patients eligibility
Patients aged 18–80 years were eligible for this study if they had a histopathology diagnosis of treatment-naive ES-SCLC. Other eligibility criteria were the measurable disease according to the standard Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 [24]; an Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0 to 2; a life expectancy of ≥ 3 months; patients with asymptomatic or treated and stable brain metastases were also allowed to be included.
Key exclusion criteria were previously participated in other clinical trials and have not terminated the trial, or combined with other pathological types of tumors except for small cell lung cancer; a history of severe allergies, allergies, or psychotropic substance abuse. In addition, patients were also ineligible if they are pregnant or breastfeeding, or had a chronic persistent infection. The researcher considered that the patients are not suitable to participate in the study.
Procedures
Anlotinib was administered orally at an initial dose of 12 mg once daily on days 1 to 14 of each 21-day cycle. Chemotherapy consisted of etoposide 100 mg/m2, was administered intravenously on days 1 to 3 of each cycle. And cisplatin 75 mg/m2 or carboplatin (AUC = 4–5) was administered intravenously on day 1 of each cycle. The choice of either cisplatin or carboplatin was at the investigator’s discretion. Additionally, patients who are sensitive to first-line chemotherapy and whose efficacy is assessed as CR or PR, whether received chest radiotherapy or craniocerebral radiotherapy (including prophylactic cranial irradiation), should be selected according to the discretion of the investigator and clinical status of patients. The combination of chemotherapy and anlotinib regimen was continued for 4 to 6 cycles, followed by maintenance anlotinib (12 mg) every 3 weeks until disease progression (PD), unacceptable toxicity, or consent withdrawal.
Dose adjustments
Adverse events (AEs) associated with anlotinib were managed with supportive care, dose reductions, interruption until they became tolerable, or permanent discontinuation of anlotinib. Toxicities were graded according to the National Cancer InstituteCommon Terminology Criteria for Adverse Events (NCI-CTCAE) version 5.0. Briefly, no dose interruption or modification of anlotinib was made for grade 1 toxicity. According to NCI-CTCAT5.0, dose interruption or modification of anlotinib for grade 2 or worse toxicity are listed in the supplement (Supplementary Table 1). The maximum duration per episode of dose interruptions during one cycle is 5 days. Otherwise, patients will no longer receive anlotinib during in this cycle. If patients required dose reduction, two levels of dose reduction per patient were allowed (12 mg reduced to 10 mg or 8 mg), and dose re-escalation was permitted. But, despite a reduced dose to 8 mg or interrupted dose at the beginning of the next cycle by more than 2 weeks, patients remained unable to tolerate anlotinib, anlotinib should be permanently discontinued.
Additionally, if platinum-etoposide had to be delayed due to its intolerable toxicity, dose reductions and interruptions were permitted, and patients continued to receive treatment with anlotinib until PD or unacceptable toxicity. Patients were also allowed to switch between carboplatin and cisplatin at the investigator’s discretion.
Tumor response and safety assessment
Tumor response was evaluated by investigators according to RECIST version 1. Tumor assessments were conducted by computed tomography (CT) and magnetic resonance imaging (MRI) at baseline, every 6 weeks (2 cycles) during the chemotherapy cycles, and thereafter every 8 weeks during maintenance treatment until PD or treatment was discontinued. Once complete response (CR) or partial response (PR) occurred, another CT and MRI will be added 4 weeks later to confirm the CR or PR. If clinical symptoms of patients aggravate during treatment, the CT and MRI were considered to take ahead of time by the investigator.
Safety was assessed by adverse events (AEs) graded according to the National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE) version 5.0. Physical examination, vital signs, and laboratory analyses (hematology, serum biochemistry, routine urine examinations, and coagulation) were assessed at baseline, every 6 weeks (2 cycles) during the chemotherapy cycles, and thereafter every 8 weeks during maintenance treatment. Throughout the treatment period, all AEs and combined medication were recorded.
