Summary
The goals of the present study were to define the anticancer activity of LFM-A13 (α-cyano-β-hydroxy-β-methyl-N-(2,5-dibromophenyl)-propenamide), a potent inhibitor of Polo-like kinase (PLK), in a mouse mammary cancer model induced by 7,12-dimethylbenz(a)anthracene (DMBA) in vivo and explore its anticancer mechanism(s). We also examined whether the inhibition of PLK by LFM-A13 would improve the efficiency of paclitaxel in breast cancer growth in vivo. To do this, female BALB/c mice received 1 mg of DMBA once a week for 6 weeks with oral gavage. LFM-A13 (50 mg/kg body weight) was administered intraperitoneally with DMBA administration and continued for 25 weeks. We found that LFM-A13, paclitaxel, and their combination have a significant effect on the DMBA-induced breast tumor incidence, mean tumor numbers, average tumor weight, and size. At the molecular level, the administration of LFM-A13 hindered mammary gland carcinoma development by regulating the expression of PLK1, cell cycle-regulating proteins cyclin D1, cyclin dependent kinase-4 (CDK-4), and the CDK inhibitor, p21. Moreover, LFM-A13 treatment upregulated the levels of IκB, the pro-apoptotic proteins Bax, and caspase-3, and down-regulated p53 and the antiapoptotic protein Bcl-2 in mammary tumors. The combination of LFM-A13 with paclitaxel was found to be more effective compared with either agent alone. Collectively, these results suggest that LFM-A13 has an anti-proliferative activity against breast cancer in vivo and that LFM-A13 and paclitaxel combination could be a strategy for the treatment of breast cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer is the most common cancer with the highest morbidity and mortality in women all over the world. It is estimated that there will be over 40,000 deaths from breast cancer in 2017 in United States of America alone [1]. As the number of breast cancer cases in many developing countries has increased [2], it is a disease that afflicts both developed and developing countries. Whilst African countries have experienced the lowest incidence rates of breast cancer, the incidence of the disease in these areas is beginning to rise [3]. The development of breast cancer is associated with alterations in cellular differentiation, increased cell proliferation and defects in apoptosis [4].
Polo-like kinases (PLK) are a family of highly conserved mammalian serine/threonine protein kinases with multiple functions during cell division, including centrosome maturation, mitotic spindle formation, they can promote mitotic exit and cytokinesis, and can regulate apoptosis [5,6,7,8]. There are five mammalian PLKs called PLK1–5 [9]. PLK1 promotes mitotic entry through the phosphorylation of Cdc25C [10]. The expression of PLK1 is induced during S phase and reaches its peak during the G2/M phase of the cell cycle [11]. Notably, PLK1 expression is increased in different types of cancer, including non-small cell lung cancer, ovarian cancer and colon cancer [7, 11]. On the other hand, PLK3 functions as an inhibitor of the Cdc25C and G2/M transition, and increased expression of PLK3 leads to rapid cell cycle arrest and apoptosis [12, 13]. Decreased expression of PLK3 has been detected in lung carcinomas [14], head and neck cancer [15], and rat colon tumors [16].
PLK inhibitors are being evaluated as a new class of anti-cancer drugs. A number of animal studies has shown that PLK inhibitors are useful in the treatment of tumors [17,18,19]. However, the molecular basis of the anti-cancer activity of these inhibitors is still unknown. In this study, we evaluated anti-tumor effects of PLK inhibitor, LFM-A13 (α-cyano-β-hydroxy-β-methyl-N-(2,5-dibromophenyl)-propenamide) on 7,12-dimethylbenz(a)anthracene (DMBA)-induced breast tumors in mice. We also evaluated the effect of LFM-A13 on the expression of PLKs, cell-cycle regulatory proteins as well as apoptosis-related proteins in DMBA-induced experimental breast cancer to find the mechanism(s) underlying anti-tumor activity in mice.
