Summary
Rolapitant is a neurokinin-1 receptor antagonist that is approved in combination with other antiemetic agents in adults for the prevention of delayed nausea and vomiting (CINV) associated with initial and repeat courses of emetogenic cancer chemotherapy, including but not limited to highly emetogenic chemotherapy. Here, we assessed the absorption, metabolism, and excretion of 14C-labeled rolapitant in healthy male subjects. Rolapitant was administered as a single 180-mg oral dose containing approximately 100 μCi of total radioactivity, with plasma, urine, and fecal samples collected at defined intervals after dosing. Rolapitant had a large apparent volume of distribution, indicating that it is widely distributed into body tissues. Rolapitant was slowly metabolized and eliminated with a mean half-life of 186 h. Exposure to the major metabolite of rolapitant, C4-pyrrolidinyl hydroxylated rolapitant or M19, was approximately 50% of rolapitant exposure in plasma. Renal clearance was not a significant elimination route for rolapitant-related entities. Total radioactivity recovered in urine accounted for 14.2% of the dose, compared to 72.7% recovery in feces. Adverse events (AEs) were generally mild; there were no serious AEs, and no clinically significant changes in laboratory or electrocardiogram parameters were observed. The combination of rolapitant safety, its long half-life, extensive tissue distribution, and slow elimination via the hepatobiliary route (rather than renal excretion) suggest suitability that a single dose of rolapitant may provide protection against CINV beyond the first 24 h after chemotherapy administration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nausea and vomiting are common, undesirable consequences of cytotoxic chemotherapy that negatively affect the quality of life of treated patients and have the potential to disrupt receipt of effective cancer treatments [1,2,3,4,5,6]. Rolapitant is an antagonist of the neurokinin-1 (NK-1) receptor [7], which mediates induction of vomiting in response to substance P release from neurons that is triggered by chemotherapy administration. This interaction occurs during the delayed phase (occurring >24 h to up to 5 days after chemotherapy) of chemotherapy-induced nausea and vomiting (CINV) [8]. Notably, NK-1 receptors are also present in the gastrointestinal tract and may contribute to acute-phase CINV (occurring within 1–2 h of chemotherapy and lasting for up to 24 h) [6].
Rolapitant significantly improved complete response (defined as no emesis and no use of rescue medication) in the delayed phase of CINV compared with controls in four clinical studies (including three randomized phase III studies [9,10,11] and one phase II study [12]) of patients receiving either highly or moderately emetogenic chemotherapy. Rolapitant was effective through multiple cycles in the delayed phase following chemotherapy [9]. As a result, rolapitant was approved for use in combination with other antiemetic agents in adults for the prevention of delayed nausea and vomiting associated with initial and repeat courses of emetogenic cancer chemotherapy, including but not limited to highly emetogenic chemotherapy [13]. Current treatment guidelines recommend NK-1 receptor antagonist use in CINV prophylaxis for highly emetogenic and select moderately emetogenic chemotherapies [2, 14, 15]; rolapitant has an added advantage of having the longest half-life among existing NK-1 antagonists, which may provide sustained efficacy [16]. Rolapitant is administered as a single 180-mg dose as part of a combination therapy within 2 h before the initiation of each chemotherapy cycle [13].
Regulatory agencies, including the US Food and Drug Administration and European Medicines Agency, have stressed the importance of studying the absorption, metabolism, and excretion of drugs [17, 18] and the utility of radiolabeled probes in these analyses [19]. In the present study, we characterized the absorption, metabolism, and excretion of 14C-labeled rolapitant in healthy male subjects.
Materials and methods
Study design
This was a two-part, non-randomized, open-label, single-center, single-dose study conducted in healthy male volunteers. Six patients enrolled in each part. The objective of Part 1 was to determine the absolute bioavailability of rolapitant; results were previously described [20]. The objective of Part 2, which is reported here, was to characterize the absorption, metabolism, and excretion of rolapitant.
Written informed consent was obtained from all subjects during pre-study screening procedures conducted within 21 days before the first dosing day. Upon enrollment, subjects were confined to the study site, under appropriate medical supervision, from Day −1 to the morning of Day 15. Additional sampling was performed in an outpatient setting.