Outcomes
The primary endpoint was progression-free survival (PFS), defined as the time from treatment initiation to PD (assessed by blinded independent central review) or death from any cause. Secondary endpoints included objective response rate (ORR), overall survival (OS), disease control rate (DCR), and safety. ORR was defined as the percentage of patients with a complete (CR) or partial response (PR). PFS and ORR were assessed by investigators according to RECIST version 1.1. OS was defined as the time from treatment initiation to death from any cause. DCR was defined as the proportion of patients who achieved a CR, PR, and stable disease (SD). CR, defined as no viable tumor on pathologic analysis, all pathological lymph nodes must reduce to ≤ 10 mm in short axis, and last at least 4 weeks. PR means the sum of the longest diameter for all target lesions was reduced by at least 30% for at least 4 weeks. SD was defined as neither sufficient tumor reduction to qualify for PR nor sufficient increase to qualify for PD.
Statistical analysis
The primary objective of this phase II study was to evaluate the median PFS. On the basis of the previous study [25], the median PFS ≥ 5.8 months is expected. The sample size estimation was completed a priori using the two-sided, one-sample log-rank test, with a two-sided alpha of 5%. A sample size of 20 included in this study provided a test power of 90.4%.
Patients’ baseline characteristics and demographics, tumor response, and safety outcomes are summarized descriptively. All analyses were based on an intention-to-treat principle. Safety analyses were conducted in all patients who received at least one dose of anlotinib and have at least one safety assessment. Efficacy was assessed in enrolled patients who were compliant with the protocol and without any major protocol violations. The Kaplan–Meier method was applied to estimate the PFS and OS, and median values were estimated with 95% confidence intervals (CI). Categorical variables were expressed as frequency (proportions) while continuous variables were expressed as means ± standard deviation (SD). All statistical tests were two-sided, and p < 0.05 was taken as significant. Statistical analyses were performed using STATA version 12.0.
Results
Patient demographics and clinical characteristics
Between Jan 15, 2019 and Dec 31, 2020, 25 patients with ES-SCLC were screened and among them, 5 patients were ineligible. A total of 20 patients were enrolled in this trial and received treatment (Fig. 1). The demographics and clinical characteristics of the 20 enrolled patients are listed in Table 1. Of the 20 patients, the median age was 66.2 years (SD = 8.1), and 17 (85%) were male. 15 (75%) patients had a smoking history and only 1 patient had quit smoking. In this study, 12 (60%) patients had a baseline ECOG PS of 1. 10 (50%) patients were at stage IVB, and 7 (35%) patients were at stage IVA. All patients had metastatic disease at study enrollment, and the most common sites of metastasis were the lymph node (90%), lung (55%), pleural (55%), and bone (25%).
Clinical activity
This study consists of the data obtained until November 3, 2021, ie, the date of the last follow-up. The median duration of treatment (including dose interruptions) was 9.4 months (IQR 5.5–15.9). The median follow-up was 14.3 months (IQR 9.6–17.7). Of the enrolled 20 patients, 14 (70%) patients receiving anlotinib combined with etoposide plus cisplatin, 6 (30%) patients received anlotinib combined with etoposide plus carboplatin (Table 1). 13 patients (65%) received the planned maximum of six cycles of anlotinib plus platinum–etoposide. The analysis of efficacy included all 20 patients assigned to treatment. The mPFS was 10.3 months (95% CI: 6.00–14.50, Fig. 2A). The mOS was 17.1 months (95% CI: 15.00–31.50, Fig. 2B) and the median duration of response (DOR) was 8.20 months (95% CI: 2.03–11.30, Fig. 2C), 5/20 of patients had a response lasting more than 12 months Fig. 3C. The response to anlotinib combined with chemotherapy treatment was presented in Table 2. All patients had a best objective response throughout the study, of these, one patient attained a CR, 17/20 patients had a PR, and 2 (10%) patients attained an SD. The ORR was 90% and DCR was 100% (Fig. 3B). As shown in the waterfall plot, which depicts the best response in patients with at least one response assessment, 90% (18/20) of patients achieved target tumor shrinkage greater than 30% (Fig. 3A). At the data cut-off, 19 patients had a PD, and the median time to progress (TTP) was 9.83 months (95% CI: 7.23–13.3, Fig. 2D). 11/20 patients died and 1 patient attained an SD.