Materials and methods
Chemicals and reagents
LFM-A13 was synthesized and characterized as defined earlier [20, 21]. LFM-A13 stock solutions were prepared in methanol and stored at −20 °C [18]. To obtain working solutions, the stocks were further diluted in 90% methanol [18]. Non-GMP grade solutions of LFM-A13 active drug were mixed with 15% DMSO/PBS solutions for testing in mice. DMBA and paclitaxel (taxol) were obtained from Sigma-Aldrich, Inc. (St. Louis, MO, USA). Phosphate-buffered formalin (10%) was purchased from Sigma (St Louis, MO). All the solvents were of high purity and analytical grade (Merck Co. Darmstadt, Germany). All antibodies were purchased from Abcam (Abcam, UK).
Animals
Fifty day old female BALB/c mice were purchased from Firat University, Elazig and housed in a constant temperature of 22 ± 2 °C and humidity of 55 ± 5% under 12 h of light and 12 h of darkness per day. The mice had an adaptation period of 1 week before the beginning of the experiment.
The research was carried out within the framework of protocols approved by the Animal Care and Use Committee of Firat University. All procedures for mice have been carried out in accordance with the relevant law, the Animal Welfare Act, the Public Health Service Policy.
Experimental design
The mice were allocated into five groups of 20 animals in each: 1) control group, animals received no DMBA and was given sesame oil, served as the negative control group; 2) DMBA group, tumor-induced animals received a single dose of DMBA dissolved in sesame oil, chosen as a positive control, 3) Paclitaxel + DMBA group, animals received paclitaxel (10 mg/kg body weight, once per week intraperitoneally) [22] after DMBA administration on day zero, 4) LFM-A13 + DMBA group, received LFM-A13 (50 mg/kg body weight, three times per week intraperitoneally), 5) Paclitaxel + LFM-A13 + DMBA group, received paclitaxel and LFM-A13. DMBA was dissolved in sesame oil to give a 10 mg/ml stock concentration and mice were gavaged p.o. with 0.1 ml (total 1 mg) DMBA once a week for 6 weeks [23]. Mice were observed daily, and all the necessary data comprising body weights and breast tumors were measured weekly. All mice were sacrificed by cervical dislocation after an overnight fast at the end of 25 week. Blood was collected and normal mammary tissue, mammary tumors, and suspicious lesions were rapidly removed, measured, and documented following by rinsing in physiological saline.
The incidence of tumors was calculated in each group. Tumors were histologically categorized as an adenocarcinoma or benign. For histological evaluation, samples were prepared in 10% buffered formalin and then embedded in paraffin. The sections were mounted on glass slides and stained with hematoxylin and eosin using routine laboratory procedures in the Pathology Laboratory of Faculty of Medicine at the University of Firat, Elazig, Turkey.
Fresh tissue was used for each experiment. Blood samples were centrifuged at 3000 x g for 10 min and the serum was carefully removed and stored at −80 °C until further analysis.
Western blotting
Western blotting was carried out as previously described [24, 25]. The primary antibodies against PLK1, Cyclin D1, cyclin dependent kinase-4 (CDK-4), p53, IκB, Bcl-2, Bax, cleaved caspase-3, and β-actin, as well as the secondary goat antirabbit horseradish-peroxidase-conjugated antibody was purchased from Abcam (Abcam Inc., UK). Blots were performed at least four times to confirm data reproducibility. Protein levels were analyzed by densitometry using an image analysis system (Image J; National Institute of Health, Bethesda, USA).
Toxicity studies in rats
Eight-week-old wistar albino rats were purchased at Firat University Laboratory Animal Research Center (Elazig, Turkey). Animals were housed in cages in a controlled environment (12-h light/12-h dark photoperiod (22 ± 2 °C, 60 ± 10% relative humidity) conditions. Study has been approved by the Committee for Animal Research and Use of Animal Care at Firat University. All procedures have been carried out in strict accordance with the applicable law, the Animal Welfare Act, the Public Health Service Policy.
In rats, acute toxicity profiles of LFM-A13 were studied as previously reported [17, 21]. Intraperitoneal injection of LFM-A13 (three times weekly) at 25, 50 and 100 mg / kg levels was administered to 8-week-old rats (groups of 10, 5 male and 5 female rats per group). Each rat was monitored daily for morbidity and mortality. Rats were sacrificed on day 30 for the determination of the toxicity of LFM-A13 through examination of blood chemistry profiles, blood counts, and evaluation of multiple organs for the presence of toxic lesions as described [21].