The protocol and supporting materials were reviewed and approved by the Medisch Ethische Toetsings Commissie van de Stichting Beoordeling Ethiek Bio-Medisch Onderzoek (Assen, Netherlands). The study was conducted in accordance with the Declaration of Helsinki (including amendments in effect at the time the study was conducted [2008]), Guidelines of the International Conference on Harmonisation on Good Clinical Practice, as well as the requirements of the Health Insurance Portability and Accountability Act of 1996 (Public Law 104-191, 104th Congress), privacy regulations, and other applicable regulatory requirements including 21 Code of Federal Regulations 312, 50, and 56.
After an overnight fast, six subjects received a single 200-mg dose of 14C-rolapitant hydrochloride monohydrate (Supplemental Fig. 1) as a suspension in 0.4% hydroxypropyl methylcellulose containing approximately 100 μCi of radioactivity administered orally and followed by 240 ml of water. Each 200 mg of rolapitant hydrochloride is equivalent to 180 mg of rolapitant free base [16]; therefore, the dose is noted as 180 mg 14C-rolapitant throughout the remainder of the manuscript. Because of the long half-life of rolapitant, it was not possible to keep subjects in confinement until >80% of total radioactivity was recovered. Subjects were followed on a weekly basis until 6 weeks postdose for 24-h urine and fecal collections to assure adequate total recovery of 14C-rolapitant.
Sample collection and analysis
Plasma, urine, and fecal samples were collected at the study site (PRA International, Zuidlaren, Netherlands) and shipped in dry ice to US research laboratories for analysis. Plasma samples were stored at −80 ± 10 °C, whereas urine and feces were stored at −20 ± 10 °C until processing.
Plasma samples were collected for metabolite profiling at predose (0 h), and at 1, 2, 3, 6, 12, 24, 72, 96, 120, 168, and 336 h postdose. To assess area under the concentration-time curve (AUC) of rolapitant and metabolites, aliquots of plasma samples were pooled across all subjects (n = 6) and time points (0–336 h) to yield a pooled sample in which the concentration of the parent drug and metabolites was proportional to the AUC for 0–336 h.
Urine samples were collected at 0–12, 12–24, 24–36, 36–48 h and in 24-h intervals up to 336 h postdose, and thereafter on Day 23, 30, 37, and 44 from six subjects. Urine was pooled over 0–336 h from all six subjects for drug metabolite profiling. Fecal homogenates were pooled over 0–336 h from all six subjects for drug metabolite profiling.
Plasma rolapitant concentrations were determined using a validated liquid chromatography-tandem mass spectrometry assay. Whole blood, plasma, urine, and feces were assayed by liquid scintillation spectrometry for total radioactivity content. Selected plasma, urine, and feces samples were used for profiling and identification of metabolites.
Pharmacokinetic parameters
Pharmacokinetic parameters determined in this study included AUC from time 0 to last quantifiable concentration (AUC0–last) and from time 0 to infinity (AUC0–inf); maximum observed plasma concentration (Cmax) and time to reach Cmax (Tmax); concentration at time of final quantifiable sample (Ctf); apparent volume of distribution (Vd); and apparent total body clearance (CL/F). Whole blood and plasma total radioactivity and concentration-time data were collected for rolapitant and its major metabolite M19 [21].
Safety
Safety was assessed based on physical examinations, adverse events (AEs), vital signs, electrocardiograms (ECGs), and clinical laboratory test results. Adverse events were classified using the Medical Dictionary for Regulatory Activities, with severity assessed using the National Cancer Institute’s Common Toxicity Criteria, and relationship to study drug was assessed by the investigator.
Results
Patients
Six adult male subjects between the ages of 37 and 64 years (mean, 55.7 years) were enrolled. All subjects were Caucasian; median body mass index was 26.8 (range: 23.4–28.5). All subjects completed the treatment phase and the required study duration.