Objective response in EC-SCLC patients treated with anlotinib combined with platinum–etoposide (November 3, 2021 data cut-off). A Waterfall plot for best response. 18 patients achieved target tumor shrinkage greater than 30%. The color indicates the type of response. B Spider graphs for response by RECIST V.1.1 criteria; C Swimmer plot of patients with an objective response. The solid square reflects the response onset for the 20 patients. At the November 3, 2021 data cut-off, 19 patients had progressed at the end of their bar, whereas 1 patient achieved disease stabilization;
All 3 patients with stable brain metastases achieved a PR as the best response, with an ORR of 100%. During follow-up period, 2 of these 3 patients had a PFS event (disease progression or death) and the mPFS was 3.00 months (95%CI: 2.00-not evaluable [NE]). At the data cutoff, 2 patients with brain metastases died and the mOS was 5.50 months (95% CI: 4.9-NE).
Safety
The safety analysis set included 20 patients. At the data cut-off, 19/20 patients had at least one treatment-related AEs (TRAEs) (Table 3). Overall, TEAEs with the grade ≥ 3 were reported in 13 patients. The common grade ≥ 3 treatment-related AEs were neutropenia (50%), leukopenia (35%), thrombocytopenia (25%), fatigue (10%), nausea (10%), hyponatremia (10%), anemia (10%). Most of low-grade toxicities were hypoproteinemia (15 [75%]), alopecia (14 [70%]), nausea (13 [65%]), dry mouth (12 [60%]), and hyponatremia (12 [60%]), which were generally reversible. No grade 5 AEs were reported and treatment-related deaths occurred.
Dose reductions were required in 6 (30%) patients. Of the 6 patients, 1 patient required modifications of the initial dose of anlotinib to 8 mg, and 5 patients required modifications to 10 mg, due to intolerance to 12 mg anlotinib. During treatment, only 3 patients required dose reductions due to AEs, including grade 3 fatigue (n = 2) and grade 4 myelosuppression (n = 1). Two patients interrupted treatment due to neutrophils and leukopenia, of whom 1 patient resumed with the support of recombinant human granulocyte colony-stimulating factor (rhG-CSF), and another patient resumed with the support of rhG-CSF and recombinant human thrombopoietin (rhTPO). Additionally, 1 patient discontinued treatment because of grade 4 thrombocytopenia.
Discussion
This phase II, single-arm study evaluated the efficacy and safety of anlotinib plus platinum–etoposide as a first-line treatment option in patients with ES-SCLC. The results demonstrated that the combination of anlotinib and chemotherapy provided encouraging efficacy with an acceptable safety profile for patients with ES-SCLC. Of the 20 patients able to be assessed in the efficacy analysis, the ORR was 90%, and the mPFS and mOS were 10.3 and 17.1 months, respectively. No unexpected treatment-related AEs were observed.
The treatment of ES-SCLC has always represented a significant challenge for oncologists [2]. As a result of recent advances, the atezolizumab plus platinum–etoposide now become the standard-of-care for ES-SCLC in the first-line setting, but it failed to significantly improve ORR [26]. Moreover, the 2-month improvement in OS was also far from satisfactory [27]. Preclinical data have shown that VEGF is overexpressed in ES-SCLC; thus angiogenesis inhibitors recently are being evaluated in the therapy of ES-SCLC, with a number of them showing early promise [28]. Here, we reported the results from a phase II trial of anlotinib (VEGF inhibitor) in combination with etoposide plus cisplatin/carboplatin as first-line therapy in patients with EC-SCLC. In the present study, the mPFS and mOS were 10.3 months 17.1 months respectively, which were comparable with the results of recent phase II studies reported by Deng et al. (mPFS, 8.02 M; mOS, 15.87 M) [29] and Liu et al. (mPFS, 6.0 M; mOS, 14.0 M) [30]. However, inconsistent with these two studies [29, 30], we enrolled elderly patients aged ≥ 75 years old and patients with a poor ECOG PS of 2, whose may have a distinct clinical manifestation and prognosis. Even so, survival benefits seen from this combination here was encouraging when compared with these studies. Notably, based on the recent lines of evidence, that is, no significant difference in PFS, OS [31], and toxicity risk [32] emerged between patients with an ECOG PS of 1 compared to those with an ECOG PS of 2, we presumed that this combination should not be reserved for only an ECOG-PS of 0–1 or younger patients but should be considered for all treatment eligible patients.