Statistical analysis
In order to verify the replicability of the data, each blotting was carried out at least four times. The discrete variables (tumor incidence, tumor type count) were analyzed by chi-square test using the PROC FREQ procedure (SAS) while continuous variables (tumor weight and volume, serum and molecular biology data) were analyzed by ANOVA using the PROC GLM procedure (SAS, 2002). Differences among groups were determined by the Tukey’s multiple comparison. Moreover, the Fisher’s Exact chi-square test option was employed in 2 × 2 cross tables due to insufficiency of sample size for each grid. Statistical significance was declared when p < 0.05. SAS software (SAS Institute Inc., Cary, NC, USA), Microsoft Excel (Microsoft, Redmond, WA, USA), and GraphPad Prism (GraphPad Software, La Jolla, CA, USA) were used for data processing.
Results
Toxicity of LFM-A13 in rats
We first tested the toxicity of LFM-A13 in vivo. Administration of three different doses of LFM-A13 to rats revealed no severe toxicity (Table S1). There was no evidence of a histopathological effect on multiple tissues from these animals (i.e. heart, kidney, pancreas, lungs, and brain) (Table S1). Consistently, biochemical and hematological assessment of toxic effects of LFM-A13 also revealed no sign of toxicity in LFM-A13-treated rats over a wide dosage range (Table S2 and S3). These data illustrate that LFM-A13 has no apparent toxicity when administered in vivo, which is consistent with the previous results [17, 21].
LFM-A13 attenuates DMBA-induced mammary tumorigenesis in mice
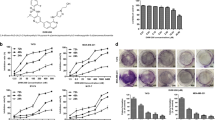
We next wanted to examine the effect of LFM-A13 as well as paclitaxel on the survival of mice with mammary cancer induced by DMBA. We found that while the survival rate is 15% in cancerous control group (DMBA), it is increased to 40% in the LFM-A13-treated group and 45% in the paclitaxel-treated group at the final day of the experiment (Fig. 1). Strikingly, the administration of both LFM-A13 and paclitaxel (LFM-A13 + P) further improved the survival rate to 50% (Fig. 1).
Percentage of survival in DMBA, LFM-A13, P, and LFM-A13 + P groups. DMBA, mice were treated with DMBA; DMBA + LFM-A13, mice were treated with LFM-A13 (50 mg/kg, three times a week, i.p.) following DMBA administration; DMBA + P, mice were treated with paclitaxel (10 mg/kg, once a week, i.p.) following DMBA administration; DMBA + LFM-A13 + P, mice were treated with combination of LFM-A13 and paclitaxel following DMBA administration. All animals were sacrificed 25 weeks following DMBA exposure
As expected, the tumor incidence in DMBA group was 100% at the end of the experimental period (Table 1) (X2 = 29.25, p < 0.0001). However, the incidence of mammary tumors caused by DMBA administration in mice, including those from animals that died or were killed during the experiment, were significantly decreased by the treatment of LFM-A13 and paclitaxel (Table 1). Administration of LFM-A13, paclitaxel, and combination of LFM-A13 and paclitaxel reduced the tumor incidence by 25%, 22%, and 30% respectively (Table 1). No tumors were detected in the control group that did not receive DMBA. Moreover, the mean number of tumors in the groups treated with LFM-A13, paclitaxel, and the combination of LFM-A13 and paclitaxel was significantly decreased by 62%, 59% and 70% compared to the DMBA group (p < 0.02; Table 1). The average weight of tumors was 2.3 g in DMBA group, 0.81 g in the LFM-A13-treated group, 1.04 g in the paclitaxel-treated group, and 0.45 g LFM-A13 + paclitaxel-treated group (p < 0.05, Table 1 and Fig. 2). The cumulative tumor size was also evaluated and found to be smaller in the treatment groups compared to the cancerous control group that was treated with DMBA only (Table 1 and Fig. 2). Interestingly, the smallest tumor size was found in the group that was treated with a combination of LFM-A13 and paclitaxel (p < 0.05, Table 1 and Fig. 2). Collectively, these data indicate that LFM-A13 attenuates DMBA-induced mammary tumorigenesis in mice, and its effect is more prominent when it is administered with paclitaxel.