Absorption, metabolism, and excretion
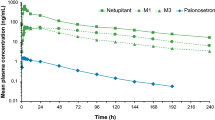
After oral administration, 14C-rolapitant was rapidly absorbed and slowly eliminated (Fig. 1). Individual peak plasma rolapitant concentrations, ranging from 531 to 1120 ng/ml, were observed at 2 to 6 h postdose (Tmax). Mean half-life was 186 h, and apparent clearance was 1.74 l/h (Table 1). Because the absolute bioavailability of orally administered rolapitant is close to 100%, the high apparent Vd (460 l) is representative of true Vd, indicating extensive tissue distribution of rolapitant. Total drug-related material in plasma was approximately two-fold higher than that of whole blood, suggesting a lack of substantial distribution of rolapitant and its metabolites into red blood cells (Table 2).
The mean AUC ratio of plasma rolapitant and plasma radioactivity was 53%. This was attributed to the formation of metabolites. Further analysis of the plasma samples for the C4-pyrrolidinyl hydroxylated metabolite M19 indicated that 25% of the increased exposure to total plasma radioactivity was due to the formation of M19; therefore, these results confirm that M19 is the major circulating metabolite of rolapitant.
Blood and/or plasma radioactivity concentrations reached maximum values within 2.5 to 3 h postdose and were less than the lower limit of quantitation by 528 h postdose. Mean blood/plasma radioactivity ratios ranged from 0.28 to 0.59 over this span. Generally, the plasma radioactivity concentration–time profile paralleled that of blood radioactivity through 528 h (the last measurable time point in blood). These data suggest minimal sequestering of drug-derived radioactivity into red blood cells.
Although desired for an absorption, metabolism, and excretion analysis, complete recovery of the radioactive dose was not expected within the 2-week collection period because of the long half-life of rolapitant. Interpolation of fecal and urine samples collected up to 6 weeks postdose (rate of elimination) was used to project the excreted dose over time. This interpolation suggested that approximately 48% of the dose would be excreted after 2 weeks postdose, and approximately 85% of the radioactive dose would have been recovered within 6 weeks, representing four half-lives if samples had been collected continuously.
Excretion of radioactivity into the urine and feces was slow. A mean measured total of 46% (range: 25.0–64.5%) of the radioactive dose was recovered in urine and fecal samples collected daily 0 to 336 h postdose after single oral administration of 14C-labeled rolapitant (Fig. 2). Total radioactivity recovered in the urine accounted for 14.2% (range: 9.1–20.0%) of the dose and total radioactivity recovered in the feces was 72.7% (range: 51.8–88.7%) of the dose at 1032 h postdose (Table 3). These data indicate that a hepatobiliary route rather than renal clearance is the major elimination route for drug-related entities.
Metabolite profiling
Unchanged rolapitant was the most prominent drug-related component in pooled plasma (0–336 h) following a single oral dose of 180 mg 14C-rolapitant (Supplemental Fig. 2). The monohydroxylated metabolite M19 was the major circulating metabolite and accounted for 28.1% of the parent drug’s exposure.
An average of 8.28% of dosed radioactivity was recovered in 0–336 h urine collection interval, which represented 93.3% of the total drug-derived material excreted in urine through Day 44. M5 and M9b were the major metabolites in urine and accounted for 1.57 and 1.64% of the dose, respectively (Supplemental Fig. 3). No other metabolite accounted for >1% of the dosed radioactivity.
An average of 90% of the total drug-derived metabolite material excreted in feces through Day 44 was present in the pooled sample and contained 37.8% of the total administered dose. The rolapitant parent drug was the most prominent drug-related component in pooled feces and accounted for 12.7% of the dose (Supplemental Fig. 4). Major fecal metabolites included M9b, M10c, and M13, which accounted for 4.27, 4.80, and 3.54% of the dose, respectively.
The most prominent biotransformation pathway of rolapitant was oxidation (Table 3 and Fig. 3). This pathway produced the major circulating metabolite M19, which represented approximately 28.1% of the parent drug exposure (0–336 h). Other major biotransformation pathways, besides mono-oxygenation to form M19, included mono-oxidation leading to the formation of M13 and multiple oxidations leading to the formation of M9b and M10c. O-dealkylation followed by glucuronidation was observed in urine. Overall, very few glucuronide conjugates were detected and accounted for <3% of the dose. Rolapitant and identified metabolites were more abundant in the feces than in the urine (37.8% vs. 8.3% of total dosed radioactivity, respectively).