More exhilaratingly, the clinical benefits in our study compared favorably with historical activity data of other angiogenesis inhibitors in the first-line setting, such as bevacizumab (mPFS, 6.7 M; mOS, 9.8 M) in GOIRC-AIFA trial [33], sorafenib (mPFS, 5.1 M; mOS, 7.4 M) [34], rh-endostatin (mPFS, 6.4 M; mOS, 12.1 M) [35], and apatinib (mPFS, 7.8 M; mOS, 12.1 M) [36]. This relatively longer survival data maybe in part be attributed to the stronger anti-angiogenic activity of anlotinib, when compared to other angiogenesis inhibitors [37]. Historically, these angiogenesis inhibitors only produced a limited benefit, even negative results in ES-SCLC when compared with first-line chemotherapy alone [28]. In SALUTE and GOIRC-AIFA trials, the addition of bevacizumab to platinum–etoposide improved PFS but unfortunately not OS [33, 38]. Thus, identifying therapeutic options that prolong OS in EC-SCLC remains critical. Considering the obvious improvement in OS observed in our study, it is suggested that the anlotinib might be a better partner for platinum-etoposide in the first-line treatment of EC-SCLC. However, as is typical of early-phase clinical trials, the efficacy analysis should be interpreted with caution due to the limitation in the sample size of participants. Thus, whether the addition of anlotinib to platinum–etoposide has better survival benefit should be investigated in a randomized controlled trial.
The combination of anlotinib plus platinum–etoposide regimen had acceptable tolerability in this study. No unexpected toxicities were observed and no treatment-related death occurred. The reported non-hematological AEs in this study were known, such as fatigue, nausea, hyponatremia, and hypokalemia similar to roniciclib or apatinib in combination with platinum–etoposide regimen [21, 39], implicating that the combination of anlotinib with platinum–etoposide would not increase non-hematological toxicities and safety. However, notably, 13 patients were experienced grade 3 or more AEs, and 11 patients were experienced hematological AEs, of these, 1 patient discontinued treatment with the grade IV thrombocytopenia, and 2 patients interrupted treatment due to neutrophils and leukopenia. The most common grade 3 or more hematological AEs were neutropenia (10/20, 50%). A pooled analysis revealed that platinum–etoposide regimen might increase the frequency of the grade 3–4 hematotoxicity, such as neutropenia, anemia, and thrombocytopenia [40]. Thus, we suspected that the neutropenia in this study may be explained by using platinum–etoposide and anlotinib would not increase hematological toxicities. Whereas, whether this increased risk of hematological toxicities was not caused by anlotinib should be confirmed in a large-scale controlled study. Additionally, the other AEs such as alanine aminotransferase elevation, hypertension, hoarseness, oral mucositis, hand-foot syndrome, were also previously reported in other tumor types and related to anlotinib [14, 41, 42]. All AEs occurred during the study were managed with dose reduction or symptomatic treatments, and no grade 5 toxicity occurred. In general, the combination of anlotinib and platinum–etoposide regimen was well-tolerated and can be delivered safely as first-line therapy in patients with ES-SCLC.
Despite these positive findings, the study still had some limitations. The primary limitation of this study is its non-randomized, single-arm design, which may cause a selection bias due to the lack of a control group. Secondly, the study with relatively small sample size and were only included patients from single center might prevent the generalizability of outcomes from this study to the broader population. Consequently, a well-designed trial with a larger scale and multiple centers is warranted.