Chemoprevention of DMBA-experimental mouse mammary carcinogenesis by LFM-A13. DMBA, mice were treated with DMBA; DMBA + LFM-A13, mice were treated with LFM-A13 (50 mg/kg, three times a week, i.p.) following DMBA administration; DMBA + P, mice were treated with paclitaxel (10 mg/kg, once a week, i.p.) following DMBA administration; DMBA + LFM-A13 + P, mice were treated with combination of LFM-A13 and paclitaxel following DMBA administration. All animals were sacrificed 25 weeks following DMBA exposure
Modulation of cell survival and cell cycle regulators by LFM-A13
In order to understand molecular mechanisms of the preventive effect of LFM-A13 on DMBA-induced breast cancer, mammary gland tumors were analyzed for the expression of PLK1, cell cycle-regulating proteins cyclin D1, cyclin dependent kinase-4 (CDK-4), and the CDK inhibitor p21 by western blotting. Administration of DMBA significantly elevated expression of PLK1 (Fig. 3a, f), cyclin D1 (Fig. 3b, f), and CDK-4 (Fig. 3c, f) compared to control. The treatment of LFM-A13 and paclitaxel or their combination to DMBA-treated animals significantly decreased PLK1, cyclin D1, and CDK-4 expression (Fig. 3). Conversely, LFM-A13 administration resulted in an increase in the expression of p21 (Fig. 3d, f), as well as IκB expression (Fig. 3e, f). Strikingly, a combination of LFM-A13 and paclitaxel was found to be more effective than any agent alone, whereas both LFM-A13 and paclitaxel had a similar effect on the expression of these proteins (Fig. 3).
Effect of LFM-A13 and paclitaxel administration on the expressions of PLK-1 (a), Cyclin D1 (b), CDK-4 (c), P21 (d) and IκB (e). DMBA, mice were treated with DMBA; DMBA + LFM-A13, mice were treated with LFM-A13 (50 mg/kg, three times a week, i.p.) following DMBA administration; DMBA + P, mice were treated with paclitaxel (10 mg/kg, once a week, i.p.) following DMBA administration; DMBA + LFM-A13 + P, mice were treated with combination of LFM-A13 and paclitaxel following DMBA administration. The relative amount of each protein was quantified by ImajeJ using β-actin as an internal control. Results are expressed as percent of control. The bar represents the standard error of the mean. Blots were repeated at least 4 times (n = 4), and a representative blot is shown (f). Actin was included to ensure equal protein loading. Small alphabet on top of each bar indicates significant difference; p < 0.05 by Fisher’s multiple comparison test
LFM-A13 treatment regulates expression of apoptosis-related proteins
We next examined whether LFM-A13 modulates the expression levels of different proteins in mammary tumors that are involved in regulation of apoptosis, such as P53 (tumor suppressor), Bcl-2 (anti-apoptotic protein), Bax (apoptotic activator), and Caspase 3. As shown in Fig. 4, administration of DMBA alone led to an increase in the expression of P53 and Bcl-2, and a decrease in the expression of Bax and Caspase 3. However, LFM-A13 and paclitaxel treatment caused a significant decrease in P53 expression (Fig. 4a, e) as well as Bcl-2 (Fig. 4b, e). There also was a concomitant increase in the expression of Bax (Fig. 4c, e) and caspase 3 (Fig. 4d, e). Similar to the findings presented above, the combination of LFM-A13 and paclitaxel was more effective than either treatment alone in the regulation of proteins that are involved in apoptosis (Fig. 4).
Effect of LFM-A13 and paclitaxel administration on the expressions P53 (a), Bcl-2 (b), Bax (c) and caspase 3 (d). DMBA, mice were treated with DMBA; DMBA + LFM-A13, mice were treated with LFM-A13 (50 mg/kg, three times a week, i.p.) following DMBA administration; DMBA + P, mice were treated with paclitaxel (10 mg/kg, once a week, i.p.) following DMBA administration; DMBA + LFM-A13 + P, mice were treated with combination of LFM-A13 and paclitaxel following DMBA administration. The relative amount of each protein was quantified by ImajeJ using β-actin as an internal control. Results are expressed as percent of control. The bar represents the standard error of the mean. Blots were repeated at least 4 times (n = 4), and a representative blot is shown (e). Actin was included to ensure equal protein loading. Small alphabet on top of each bar indicates significant difference; p < 0.05 by Fisher’s multiple comparison test
Discussion
This study highlights that LFM-A13 possesses a potent activity to ameliorate DMBA-induced mammary carcinogenesis in a mouse model, and its mechanistic effect is through the inhibition of cell proliferation and promotion of apoptosis in cancer cells.