Safety
Four of six (67%) subjects reported AEs: somnolence was reported by three (50%) subjects, diarrhea and headache were reported by two (33%) subjects each, and viral gastroenteritis was reported by one (17%) subject. Four of these AEs (diarrhea and headache, n = 1 each; somnolence, n = 2) were considered to be treatment related. All events, except viral gastroenteritis, were mild. There were no serious AEs, and no clinically significant changes in blood chemistry and hematology parameters, vital signs, or ECGs.
Discussion
Rolapitant is a selective NK-1 antagonist able to effectively prevent delayed CINV [9,10,11,12]. This study showed that rolapitant and its active metabolite are slowly metabolized and eliminated, with a mean half-life of 186 h (>1 week). This half-life is longer than that of other NK-1 antagonists [16] and supports clinical data in which a single-dose regimen prevented CINV beyond the first 24 h after chemotherapy administration in the majority of patients [13].
The Vd observed in this study indicates that rolapitant is widely distributed in the human body. This high Vd is desirable and consistent with the mechanism of target action of rolapitant, which was demonstrated in a brain NK-1 receptor occupancy study in healthy volunteers [20]. The high Vd permits adequate brain distribution, ensuring an antagonistic interaction with the target (NK-1) receptor.
Elimination of rolapitant and its metabolites predominantly occurred via the hepatobiliary route, rather than renal clearance. This characteristic is advantageous because medications that are likely to be co-administered with rolapitant, including cisplatin, carboplatin, methotrexate, and other chemotherapeutic drugs, are principally eliminated renally and known to be nephrotoxic [22,23,24].
Metabolic profiling results from this study were consistent with those observed in vitro (data on file). In vitro, CYP3A4-mediated oxygenation, primarily the formation of an active metabolite M19, is the major biotransformation pathway [13]. Therefore, M19 was evaluated in a series of clinical pharmacology studies including those assessing drug-drug interactions [25, 26]. Similar to rolapitant, this predominant circulating metabolite elicited a compelling drug profile, with minimal potential for drug-drug interactions.
A single oral dose of 14C-labeled rolapitant was well-tolerated, consistent with the results observed with rolapitant across clinical studies [9,10,11,12]. The combination of rolapitant safety, its long half-life, extensive tissue distribution, and slow elimination via the hepatobiliary route (rather than renal excretion) suggest that single-dose rolapitant administration is suitable to prevent CINV in patients beyond the first 24 h after chemotherapy administration.
References
Grunberg SM, Deuson RR, Mavros P, Geling O, Hansen M, Cruciani G, Daniele B, De Pouvourville G, Rubenstein EB, Daugaard G (2004) Incidence of chemotherapy-induced nausea and emesis after modern antiemetics. Cancer 100(10):2261–2268. https://doi.org/10.1002/cncr.20230
Berger MJ, Ettinger DS, Aston J, Barbour S, Bergsbaken J, Bierman PJ, Brandt D, Dolan DE, Ellis G, Kim EJ, Kirkegaard S, Kloth DD, Lagman R, Lim D, Loprinzi C, Ma CX, Maurer V, Michaud LB, Nabell LM, Noonan K, Roeland E, Rugo HS, Schwartzberg LS, Scullion B, Timoney J, Todaro B, Urba SG, Shead DA, Hughes M (2017) NCCN guidelines insights: antiemesis, version 2.2017. J Natl Compr Cancer Netw 15(7):883–893. https://doi.org/10.6004/jnccn.2017.0117
Cohen L, de Moor CA, Eisenberg P, Ming EE, Hu H (2007) Chemotherapy-induced nausea and vomiting—incidence and impact on patient quality of life at community oncology settings. Support Care Cancer 15(5):497–503. https://doi.org/10.1007/s00520-006-0173-z