Conclusion
In conclusion, the findings of this study reflect that the combination of anlotinib and platinum–etoposide regimen has promising efficacy and acceptable toxicities in patients with ES-SCLC as first-line therapy, suggesting that this combination might become a potential early treatment option for patients and physicians. On basis of the findings in this study, the combination regimen warrants further investigation in the large-scale phase 2 and randomized phase 3 trial.
Data availability
All data included in this study are available upon request by contact with the corresponding author.
Abbreviations
- ES-SCLC:
-
Extensive-stage small-cell lung cancer
- PFS:
-
Progression-free survival
- ORR:
-
Objective response rate
- DCR:
-
Disease control rate
- OS:
-
Overall survival
- mOS:
-
Median overall survival
- NSCLC:
-
Non-small cell lung cancer
- RECIST:
-
Response Evaluation Criteria in Solid Tumors
- ECOG:
-
Eastern Cooperative Oncology Group
- AEs:
-
Adverse events
- NCI:
-
National Cancer Institute
- MRI:
-
Magnetic resonance imaging
- CTCAE:
-
Common Terminology Criteria for Adverse Events
- SD:
-
Stable disease
- PD:
-
Disease progression
- CT:
-
Computed tomography
- AEs:
-
Adverse events
- DOR:
-
Duration of response
- TRAEs:
-
Treatment- related AEs
- TTP:
-
Time to progress
- rhG-CSF:
-
Recombinant human granulocyte colony-stimulating factor
- rhTPO:
-
Recombinant human thrombopoietin
- TKI:
-
Tyrosine kinase inhibitor
References
Facchinetti F, Di Maio M, Tiseo M (2020) Adding PD-1/PD-L1 inhibitors to chemotherapy for the first-line treatment of extensive Stage Small Cell Lung Cancer (SCLC): A meta-analysis of randomized trials. Cancers (Basel). https://doi.org/10.3390/cancers12092645
Morabito A, Rolfo C (2021) Small cell lung cancer: A new era is beginning? Cancers (Basel). https://doi.org/10.3390/cancers13112646
Rudin CM, Brambilla E, Faivre-Finn C, Sage J (2021) Small-cell lung cancer. Nat Rev Dis Primers 7:3. https://doi.org/10.1038/s41572-020-00235-0
Paz-Ares L, Dvorkin M, Chen Y, Reinmuth N, Hotta K, Trukhin D, Statsenko G, Hochmair MJ, Ozguroglu M, Ji JH et al (2019) Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet 394:1929–1939. https://doi.org/10.1016/S0140-6736(19)32222-6
Domine M, Moran T, Isla D, Marti JL, Sullivan I, Provencio M, Olmedo ME, Ponce S, Blasco A, Cobo M (2020) SEOM clinical guidelines for the treatment of small-cell lung cancer (SCLC) (2019). Clin Transl Oncol 22:245–255. https://doi.org/10.1007/s12094-020-02295-w
Sun A, Durocher-Allen LD, Ellis PM, Ung YC, Goffin JR, Ramchandar K, Darling G (2019) Initial management of small-cell lung cancer (limited- and extensive-stage) and the role of thoracic radiotherapy and first-line chemotherapy: a systematic review. Curr Oncol 26:e372–e384. https://doi.org/10.3747/co.26.4481
Ettinger DS, Wood DE, Aisner DL, Akerley W, Bauman J, Chirieac LR, D’Amico TA, DeCamp MM, Dilling TJ, Dobelbower M et al (2017) Non-small cell lung cancer, version 5.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 15:504–535. https://doi.org/10.6004/jnccn.2017.0050
Rudin CM, Ismaila N, Hann CL, Malhotra N, Movsas B, Norris K, Pietanza MC, Ramalingam SS, Turrisi AT 3rd, Giaccone G (2015) Treatment of small-cell lung cancer: American society of clinical oncology endorsement of the American college of chest physicians guideline. J Clin Oncol 33:4106–4111. https://doi.org/10.1200/JCO.2015.63.7918