Uncontrolled cellular proliferation resulting from changes in the expression of proteins involved in the cell cycle is linked to the development and progression of many types of cancer. For this reason, it is critical to develop drugs that will prevent the efficacy of these proteins for the targeted treatment of different types of cancer. LFM-A13 was developed as a specific inhibitor for both PLKs [17] and BTKs [20]. It has been well demonstrated that LFM-A13 blocks the proliferative activity of cells in human breast cancer cells and glioblastoma cells as well as in a zebrafish embryo model [17]. The tumor progression was also delayed by the use of LFM-A13 in the MMTV/Neu transgenic mouse model of HER2 positive breast cancer [17], and it was further delayed when LFM-A13 was used in combination with paclitaxel [18]. Similarly, the present study also demonstrated an anti-proliferative effect of LFM-A13 on the development of DMBA-induced mammary tumorigenesis in mice with being more effective together with paclitaxel. We showed mechanistically that these drugs impact on the expression of proteins that control cellular proliferation and survival of cancer cells.
Because PLK1, the best-characterized member of the PLK family, has a non-redundant role in the cell cycle, and dysfunction of PLK1 may contribute to the development and progression of various cancers [11, 26]. Consistent with this notion, over-expression of PLK1 has been shown in many types of human cancer including lung, melanoma, renal cancer and hepatocellular carcinoma [26]. The elevated PLK1 expression has also been reported in human breast cancer [27,28,29,30]. In agreement with this, we showed that PLK1 expression is increased upon DMBA administration in mice. Importantly, our results demonstrated that the increased level of PLK1 expression could be dampened by the use of LFM-A13 alone or with paclitaxel. This suggests that inhibition of PLK1 through LFM-A13 contributes to the amelioration of mammary tumorigenesis in mice.
Cyclin D1 is a member of the highly-conserved cyclin proteins that function as controllers in the cell cycle by activating cyclin-dependent kinases (CDKs). Cyclin D1 may form a complex with CDK4 and CDK6 to intiate a signaling cascade that directs the cell cycle progression. Overexpression of cyclin D1 has been observed in many types of human cancer [31]. Despite the importance of cyclin D1 in tumorigenesis, the potent inhibitor of cyclin D1 has not yet been discovered. The control of cyclin D1 is generally being through the inhibition of its associated kinases CDK4/CDK6 [31]. In addition to its role in the control of PLK1 expression, we observed that LFM-A13 was able to decrease the DMBA-induced expression of cyclin D1 and CDK4, and there was a corresponding increase in expression of p21, a cell cycle inhibitor [32, 33]. Moreover, the expression of IκB, an inhibitory protein that regulates NF-κB activity [34], was also increased upon LFM-A13 and paclitaxel supplementation. Thus, a reduction in PLK1 and cyclin D1 expression and a corresponding increase in p21 and IκB expression possibly contribute to the suppression of mammary carcinogenesis in mice.
One of the aims for cancer treatment is to develop drugs for inhibition of aberrantly-expressed proteins in order to sensitize cancer cells to apoptosis. Small inhibitory molecules of PLKs have emerged as to hinder the proliferation and survival of various cancer types [7, 35]. Supporting this notion is the observation that an inhibition of the cell proliferation and an induction of apoptosis were observed in HeLa cells that lack PLK1 expression [8]. Similarly, LFM-A13 has been reported to induce apoptosis in multiple myeloma cells [36]. Moreover, BI2536, another PLK inhibitor, has been shown to increase the efficacy of paclitaxel in breast cancer cells through the promotion of apoptosis [37]. The authors showed an increase in the expression of Bax and cleaved caspase 9 and a decrease in Bcl-2 expression in breast cancer cells upon administration of the PLK inhibitor [37]. Similarly, we found here that LFM-A13 inhibited proliferation of cancer cells through the induction of apoptosis upon drug administration as evident by the observation of increased detection of apoptotic proteins, activated caspase 3 and Bax, and of decreased detection of Bcl-2. Thus, LFM-A13 has an ability to sensitize cancer cells to apoptosis.