Bloechl-Daum B, Deuson RR, Mavros P, Hansen M, Herrstedt J (2006) Delayed nausea and vomiting continue to reduce patients’ quality of life after highly and moderately emetogenic chemotherapy despite antiemetic treatment. J Clin Oncol 24(27):4472–4478. https://doi.org/10.1200/JCO.2006.05.6382
Hilarius DL, Kloeg PH, van der Wall E, van den Heuvel JJ, Gundy CM, Aaronson NK (2012) Chemotherapy-induced nausea and vomiting in daily clinical practice: a community hospital-based study. Support Care Cancer 20(1):107–117. https://doi.org/10.1007/s00520-010-1073-9
Hesketh PJ (2008) Chemotherapy-induced nausea and vomiting. N Engl J Med 358(23):2482–2494. https://doi.org/10.1056/NEJMra0706547
Duffy RA, Morgan C, Naylor R, Higgins GA, Varty GB, Lachowicz JE, Parker EM (2012) Rolapitant (SCH 619734): a potent, selective and orally active neurokinin NK1 receptor antagonist with centrally-mediated antiemetic effects in ferrets. Pharmacol Biochem Behav 102(1):95–100. https://doi.org/10.1016/j.pbb.2012.03.021
Garcia-Recio S, Gascon P (2015) Biological and pharmacological aspects of the NK1-receptor. Biomed Res Int 2015:495704. https://doi.org/10.1155/2015/495704
Rapoport B, Schwartzberg L, Chasen M, Powers D, Arora S, Navari R, Schnadig I (2016) Efficacy and safety of rolapitant for prevention of chemotherapy-induced nausea and vomiting over multiple cycles of moderately or highly emetogenic chemotherapy. Eur J Cancer 57:23–30. https://doi.org/10.1016/j.ejca.2015.12.023
Rapoport BL, Chasen MR, Gridelli C, Urban L, Modiano MR, Schnadig ID, Poma A, Arora S, Kansra V, Schwartzberg LS, Navari RM (2015) Safety and efficacy of rolapitant for prevention of chemotherapy-induced nausea and vomiting after administration of cisplatin-based highly emetogenic chemotherapy in patients with cancer: two randomised, active-controlled, double-blind, phase 3 trials. Lancet Oncol 16(9):1079–1089. https://doi.org/10.1016/S1470-2045(15)00035-2
Schwartzberg LS, Modiano MR, Rapoport BL, Chasen MR, Gridelli C, Urban L, Poma A, Arora S, Navari RM, Schnadig ID (2015) Safety and efficacy of rolapitant for prevention of chemotherapy-induced nausea and vomiting after administration of moderately emetogenic chemotherapy or anthracycline and cyclophosphamide regimens in patients with cancer: a randomised, active-controlled, double-blind, phase 3 trial. Lancet Oncol 16(9):1071–1078. https://doi.org/10.1016/S1470-2045(15)00034-0
Rapoport B, Chua D, Poma A, Arora S, Wang Y, Fein LE (2015) Study of rolapitant, a novel, long-acting, NK-1 receptor antagonist, for the prevention of chemotherapy-induced nausea and vomiting (CINV) due to highly emetogenic chemotherapy (HEC). Support Care Cancer 23(11):3281–3288. https://doi.org/10.1007/s00520-015-2738-1
Levade M, David E, Garcia C, Laurent PA, Cadot S, Michallet AS, Bordet JC, Tam C, Sie P, Ysebaert L, Payrastre B (2014) Ibrutinib treatment affects collagen and von Willebrand factor-dependent platelet functions. Blood 124(26):3991–3995. https://doi.org/10.1182/blood-2014-06-583294
Roila F, Molassiotis A, Herrstedt J, Aapro M, Gralla RJ, Bruera E, Clark-Snow RA, Dupuis LL, Einhorn LH, Feyer P, Hesketh PJ, Jordan K, Olver I, Rapoport BL, Roscoe J, Ruhlmann CH, Walsh D, Warr D, van der Wetering M, participants of the MECCC (2016) 2016 MASCC and ESMO guideline update for the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting and of nausea and vomiting in advanced cancer patients. Ann Oncol 27(suppl 5):v119–v133. https://doi.org/10.1093/annonc/mdw270
Hesketh PJ, Bohlke K, Lyman GH, Basch E, Chesney M, Clark-Snow RA, Danso MA, Jordan K, Somerfield MR, Kris MG, American Society of Clinical O (2016) Antiemetics: American Society of Clinical Oncology focused guideline update. J Clin Oncol 34(4):381–386. https://doi.org/10.1200/JCO.2015.64.3635
Rapoport BL (2017) Differential pharmacology and clinical utility of rolapitant in chemotherapy-induced nausea and vomiting. Cancer Manag Res 9:41–50. https://doi.org/10.2147/CMAR.S97543