Pietanza MC, Byers LA, Minna JD, Rudin CM (2015) Small cell lung cancer: will recent progress lead to improved outcomes? Clin Cancer Res 21:2244–2255. https://doi.org/10.1158/1078-0432.CCR-14-2958
Zhou T, Zhang Z, Luo F, Zhao Y, Hou X, Liu T, Wang K, Zhao H, Huang Y, Zhang L (2020) Comparison of first-line treatments for patients with extensive-stage small cell lung cancer: A systematic review and network meta-analysis. JAMA Netw Open 3:e2015748. https://doi.org/10.1001/jamanetworkopen.2020.15748
Fruh M, De Ruysscher D, Popat S, Crino L, Peters S, Felip E, Group EGW (2013) Small-cell lung cancer (SCLC): ESMO Clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 24(Suppl 6):vi99–vi105. https://doi.org/10.1093/annonc/mdt178
Liu SV, Reck M, Mansfield AS, Mok T, Scherpereel A, Reinmuth N, Garassino MC, De Castro CJ, Califano R, Nishio M et al (2021) Updated overall survival and PD-L1 subgroup analysis of patients with extensive-stage small-cell lung cancer treated with atezolizumab, carboplatin, and etoposide (IMpower133). J Clin Oncol 39:619–630. https://doi.org/10.1200/JCO.20.01055
Shen G, Zheng F, Ren D, Du F, Dong Q, Wang Z, Zhao F, Ahmad R, Zhao J (2018) Anlotinib: a novel multi-targeting tyrosine kinase inhibitor in clinical development. J Hematol Oncol 11:120. https://doi.org/10.1186/s13045-018-0664-7
Syed YY (2018) Anlotinib: First global approval. Drugs 78:1057–1062. https://doi.org/10.1007/s40265-018-0939-x
Sun Y, Du F, Gao M, Ji Q, Li Z, Zhang Y, Guo Z, Wang J, Chen X, Wang J et al (2018) Anlotinib for the treatment of patients with locally advanced or metastatic medullary thyroid cancer. Thyroid 28:1455–1461. https://doi.org/10.1089/thy.2018.0022
Zhou A-P, Bai Y, Song Y, Li H, Xie X, Ren X-B, Ye D, Liu J, Luo H, Bai X (2016) Anlotinib in metastatic renal cell carcinoma (mRCC) with a previous anti-VEGFR TKI: Preliminary results from a multicenter, phase II trial. Am Soc Clin Oncol
Huang J, Xiao J, Fang W, Lu P, Fan Q, Shu Y, Feng JF, Zhang S, Ba Y, Liu Y (2019) Anlotinib in chemotherapy-refractory metastatic esophageal squamous cell carcinoma (ESCC): A randomized, double-blind, multicenter phase II trial. Am Soc Clin Oncol
Xie C, Wan X, Quan H, Zheng M, Fu L, Li Y, Lou L (2018) Preclinical characterization of anlotinib, a highly potent and selective vascular endothelial growth factor receptor-2 inhibitor. Cancer Sci 109:1207–1219. https://doi.org/10.1111/cas.13536
Liang L, Hui K, Hu C, Wen Y, Yang S, Zhu P, Wang L, Xia Y, Qiao Y, Sun W et al (2019) Autophagy inhibition potentiates the anti-angiogenic property of multikinase inhibitor anlotinib through JAK2/STAT3/VEGFA signaling in non-small cell lung cancer cells. J Exp Clin Cancer Res 38:71. https://doi.org/10.1186/s13046-019-1093-3
Ruan X, Shi X, Dong Q, Yu Y, Hou X, Song X, Wei X, Chen L, Gao M (2019) Antitumor effects of anlotinib in thyroid cancer. Endocr Relat Cancer 26:153–164. https://doi.org/10.1530/ERC-17-0558
He Z, Zhou H, Wang J, Li D, Zhang X, Wang P, Ma T, Zhang Y, Tian C, Chen Y et al (2021) Apatinib with etoposide capsules as a third- or further-line therapy for extensive-stage small cell lung cancer: an open-label, multicenter, single-arm phase II trial. Transl Lung Cancer Res 10:889–899. https://doi.org/10.21037/tlcr-20-1235
Cheng Y, Wang Q, Li K, Shi J, Liu Y, Wu L, Han B, Chen G, He J, Wang J (2019) Overall survival (OS) update in ALTER 1202: Anlotinib as third-line or further-line treatment in relapsed small-cell lung cancer (SCLC). Ann Oncol 30:v711