In summary, our findings reveal that the inhibitor of PLKs, LFM-A13, attenuates DMBA-induced mammary tumorigenesis in mice through regulation of multiple factors that are involved in cell cycle, survival and apoptosis. Importantly, LFM-A13 had no damage to cells of the immune system and other tissues, and its effect was found to be more effective when it is administered together with paclitaxel. Our results suggest that inhibition of PLK activity by LFM-A13 could be a beneficial approach for the treatment of breast cancer.
References
Siegel RL, Miller KD, Jemal A (2017) Cancer statistics, 2017. CA Cancer J Clin 67(1):7–30. https://doi.org/10.3322/caac.21387
Shulman LN, Willett W, Sievers A, Knaul FM (2010) Breast cancer in developing countries: opportunities for improved survival. J Oncol 2010:595167. https://doi.org/10.1155/2010/595167
Vanderpuye V, Grover S, Hammad N, PoojaPrabhakar SH, Olopade F, Stefan DC (2017) An update on the management of breast cancer in Africa. Infect Agent Cancer 12:13. https://doi.org/10.1186/s13027-017-0124-y
Kumaraguruparan R, Seshagiri PB, Hara Y, Nagini S (2007) Chemoprevention of rat mammary carcinogenesis by black tea polyphenols: modulation of xenobiotic-metabolizing enzymes, oxidative stress, cell proliferation, apoptosis, and angiogenesis. Mol Carcinog 46(9):797–806. https://doi.org/10.1002/mc.20309
Donaldson MM, Tavares AA, Hagan IM, Nigg EA, Glover DM (2001) The mitotic roles of polo-like kinase. J Cell Sci 114(Pt 13):2357–2358
Archambault V, Glover DM (2009) Polo-like kinases: conservation and divergence in their functions and regulation. Nat Rev Mol Cell Biol 10(4):265–275. https://doi.org/10.1038/nrm2653
Lee SY, Jang C, Lee KA (2014) Polo-like kinases (plks), a key regulator of cell cycle and new potential target for cancer therapy. Dev Reprod 18(1):65–71. 10.12717/DR.2014.18.1.065
Liu X, Erikson RL (2003) Polo-like kinase (Plk)1 depletion induces apoptosis in cancer cells. Proc Natl Acad Sci U S A 100(10):5789–5794. https://doi.org/10.1073/pnas.1031523100
de Carcer G, Manning G, Malumbres M (2011) From Plk1 to Plk5: functional evolution of polo-like kinases. Cell Cycle 10(14):2255–2262. https://doi.org/10.4161/cc.10.14.16494
Toyoshima-Morimoto F, Taniguchi E, Nishida E (2002) Plk1 promotes nuclear translocation of human Cdc25C during prophase. EMBO Rep 3(4):341–348. https://doi.org/10.1093/embo-reports/kvf069
Takai N, Hamanaka R, Yoshimatsu J, Miyakawa I (2005) Polo-like kinases (Plks) and cancer. Oncogene 24(2):287–291. https://doi.org/10.1038/sj.onc.1208272
Conn CW, Hennigan RF, Dai W, Sanchez Y, Stambrook PJ (2000) Incomplete cytokinesis and induction of apoptosis by overexpression of the mammalian polo-like kinase, Plk3. Cancer Res 60(24):6826–6831
Wang Q, Xie S, Chen J, Fukasawa K, Naik U, Traganos F, Darzynkiewicz Z, Jhanwar-Uniyal M, Dai W (2002) Cell cycle arrest and apoptosis induced by human polo-like kinase 3 is mediated through perturbation of microtubule integrity. Mol Cell Biol 22(10):3450–3459
Li B, Ouyang B, Pan H, Reissmann PT, Slamon DJ, Arceci R, Lu L, Dai W (1996) Prk, a cytokine-inducible human protein serine/threonine kinase whose expression appears to be down-regulated in lung carcinomas. J Biol Chem 271(32):19402–19408