Food and Drug Administration (Center for Drug Evaluation and Research) (2017) Guidance for industry: safety testing of drug metabolites. https://www.fda.gov/downloads/Drugs/.../Guidances/ucm079266.pdf
Committee for Medicinal Products for Human Use (2016) Guideline on evaluation of anticancer medicinal products in man. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2016/03/WC500203320.pdf
Nijenhuis CM, Schellens JH, Beijnen JH (2016) Regulatory aspects of human radiolabeled mass balance studies in oncology: concise review. Drug Metab Rev 48(2):266–280. https://doi.org/10.1080/03602532.2016.1181081
Wang X, Zhang ZY, Powers D, Wang J, Lu S, Kansra V (2017) Rolapitant absolute bioavailability and PET imaging studies in healthy adult volunteers. Clin Pharmacol Ther 102(2):332–339. https://doi.org/10.1002/cpt.637
Poma A, Christensen J, Pertikis H et al (2013) Rolapitant and its major metabolite do not affect the pharmacokinetics of midazolam, a sensitive cytochrome P450 3A4 substrate [abstract 441]. Support Care Cancer 21:S154
Hartlev LB, Boeje CR, Bluhme H, Palshof T, Rehling M (2012) Monitoring renal function during chemotherapy. Eur J Nucl Med Mol Imaging 39(9):1478–1482. https://doi.org/10.1007/s00259-012-2158-0
Tanabe N, Goto M, Morita H, Gotu T, Inagaki J, Yamanaki N, Kimura K (1991) Pharmacokinetics of cis-diammine-dichlor-platin in a hemodialysis patient. Cancer Investig 9(6):629–635. https://doi.org/10.3109/07357909109039874
Widemann BC, Adamson PC (2006) Understanding and managing methotrexate nephrotoxicity. Oncologist 11(6):694–703. https://doi.org/10.1634/theoncologist.11-6-694
Wang X, Zhang ZY, Powers D, Wang J, Lu S, Arora S, Hughes L, Christensen J, Kansra V (2017) Bioequivalence of intravenous and oral rolapitant: results from a randomized, open-label pivotal study. J Clin Pharmacol 57(12):1600–1606. https://doi.org/10.1002/jcph.966
Wang X, Zhang ZY, Arora S, Hughes L, Wang J, Powers D, Christensen J, Lu S, Kansra V (2018) Effects of rolapitant administered intravenously or orally on the pharmacokinetics of digoxin (P-glycoprotein substrate) and sulfasalazine (breast cancer resistance protein substrate) in healthy volunteers. J Clin Pharmacol 58(2):202–211. https://doi.org/10.1002/jcph.1005
Acknowledgments
The authors wish to acknowledge the contribution of the scientists at Schering-Plough Corporation in the execution of the study; to thank Jan Jaap van Lier, MD, who was the primary investigator for this study; and to thank Pearl Pathways, LLC for initial manuscript drafting. Writing and editorial support, funded by TESARO, Inc. (Waltham, MA, USA) and coordinated by Hemant Vyas, PhD of TESARO Inc., was provided by Tiffany Brake, PhD and Beverly Stanley, ELS of Team 9 Science, Vaniam Group (Chicago, IL, USA). All authors were involved in the collection, analysis, and interpretation of data, the writing of the manuscript, and the decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Funding for this study and for the preparation of this manuscript was provided by TESARO, Inc. All authors are employees or former employees of the study sponsor. The authors have indicated that they have no other conflicts of interest regarding the content of this article. Professional medical writing support was used to develop this article.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
ESM 1
(DOCX 450 kb)
Rights and permissions
About this article
Cite this article
Zhang, ZY., Wang, J., Kansra, V. et al. Absorption, metabolism, and excretion of the antiemetic rolapitant, a selective neurokinin-1 receptor antagonist, in healthy male subjects. Invest New Drugs 37, 139–146 (2019). https://doi.org/10.1007/s10637-018-0638-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-018-0638-1