Li Y, Sun Z, Sun W, Wang H, Zu J (2022) Effectiveness and Safety of anlotinib monotherapy for patients with extensive-stage small-cell lung cancer who progressed to chemotherapy: A real-world exploratory study. Clin Med Insights Oncol 16:11795549211067184. https://doi.org/10.1177/11795549211067184
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247
Lara PN Jr, Natale R, Crowley J, Lenz HJ, Redman MW, Carleton JE, Jett J, Langer CJ, Kuebler JP, Dakhil SR et al (2009) Phase III trial of irinotecan/cisplatin compared with etoposide/cisplatin in extensive-stage small-cell lung cancer: clinical and pharmacogenomic results from SWOG S0124. J Clin Oncol 27:2530–2535. https://doi.org/10.1200/JCO.2008.20.1061
Wang S, Zimmermann S, Parikh K, Mansfield AS, Adjei AA (2019) Current diagnosis and management of small-cell lung cancer. Mayo Clin Proc 94:1599–1622. https://doi.org/10.1016/j.mayocp.2019.01.034
Horn L, Mansfield AS, Szczesna A, Havel L, Krzakowski M, Hochmair MJ, Huemer F, Losonczy G, Johnson ML, Nishio M et al (2018) First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med 379:2220–2229. https://doi.org/10.1056/NEJMoa1809064
Montanino A, Manzo A, Carillio G, Palumbo G, Esposito G, Sforza V, Costanzo R, Sandomenico C, Botti G, Piccirillo MC et al (2021) Angiogenesis inhibitors in small cell lung cancer. Front Oncol. https://doi.org/10.3389/fonc.2021.655316
Deng P, Hu C, Chen C, Cao L, Gu Q, An J, Qin L, Li M, He B, Jiang J et al (2022) Anlotinib plus platinum-etoposide as a first-line treatment for extensive-stage small cell lung cancer: A single-arm trial. Cancer Med n/a. https://doi.org/10.1002/cam4.4736
Liu C, Liao J, Wu X, Zhao X, Sun S, Wang H, Hu Z, Zhang Y, Yu H, Wang J (2022) A phase II study of anlotinib combined with etoposide and platinum-based regimens in the first-line treatment of extensive-stage small cell lung cancer. Thorac Cancer 13:1463–1470. https://doi.org/10.1111/1759-7714.14414
Almquist D, Langlais B, Yu NY, Sio TTW, Savvides P, Yang P, Schild SE, Mansfield AS, Ernani V (2021) Chemoimmunotherapy for the treatment of extensive-stage small cell lung cancer (ES-SCLC) in patients with an Eastern Cooperative Group (ECOG) performance status (PS) of two or greater. J Clin Oncol 39:8569–8569. https://doi.org/10.1200/JCO.2021.39.15_suppl.8569
Broderick JM, Hussey J, Kennedy MJ, O’ Donnell DM (2014) Patients over 65years are assigned lower ECOG PS scores than younger patients, although objectively measured physical activity is no different. J Geriatr Oncol 5:49–56. https://doi.org/10.1016/j.jgo.2013.07.010
Tiseo M, Boni L, Ambrosio F, Camerini A, Baldini E, Cinieri S, Brighenti M, Zanelli F, Defraia E, Chiari R et al (2017) Italian, multicenter, phase III, randomized study of cisplatin plus etoposide with or without bevacizumab as first-line treatment in extensive-disease small-cell lung cancer: The GOIRC-AIFA FARM6PMFJM Trial. J Clin Oncol 35:1281–1287. https://doi.org/10.1200/JCO.2016.69.4844
Sharma N, Pennell N, Nickolich M, Halmos B, Ma P, Mekhail T, Fu P, Dowlati A (2014) Phase II trial of Sorafenib in conjunction with chemotherapy and as maintenance therapy in extensive-stage small cell lung cancer. Invest New Drugs 32:362–368. https://doi.org/10.1007/s10637-013-0061-6