Dai W, Li Y, Ouyang B, Pan H, Reissmann P, Li J, Wiest J, Stambrook P, Gluckman JL, Noffsinger A, Bejarano P (2000) PRK, a cell cycle gene localized to 8p21, is downregulated in head and neck cancer. Genes Chromosom Cancer 27(3):332–336
Dai W, Liu T, Wang Q, Rao CV, Reddy BS (2002) Down-regulation of PLK3 gene expression by types and amount of dietary fat in rat colon tumors. Int J Oncol 20(1):121–126
Uckun FM, Dibirdik I, Qazi S, Vassilev A, Ma H, Mao C, Benyumov A, Emami KH (2007) Anti-breast cancer activity of LFM-A13, a potent inhibitor of polo-like kinase (PLK). Bioorg Med Chem 15(2):800–814. https://doi.org/10.1016/j.bmc.2006.10.050
Uckun FM (2007) Chemosensitizing anti-cancer activity of LFM-A13, a leflunomide metabolite analog targeting polo-like kinases. Cell Cycle 6(24):3021–3026. https://doi.org/10.4161/cc.6.24.5096
Rudolph D, Steegmaier M, Hoffmann M, Grauert M, Baum A, Quant J, Haslinger C, Garin-Chesa P, Adolf GR (2009) BI 6727, a polo-like kinase inhibitor with improved pharmacokinetic profile and broad antitumor activity. Clin Cancer Res 15(9):3094–3102. https://doi.org/10.1158/1078-0432.CCR-08-2445
Mahajan S, Ghosh S, Sudbeck EA, Zheng Y, Downs S, Hupke M, Uckun FM (1999) Rational design and synthesis of a novel anti-leukemic agent targeting Bruton's tyrosine kinase (BTK), LFM-A13 [alpha-cyano-beta-hydroxy-beta-methyl-N-(2, 5-dibromophenyl)propenamide]. J Biol Chem 274(14):9587–9599
Uckun FM, Zheng Y, Cetkovic-Cvrlje M, Vassilev A, Lisowski E, Waurzyniak B, Chen H, Carpenter R, Chen CL (2002) In vivo pharmacokinetic features, toxicity profile, and chemosensitizing activity of alpha-cyano-beta-hydroxy-beta- methyl-N-(2,5-dibromophenyl)propenamide (LFM-A13), a novel antileukemic agent targeting Bruton's tyrosine kinase. Clin Cancer Res 8(5):1224–1233
Fukui M, Yamabe N, Zhu BT (2010) Resveratrol attenuates the anticancer efficacy of paclitaxel in human breast cancer cells in vitro and in vivo. Eur J Cancer 46(10):1882–1891. https://doi.org/10.1016/j.ejca.2010.02.004
Hua F, Li K, JJ Y, Lv XX, Yan J, Zhang XW, Sun W, Lin H, Shang S, Wang F, Cui B, Mu R, Huang B, Jiang JD, ZW H (2015) TRB3 links insulin/IGF to tumour promotion by interacting with p62 and impeding autophagic/proteasomal degradations. Nat Commun 6:7951. https://doi.org/10.1038/ncomms8951
Sahin K, Tuzcu M, Sahin N, Akdemir F, Ozercan I, Bayraktar S, Kucuk O (2011) Inhibitory effects of combination of lycopene and genistein on 7,12- dimethyl benz(a)anthracene-induced breast cancer in rats. Nutr Cancer 63(8):1279–1286. https://doi.org/10.1080/01635581.2011.606955
Tuzcu M, Aslan A, Tuzcu Z, Yabas M, Bahcecioglu IH, Ozercan IH, Kucuk O, Sahin K (2012) Tomato powder impedes the development of azoxymethane-induced colorectal cancer in rats through suppression of COX-2 expression via NF-kappaB and regulating Nrf2/HO-1 pathway. Mol Nutr Food Res 56(9):1477–1481. https://doi.org/10.1002/mnfr.201200130
Liu Z, Sun Q, Wang X (2016) PLK1, a potential target for cancer therapy. Transl Oncol 10(1):22–32. https://doi.org/10.1016/j.tranon.2016.10.003
Wolf G, Hildenbrand R, Schwar C, Grobholz R, Kaufmann M, Stutte HJ, Strebhardt K, Bleyl U (2000) Polo-like kinase: a novel marker of proliferation: correlation with estrogen-receptor expression in human breast cancer. Pathol Res Pract 196(11):753–759. https://doi.org/10.1016/S0344-0338(00)80107-7