Lu S, Li L, Luo Y, Zhang L, Wu G, Chen Z, Huang C, Guo S, Zhang Y, Song X et al (2015) A multicenter, open-label, randomized phase II controlled study of rh-endostatin (Endostar) in combination with chemotherapy in previously untreated extensive-stage small-cell lung cancer. J Thorac Oncol 10:206–211. https://doi.org/10.1097/JTO.0000000000000343
Luo H, Zhang L, Yang B, Feng Y, Xiong Y, Zhang S, Li X, Qian C, Dong W, Dai N (2020) A randomized phase 2 trial of apatinib vs observation as maintenance treatment following firstline induction chemotherapy in extensive stage small cell lung cancer. Invest New Drugs 38:148–159. https://doi.org/10.1007/s10637-019-00828-x
Lin B, Song X, Yang D, Bai D, Yao Y, Lu N (2018) Anlotinib inhibits angiogenesis via suppressing the activation of VEGFR2, PDGFRbeta and FGFR1. Gene 654:77–86. https://doi.org/10.1016/j.gene.2018.02.026
Spigel DR, Townley PM, Waterhouse DM, Fang L, Adiguzel I, Huang JE, Karlin DA, Faoro L, Scappaticci FA, Socinski MA (2011) Randomized phase ii study of bevacizumab in combination with chemotherapy in previously untreated extensive-stage small-cell lung cancer: Results from the SALUTE Trial. J Clin Oncol 29:2215–2222. https://doi.org/10.1200/jco.2010.29.3423
Reck M, Horn L, Novello S, Barlesi F, Albert I, Juhasz E, Kowalski D, Robinet G, Cadranel J, Bidoli P et al (2019) Phase II study of roniciclib in combination with cisplatin/etoposide or carboplatin/etoposide as first-line therapy in patients with extensive-disease small cell lung cancer. J Thorac Oncol 14:701–711. https://doi.org/10.1016/j.jtho.2019.01.010
Jiang S, Huang L, Zhen H, Jin P, Wang J, Hu Z (2021) Carboplatin versus cisplatin in combination with etoposide in the first-line treatment of small cell lung cancer: a pooled analysis. BMC Cancer 21:1308. https://doi.org/10.1186/s12885-021-09034-6
Gao Y, Liu P, Shi R (2020) Anlotinib as a molecular targeted therapy for tumors. Oncol Lett 20:1001–1014. https://doi.org/10.3892/ol.2020.11685
Zhou AP, Bai Y, Song Y, Luo H, Ren XB, Wang X, Shi B, Fu C, Cheng Y, Liu J et al (2019) Anlotinib versus sunitinib as first-line treatment for metastatic renal cell carcinoma: a randomized phase ii clinical trial. Oncologist 24:e702–e708. https://doi.org/10.1634/theoncologist.2018-0839
Acknowledgements
We thank all participants and their families.
Funding
This study was supported by Technologies R & D Program of Henan Province (202102310456), and Medical Science and Technology Joint construction Project of Henan Province (LHGJ20210730).
Author information
Authors and Affiliations
Contributions
Tiandong kong and Danna Liu contributed to the study conception and design. Administrative support was provided by Lei Wang. Hanli Zhou and Fangfang Duan provided materials and samples, Data collection, analysis and interpretation were performed by Xiaoli Zhao, Lu Chen, and Tiandong Kong. All authors have read and approved the manuscript.
Corresponding authors
Ethics declarations
Ethical approval
This study was conducted according to the "Declaration of Helsinki". Ethics approval is obtained from the medical ethics committee of the Third People's Hospital of Zhengzhou city.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kong, T., Chen, L., Zhao, X. et al. Anlotinib plus etoposide and cisplatin/carboplatin as first-line therapy for extensive-stage small cell lung cancer (ES-SCLC): a single-arm, phase II study. Invest New Drugs 40, 1095–1105 (2022). https://doi.org/10.1007/s10637-022-01279-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-022-01279-7