Weichert W, Kristiansen G, Winzer KJ, Schmidt M, Gekeler V, Noske A, Muller BM, Niesporek S, Dietel M, Denkert C (2005) Polo-like kinase isoforms in breast cancer: expression patterns and prognostic implications. Virchows Arch 446(4):442–450. https://doi.org/10.1007/s00428-005-1212-8
King SI, Purdie CA, Bray SE, Quinlan PR, Jordan LB, Thompson AM, Meek DW (2012) Immunohistochemical detection of polo-like kinase-1 (PLK1) in primary breast cancer is associated with TP53 mutation and poor clinical outcom. Breast Cancer Res 14(2):R40. https://doi.org/10.1186/bcr3136
Donizy P, Halon A, Surowiak P, Kaczorowski M, Kozyra C, Matkowski R (2016) Augmented expression of polo-like kinase 1 is a strong predictor of shorter cancer-specific overall survival in early stage breast cancer at 15-year follow-up. Oncol Lett 12(3):1667–1674. https://doi.org/10.3892/ol.2016.4890
Musgrove EA, Caldon CE, Barraclough J, Stone A, Sutherland RL (2011) Cyclin D as a therapeutic target in cancer. Nat Rev Cancer 11(8):558–572. https://doi.org/10.1038/nrc3090
Gartel AL, Tyner AL (2002) The role of the cyclin-dependent kinase inhibitor p21 in apoptosis. Mol Cancer Ther 1(8):639–649
Abbas T, Dutta A (2009) p21 in cancer: intricate networks and multiple activities. Nat Rev Cancer 9(6):400–414. https://doi.org/10.1038/nrc2657
Gilmore TD (2006) Introduction to NF-kappaB: players, pathways, perspectives. Oncogene 25(51):6680–6684. https://doi.org/10.1038/sj.onc.1209954
Degenhardt Y, Lampkin T (2010) Targeting polo-like kinase in cancer therapy. Clin Cancer Res 16(2):384–389. https://doi.org/10.1158/1078-0432.CCR-09-1380
Rushworth SA, Bowles KM, Barrera LN, Murray MY, Zaitseva L, MacEwan DJ (2013) BTK inhibitor ibrutinib is cytotoxic to myeloma and potently enhances bortezomib and lenalidomide activities through NF-kappaB. Cell Signal 25(1):106–112. https://doi.org/10.1016/j.cellsig.2012.09.008
Prashanth Kumar BN, Rajput S, Bharti R, Parida S, Mandal M (2015) BI2536--a PLK inhibitor augments paclitaxel efficacy in suppressing tamoxifen induced senescence and resistance in breast cancer cells. Biomed Pharmacother 74:124–132. https://doi.org/10.1016/j.biopha.2015.07.005
Acknowledgements
The authors thank Prof. Fatih Uckun (USC-USA) for kindly providing LFM-A13, and Prof. Gerard F. Hoyne (The University of Notre Dame Australia) for carefully reading and correcting grammar of the manuscript. The work was supported in part by the Turkish Academy of Sciences.
Funding
The work was supported in part by the Turkish Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
All authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Informed consent
For this type of study, formal consent is not required.
Electronic supplementary material
ESM 1
(PDF 85 kb)
Rights and permissions
About this article
Cite this article
Sahin, K., Tuzcu, M., Yabas, M. et al. LFM-A13, a potent inhibitor of polo-like kinase, inhibits breast carcinogenesis by suppressing proliferation activity and inducing apoptosis in breast tumors of mice. Invest New Drugs 36, 388–395 (2018). https://doi.org/10.1007/s10637-017-0540-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-017-0540-2